Abstract
Background
The left atrial posterior wall (PW) often contains sites required for maintenance of atrial fibrillation (AF). Electrical isolation of the PW is an important feature of all open surgeries for AF. This study assessed the ability of current ablation techniques to achieve PW isolation (PWI) and its effect on recurrent AF.
Methods and Results
Fifty‐seven consecutive patients with persistent or high‐burden paroxysmal AF underwent catheter ablation, which was performed using an endocardial‐only (30) or a hybrid endocardial–epicardial procedure (27). The catheter ablation lesion set included pulmonary vein antral isolation and a box lesion on the PW (roof and posterior lines). Success in creating the box lesion was assessed as electrical silence of the PW (voltage <0.1 mV) and exit block in the PW with electrical capture. Cox proportional hazards models were used for analysis of AF recurrence. PWI was achieved in 21 patients (36.8%), more often in patients undergoing hybrid ablation than endocardial ablation alone (51.9% versus 23.3%, P=0.05). Twelve patients underwent redo ablation. Five of 12 had a successful procedural PWI, but all had PW reconnection at the redo procedure. Over a median follow‐up of 302 days, 56.1% of the patients were free of atrial arrhythmias. No parameter including procedural PWI was a statistically significant predictor of recurrent atrial arrhythmias.
Conclusions
PWI during catheter ablation for AF is difficult to achieve, especially with endocardial ablation alone. Procedural achievement of PWI in this group of patients was not associated with a reduction in recurrent atrial arrhythmias, but reconnection of the PW was common.
Keywords: atrial fibrillation, catheter ablation, hybrid ablation, posterior wall box, posterior wall isolation
Subject Categories: Arrhythmias, Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator
Introduction
Isolation of the left atrial (LA) posterior wall (PW) as a strategy for management of complex atrial fibrillation (AF), in addition to standard pulmonary vein antral isolation, has been investigated with variable results.1, 2, 3, 4, 5, 6 LA PW isolation (PWI) is attempted with the intent of eliminating triggers and drivers often found on the PW and with the goal of debulking the atrial tissue. LA PWI has been part of the surgical Maze procedure,7, 8 hybrid endocardial–epicardial ablation, and endocardial‐only ablation of AF.9, 10, 11, 12, 13 It has yielded good results in open surgical procedures, but results from previous endocardial‐only ablation studies are conflicting.1, 4
LA PWI is challenging to achieve with the currently available endocardial radiofrequency catheter ablation procedure because of the need for long linear lesions with conduction block across the LA roof and lower PW. Creating a lower PW linear lesion without a gap is particularly challenging because ablation over the esophagus is frequently required. Moreover, durability of LA PWI is not guaranteed by procedural success with current ablation technology. In this paper, we presented our experience with PWI and its efficacy as a strategy for management of patients with complex AF substrates, using endocardial‐only or hybrid surgical epicardial and endocardial ablation procedures.
Methods
Patient Population
Consecutive patients with persistent or high‐burden paroxysmal AF underwent ablation with a catheter‐based endocardial‐only or hybrid endocardial–epicardial ablation procedure with a plan for LA PWI over a period of August 2012 to November 2013. Patients selected for hybrid ablation were considered unlikely to benefit from endocardial ablation alone because of longstanding persistent AF, the presence of significant structural heart disease (especially LA dilatation), and failure after ≥1 endocardial ablation session. The endocardial PWI was performed in patients with LA dilatation, with long‐duration AF, or with AF that did not terminate after pulmonary vein isolation alone.
Our institutional review board approved acquisition of data for the study, and all patients provided informed consent.
Radiofrequency Ablation
Radiofrequency ablation was performed on all patients, using either an endocardial‐only or a combined epicardial–endocardial procedure. All procedures were performed at the University of North Carolina Hospitals electrophysiology laboratory.
Endocardial ablation
All patients with persistent AF or with paroxysmal AF and a CHADS2 score ≥1 underwent preprocedural transesophageal echocardiography to rule out intracardiac thrombus before the procedure, and intravenous heparin was administered with a target activated clotting time of >350 seconds before transseptal access. LA access was achieved by double transseptal puncture. A decapolar circular mapping catheter was used to acquire an anatomic map of the pulmonary veins and the left atrium (EnSite/NaVx [St Jude Medical] or Carto [Biosense Webster]).
