Abstract
Background
In adult Fontan patients, ventricular or arterial dysfunction may impact homeostasis of the Fontan circulation and predispose to heart failure. We sought to characterize ventricular‐arterial (VA) properties in adult Fontan patients.
Methods and Results
Adult Fontan patients (n=170), including those with right (SRV, n=57) and left (SLV, n=113) dominant ventricular morphology, were compared to age, sex, and body size matched controls (n=170). Arterial function, load‐insensitive measures of contractility, VA coupling, diastolic function, and ventricular efficiency were assessed. Compared to controls, Fontan patients had similar arterial (Ea), but lower end‐systolic ventricular (Ees), elastance, preload recruitable stroke work and peak power index, impaired VA coupling, eccentric remodeling, reduced ventricular efficiency and increased diastolic stiffness (P<0.05 for all). Ventricular efficiency declined steeply with higher heart rate in Fontan, but not control, patients. Among Fontan patients (n=123) and controls (n=162) with preserved cardiac index (CI; ≥2.5 L/min per m2), Fontan patients had worse contractility than controls, but CI was preserved owing to relative tachycardia, lower afterload, and eccentric remodeling. However, 25% of Fontan patients had reduced CI and were distinguished from those with preserved CI by less‐eccentric remodeling and worse diastolic function, rather than more‐impaired contractility.
Conclusions
Adult Fontan patients have contractile and diastolic dysfunction with normal afterload, impaired VA coupling, and reduced ventricular efficiency with heightened sensitivity to heart rate. Maintenance of CI is dependent on lower afterload, eccentric remodeling, and relative preservation of diastolic function. These data contribute to our understanding of circulatory physiology in adult Fontan patients.
Keywords: adult Fontan circulation, single ventricular heart, ventricular arterial coupling, ventricular efficiency, ventricular function
Subject Categories: Cardiovascular Surgery, Congenital Heart Disease
Introduction
Adults with congenital heart disease (CHD) represent a growing heart failure (HF) population.1 Incidence of HF among Fontan palliated patients with univentricular physiology is high, but the etiologies and manifestations of a failing Fontan circulation are diverse.1 Even mild systolic or diastolic ventricular dysfunction or excessive ventricular afterload may elevate filling pressures and/or limit the capacity of the Fontan circulation to maintain cardiac output. Although conventional echo parameters in pediatric Fontan patients relative to normative values has been described,2 few data regarding cardiac function are available, particularly in the adult Fontan population. Given that adult Fontan survivors may be free of the factors associated with earlier mortality after Fontan surgery, ventricular arterial (VA) function in adults may be quite different from pediatric Fontan patients.1, 3 The objective of this study was to characterize VA function and coupling in a large, generally stable cohort of adult Fontan survivors of both right and left dominant ventricular morphology and age, sex, and body size matched controls. As Fontan patients may have altered ventricular loading conditions, ejection fraction (EF) may not accurately reflect systolic ventricular function. Thus, validated noninvasive load‐insensitive indices of ventricular performance and VA coupling were assessed. The relationship of VA properties to underlying ventricular morphology, an index of circulatory success (resting cardiac index [CI]), and medical therapy were evaluated.
Methods
Identification of Study Cohort
This study was approved by the Mayo Institutional Review Board and restricted to appropriately consented patients. Of 1134 patients who underwent a Fontan procedure at the Mayo Clinic (Rochester, MN) between 1973 and 2012, 658 had a clinical visit as an adult (aged ≥18 years) when archived echocardiographic images were potentially available (1985 to present). Of these, the operative and echocardiogram diagnosis indicated single right ventricle (SRV) in 111, single left ventricle (SLV) in 385, and indeterminate cardiac morphology or past biventricular candidacy in 162. Among adult SRV patients, 57 (51%) had an echocardiogram and were included in this study. Adult SLV patients with available echocardiograms were randomly assigned (n=113), using 2:1 matching to SRV patients for sex, age at operation (aged <6 years; 3 year, aged 6–12 years; 5 year, aged >12 years; 8‐year intervals), and surgical era (10‐year intervals). Echocardiograms from the initial adult clinical visit were analyzed (ProSolv software; ProSolv CardioVascular, Indianapolis, IN).
Patients undergoing echocardiography but proven to have no cardiovascular diseases, normal electrocardiogram and echocardiograms, which were defined by normal left and right ventricular size, left ventricular (LV) EF ≥50%, no significant valvular disease, and normal diastolic functional class with no two‐dimensional (2D) or Doppler evidence of pulmonary hypertension, were considered to be potential controls. No requirement for specific stroke volume (SV) or CI was imposed. Potential controls were randomly matched (1:1) to Fontan patients for sex, age (within 5 years), and body surface area (within 0.15 m2).
Because New York Heart Association functional class was not described in the medical record of most Fontan patients, clinical status (stable or impaired) was assessed using the available descriptions of clinical stability in the medical record. Stable clinical status reflects description of the patient as doing well with no clinical instability warranting admission or close outpatient follow‐up. If the general condition was stable, but an arrhythmia issue not resulting in clinical instability was being evaluated or treated, the condition was also considered stable. If patients were deemed to require close outpatient follow‐up or hospitalization for a clinical issue, the clinical status was considered impaired. Presence and clinical severity of protein losing enteropathy and liver cirrhosis were assessed.
Assessment of Vascular Function
Arterial elastance (Ea), a measure of ventricular afterload, was calculated as end‐systolic pressure (ESP) divided by SV. SV was calculated as the velocity‐time integral of ventricular outflow tract multiplied by the subaortic cross‐sectional area. Although Fontan anatomy may limit accuracy of this method for calculation of SV, it has been validated both in the right and left ventricle for the assessment of Qp/Qs.4 End‐systolic pressure was estimated by systolic arterial pressure×0.9.5 Total arterial compliance was calculated as SV divided by systemic pulse pressure.6 Systemic vascular resistance (SVR) index was calculated as mean arterial blood pressure divided by CI.
