Abstract
Background
Participant‐reported health status is a key indicator of cardiovascular health, but its predictive value relative to traditional and nontraditional risk factors is unknown. We evaluated whether participant‐reported health status, as indexed by self‐rated health, predicted cardiovascular disease, and all‐cause mortality risk excess of 10‐year atherosclerotic cardiovascular disease (ASCVD) risk scores and 5 nontraditional risk biomarkers.
Methods and Results
Analyses used prospective observational data from the 1999–2002 National Health and Nutrition Examination Surveys among those aged 40 to 79 years (N=4677). Vital status was ascertained through 2011, during which there were 850 deaths, 206 from cardiovascular disease (CVD). We regressed CVD and all‐cause mortality on standardized values of self‐rated health in survival models, adjusting for age, sex, education, existing chronic disease, race/ethnicity, ASCVD risk, and standardized biomarkers (fibrinogen, C‐reactive protein [CRP], triglycerides, albumin, and uric acid). In sociodemographically adjusted models, a 1‐SD decrease in self‐rated health was associated with increased risk of CVD mortality (hazard ratio [HR], 1.92; 95% CI, 1.51–2.45; P<0.001), and this hazard remained strong after adjusting for ASCVD risk and nontraditional biomarkers (HR, 1.79; 95% CI, 1.42–2.26; P<0.001). Self‐rated health also predicted all‐cause mortality even after adjustment for ASCVD risk and nontraditional biomarkers (HR, 1.50; 95% CI, 1.35–1.66; P<0.001).
Conclusions
Self‐rated health provides prognostic information beyond that captured by traditional ASCVD risk assessments and by nontraditional CVD biomarkers. Consideration of self‐rated health in combination with traditional risk factors may facilitate risk assessment and clinical care.
Keywords: biological markers, cardiovascular disease risk factors, epidemiology, health policy and outcomes research, mortality, patient reported outcomes, quality of life
Subject Categories: Cardiovascular Disease, Quality and Outcomes, Epidemiology, Risk Factors, Mortality/Survival
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the United States.1 As a complement to traditional CVD risk factors, such as smoking status, hypertension, diabetes mellitus, and cholesterol, current national guidelines recommend assessment of participant‐reported health status.2 Participant or self‐reported health status is considered a key indicator of cardiovascular health,2 predicting morbidity,3, 4 and mortality,5, 6 above and beyond traditional and nontraditional risk factors.7, 8, 9 In addition to predicting mortality risk among healthly individuals, self‐reported health status remains a robust predictor of cardiovascular mortality within diseased populations.10 In 2014, CVD risk formulations were updated to more optimally model the impact of multiple risk factors, now known as the 10‐year atherosclerotic cardiovascular disease (ASCVD) risk calculation. Importantly, this model's risk prediction is not duplicated by, or achievable through, consideration of traditional risk factors individually.11 No studies to date have evaluated the prognostic value of self‐reported health status relative to current CVD risk formulations.11 Comparisons of self‐reported health status with nontraditional risk factors are also sparse. Hence, the prognostic value of self‐reported health over optimized consideration of traditional and nontraditional risk factors together is unknown.
Our objective was to evaluate the prognostic value of participant‐reported health status, as indexed by self‐rated health, for risk of incident cardiovascular and all‐cause mortality after controlling for 10‐year ASCVD risk. We also examined this association after further adjustment for 5 nontraditional CVD risk factors (fibrinogen, C‐reactive protein [CRP], triglycerides, albumin, and uric acid).12 We evaluated these associations in a probability sample of US residents aged 40 to 79 years (N=4677) followed for 9 to 12 years.
Methods
Data Source
We analyzed data from the combined 1999–2002 National Health and Nutrition Examination Surveys (NHANES), a representative sample of US residents aged 1 month to 85 years and older.13, 14 Participants were interviewed in their homes and a subset completed a physical examination in a mobile examination center. Among all participants in the 1999–2002 survey years, 83% of those screened participated in the interview and 93% of interviewed participants were examined.15 Examined participants are weighted to represent the civilian noninstitutionalized US population.15 Current CVD risk assessment formulae are relevant to adults aged 40 to 79 years of age, so we restricted our analyses to this age range (N=5701). Participants missing data on ASCVD risk variables, self‐rated health, education (coded less than high school, high school diploma, some college, or college graduate or higher) and the other biomarkers reduced the analytic sample to 4677 (82% of those aged 40–79 who were examined; Figure 1). All participants provided informed consent, and the study was approved by the National Center for Health Statistics (NCHS) Ethical Review Board.