Endocardial ablation was performed using an irrigated ablation catheter (Chili II [Boston Scientific], Thermocool SF [Biosense Webster], or Safire Blu [St Jude Medical]) guided by a circular mapping catheter. Power of 25 to 40 W was used and was reduced to 20 to 30 W while ablating on the PW. Esophageal temperature was monitored, and power delivery was interrupted for temperature elevations ≥0.4°C. All patients underwent pulmonary vein antral isolation. Lesion placement was continued until entrance block was demonstrated either as loss of the signals within the pulmonary vein or as the appearance of a dissociated pulmonary vein potential. A roof line was created anteriorly across the LA roof that connected the 2 superior pulmonary veins. A line of lesions was created along the endocardial posterior left atrium to connect the left and right inferior pulmonary veins to isolate the PW between the pulmonary vein pedicles. Additional linear ablation or ablation guided by complex fractionated atrial electrograms was performed at the operator's discretion in patients who remained in AF. When the arrhythmia organized into either an atrial flutter or a focal atrial tachycardia, this was mapped and ablated. If AF persisted, ibutilide was administered intravenously as necessary and an external shock was delivered as necessary to cardiovert the patient to normal sinus rhythm. Cavotricuspid isthmus ablation was performed in all patients with persistent AF and in those with paroxysmal AF who had a history of typical atrial flutter.
After conversion to sinus rhythm, a voltage map of the left atrium was acquired, and the area of electrical silence encompassed by the ablation lines (defined as voltage <0.1 mV) was assessed on the map. Additional lesions along the ablation lines were placed if the PW still had electrical activity. Electrical block was defined as pacing from the PW with PW capture and exit block to the remainder of the atrium and/or the appearance of a dissociated potential on the PW.
Hybrid epicardial–endocardial procedure
Hybrid epicardial–endocardial procedures were performed in a hybrid electrophysiology/operating room suite with integrated fluoroscopy and a 3‐dimensional mapping and imaging system along with endoscopy and general anesthesia. The epicardial procedure was initially performed by a cardiothoracic surgery team through either a subxiphoid transdiaphragmatic access or a right minithoracotomy/thoracoscopic access. The details of the epicardial part of the procedure were described previously.9, 14
Subsequent to the epicardial portion of the procedure, the endocardial procedure was performed in a manner similar to the endocardial‐only ablation procedure. After transseptal access, a 3‐dimensional electroanatomic map with voltage mapping was acquired to define the ablation lines made during the epicardial part of the procedure and to check for silence and isolation of the PW box and the pulmonary veins. If the pulmonary veins were not isolated, endocardial lesions were placed as necessary to achieve block guided by a circular mapping catheter. The integrity of the roof line and the low posterior line was checked with the voltage map, and documentation of entrance block into the PW box and demonstration of exit block were performed once the patient was in sinus rhythm. Additional ablation lesions were then placed in a stepwise fashion in a manner similar to the endocardial‐only procedure.
Postablation care
All patients were observed overnight in the hospital, with clinical assessment immediately following the procedure and before discharge. Length of hospitalization was prolonged for antiarrhythmic drug loading if indicated. Recovery and discharge of patients after a hybrid ablation procedure took longer, and recovery from the surgical epicardial part of the ablation procedure and management of the drain were additional determinants of length of hospital stay.
After ablation, anticoagulation with warfarin or one of the newer oral anticoagulants was reinitiated with bridging enoxaparin and/or intravenous heparin, as appropriate. Oral anticoagulant was administered for at least 6 weeks in all patients and was continued subsequently based on risk factors for cerebrovascular accident. A class I or III antiarrhythmic medication was initiated in all patients after ablation. The choice and duration of antiarrhythmic medication was made on an individual basis taking clinical factors into consideration but was not discontinued at <6 weeks.
Follow‐up
Patients were followed at 6 weeks and at 3, 6, and 12 months following the ablation procedure. A clinical interview, a full clinical examination, and electrocardiography were performed at each follow‐up. Patients with an implanted pacemaker or implantable cardioverter‐defibrillator had interrogation of their device to look for any evidence of AF at each visit. Long‐term electrocardiographic cardiac monitoring for recurrence was done at 3 and 12 months to assess for asymptomatic AF. All patients with combined epicardial–endocardial ablation had implantation of a looping ECG event recorder (Medtronic Reveal) to monitor recurrent AF. Recurrence was defined as >30 seconds of documented AF or atrial flutter/atrial tachycardia occurring after a blanking period of 3 months after ablation or >2 minutes in patients with implanted recorders (the lower limit of detection of the device).
Statistical Analysis
Continuous variables were summarized as means and standard deviations, and categorical variables were summarized as percentages. The means of the continuous variables were compared using the t test, and the frequencies between groups were compared with the chi‐squared test or Fisher's exact test, as appropriate. The predictors of recurrent AF after catheter ablation were analyzed with Cox proportional hazards models using univariate and multivariate models. Kaplan–Meier curves were generated for estimated probability of recurrence of AF on follow‐up. The open source software R (R Foundation for Statistical Computing) was used for all statistical analysis.