Assessment of Ventricular Function and VA Coupling
In Fontan and control patients, EF was evaluated by quantitative and integrated semiquantitative methods. Techniques for assessment of EF in the Mayo Echocardiographic Laboratory evolved during the study period. Given this evolution, we reanalyzed all Fontan patient images using the single‐plane Simpson's method to provide a consistent quantitative EF assessment in all patients. A constant feature of EF assessment over time has been the integration of quantitative measures and their potential limitations attributed to imaging specifics in each patient with the visual estimation of EF provided by an experienced echocardiographer. The clinically reported, integrated semiquantitative EF assessment showed good correlation and agreement with the Simpson's method (Figure 1A). In Fontan patients, agreement and correlation between EF measures was similar irrespective of ventricular morphology (Figure 1E and 1F). Similarly, in controls, the integrated semiquantitative EF assessment was available in all patients and was used in this study. This EF assessment showed good correlation and agreement with the most commonly reported quantitative method used during the study era (2D modified Quinones method; Figure 1C).
Figure 1.
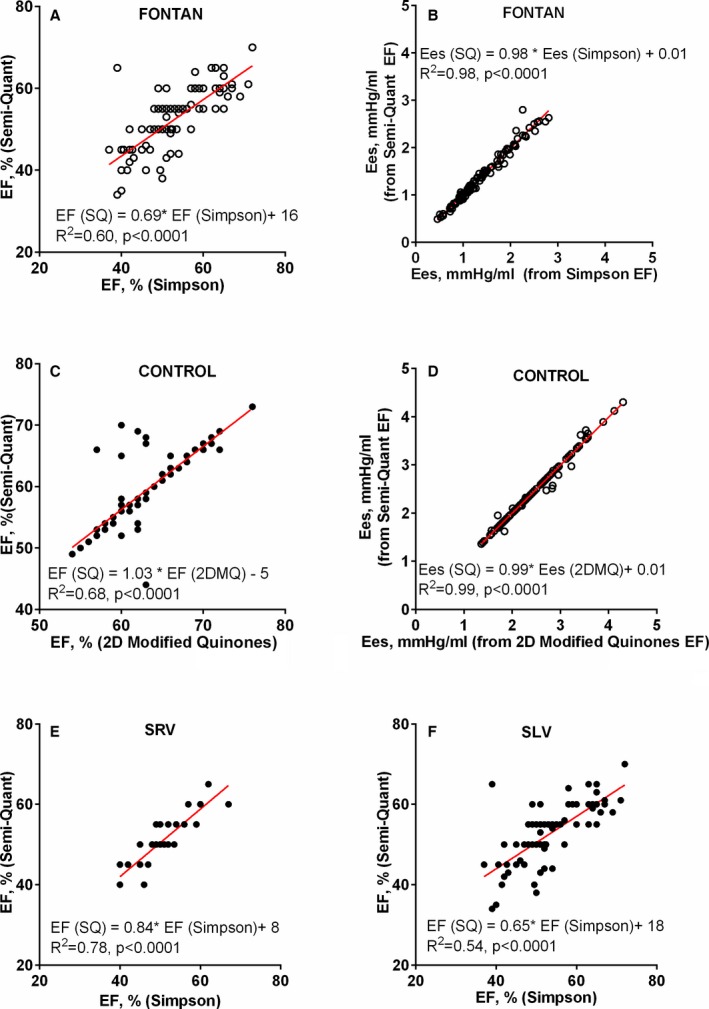
Ejection fraction (EF) methods and impact on end‐systolic elastance (Ees) in Fontan and control patients. A, Correlation between Simpson's EF and the clinically reported, integrated semiquantitative (SQ) EF assessment in Fontan patients. B, Correlation between Ees calculated from Simpson's EF or SQ EF in Fontan patients. C, Correlation between two‐dimensional (2D) modified Quinones (2DMQ) EF and the clinically reported, integrated semiquantitative EF assessment in control patients. D, Correlation between Ees calculated from 2DMQ or SQ method in control patients. E and F, Correlation between Simpson's EF and the clinically reported, integrated semiquantitative EF assessment in Fontan patients with right (SRV) or left (SLV) dominant ventricular morphology.
End‐systolic elastance (Ees), a load‐insensitive measure of ventricular contractility, was calculated by the modified single‐beat method as previously described.7, 8, 9, 10 The physiological concepts underlying the single‐beat estimation of Ees are based on the consistency of normalized time‐varying elastance, and this fundamental concept was validated in conditions with diverse ventricular geometry and function, including patients with hypertrophic or dilated cardiomyopathy, ventricular aneurysms,9 and SLV and SRV Fontan patients.10 Extrapolation of this concept allows the use of noninvasive blood pressures for systolic (SBP), diastolic (DBP) and end‐systolic (ESP) blood pressures, echo Doppler derived SV, pre‐ejection (PEP) and total systolic periods (SEP) and EF, and a normalized ventricular elastance at arterial end‐diastole (calculated from normalized time‐varying elastance curve) to derive Ees and V0 noninvasively as previously described7 and outlined below:
where E(Nd(est))=0.0275−0.165×EF+0.3656×(DBP/ESP)+0.515×E(Nd(avg)) and E(Nd(avg))=0.35695−7.2266×(PEP/SEP)+74.249×(PEP/SEP)2−307.39×(PEP/SEP)3+684.54×(PEP/SEP)4−856.92×(PEP/SEP)5+571.95×(PEP/SEP)6−159.1×(PEP/SEP)7.