Figure 1.
Participant selection flow diagram, 1999–2001 National Health and Nutrition Examination Surveys. ASCVD indicates atherosclerotic cardiovascular disease.
Vital Status
Vital status on all participants in this analysis was ascertained through December 31, 2011 using the National Death Index.16 Specific causes of death were classified using the 10th revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death and these categories were collapsed into the 10 leading causes of death in the public use file. Cardiovascular deaths included combined deaths from diseases of heart (I00–I09, I11, I13, I20–I51) and cerebrovascular diseases (I60–I69).
Risk Factors
Traditional CVD risk factors
Blood pressure was measured up to 4 times by trained personnel using a mercury sphygmomanometer. Blood pressure was defined by the average of values excluding the first reading unless there was only 1 measurement, in which case the single reading was used as the average. Diabetes mellitus was defined by glucose ≥126 mg/dL or taking insulin.17 Current smoking status was determined by affirmative answers to both “have you ever smoked at least 100 cigarettes in your life?” and “do you currently smoke?” Total cholesterol and high‐density lipoprotein (HDL) were assessed at the Johns Hopkins University Lipoprotein Analytical Laboratory (Baltimore, MD) using standard reference methods.18, 19
ASCVD risk calculation
Ten‐year risk for an ASCVD event was derived for each participant following described methods11, 20 and specifically used sex‐ and race‐specific regression coefficients for age, treated or untreated systolic blood pressure, total and low‐density lipoprotein (LDL) cholesterol, current smoking (yes/no), and history of diabetes mellitus (yes/no) to calculate ASCVD risk. Our purpose in calculating ASCVD risk scores was to optimally partition health risk among our participants rather than to make treatment decisions. Therefore, we included ASCVD risk calculations risk for the few participants with systolic blood pressure higher than 200 mm Hg (N=27) and with LDL ≥190 mg/dL (N=94); these values are precluded from online risk calculators. Similarly, we calculated ASCVD risk for those reporting a past diagnosis of stroke, heart attack, or other coronary heart disease.
Nontraditional risk biomarkers
NHANES includes a number of laboratory assays. We selected CRP, fibrinogen, urinary albumin, triglycerides, and uric acid because they represent 5 of the most studied nontraditional CVD risk factors.12 Quantitative CRP (sensitive to 0.2 mg/dL) was assessed with latex enhanced nephlometry using a Behring Nephelometer. Fibrinogen concentration was assayed using a STA‐Compact (Diagnostica Stago, Inc., Parsippany, NJ).21, 22 Albumin, triglycerides, and uric acid concentrations were determined with a Hitachi Model 704 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN).23, 24 NHANES laboratory staff complete comprehensive laboratory procedure training and formal retraining is conducted annually. Examination protocol fidelity and quality assurance is monitored regularly during unscheduled evaluations by NCHS staff and contractors.23
Diagnosed Chronic Diseases
Participants were asked whether a doctor or other health professional ever told them they had had a heart attack, a stroke, coronary heart disease, congestive heart failure, or cancer. Diabetes mellitus history is captured in the ASCVD risk calculation.
Participant Reported Outcome
Self‐rated health was assessed by the question, “Would you say your health in general is excellent, very good, good, fair or poor?” This global rating reflects subjective and objective perceptions of mental and physical disease burden25 and is shaped by individual and social characteristics.26, 27 We chose this item because of its importance in population health surveillance28 (http://www.cdc.gov/hrqol/overview.htm) and because it complements other assessments, such as the medical history and laboratory tests.2 This single‐item assessment has comparable predictive validity for mortality irrespective of wording and when compared to more‐complex multi‐item assessments.6, 29 We did not evaluate change in health status over the last year or subjective health questions that were not assessed across both survey cycles.