Results
Clinical Characteristics
Fifty‐seven patients undergoing attempted PWI were included in the analysis. In total, 27 patients (47.4%) underwent hybrid ablation, and the remainder underwent endocardial‐only ablation. Clinical characteristics are summarized in Tables 1 and 2. The age of the study cohort was 62.4±8.8 years, and 78.9% of the cohort was male. LA size for the cohort was 4.8±0.6 cm, and left ventricular ejection fraction was 53.4±10.0%. The CHA2DS2‐VaSc score was 2.6±1.4. Six patients (10.5%) had high‐burden paroxysmal AF, and the rest had persistent AF. The cohort had significant comorbidities predisposing to AF, including hypertension (82.5%), heart failure (38.6%), diabetes mellitus (29.8%), and history of coronary artery disease (24.6%). Overall, 15 patients (9 in the hybrid ablation group and 6 in the endocardial‐only ablation group) had a history of ≥1 previous ablation procedure for AF. Hybrid and endocardial‐only ablation patients were clinically similar except for larger LA size in the hybrid group (5.0±0.7 versus 4.5±0.5 cm, P=0.005) (Table 1).
Table 1.
Summary Clinical Data in Hybrid and Endocardial‐Only AF Ablation
| Variables | All | Endocardial‐Only Group | Hybrid Group | P Value |
|---|---|---|---|---|
| Age, y | 62.4±8.8 | 63.7±8.8 | 60.9±8.7 | 0.11 |
| Male sex, % | 78.9 | 73.3 | 85.2 | 0.44 |
| BMI | 33.4±5.9 | 32.5±6.4 | 34.4±5.1 | 0.11 |
| LVEF, % | 53.4±10.0 | 53.6±9.3 | 53.3±10.9 | 0.45 |
| Left atrial diameter, cm | 4.8±0.6 | 4.5±0.5 | 5.0±0.7 | 0.005 |
| History (%) | ||||
| Hypertension | 82.5 | 80.0 | 85.2 | 1.00 |
| Diabetes mellitus | 29.8 | 36.7 | 22.2 | 0.37 |
| Coronary artery disease | 24.6 | 23.3 | 29.6 | 0.81 |
| Congestive heart failure | 38.6 | 30.0 | 48.1 | 0.26 |
| CHA2DS2‐VaSc | 2.6±1.4 | 2.7±1.4 | 2.5±1.4 | 0.31 |
AF indicates atrial fibrillation; BMI, body mass index; LVEF, left ventricular ejection fraction.
Table 2.
Summary of Baseline Clinical Data in Patients With and Without Recurrent AF
| Variables | All | Patients With Recurrence | Patients Without Recurrence | P Value |
|---|---|---|---|---|
| Age, y | 62.4±8.8 | 60.2±8.5 | 64.1±8.8 | 0.10 |
| Male sex, % | 78.9 | 76.0 | 81.3 | 0.87 |
| BMI | 33.4±5.9 | 34.7±6.3 | 32.3±5.4 | 0.14 |
| LVEF, % | 53.4±10.0 | 53.0±11.0 | 53.8±9.3 | 0.76 |
| Left atrial diameter, cm | 4.8±0.6 | 4.9±0.7 | 4.7±0.5 | 0.30 |
| History (%) | ||||
| Hypertension | 82.5 | 84.0 | 81.3 | 1.00 |
| Diabetes mellitus | 29.8 | 24.0 | 34.4 | 0.58 |
| Coronary artery disease | 24.6 | 24.0 | 25.0 | 1.00 |
| Congestive heart failure | 38.6 | 36.0 | 40.6 | 0.93 |
| CHA2DS2‐VaSc | 2.6±1.4 | 2.3±1.5 | 2.8±1.3 | 0.22 |
AF indicates atrial fibrillation; BMI, body mass index; LVEF, left ventricular ejection fraction.
Catheter Ablation
Figure 1 shows endocardial voltage maps for a patient with electrical silence of the PW (Figure 1A) (defined as no measured voltage >0.1 mV) after endocardial‐only ablation and a patient for whom silence of the PW could not be achieved despite exhaustive ablation (Figure 1B). Figure 2 shows an example of entrance and exit block in the PW. In Figure 2A, an isolated PW potential is evident during sinus rhythm, and this potential could be captured by PW pacing (Figure 2B), confirming entrance and exit block in the PW in this patient. An electrically silent PW by voltage map was achieved in 44% of the patients, but PWI with documented exit block was achieved in only 37% of the patients. PWI was achieved more frequently in patients undergoing hybrid ablation than endocardial ablation alone (51.9% versus 23.3%, P=0.05). LA size, left ventricular systolic function, and the presence of hypertension, diabetes mellitus, or coronary artery disease did not predict the likelihood of successful PWI.