These methods have been used to characterize Ees and V0 in a variety of clinical conditions.8, 11, 12 Importantly, given the multiple variables integrated into the single‐beat Ees assessment, the effect of EF on Ees is relatively small. In all patients in this study, there was a decrease in Ees of 0.015 (SE, 0.005) mm Hg/mL per 1‐unit decrease in EF. Furthermore, Ees calculated with the quantitative (Simpson's) or the integrated semiquantitative EF assessment showed excellent agreement and correlation (Figure 1B). Ees in controls calculated with semiquantitative or quantitative methods also showed good agreement and correlation (Figure 1D). VA coupling, the index of appropriateness of cardiac contractility in the context of afterload, was expressed as Ees/Ea.7, 8, 9
Given that the Ees single‐beat estimation provides V0,7 an assumed relaxed ventricular volume corresponding to the X intercept of the end‐systolic pressure‐volume relationship, calculation of ventricular mechanical and energetic efficiencies is possible. Ventricular mechanical efficiency was calculated as external work (EW) divided by pressure volume area (PVA), where EW=SV×MAP and PVA=EW+1/2×Pes×(ESV−V0) with MAP (mean arterial pressure) and Pes (end‐systolic pressure; 0.9×SBP) derived from echo and brachial blood pressure. Ventricular energetic efficiency was calculated by the method of Burkoff and Sagawa, which assumes that myocardial oxygen consumption is proportional to the PVA, whereas the intercept of the PVA‐oxygen cost relationship (ie, oxygen cost at PVA equals zero) is a factor of Ees.13 Accordingly, myocardial oxygen consumption can be estimated from Ees and PVA, with ventricular efficiency (%) calculated as stroke work/myocardial oxygen consumption. Both EW and myocardial oxygen consumption are expressed in Joules,13 and energetic efficiency is expressed as percent.
Additional load‐insensitive measures of ventricular systolic performance, including preload recruitable stroke work (PRSW) and peak power index (PPI), were calculated. PRSW is a load‐insensitive index of contractile performance derived invasively as the slope of the linear relationship between stroke work (SW, Y) and end‐diastolic volume (EDV, X) with an X intercept of Vw. Thus, PRSW=SW/(EDV−Vw). Single‐beat PRSW was calculated using allometric principles, as previously validated, against invasive assessment in patients with variable LV geometry and function, including patients with regional wall motion abnormalities14:
where SW=MAP×SV, EDV=SV/EF, LV wall volume=LV mass/1.05, and k is estimated from the previously defined relationship between invasively measured k and LV mass as k=(0.0004×LV mass)+0.6408.
The PPI characterizes cardiac performance by total cardiac hydraulic power output calculated as the product of maximal flow and maximal developed pressure. Maximal flow and developed pressure occur early in ejection and are relatively load independent. Noninvasively, PPI is calculated using pulsed wave and 2D echocardiography and blood pressures as15:
Assessment of Diastolic Function
Diastolic wall strain (DWS) was calculated in Fontan patients and controls as an index of ventricular myocardial passive stiffness, as previously described, and validated against invasive assessment.16, 17 DWS is based on the linear elastic theory, which predicts that in the presence of similar EF, impaired diastolic wall thinning reflects resistance to deformation in diastole and thus increased diastolic myocardial stiffness. DWS was calculated as (PWs−PWd)/PWs, where PWs and PWd are ventricular systolic and posterior wall thicknesses. Doppler diastolic indices available throughout the study period were reported, when available. As Doppler assessment of diastolic function evolved over the accrual period of this study, contemporary Doppler indices for estimating relaxation (E′) and filling pressures (E/E′) were available in only 7% of SRV and 24% of SLV patients and thus are not reported. Older standard Doppler indices are reflective of the summed effect of LV relaxation and filling pressures, but provide some information regarding diastolic function and are presented.
Assessment of Ventricular Geometry
Ventricular volume was calculated by dividing SV by EF.12, 18, 19 Ventricular mass was calculated from end‐diastolic volume (EDV) and posterior wall thickness assuming a prolate ellipse model.20
Statistical Analysis
Data are shown as percent or mean±SD. Comparison between groups was performed by Student t test or chi‐squared test, as appropriate. Comparison of variables between groups, adjusting for pertinent covariates, used multivariable least squares regression with dummy variables for group and assessment for group‐covariate interactions. A 2‐sided alpha value less than 0.05 was considered statistically significant. All analyses were performed by JMP software (version 9; SAS institute Inc., Cary, NC).
Results
Anatomic and Surgical Fontan Patient Characteristics
Matching resulted in similar sex, age at operation, and operative era between SRV versus SLV Fontan patients (Table 1). The original Fontan procedure type, palliative surgical procedures, fenestration, Fontan revision, or frequency of atrioventricular or aortic valve regurgitation were similar between SLV and SRV (Table 1). No patient had residual coarctation of the aorta.
Table 1.
Fontan Anatomic and Surgical Characteristics
| SRV (n=57) | SLV (n=113) | P Value | |
|---|---|---|---|
| Sex, female, n (%) | 25 (44) | 50 (44) | 0.96 |
| Age at operation, y, n (%) | 12.3±9.1 | 13.1±11.0 | 0.65 |
| 0–3 | 6 (10) | 19 (17) | |
| 4–6 | 13 (23) | 28 (25) | |
| 7–15 | 20 (35) | 25 (22) | |
| >15 | 18 (32) | 41 (36) | |
| Era of operation, n (%) | 0.41 | ||
| Before 1984 | 3 (5) | 8 (7) | |
| 1985–1989 | 26 (46) | 62 (55) | |
| 1990–1994 | 15 (26) | 28 (25) | |
| 1995–2012 | 13 (23) | 15 (13) | |
| Anatomical features, n (%) | NA | ||
| Univentricular heart | 28 (49) | 49 (43) | |
| Complex | 26 (46) | 16 (14) | |
| Tricuspid atresia | 48 (43%) | ||
| Hypoplastic left heart syndrome (classical) | 3 (5%) | ||
| Initial Fontan type, n (%) | 0.36 | ||
| Atrial pulmonary connectiona | 22 (39) | 52 (46) | |
| TCPC (lateral tunnel, IAC, ECC) | 35 (61) | 61 (54) | |
| History of palliative surgery, n (%) | |||
| Pulmonary arterial banding | 16 (28) | 28 (25) | 0.64 |
| Aortopulmonary shunt | 27 (47) | 70 (62) | 0.07 |
| Staged Glenn | 20 (35) | 51 (45) | 0.21 |
| Coarctation repair or aortic reconstruction | 8 (14) | 8 (7) | 0.14 |
| Number of open operations pre‐Fontan, n (%) | 0.15 | ||
| 0 | 11 (19) | 11 (10) | |
| 1 | 31 (54) | 55 (49) | |
| 2 | 13 (23) | 41 (36) | |
| 3 or more | 2 (4) | 6 (5) | |
| History of Fontan revision, n (%) | 4 (7) | 17 (15) | 0.15 |
| Fenestration at operation, n (%) | 7 (12) | 16 (14) | 0.73 |
| Additional fenestration post‐Fontan, n (%) | 2 (4) | 10 (9) | 0.17 |
| Aortic regurgitation | 0.76 | ||
| ≤Mild | 55 (96) | 110 (97) | |
| >Mild | 2 (4) | 3 (3) | |
| Aortic stenosis | 0.99 | ||
| Peak instantaneous gradient ≤30 mm Hg | 55 (96) | 109 (96) | |
| Peak instantaneous gradient >30 mm Hg | 2 (4) | 4 (4) | |
| Atrioventricular valve regurgitation | 0.17 | ||
| ≤Mild | 50 (88) | 106 (94) | |
| >Mild | 7 (12) | 7 (6) | |
ECC indicates extracardiac conduit; IAC, intra‐atrial conduit; NA, not applicable; SLV, left‐dominant single ventricle; SRV, right‐dominant single ventricle; TCPC, total cavopulmonary connection; Vasodilator, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker.