Statistical Analysis
Self‐rated health (in 5 categories) and biomarkers were standardized within the analytical sample to provide comparable unit scaling for comparison.30 CRP was log transformed before standardizing. Thus, unless otherwise noted, all reported hazard ratios reflect a 1‐SD change in the predictor. We used Cox regression to estimate the association between self‐rated health and the 2 mortality outcomes. Participants who died from non‐CVD causes (N=644) were excluded from CVD mortality analyses. We used participants’ attained age as the time scale to correctly specify age‐dependent mortality risk31 and also stratified on 5‐year birth cohorts to adjust for study entry age.32 Models including education and sex as covariates failed to meet proportional hazards assumptions. Therefore, education and sex were modeled as stratification variables, an approach that permits adjustment for these characteristics without explicitly modeling their hazard functions.33, 34 We further examined model specification by evaluating squared predicted scores generated from fully adjusted hazard models. These squared terms were not statistically significant, providing additional evidence of adequately specified regression models.35
To evaluate the prognostic value of self‐rated health, we entered self‐rated health in survival models that cumulatively adjusted for (1) race/ethnicity and diagnosed chronic diseases (summed and categorized into 0, 1, 2, or more), (2) ASCVD risk, and (3) the 5 nontraditional CVD biomarkers. We also report parallel analyses for each of the 5 biomarkers (entering self‐rated health in the third model) to compare the prognostic strength of each to self‐rated health. Finally, we report sensitivity analyses for self‐rated health restricting the sample to participants free of baseline CVD as well as comparisons of categorical self‐rated health.
We used StataMP software (version 13.1; Stata Corp LP, College Station, TX) for all estimates and incorporated NHANES clustering, stratification, and 4‐year exam weights.15 The analytical sample was identified with the subpop option to preserve the integrity of the complex survey design. CIs that did not include 1.0 were considered statistically significant.
Results
Participant characteristics by vital status are presented in Table 1. During follow‐up, there were 850 deaths (18.2%), including 206 (5.1%) from CVD.
Table 1.
Baseline Characteristics of 1999–2002 NHANES Participants
| Characteristic | Total | Alive | Deceased |
|---|---|---|---|
| Participants, n | 4677 | 3827 | 850 |
| Age y, mean (SE) | 55.0 (0.24) | 53.5 (0.22) | 64.3 (0.46) |
| Female sex, % (N) | 52 (2321) | 53 (1982) | 45 (339) |
| Race/ethnicity, % (N) | |||
| Mexican American | 5 (1122) | 5 (933) | 4 (189) |
| Other Hispanic | 6 (215) | 6 (183) | 6 (32) |
| White (non‐Hispanic) | 77 (2347) | 77 (1920) | 77 (427) |
| Black (non‐Hispanic) | 9 (871) | 9 (683) | 11 (188) |
| Other race | 4 (122) | 4 (108) | 2 (14) |
| Education level, % (N) | |||
| <9th grade | 8 (885) | 6 (643) | 15 (242) |
| 9th–11th grade, no diploma | 14 (827) | 13 (638) | 23 (189) |
| High school diploma | 25 (1010) | 25 (838) | 25 (172) |
| Some college | 27 (1070) | 28 (909) | 25 (161) |
| College graduate or higher | 26 (885) | 29 (799) | 12 (86) |
| Diagnosed chronic diseases, % (N) | |||
| Myocardial infarction | 5.0 (270) | 3.7 (152) | 12.6 (118) |
| Other coronary heart disease | 5.1 (260) | 3.7 (152) | 13.3 (108) |
| Congestive heart failure | 3.0 (168) | 1.8 (78) | 10.0 (90) |
| Stroke | 2.9 (174) | 1.9 (92) | 8.8 (82) |
| Cancer | 7.3 (358) | 5.8 (229) | 16.3 (129) |
| Self‐rated health, % (N) | |||
| Poor | 4 (275) | 3 (157) | 12 (118) |
| Fair | 15 (988) | 13 (733) | 27 (255) |
| Good | 30 (1493) | 29 (1217) | 35 (276) |