Figure 1.
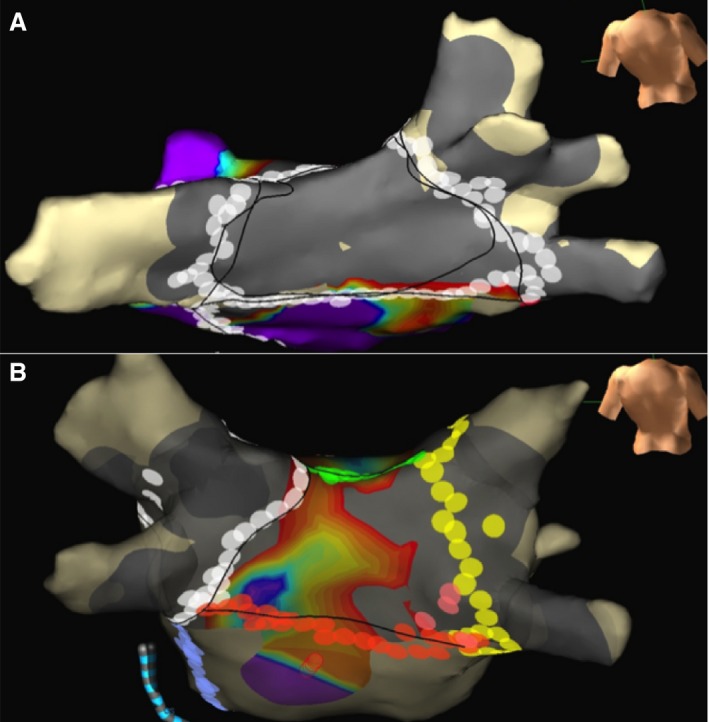
A, Lesion sets to an isolated posterior wall, with a silent posterior wall on voltage mapping after completion of ablation. B, A patient unable to isolate the posterior wall despite lesion sets for posterior wall isolation, with electrical activity in areas of the posterior wall. Gray areas show bipolar peak‐to‐peak voltage during sinus rhythm <0.1 mV, and purple areas show bipolar voltage during sinus rhythm of >0.5 mV.
Figure 2.
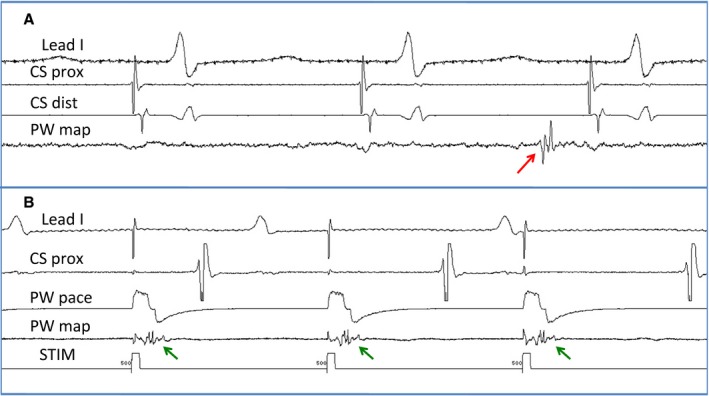
An example of entrance and exit block in the PW. A, An isolated PW potential (red arrow) evident during sinus rhythm. B, Capture of PW (green arrows) with pacing without capturing the rest of the atrium, proving exit block from the PW box. CS indicates coronary sinus; CS dist, bipole recording distal coronary sinus; CS prox, bipole recording proximal coronary sinus; PW, posterior wall; PW map, bipole recording potential inside the posterior wall box; PW pace, bipole used to pace from the posterior wall box. STIM, stimulation channel.
The total radiofrequency duration for endocardial ablation was 89.4±39.0 minutes, total fluoroscopy time was 64.4±20.5 minutes, and a total of 150±62 endocardial lesions were placed. Although fluoroscopy times were similar in hybrid and endocardial‐only procedures (63±20 versus 65±21 minutes, P=0.79), total endocardial radiofrequency duration was considerably lower in patients undergoing hybrid ablation (62±19 versus 114±35 minutes, P<0.001). The number of endocardial lesions placed was also lower in patients undergoing hybrid procedures (107±34 versus 188±57, P<0.001). Ablation of complex fractionated atrial electrograms was performed in 28 patients (49.1% of patients, 60.0% in the endocardial‐only group versus 37.0% in the hybrid ablation group; P=0.14), and mitral isthmus ablation was performed in 30 patients (52.6% of patients, 60.0% in the endocardial‐only group versus 44.4% in the hybrid ablation group; P=0.36).