Including Bjork modification.
Clinical Characteristics of Adult Fontan Patients and Controls
Clinical characteristics were similar in SRV and SLV (P>0.10 for all), and thus data are shown for the entire Fontan group. Mean age was 26 years, and patients were over 12 years out from Fontan procedure (Table 2). Treatment with renin‐angiotensin system (RAS) antagonists (35%) or beta‐blockers (15%) was relatively uncommon. Nearly 25% of patients were undergoing evaluation for supraventricular arrhythmias (Table 2).
Table 2.
Clinical Characteristics of Adult Fontan Patients
| All Fontan (n=170) | Control (n=170) | |
|---|---|---|
| Age at echocardiogram, y | 26±8 | 26±8 |
| Sex, female, n (%) | 75 (44) | 75 (44) |
| Body surface area, m2 | 1.75±0.21 | 1.79±0.23 |
| Interval from Fontan, y | 12.4±7.7 | NA |
| RAS antagonist, n (%) | 60 (35) | 0 |
| Beta‐blocker, n (%) | 25 (15) | 0 |
| Current arrhythmia evaluation, n (%) | 45 (26) | 0 |
| Rhythm (n=169), n (%) | ||
| Sinus | 107 (63) | 170 (100) |
| Paroxysmal AF or SVT | 17 (10) | |
| Persistent AF | 8 (5) | |
| Junctional rhythm | 14 (8) | |
| Paced | 23 (14) | |
| Global clinical status (n=169), n (%) | NA | |
| Stable | 130 (77) | |
| Impaired | 39 (23) | |
| Fontan obstruction, n (%) | NA | |
| None | 164 (97) | |
| Present (2 mild, 3 >mild) | 5 (3) | |
| Protein losing enteropathy, n (%) | 15 (9) | NA |
| Needing interventions | 10 (6) | |
| Cirrhosis, n (%) | 6 (4) | NA |
| Needing interventions | 2 (1) | |
| Dyspnea, n (%) | NA | |
| None | 139 (82) | |
| Present (24 mild, 7 more than mild) | 31 (18) | |
| Fatigue n (%) | NA | |
| None | 129 (76) | |
| Present | 41 (24) | |
| Edema n (%) | NA | |
| None | 155 (91) | |
| Present (13 mild, 2 more than mild) | 15 (9) | |
AF indicates atrial flutter/fibrillation; NA, not applicable; RAS antagonist, angiotensin‐converting enzyme inhibitor and angiotensin blocker; SVT, supraventricular tachycardia.
Vascular Function
As compared to controls, heart rate (HR) was higher and SBP was lower in Fontan patients (Table 3). As expected, in both Fontan and controls, afterload (Ea) increased with HR and similarly in Fontan patients and controls (Figure 2A). Indices of vascular properties were similar in the 2 groups (Table 3). As compared to SLV, HR, SBP, and aortic stiffness, SVR and Ea were similar in SRV Fontan patients (Table 3).
Table 3.
Arterial and Ventricular Properties in Fontan and Control Patients
| All Fontan | SRV | SLV | Controls | |
|---|---|---|---|---|
| n | 170 | 57 | 113 | 170 |
| Heart rate, bpm | 74±15a | 74±15a | 74±15a | 70±11 |
| Vascular properties | ||||
| Systolic blood pressure, mm Hg | 110±15a | 109±14a | 110±15a | 115±14 |
| Pulse pressure, mm Hg | 43±12 | 43±12 | 43±12 | 44±9 |
| Aortic compliance, mL/mm Hg | 2.05±0.92 | 2.03±0.83 | 2.05±0.97 | 1.90±0.49 |
| SVRI, dyne·s·cm−5·m2 | 2128±794 | 2020±548a | 2184±892 | 2216±403 |
| Effective arterial elastance (Ea), mm Hg/mL | 1.35±0.55 | 1.30±0.39 | 1.38±0.61 | 1.33±0.30 |
| Ventricular geometry | ||||
| Ventricular end‐diastolic volume index, mL/m2 | 96±34a | 101±26a | 94±38a | 73±13 |
| Posterior wall thickness, mm | 9.1±2.3a | 8.6±2.1 | 9.3±2.3a | 8.1±1.4 |
| Ventricular mass index, g/m2 | 92±32a | 91±28a | 92±34a | 69±15 |
| Systolic function, VA coupling and efficiency | ||||
| Ejection fraction, % | 50±9a | 48±8a, b | 51±10a | 63±5 |
| Stroke volume index, mL/m2 | 47±15 | 48±13 | 46±16 | 45±7 |
| Cardiac index, L/min per m2 | 3.35±1.01a | 3.36±0.85a | 3.34±1.09 | 3.16±0.49 |
| End‐systolic elastance (Ees), mm Hg/mL | 1.39±0.67a | 1.19±0.41a, b | 1.48±0.75a | 2.37±0.63 |
| Ees×mass, mm Hg·g/mL | 215±115a | 174±64a, b | 229±125a | 283±79 |
| V0, mL | −0.1±35 | −1.0±31.9 | 0.3±37.2 | 2.4±11 |