| Very good | 30 (1160) | 32 (1023) | 19 (137) |
| Excellent | 20 (761) | 22 (697) | 8 (64) |
| Mean (SD) self‐rated health | 3.24 (1.13) | 3.36 (1.11) | 2.73 (1.12) |
| ASCVD risk, % (95% CI)a | 8.9 (8.5–9.4) | 7.5 (7.1–7.9) | 17.8 (17.0–18.7) |
| Fibrinogen, mg/dL (95% CI) | 364 (358–370) | 359 (352–365) | 396 (388–405) |
| CRP mg/dL (95% CI) | 0.45 (0.42–0.49) | 0.42 (0.39–0.46) | 0.63 (0.55–0.71) |
| Triglycerides, mg/dL (95% CI) | 160 (151–168) | 155 (146–164) | 187 (157–218) |
| Albumin, μg/mL (95% CI) | 42.0 (31.8–52.2) | 27.6 (17.6–37.7) | 129.1 (90.1–168.1) |
| Uric acid, mg/dL (95% CI) | 5.43 (5.37–5.50) | 5.37 (5.29–5.44) | 5.84 (5.72–5.97) |
All values except for mean self‐rated health are weighted to represent the civilian noninstitutionalized population ages 40 to 79 years. Some percentages may not sum to 100 because of rounding. ASCVD indicates atherosclerotic cardiovascular disease; CRP, C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
Ten‐year risk based upon national guidelines.11
CVD Mortality
In a stratified model with race/ethnicity and chronic disease covariates, self‐rated health was inversely associated with CVD mortality (Table 2, CVD mortality, model 1). This association persisted after further adjusting for ASCVD risk (Table 2, CVD mortality, model 2) and the 5 nontraditional biomarkers (hazard ratio [HR], 1.79; 95% CI, 1.42–2.26; Figure 2).36 This fully adjusted association was observed when analyzing women (HR, 2.31; 95% CI, 1.71–3.11) and men (HR, 1.62; 95% CI, 1.18–2.23) separately. We repeated the fully adjusted regressions for participants who did not report a previous diagnosis of stroke, heart attack, congestive heart failure, or other coronary heart disease. The inverse association between self‐rated health and CVD mortality risk persisted among participants free of baseline CVD (HR, 1.67; 95% CI, 1.27–2.18; N=3627; 124 CVD deaths). Using self‐rated health categories referenced to those reporting very good or excellent heath, CVD mortality for the full sample was progressively higher for those reporting good (HR, 2.09; 95% CI, 1.15–3.81), fair (HR, 2.72; 95% CI, 1.51–4.90), and poor health (HR, 6.46; 95% CI, 3.09–13.53).
Table 2.
CVD and All‐Cause Mortality Hazard Ratios (95% CI) for Self‐Rated Health and Nontraditional Biomarkers
| Variablea | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| CVD mortality (N=4033) | ||||||
| Self‐rated health | 1.92 | 1.51 to 2.45 | <0.001 | 1.84 | 1.45 to 2.35 | <0.001 |
| Log CRP | 1.22 | 0.97 to 1.52 | 0.086 | 1.17 | 0.92 to 1.50 | 0.189 |
| Urinary albumin | 1.22 | 1.12 to 1.32 | <0.001 | 1.20 | 1.11 to 1.30 | <0.001 |
| Triglycerides | 1.16 | 1.06 to 1.27 | 0.002 | 1.10 | 1.00 to 1.21 | 0.056 |
| Uric acid | 1.10 | 0.84 to 1.44 | 0.475 | 1.08 | 0.84 to 1.39 | 0.522 |
| Fibrinogen | 1.52 | 1.26 to 1.82 | <0.001 | 1.48 | 1.22 to 1.81 | <0.001 |
| All‐cause mortality (N=4677) | ||||||
| Self‐rated health | 1.58 | 1.43 to 1.75 | <0.001 | 1.54 | 1.38 to 1.71 | <0.001 |
| Log CRP | 1.19 | 1.09 to 1.30 | <0.001 | 1.15 | 1.06 to 1.26 | 0.003 |
| Urinary albumin | 1.13 | 1.07 to 1.19 | <0.001 | 1.11 | 1.05 to 1.17 | 0.001 |
| Triglycerides | 1.13 | 1.06 to 1.21 | 0.001 | 1.08 | 1.02 to 1.15 | 0.017 |
| Uric acid | 1.11 | 1.00 to 1.22 | 0.045 | 1.10 | 1.00 to 1.22 | 0.049 |
| Fibrinogen | 1.19 | 1.08 to 1.30 | 0.001 | 1.16 | 1.06 to 1.28 | 0.003 |
Both models stratify on 5‐year age cohort, sex, and education. Model 1 includes race/ethnicity and chronic disease as covariates; model 2 also includes 10‐year atherosclerotic cardiovascular disease risk. Each hazard ratio reflects a 1‐SD change in the predictor. CRP indicates C‐reactive protein; CVD, cardiovascular disease.
Standardized mean=0, standard deviation=1.0.
Figure 2.