Four patients were in sinus rhythm at the start of the procedure. One of these patients was planned for endocardial‐only ablation, and 3 were planned for hybrid ablation; all of the latter had recurred after 2 previous endocardial ablations. Of the 24 hybrid ablation patients in AF, termination of AF was seen in 4 with epicardial ablation alone (all of them into sinus rhythm), and AF terminated in 11 with additional endocardial ablation (9 into sinus rhythm and 2 into atrial flutter). Of 29 endocardial‐only ablation patients in AF at the start of the procedure, AF terminated in 10 (8 into sinus rhythm and 2 into atrial flutter). Termination into either sinus rhythm or atrial flutter at the end of the procedure in patients not presenting in sinus rhythm was seen more often in patients undergoing the hybrid ablation procedure (15 of 24 patients, 62.5%) compared with patients undergoing the endocardial‐only procedure (10 of 29 patients, 34.5%; P=0.04).
Recurrence of AF
Over a median postablation follow‐up of 302 days, recurrent atrial arrhythmia was seen in 25 patients (43.9%). Of these 25 patients, 7 had atrial tachycardia, and the rest had AF as the mode of recurrent arrhythmia. Only 12 of these patients opted for a redo ablation; the symptoms were reasonably well controlled with antiarrhythmic medications in the remainder. Patients with and without recurrent atrial arrhythmias did not have statistically significant differences in various clinical parameters (Table 2). No clinical factor or procedural factor, including PWI and LA size, was a statistically significant predictor of recurrent AF (Table 3). PWI did not affect the risk of recurrence in a univariate model in the entire cohort (Figure 3). Cox regression for subgroups of patients undergoing endocardial‐only ablation and hybrid ablation did not show any statistically significant predictor for recurrence in the multivariate model except for LA size in the hybrid ablation subgroup (hazard ratio 4.26, 95% CI 1.09–16.74; P=0.038).
Table 3.
Cox Univariate and Multivariate Analysis of Predictors of Recurrent Atrial Arrhythmias
| Variable | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|
| Posterior wall isolation | 1.53 (0.66–3.55) | 0.32 | 1.33 (0.50–3.56) | 0.57 |
| LA size | 1.69 (0.83–3.42) | 0.15 | 2.06 (0.89–4.75) | 0.09 |
| BMI | 1.05 (0.99–1.12) | 0.13 | 1.04 (0.95–1.13) | 0.44 |
| Age | 0.948 (0.906–0.992) | 0.02 | 0.95 (0.906–1.003) | 0.066 |
| Previous AF ablation | 1.28 (0.53–3.06) | 0.59 | 1.10 (0.39–3.08) | 0.85 |
| Hybrid ablation | 1.21 (0.55–2.66) | 0.64 | 0.73 (0.29–1.80) | 0.49 |
AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio; LA, left atrium.
Figure 3.
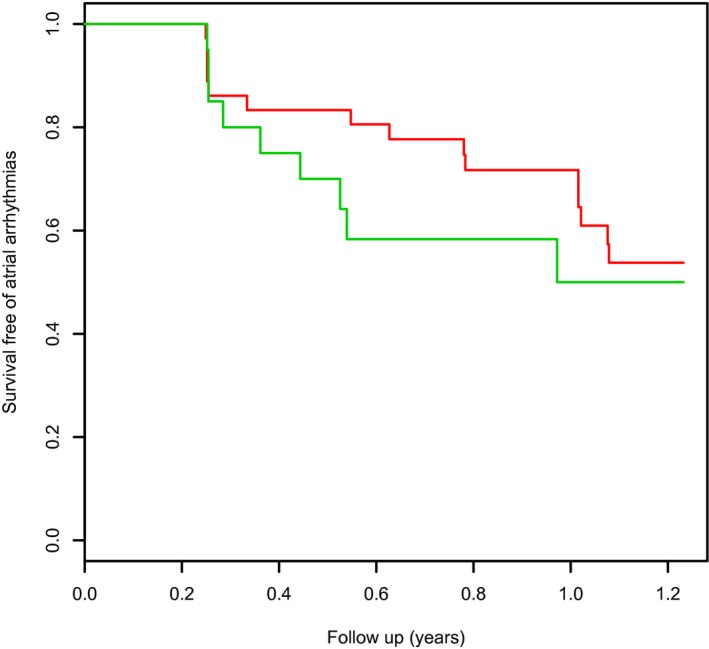
Kaplan–Meier curve showing freedom from atrial arrhythmias after index ablation procedure in patients with (green line) and without (red line) procedural isolation of the posterior wall (no statistically significant difference in survival free of atrial arrhythmia).