| VA coupling ratio, Ees/Ea | 1.05±0.35a | 0.97±0.34a, b | 1.09±0.36a | 1.80±0.34 |
| PRSW, erg·cm−3·103 | 72±17a | 70±18a | 72±17a | 91±14 |
| Peak power index, mm Hg/s | 320±94a | 307±109a | 326±86a | 369±77 |
| Mechanical efficiency (EW/PVA) | 0.62±0.08a | 0.60±0.09a, b | 0.63±0.08a | 0.74±0.04 |
| Energetic efficiency, % | 19.8±2.6a | 19.4±2.8a | 20.1±2.4a | 23.4±1.2 |
| Diastolic function | ||||
| DWS | 0.27±0.11a | 0.23±0.12a, b | 0.28±0.11a | 0.39±0.07 |
| E/A ratio (n with data) | 1.50±0.67a (154) | 1.46±0.65a (49) | 1.52±0.68a (105) | 1.88±0.54 (164) |
| Deceleration time, ms (n with data) | 182±48 (146) | 185±52 (47) | 181±46 (99) | 184±27 (161) |
| PV atrial velocity, m/s (n with data) | 0.30±0.14a (90) | 0.28±0.11a (26) | 0.31±0.15a (64) | 0.25±0.09 (151) |
| PV S/D flow velocity ratio (n with data) | 0.84±0.54a (61) | 0.91±0.66 (20) | 0.81±0.48a (41) | 0.98±0.35 (160) |
| IVRT, ms (n with data) | 69±32a (92) | 73±34a (29) | 66±31a (63) | 54±22 (170) |
bpm indicates beats per minute; DWS, diastolic wall strain; IVRT, isovolumic relaxation time; PRSW, preload recruitable stroke work; PV, pulmonary vein; S/D, systolic/diastolic; SLV, single left ventricle; SRV, single right ventricle; SVRI, systemic vascular resistance index; VA coupling, ventricular‐arterial coupling; V0, ventricular volume axis intercept of end‐systolic pressure volume relationship.
P<0.05 versus controls.
P<0.05 SRV versus SLV.
Figure 2.
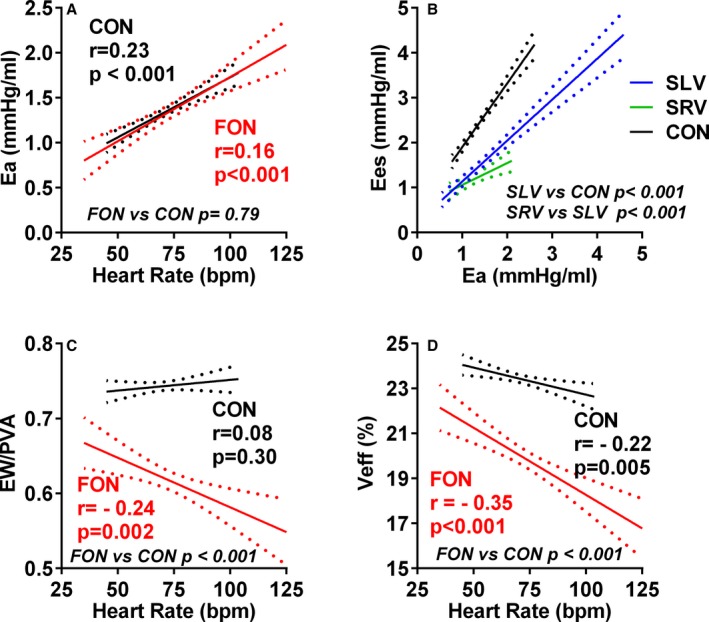
Ventricular and arterial properties in Fontan (FON) patients and controls (CON). The regression lines and the 95% CIs for the regression are shown. Arterial elastance (Ea) varies similarly with heart rate in control and Fontan patients (A). The increase of end‐systolic elastance (Ees) with Ea in single left ventricle (SLV) and single right ventricle (SRV) Fontan patients was blunted compared to control (B). Ventricular efficiency decreases more dramatically with heart rate in Fontan (P for slope difference, <0.05 for both) than control patients (C and D). bpm, beats per minute.
Ventricular Geometry
EDV index, wall thickness, and ventricular mass were greater in Fontan, as compared to control, patients. In Fontan patients, 39% had greater EDV index than 2 SDs above the mean (>99 mL/m2) for EDV index in controls. Ventricular geometry was similar in SLV and SRV Fontan patients (Table 3).
Ventricular Function, VA Coupling, and Ventricular Efficiency
Compared to controls, EF, Ees, Ees normalized to ventricular mass, PRSW, PPI, and mechanical and energetic efficiency were all lower in Fontan patients whereas V0 was similar (Table 3). VA coupling (Ees/Ea) was more impaired in Fontan patients than controls (Figure 2B and Table 3). Mechanical and energetic efficiency declined more dramatically with HR in Fontan patients (P for interaction, <0.05 for both) than controls and were lower than controls, even after adjusting for HR (Figure 2C and 2D). As compared to SLV, most (EF, Ees, Ees normalized to ventricular mass, Ees/Ea, and mechanical efficiency) of ventricular functional indices were significantly lower in SRV.
Among Fontan patients, EF was severely (<40%) reduced in 16 (10%), mildly (40–49%) reduced in 60 (37%), and preserved (≥50%) in 87 (53%). EF is a load‐dependent index of contractility, and thus many load‐independent indices of contractile performance may show some correlation with EF. However, Ees/Ea, PRSW, and mechanical and energetic efficiency were all lower in Fontan patients than controls after adjusting for EF and lower in Fontan than control patients across the overlapping (EF, >50%) EF range, demonstrating significant contractile dysfunction even in the presence of preserved EF in Fontan patients (Figure 3).
Figure 3.