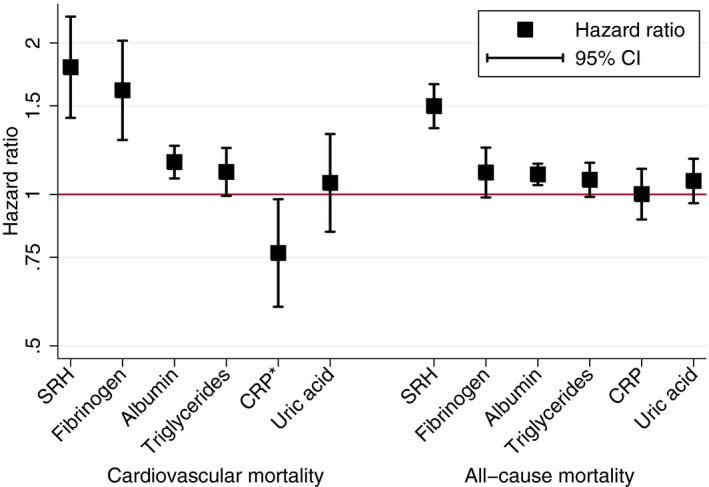
Hazard ratios for cardiovascular and all‐cause mortality by self‐rated health and nontraditional biomarkers, National Health and Nutrition Examination Surveys 1999–2002. Models are simultaneously adjusted for all predictors as well as age, race/ethnicity, diagnosed chronic disease (heart disease, stroke, and cancer), and 10‐year atherosclerotic cardiovascular disease risk. All predictors are standardized to unit variance. *The CRP coefficient for cardiovascular disease (CVD) mortality reverses because fibrinogen is included in this model. CRP and fibrinogen are correlated 0.55 and correlated predictors in simultaneous regression models can cause a reversal of one of the regression coefficients.36 CRP is positively associated with CVD mortality risk when excluding fibrinogen from the model (Table 2). CRP indicates C‐reactive protein; SRH, self‐rated health.
All‐Cause Mortality
Self‐rated health showed a similar inverse association with deaths from all causes. In the stratified model adjusted for race/ethnicity and chronic disease, self‐rated health was inversely associated with mortality (HR, 1.58; 95% CI, 1.43–1.75; N=4677; 850 deaths) and this association persisted after adjustment for ASCVD risk (Table 2, All‐cause mortality, model 2) and when further adjusting for the 5 nontraditional biomarkers (HR, 1.50; 95% CI, 1.35–1.66; Figure 2). This fully adjusted association was observed when analyzing women (HR, 1.59; 95% CI, 1.36–1.87) and men (HR, 1.45; 95% CI, 1.26–1.66) separately.
This inverse association with all‐cause mortality risk was also observed when restricting participants to those free of baseline CVD (HR, 1.56; 95% CI, 1.37–1.77; N=4119; 616 events). Using self‐rated health categories, all‐cause mortality risk increased in a dose‐response manner for those reporting good (HR, 1.51; 95% CI, 1.15–1.98), fair (HR, 2.34; 95% CI, 1.86–2.94), and poor (HR, 3.16; 95% CI, 2.19–4.55) health relative to those with very good/excellent health. Biomarkers and ASCVD risk were strongly stratified by self‐rated health categories (Table 3). Higher self‐rated health was consistently associated with lower ASCVD risk and more‐favorable nontraditional biomarker concentrations.
Table 3.
Nontraditional Biomarkers and ASCVD Risk (95% CI) by Category of Self‐Rated Health, NHANES 1999–2002
| Variablea | Self‐Rated Health (n) | P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Poor (275) | Fair (988) | Good (1493) | Very Good (1160) | Excellent (761) | |||||||
| Fibrinogen | 417 | 399 to 434 | 381 | 373 to 390 | 371 | 364 to 378 | 356 | 349 to 362 | 342 | 331 to 353 | <0.001 |
| CRP | 0.92 | 0.68 to 1.16 | 0.60 | 0.55 to 0.66 | 0.49 | 0.45 to 0.53 | 0.39 | 0.35 to 0.43 | 0.30 | 0.25 to 0.34 | <0.001 |
| Triglycerides | 187 | 164 to 211 | 178 | 165 to 192 | 173 | 156 to 189 | 148 | 135 to 160 | 138 | 123 to 152 | <0.001 |
| Urinary albumin | 206 | 49 to 363 | 76 | 47 to 105 | 41 | 22 to 60 | 21 | 13 to 29 | 16 | 11 to 22 | <0.001 |
| Uric acid | 5.9 | 5.6 to 6.3 | 5.5 | 5.3 to 5.7 | 5.6 | 5.5 to 5.6 | 5.4 | 5.3 to 5.5 | 5.2 | 5.1 to 5.3 | <0.001 |
| ASCVD risk, % | 14 | 13 to 16 | 11 | 10 to 12 | 10 | 9 to 11 | 8 | 7 to 8 | 6 | 6 to 7 | <0.001 |
All estimates incorporate the complex survey design. P values were obtained by regressing each variable on self‐rated health. ASCVD indicates atherosclerotic cardiovascular disease; CRP, C‐reactive protein; NHANES, National Health and Nutrition Examination Survey.