Eight patients in the endocardial‐only ablation group and 4 patients in the hybrid ablation group had redo ablation procedures. Three of 4 in the hybrid group and 2 of 8 in the endocardial‐only group had PWI at the end of the index procedure. The PW was not isolated at the beginning of the redo procedure for any of these 5 patients.
Complications
None of the patients had stroke, pericardial tamponade, or any major bleed requiring transfusion.
Discussion
In this study we investigated PWI as a strategy for radiofrequency ablation for AF. We assessed the achievement of procedural isolation of the PW by both voltage mapping and pacing techniques and declared procedural success only when there were demonstrable exit and entrance blocks. With our rigorous assessment for isolation, the rate of successful PWI was low. Even the patients who achieved procedural PWI did not show lower recurrence of AF on follow‐up after the procedure. Importantly, in a small subset of patients who underwent redo procedures, reconnection of the PW was common. For this reason, it could be argued that the efficacy of PWI in catheter‐based and hybrid procedures remains an open question. Its apparent efficacy in surgical Maze procedures would argue that our somewhat disappointing results occurred because of difficulty in achieving durable PWI with current radiofrequency ablation techniques.
The PW may be important in the initiation and maintenance of AF for multiple reasons. Embryologically, the LA PW pedicle between the pulmonary veins has the same origin as the pulmonary veins and thus might share a similar propensity for housing triggers. The PW and roof contain favorable conditions for reentry. Complex fiber orientation within the PW creates anisotropy, allowing conduction block, reentry, and wavebreak.15 The pulmonary vein antra and PW may be subject to increased wall stress compared with other areas of the atria.16 In chronic AF related to mitral valve disease, histopathological examination reveals greater myocytolysis and interstitial changes in the PW.17 This fibrosis in the PW promotes slow conduction and reentry and may provide stabilizing anchor points for spiral waves.18 Consequently, it is not surprising that rotors, focal sources of AF, and important complex fractionated atrial electrograms are often localized to the PW and roof.19, 20, 21 Electrical isolation of the PW would eliminate these triggers and drivers of AF while debulking the atria, leaving less remaining available substrate for fibrillation.
In addition to the mechanistic considerations to support PWI as a strategy for ablation of AF, the efficacy of the Cox‐Maze procedure in the surgical treatment of AF also suggests the importance of the LA PW in the pathogenesis of AF. All variants of a Maze‐like procedure, including modified Maze procedures performed by minimally invasive surgical approaches, have isolation of LA PW as the end result of the procedure.7, 8, 22, 23 Our hypothesis also stems from our previous observation9 of successful elimination of signals on the PW during a hybrid epicardial–endocardial procedure with a combination of minimally invasive surgical epicardial ablation and catheter‐based endocardial ablation and favorable results in controlling AF.
In our study, we could isolate the PW with endocardial ablation in only 23% of the patients. Even with hybrid ablation, the rate of achieving PWI was relatively low at 52%. Achieving PWI requires aggressive ablation on the PW over the esophagus, and often, adequate lesion placement may be limited by esophageal temperature rises. Previous studies evaluating PWI have shown variable success in achieving PWI, with success rates ranging from 19% to as high as 96%.1, 3, 4, 5, 6, 24 Hybrid ablation series have not documented PWI except for Muneretto et al, with isolation after epicardial ablation alone in 83% and completion of isolation in the rest of the patients with endocardial ablation.12 Nevertheless, the criteria and rigor of demonstrating isolation in these studies have been variable. In our series, PWI was rigorously evaluated in each patient, as described in the Methods.
In this cohort, no statistically significant difference was seen in recurrence of atrial arrhythmias between those with complete PWI and those with incomplete lines. Although this finding is unexpected, it is not inexplicable. Two questions need attention in this regard. The first is related to the durability of PWI, and the second is the question of incremental benefit of PWI in treating AF over and above the rest of the ablation procedure.
Placement of a durable lesion with durable PWI using radiofrequency catheter ablation is itself challenging. Acute procedural success of PWI in many such patients may be limited by tissue edema developing during the procedure. In contrast, development of edema and inflammation during placement of ablation lesions may make the damage reversible on subsidence of edema and inflammation, and thus lesions may not be durable. Lack of durable isolation with current catheter ablation techniques has been very well demonstrated in multiple pulmonary vein isolation series.25, 26 In this series, 37% of the patients had dissociated PWs at the end of index procedure, with questionable durability of isolation. Of the 5 patients with PWI who underwent a redo ablation later, conduction to the PW recurred in all. Similar findings were seen in other studies of PWI, with a large fraction of patients studied for redo procedures having a reconnected PW. This may be due to overrepresentation of PW reconnection in patients undergoing redo procedures. Nevertheless, it should be noted that even with incomplete lines, it is possible that sites containing driver mechanisms in the roof or low PW may have been coincidentally interrupted if they were in the path of incomplete lines.