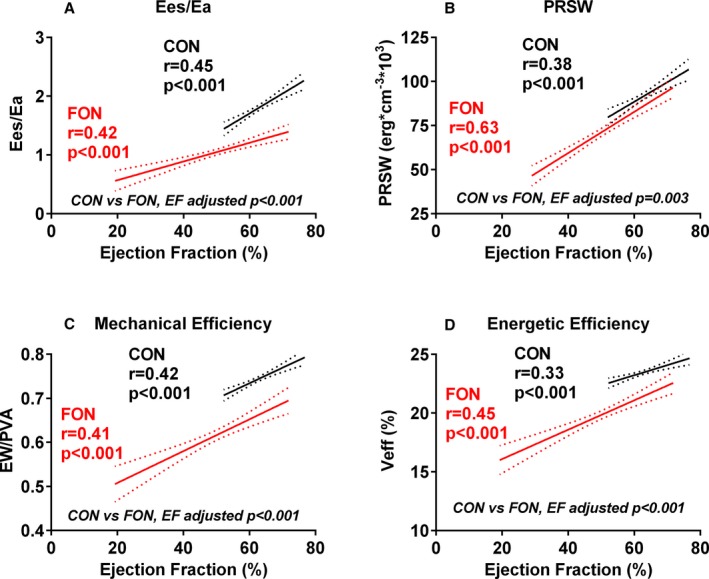
Ventricular function in Fontan (FON) patients and controls (CON). Relationship to ejection fraction. Whereas ventricular arterial coupling (Ees/Ea; A), preload recruitable stroke work (PRSW; B) and mechanical (C) and energetic (D) efficiency all decrease with decreasing EF in Fontan and control patients, all these indices are lower in Fontan patients versus controls across the overlapping range of EFs. Ea indicates arterial elastance; Ees, end‐systolic elastance.
Aortic reconstruction can disrupt the relationship between central and brachial blood pressure. Relatively few Fontan patients (n=16) had a history of aortic reconstruction and none had residual coarctation (Table 1). Excluding Fontan patients with a history of aortic reconstruction (and their matched controls), our primary findings (average Ea, Ees, and Ees/Ea in Fontan patients vs controls) were essentially identical, and thus Ea was still similar in Fontan and controls and Ees and Ees/Ea were still significantly lower (P<0.05 for both) in Fontan patients than controls.
Diastolic Function
Compared to controls, DWS was lower in Fontan patients, suggesting increased myocardial diastolic stiffness (Table 3). Adjusting for EF, DWS remained lower in Fontan patients (Figure 4). Conventional Doppler indices supported the presence of impaired relaxation (lower E/A and longer isovolumic relaxation time) and elevated LV filling pressures (lower S/D ratio of pulmonary venous flow and higher pulmonary reverse flow velocity during atrial contraction; Table 3).
Figure 4.
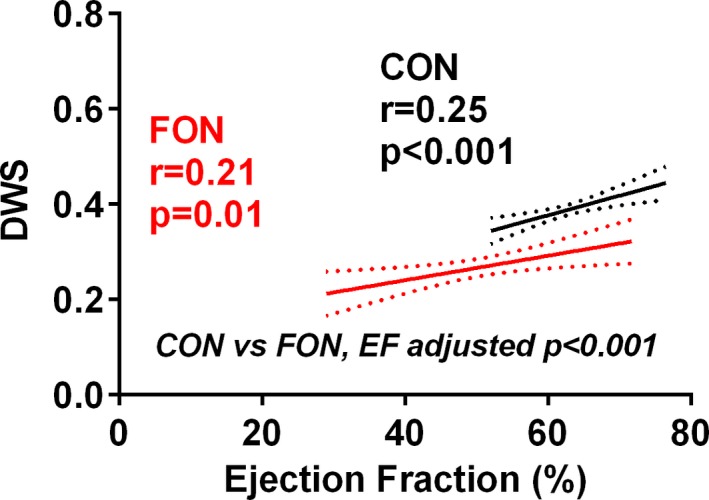
Diastolic wall strain (DWS) is lower in Fontan (FON) than control (CON) patients even at similar levels of ejection fraction (EF). The relationship between EF and DWS in Fontan and control patients and the results of multivariable linear regression analysis comparing DWS between groups while adjusting for the covariate (EF) are shown. DWS is lower (greater myocardial stiffness) in Fontan patients, even adjusting for EF. This figure demonstrates that over a range of overlapping EF, DWS was still lower in Fontan than control patients.
Compared to SLV, DWS was lower in SRV Fontan patients (Table 3). Adjusting for EF, this difference was not significant (P=0.11). Doppler parameters were not significantly different between SLV and SRV patients. Whereas blood pressure in non‐TA (tricuspid atresia) morphology was higher than those with tricuspid atresia, there were no differences in load‐insensitive cardiovascular properties among SLV Fontan patients (Table 4).
Table 4.