Values are mg/dL except for urinary albumin (μg/mL) and ASCVD risk.
Discussion
We evaluated whether self‐rated health was associated with CVD and all‐cause mortality in a probability sample of US adults aged 40 to 79 years of age. Poor self‐rated health was associated with a 79% greater risk of CVD mortality and a 50% greater risk of all‐cause mortality after statistically controlling for ASCVD risk11 and 5 nontraditional biomarkers.12 Self‐rated health had the strongest association with mortality relative to the nontraditional biomarkers and, in absolute terms, was large in magnitude. Thus, self‐rated health meets several requirements for a prognostic variable—it is statistically associated with mortality, is independent of other established predictors,37, 38 and the magnitude of association is large enough to discount residual confounding.12
This study is the first to show that self‐rated health predicts CVD mortality risk beyond that captured in current CVD risk formulations. We also show that this predictive capability is independent of, and generally larger than, a set of popular nontraditional CVD biomarkers. With the exception of fibrinogen for CVD mortality, mortality hazards for a 1‐SD change in self‐rated health were consistently of larger magnitude than all other biomarkers for both mortality outcomes. Our risk estimates for nontraditional biomarkers are consistent with previous studies12, 39;12, 39 however, the utility of nontraditional biomarkers for improved risk prediction40 remains controversial.39, 41
Unlike laboratory data, self‐rated health does not reflect a biological pathway to mortality risk. Instead, we view self‐rated health as an integrated or synthesized summary of conditions that are on the path.27 That is, the layperson's perspective on their health draws upon a knowledge base that overlaps with, but extends beyond, biomedical assessments.42 Our study provides further support for this view because both 10‐year ASCVD risk and poorer biomarker status robustly worsen in parallel with poorer self‐rated health. However, adjusting for biomarkers did little to weaken the association between self‐rated health and mortality. This pattern has been observed elsewhere7, 9 and challenges the notion that self‐rated health judgments are necessarily informed by—and entirely accounted for by—objective clinical and physiological states.27
No assessment battery perfectly reflects health status and therein lies the advantage of self‐rated health—it empirically captures a wide range of health‐relevant domains beyond any biomarker panel yet available.7, 9, 43 In addition to single baseline assessments, trajectories of self‐rated health predict recurrent events within clinical samples, such as myocardial infarction patients.10 The validity of self‐rated health is further supported by the independence of self‐rated health from extraneous influences, such as transient moods.44 Beyond its utility as a global indicator of health risk and health status, self‐rated health is intrinsically valuable as a gold‐standard indicator of health‐related quality of life.2, 26 Self‐rated health may also improve mortality risk classification vis‐à‐vis ASCVD risk scores, but evaluating this potential requires a much larger number of CVD events than observed here.
Strengths of this study include objective assessment of a number of traditional and nontraditional biomarkers in a large, diverse representative sample followed for over a decade. We adopted recommendations to concurrently evaluate multiple risk factors using standardized scaling for biomarkers.12 This head‐to‐head approach provides a less‐biased determination of which risk factors are most important12 and shows that self‐rated health is among the most potent indicators of survival over 9 to 12 years. This taxonomy of the most potent health indicators can help clinicians identify patients that may benefit from more‐aggressive risk factor management or other clinical interventions. Another possible application is to include self‐rated health in the primary care examination. Poor health evaluations could be used to initiate further discussion of why patients expressing low self‐rated health hold that opinion (ie, family history, other health problems, functional limitations, etc). Such discussions may unveil information unknown to the provider and could lead to altered risk factor interventions. Currently, guidelines for integration of self‐rated health and other patient‐reported outcomes into clinical decision making are poorly developed and represent an important area for future research.45
This study has several limitations. We lacked data on nonfatal CVD outcomes, and we did not examine complementary participant‐reported health domains, such as symptom burden, functional status, health behaviors, and other diagnostic labels (eg, arthritis). These theoretically important46 assessments are associated with self‐rated health47, 48, 49, 50, 51, 52 and may provide a more‐complete picture of CVD burden.2 We included ASCVD risk as a covariate in models beyond the context in which they were developed (ie, extending to persons with existing CVD at baseline and considering nonatherosclerotic causes of death). Nonetheless, survival models with ASCVD risk scores are more likely to accurately reflect risk and are more parsimonious relative to traditional risk factors modeled individually.