Inability to achieve durable block across linear lesions may also explain the findings of the STAR‐AF II trial, which did not demonstrate any benefit of additional linear ablation beyond pulmonary vein isolation for AF‐free survival. It would not be surprising that with the use of similar ablation technology, the chances of achievement of durable block across the linear lesions in this study also would have been low and thus translated into no benefit of additional linear ablation in these patients.27
Although the additional efficacy of adding PWI to pulmonary vein isolation was not assessed in this study, it has been evaluated in 2 previous studies. Tamborero et al found no additional benefit in completing PWI by adding a low posterior line to a roof line compared with a roof line alone.1 Lim et al compared single‐ring PWI to wide‐area pulmonary vein isolation and saw no reduction in recurrent atrial arrhythmias. Although rates of AF were reduced, recurrent macroreentry was greater in the single‐ring group.4 Nevertheless, concerns about the durability of PWI leave these data open to question.
Limitations
These data are from a relatively small series of patients at a single center. A significant fraction of the patients included in the study underwent an epicardial ablation procedure as part of a hybrid surgical epicardial and catheter‐based endocardial ablation procedure. The numbers are not large enough to allow subgroup analysis, especially with a small fraction of the patients achieving PWI. Moreover, durability of PWI with the available ablation techniques is an open question, and reassessment of durable isolation has been documented in only a small fraction of patients returning for redo ablation and thus with inherent selection bias.
Conclusions
When the outcome of LA PWI is assessed rigorously using pacing techniques in addition to voltage mapping, PWI during AF ablation procedure is difficult to achieve with current ablation techniques, especially with endocardial‐only ablation. Procedural isolation of the LA PW does not appear to influence recurrence of AF in this patient cohort; however, with questionable durability of isolation with current radiofrequency ablation techniques, the value of PWI as a strategy for AF ablation remains an open question.
Disclosures
Kiser is consultant for Karl Storz Endoskope, Tuttlingen, Germany and Atricure Inc, West Chester OH USA, and is an owner of ACATI, West End, NC USA. Mounsey is consultant for Medtronic, Inc, Minneapolis, MN USA, St. Jude Medical, St Paul, MN USA, Boston Scientific Inc, Marlborough, MA USA and Catheter Robotics Inc., Mt Olive, NJ USA, and has an honorarium relationship with Atricure Inc, West Chester OH USA. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2016;5:e003885 doi: 10.1161/JAHA.116.003885)
References
- 1. Tamborero D, Mont L, Berruezo A, Matiello M, Benito B, Sitges M, Vidal B, de Caralt TM, Perea RJ, Vatasescu R, Brugada J. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol. 2009;2:35–40. [DOI] [PubMed] [Google Scholar]
- 2. Thomas SP, Lim TW, McCall R, Seow SC, Ross DL. Electrical isolation of the posterior left atrial wall and pulmonary veins for atrial fibrillation: feasibility of and rationale for a single‐ring approach. Heart Rhythm. 2007;4:722–730. [DOI] [PubMed] [Google Scholar]
- 3. Lim TW, Koay CH, McCall R, See VA, Ross DL, Thomas SP. Atrial arrhythmias after single‐ring isolation of the posterior left atrium and pulmonary veins for atrial fibrillation: mechanisms and management. Circ Arrhythm Electrophysiol. 2008;1:120–126. [DOI] [PubMed] [Google Scholar]
- 4. Lim TW, Koay CH, See VA, McCall R, Chik W, Zecchin R, Byth K, Seow SC, Thomas L, Ross DL, Thomas SP. Single‐ring posterior left atrial (box) isolation results in a different mode of recurrence compared with wide antral pulmonary vein isolation on long‐term follow‐up: longer atrial fibrillation‐free survival time but similar survival time free of any atrial arrhythmia. Circ Arrhythm Electrophysiol. 2012;5:968–977. [DOI] [PubMed] [Google Scholar]
- 5. Kumagai K, Muraoka S, Mitsutake C, Takashima H, Nakashima H. A new approach for complete isolation of the posterior left atrium including pulmonary veins for atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1047–1052. [DOI] [PubMed] [Google Scholar]
- 6. Chilukuri K, Scherr D, Dalal D, Cheng A, Spragg D, Nazarian S, Barcelon BD, Marine JE, Calkins H, Henrikson CA. Conventional pulmonary vein isolation compared with the “box isolation” method: a randomized clinical trial. J Interv Card Electrophysiol. 2011;32:137–146. [DOI] [PubMed] [Google Scholar]