Ventricular and Arterial Properties in SLV Fontan Patients With Tricuspid Atresia (TA) Versus Non‐TA Morphology
| SLV:TA | SLV:Non‐TA | P Value | |
|---|---|---|---|
| n | 48 | 65 | |
| Age, y | 28.8±8.5 | 25.4±7.1 | 0.023 |
| Time post‐Fontan, years | 12.6±8.8 | 12.8±7.5 | 0.88 |
| Body surface area, m2 | 1.73±0.20 | 1.78±0.22 | 0.25 |
| Stable clinical status, n (%) | 33 (69) | 53 (83) | 0.21 |
| Heart rate, bpm | 73±13 | 75±17 | 0.45 |
| Vascular properties | |||
| Systolic blood pressure, mm Hg | 106±14 | 112±15 | 0.045 |
| Pulse pressure, mm Hg | 40±12 | 45±12 | 0.027 |
| Aortic compliance, mm Hg/mL | 2.08±1.00 | 2.04±0.95 | 0.82 |
| SVRI, dyne·s·cm−5·m2 | 2235±761 | 2147±980 | 0.62 |
| Effective arterial elastance (Ea), mm Hg/mL | 1.42±0.60 | 1.35±0.62 | 0.56 |
| Ventricular geometry | |||
| Ventricular end‐diastolic volume index, mL/m2 | 87±32 | 99±41 | 0.09 |
| Ventricular mass index, g/m2 | 90±37 | 94±32 | 0.61 |
| Systolic function, VA coupling and efficiency | |||
| Ejection fraction, % | 52±10 | 50±10 | 0.30 |
| Stroke volume index, mL/m2 | 44±15 | 48±16 | 0.19 |
| Cardiac index, L/min per m2 | 3.2±1.0 | 3.5±1.1 | 0.14 |
| End‐systolic elastance (Ees), mm Hg/mL | 1.58±0.73 | 1.41±0.76 | 0.24 |
| VA coupling ratio, Ees/Ea | 1.14±0.36 | 1.05±0.35 | 0.21 |
| PRSW, erg·cm−3·103 | 70.6±15.9 | 73.5±18.1 | 0.41 |
| Peak power index, mm Hg/mL | 320±90 | 331±83 | 0.52 |
| Energetic efficiency, % | 20.4±2.3 | 19.8±2.5 | 0.22 |
| Diastolic function | |||
| DWS | 0.28±0.11 | 0.28±0.11 | 0.83 |
bpm indicates beats per minute; DWS, diastolic wall strain; PRSW, preload recruitable stroke work; SLV, single left ventricle; SVRI, systemic vascular resistance index; VA coupling, ventricular‐arterial coupling.
Cardiac Output
CI was available in 163 Fontan and all control patients. On average, CI was within normal range in both groups, but higher in Fontan than control patients and similar in SLV and SRV Fontan patients (Table 3). However, 40 (25%) Fontan patients had a reduced (<2.5 L/min per m2) CI whereas just 8 (5%) control patients had a CI just below the partition value.
Among patients with preserved CI (123 Fontan, 162 control), CI was higher in Fontan than control patients (Figure 5A). This was attributed both to higher HR and SV index in Fontan patients (Figure 5B and 5C). The higher SV index was, in part, attributed to lower SVR and total afterload (Figure 5D and 5E). However, VA coupling was impaired with lower Ees at any Ea and thus lower EF in Fontan patients (Figure 5F and 5G). Despite lower contractility, eccentric remodeling with a higher EDV index for any EF in Fontan patients (Figure 5H) maintained SV.
Figure 5.
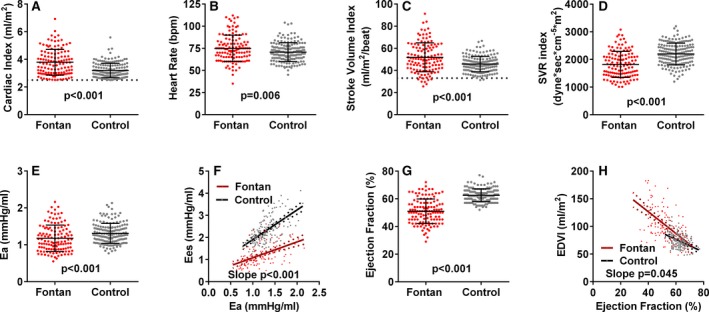
Ventricular arterial function in Fontan and control patients with normal cardiac index. Cardiac index (A) was higher in Fontan (n=123) than control patients (n=168). This was attributed both to higher heart rate (B) and stroke volume index (C) in Fontan patients. The higher stroke volume index was, in part, attributed to lower systemic vascular resistance (SVR) index (D) and total afterload (E) as assessed by arterial elastance (Ea). However, ventricular‐arterial (VA) coupling (F) was impaired with lower ventricular systolic elastance (Ees) at any Ea and thus lower EF in Fontan patients (G). Despite lower contractility, eccentric remodeling with a higher end‐diastolic volume index (EDVI) for any EF (H) in Fontan patients maintained stroke volume. Dotted lines indicate lower limit of normal for cardiac index (A) and stroke volume index (C). bpm indicates beats per minute; CI, cardiac index.
Several differences were observed in Fontan patients with preserved versus reduced CI (Figure 6A). The lower CI was not attributed to lower HR, but rather a lower SV index associated with higher SVR and total afterload (Figure 6B through 6E). However, contractility was not different among Fontan patients with reduced versus preserved CI given that Ees was similar at any Ea (Figure 6F) and EF was similar (48±10 vs 51±9; P=0.07). Rather, Fontan patients with reduced CI had a smaller EDV index for any EF (Figure 6G) and lower diastolic wall strain (Figure 6H), suggesting unique remodeling and worse diastolic dysfunction.
Figure 6.
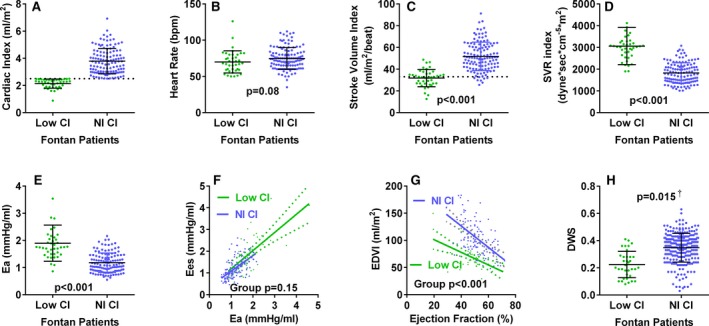
Ventricular arterial properties in Fontan patients with preserved versus reduced cardiac index. As compared to those with preserved cardiac index, Fontan patients with reduced (<2.5 L/min per m2) cardiac index (A) had similar heart rate (B), but lower stroke volume index (C), and higher systemic vascular resistance (SVR) index (D) and total afterload (E). Ventricular systolic elastance (Ees) was similar at any afterload (F) indicating similar ventricular arterial coupling. Fontan patients with reduced CI had a smaller end‐diastolic volume index (EDVI) for any ejection fraction (EF; G) and lower diastolic wall strain (DWS; H), suggesting greater diastolic myocardial stiffness. † P value adjusted for EF. Dotted lines indicate lower limit of normal for cardiac index (A) and stroke volume index (C). bpm indicates beats per minute; CI, cardiac index; Nl, normal.