In summary, self‐rated health had the strongest associations with CVD mortality risk beyond that captured in current CVD risk formulations and after accounting for traditional and nontraditional CVD risk factors. In addition to the predictive power of self‐rated health for CVD mortality, asking patients about their general health status is exceedingly simple, inexpensive, and safe to measure. These advantages reinforce the value of self‐rated health as a key metric of cardiovascular health2 and align with broader national trends to emphasize patient‐centered approaches to the measurement of health and the delivery of health care.
Sources of Funding
This work was partially funded by NIH T32 # HL082610‐07 (Cribbet).
Disclosures
None. The views expressed are the authors’ and do not represent those of the Centers for Disease Control or the National Center for Health Statistics.
Acknowledgments
We are grateful to the study participants and to Centers for Disease Control and Prevention and the National Center for Health Statistics for providing these data.
(J Am Heart Assoc. 2016;5:e003741 doi: 10.1161/JAHA.116.003741)
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. [DOI] [PubMed] [Google Scholar]
- 3. Barger SD, Muldoon MF. Hypertension labelling was associated with poorer self‐rated health in the Third US National Health and Nutrition Examination Survey. J Hum Hypertens. 2006;20:117–123. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg P, Gueguen A, Schmaus A, Nakache JP, Goldberg M. Longitudinal study of associations between perceived health status and self reported diseases in the French Gazel cohort. J Epidemiol Community Health. 2001;55:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benyamini Y, Idler EL. Community studies reporting association between self‐rated health and mortality: additional studies, 1995 to 1998. Res Aging. 1999;21:392–401. [Google Scholar]
- 6. Idler EL, Benyamini Y. Self‐rated health and mortality: a review of twenty‐seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 7. Haring R, Feng YS, Moock J, Volzke H, Dorr M, Nauck M, Wallaschofski H, Kohlmann T. Self‐perceived quality of life predicts mortality risk better than a multi‐biomarker panel, but the combination of both does best. BMC Med Res Methodol. 2011;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jylha M, Volpato S, Guralnik JM. Self‐rated health showed a graded association with frequently used biomarkers in a large population sample. J Clin Epidemiol. 2006;59:465–471. [DOI] [PubMed] [Google Scholar]
- 9. Lima‐Costa MF, Cesar CC, Chor D, Proietti FA. Self‐rated health compared with objectively measured health status as a tool for mortality risk screening in older adults: 10‐year follow‐up of the Bambui Cohort Study of Aging. Am J Epidemiol. 2012;175:228–235. [DOI] [PubMed] [Google Scholar]
- 10. Benyamini Y, Gerber Y, Molshatzki N, Goldbourt U, Drory Y. Recovery of self‐rated health as a predictor of recurrent ischemic events after first myocardial infarction: a 13‐year follow‐up. Health Psychol. 2014;33:317–325. [DOI] [PubMed] [Google Scholar]
- 11. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 12. Ioannidis JPA, Tzoulaki I. Minimal and null predictive effects for the most popular blood biomarkers of cardiovascular disease. Circ Res. 2012;110:658–662. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . NHANES 1999–2000 Public Data Release. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 14. Centers for Disease Control and Prevention . NHANES 2001–2002 Public Data Release. Hyattsville, MD: National Center for Health Statistics; 2009. [Google Scholar]
- 15. Johnson CL, Paulose‐Ram R, Ogden CL, Carroll MD, Kruszon‐Moran D, Dohrmann SM, Curtin LR. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 16. National Center for Health Statistics . Public‐Use Linked Mortality File, 2015. Hyattsville, MD: National Center for Health Statistics; 2015. [Google Scholar]
- 17. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 2001–2002 Data Documentation, Total Cholesterol and HDL. 2010th ed Hyattsville, MD: National Center for Health Statistics; 2010. [Google Scholar]
- 19. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 1999–2000 Data Documentation, Cholesterol—Total & HDL. 2010th ed Hyattsville, MD: National Center for Health Statistics; 2010. [Google Scholar]
- 20. Barger SD, Cribbet MR, Muldoon MF. Leukocyte telomere length and cardiovascular risk scores for prediction of cardiovascular mortality. Epidemiology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 2001–2002 Data Documentation, C‐Reactive Protein (CRP), Fibrinogen, Bone Alkaline Phosphatase & Urinary N‐Telopeptides (L11_B). 2006th ed Hyattsville, MD: National Center for Health Statistics; 2007. [Google Scholar]