- 7. Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, Damiano NR, Bloch JB, Moon MR, Damiano RJ Jr. A prospective, single‐center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. [DOI] [PubMed] [Google Scholar]
- 8. Lall SC, Melby SJ, Voeller RK, Zierer A, Bailey MS, Guthrie TJ, Moon MR, Moazami N, Lawton JS, Damiano RJ Jr. The effect of ablation technology on surgical outcomes after the Cox‐maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133:389–396. [DOI] [PubMed] [Google Scholar]
- 9. Gehi AK, Mounsey JP, Pursell I, Landers M, Boyce K, Chung EH, Schwartz J, Walker TJ, Guise K, Kiser AC. Hybrid epicardial‐endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm. 2013;10:22–28. [DOI] [PubMed] [Google Scholar]
- 10. Kumar N, Pison L, La Meir M, Maessen J, Crijns HJ. Hybrid approach to atrial fibrillation ablation using bipolar radiofrequency devices epicardially and cryoballoon endocardially. Interact Cardiovasc Thorac Surg. 2014;19:590–594. [DOI] [PubMed] [Google Scholar]
- 11. La Meir M, Gelsomino S, Lorusso R, Luca F, Pison L, Parise O, Wellens F, Gensini GF, Maessen J. The hybrid approach for the surgical treatment of lone atrial fibrillation: one‐year results employing a monopolar radiofrequency source. J Cardiothorac Surg. 2012;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muneretto C, Bisleri G, Bontempi L, Curnis A. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long‐standing atrial fibrillation: a 30‐month assessment with continuous monitoring. J Thorac Cardiovasc Surg. 2012;144:1460–1465; discussion 1465. [DOI] [PubMed] [Google Scholar]
- 13. Pison L, La Meir M, van Opstal J, Blaauw Y, Maessen J, Crijns HJ. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60:54–61. [DOI] [PubMed] [Google Scholar]
- 14. Muneretto C, Bisleri G, Bontempi L, Cheema FH, Curnis A. Successful treatment of lone persistent atrial fibrillation by means of a hybrid thoracoscopic‐transcatheter approach. Innovations (Phila). 2012;7:254–258. [DOI] [PubMed] [Google Scholar]
- 15. Markides V, Schilling RJ, Ho SY, Chow AW, Davies DW, Peters NS. Characterization of left atrial activation in the intact human heart. Circulation. 2003;107:733–739. [DOI] [PubMed] [Google Scholar]
- 16. Hunter RJ, Liu Y, Lu Y, Wang W, Schilling RJ. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:351–360. [DOI] [PubMed] [Google Scholar]
- 17. Corradi D, Callegari S, Benussi S, Maestri R, Pastori P, Nascimbene S, Bosio S, Dorigo E, Grassani C, Rusconi R, Vettori MV, Alinovi R, Astorri E, Pappone C, Alfieri O. Myocyte changes and their left atrial distribution in patients with chronic atrial fibrillation related to mitral valve disease. Hum Pathol. 2005;36:1080–1089. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS, Landas S, Guiraudon G, Jalife J, Berenfeld O, Kalifa J. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. [DOI] [PubMed] [Google Scholar]
- 19. Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on‐treatment analysis of the CONFIRM trial (conventional ablation for AF with or without focal impulse and rotor modulation). J Am Coll Cardiol. 2013;62:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. [DOI] [PubMed] [Google Scholar]
- 21. Oral H, Chugh A, Yoshida K, Sarrazin JF, Kuhne M, Crawford T, Chalfoun N, Wells D, Boonyapisit W, Veerareddy S, Billakanty S, Wong WS, Good E, Jongnarangsin K, Pelosi F Jr, Bogun F, Morady F. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long‐lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782–789. [DOI] [PubMed] [Google Scholar]
- 22. Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 23. Cox JL, Boineau JP, Schuessler RB, Jaquiss RD, Lappas DG. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg. 1995;110:473–484. [DOI] [PubMed] [Google Scholar]
- 24. Sohns C, Bergau L, Seegers J, Luthje L, Vollmann D, Zabel M. Single‐ring ablation compared with standard circumferential pulmonary vein isolation using remote magnetic catheter navigation. J Interv Card Electrophysiol. 2014;41:75–82. [DOI] [PubMed] [Google Scholar]
- 25. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, Schaumann A, Chun J, Falk P, Hennig D, Liu X, Bansch D, Kuck KH. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005;111:127–135. [DOI] [PubMed] [Google Scholar]
- 26. Verma A, Kilicaslan F, Pisano E, Marrouche NF, Fanelli R, Brachmann J, Geunther J, Potenza D, Martin DO, Cummings J, Burkhardt JD, Saliba W, Schweikert RA, Natale A. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–635. [DOI] [PubMed] [Google Scholar]
- 27. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P; STAR AF II Investigators . Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]