- 22. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 1999–2000 Data Documentation, C‐Reactive Protein (CRP) (LAB11). 2006th ed Hyattsville, MD: National Center for Health Statistics; 2008. [Google Scholar]
- 23. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 1999–2000 Data Documentation, Biochemistry Profile and Hormones (LAB18). 2006th ed Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 24. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey 2001–2002 Data Documentation, Standard Biochemistry Profile (L40_B). 2006th ed Hyattsville, MD: National Center for Health Statistics; 2006. [Google Scholar]
- 25. Tissue T. Another look at self‐rated health among the elderly. J Gerontol. 1972;27:91–94. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention . Measuring Healthy Days: Population Assessment of Health‐Related Quality of Life. Atlanta, GA: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 27. Jylha M. What is self‐rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009;69:307–316. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention . Health‐related quality‐of‐life measures—United States, 1993. Morb Mortal Wkly Rep. 1995;44:195–200. [PubMed] [Google Scholar]
- 29. DeSalvo KB, Fan VS, McDonell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40:1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rao SR, Schoenfeld DA. Survival methods. Circulation. 2007;115:109–113. [DOI] [PubMed] [Google Scholar]
- 31. Cologne J, Hsu WL, Abbott RD, Ohishi W, Grant EJ, Fujiwara S, Cullings HM. Proportional hazards regression in epidemiologic follow‐up studies: an intuitive consideration of primary time scale. Epidemiology. 2012;23:565–573. [DOI] [PubMed] [Google Scholar]
- 32. Pencina MJ, Larson MG, D'Agostino RB. Choice of time scale and its effect on significance of predictors in longitudinal studies. Stat Med. 2007;26:1343–1359. [DOI] [PubMed] [Google Scholar]
- 33. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 34. Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time‐to‐Event Data. 2nd ed Hoboken, NJ: Wiley; 2008. [Google Scholar]
- 35. Cleves M, Gutierrez RG, Gould W, Marchenko YV. An Introduction to Survival Analysis Using Stata. 3rd ed College Station, TX: Stata Press; 2010. [Google Scholar]
- 36. Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analyses for the Behavioral Sciences. 3rd ed Mawah, NJ: Lawrence Erlbaum; 2003. [Google Scholar]
- 37. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 39. Tzoulaki I, Siontis KC, Evangelou E, Ioannidis JP. Bias in associations of emerging biomarkers with cardiovascular disease. JAMA Intern Med. 2013;173:664–671. [DOI] [PubMed] [Google Scholar]
- 40. Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC Jr, Psaty BM, Greenland P, Herrington DM. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nissen SE. Biomarkers in cardiovascular medicine: the shame of publication bias. JAMA Intern Med. 2013;173:671–672. [DOI] [PubMed] [Google Scholar]
- 42. Idler EL. Self‐assessments of health: the next stage of studies. Res Aging. 1999;21:387–391. [Google Scholar]
- 43. Robinson‐Cohen C, Hall YN, Katz R, Rivara MB, de Boer IH, Kestenbaum BR, Himmelfarb J. Self‐rated health and adverse events in CKD. Clin J Am Soc Nephrol. 2014;9:2044–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barger SD, Burke SM, Limbert MJ. Do induced moods really influence health perceptions? Health Psychol. 2007;26:85–95. [DOI] [PubMed] [Google Scholar]
- 45. Bradley SM. The routine clinical capture of patient‐reported outcomes: how competition on value will lead to change. Circ Cardiovasc Qual Outcomes. 2014;7:635–636. [DOI] [PubMed] [Google Scholar]
- 46. Wilson IB, Cleary PD. Linking clinical variables with health‐related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 47. Barger SD. Do psychological characteristics explain socioeconomic stratification of self‐rated health? J Health Psychol. 2006;11:21–35. [DOI] [PubMed] [Google Scholar]
- 48. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self‐assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55:P107–P116. [DOI] [PubMed] [Google Scholar]
- 49. Benyamini Y, Leventhal EA, Leventhal H. Self‐assessments of health: what do people know that predicts their mortality? Res Aging. 1999;21:477–500. [Google Scholar]
- 50. Gonzalez JS, Chapman GB, Leventhal H. Gender differences in the factors that affect self‐assessments of health. J Appl Biobehav Res. 2002;7:133–155. [Google Scholar]
- 51. Krause NM, Jay GM. What do global self‐rated health items measure? Med Care. 1994;32:930–942. [DOI] [PubMed] [Google Scholar]
- 52. Mora PA, DiBonaventura MD, Idler E, Leventhal EA, Leventhal H. Psychological factors influencing self‐assessments of health: toward an understanding of the mechanisms underlying how people rate their own health. Ann Behav Med. 2008;36:292–303. [DOI] [PubMed] [Google Scholar]