Ventricular and Arterial Properties According to Clinical Status and Medical Therapy
Differences between controls and Fontan patients were similar when analysis was restricted to Fontan patients with stable clinical status (Table 5). The cause of clinical instability varied (Table 6). Compared to those not treated, Fontan patients treated with RAS antagonists or beta‐blockers had better vascular function, but similar ventricular geometry and ventricular function (Tables 7 and 8).
Table 5.
Ventricular and Arterial Properties in Fontan Patients With Stable Clinical Status Versus Age, Sex, and Body Size Matched Controls
| Stable Fontan | Matched Control | P Value | |
|---|---|---|---|
| n | 130 | 130 | |
| Age, y | 25.7±8.0 | 26.0±7.8 | 0.74 |
| Body surface area, m2 | 1.75±0.22 | 1.79±0.24 | 0.15 |
| Time post‐Fontan, year | 12.3±7.5 | NA | NA |
| Single right ventricle, n (%) | 44 (34) | NA | NA |
| Protein losing enteropathy/cirrhosis, n (%) | 8/1 (7%) | NA | NA |
| Any RAS antagonist, n (%) | 51 (39) | 0 | NA |
| Any beta‐blocker, n (%) | 17 (13) | 0 | NA |
| Heart rate, beat/min | 74±14 | 71±11 | 0.068 |
| Vascular properties | |||
| Systolic blood pressure, mm Hg | 110±14 | 116±14 | 0.0018 |
| Pulse pressure, mm Hg | 43±12 | 44±9 | 0.64 |
| Aortic compliance, mm Hg/mL | 2.08±0.94 | 1.89±0.47 | 0.043 |
| SVRI, dyne·s·cm−5·m2 | 2096±798 | 2224±411 | 0.11 |
| Effective arterial elastance (Ea), mm Hg/mL | 1.33±0.51 | 1.34±0.30 | 0.78 |
| Ventricular geometry | |||
| Ventricular end‐diastolic volume index, mL/m2 | 99±36 | 73±13 | <0.0001 |
| Ventricular mass index, g/m2 | 94±33 | 69±16 | <0.0001 |
| Systolic function, VA coupling and efficiency | |||
| Ejection fraction, % | 50±9 | 63±5 | <0.0001 |
| Stroke volume index, mL/m2 | 48±15 | 45±7 | 0.058 |
| Cardiac index, L/min per m2 | 3.41±1.00 | 3.17±0.51 | 0.016 |
| End‐systolic elastance (Ees), mm Hg/mL | 1.37±0.65 | 2.39±0.63 | <0.0001 |
| VA coupling ratio, Ees/Ea | 1.05±0.35 | 1.80±0.33 | <0.0001 |
| PRSW, erg·cm−3·103 | 72±17 | 92±14 | <0.0001 |
| Peak power index, mm Hg/s | 315±88 | 371±76 | <0.0001 |
| Ventricular efficiency, % | 19.9±2.5 | 23.5±1.2 | <0.0001 |
| Diastolic function | |||
| DWS | 0.27±0.11 | 0.39±0.07 | <0.0001a |
DWS indicates diastolic wall strain; NA, not applicable; PRSW, preload recruitable stroke work; RAS antagonist, angiotensin‐converting enzyme inhibitor and angiotensin blocker; SVRI, systemic vascular resistance index; VA coupling, ventricular‐arterial coupling.
P<0.001 adjusting for ejection fraction in mulitivariable linear regression.
Table 6.
Reason for Impaired Clinical Status in Fontan Patients
| Clinical Condition | n |
|---|---|
| Heart failure | 2 |
| Heart failure+atrial arrhythmia | 6 |
| Heart failure+othera | 4 |
| Thrombus | 3 |
| Symptomatic atrial arrhythmia alone | 14 |
| Atrial arrhythmia+lung disease (amiodarone) | 2 |
| Atrial arrhythmia+cirrhosis | 2 |
| Atrial arrhythmia+thrombus | 1 |
| Fontan obstruction/conversion | 3 |
| Protein losing enteropathy | 3 |
| Cirrhosis | 1 |
Lung disease (n=2), Fontan obstruction (n=1), cirrhosis, and atrial arrhythmia (n=1).
Table 7.
Ventricular and Arterial Properties in Fontan Patients According to Renin Angiotensin System (RAS) Antagonist Therapy
| RAS Antagonist | No RAS Antagonist | P Value | |
|---|---|---|---|
| n | 60 | 110 | |
| Sex, female n (%) | 27 (45) | 48 (44) | 0.86 |
| Age, y | 25.5±9.3 | 26.6±7.2 | 0.40 |
| Body surface area, m2 | 1.75±0.25 | 1.74±0.19 | 0.75 |
| Time post‐Fontan, y | 15.4±5.0 | 10.8±8.4 | 0.0001 |
| Single right ventricle, n (%) | 24 (40) | 33 (30) | 0.19 |
| Stable clinical status, n (%) | 51 (85) | 79 (72) | 0.28 |
| Heart rate, beat/min | 73±14 | 75±16 | 0.34 |
| Vascular properties | |||
| Systolic blood pressure, mm Hg | 110±14 | 109±15 | 0.68 |
| Pulse pressure, mm Hg | 44±11 | 43±12 | 0.47 |
| Aortic compliance, mL/mm Hg | 2.13±0.85 | 2.00±0.96 | 0.39 |
| SVRI, dyne·s·cm−5·m2 | 1944±620 | 2237±865 | 0.023 |
| Effective arterial elastance (Ea), mm Hg/mL | 1.23±0.41 | 1.42±0.60 | 0.030 |
| Ventricular geometry | |||
| Ventricular end‐diastolic volume index, mL/m2 | 99±32 | 95±36 | 0.45 |
| Ventricular mass index, g/m2 | 94±38 | 90±29 | 0.52 |
| Systolic function, VA coupling and efficiency | |||
| Ejection fraction, % | 52±8 | 49±10 | 0.037 |
| Stroke volume index, mL/m2 | 51±15 | 45±14 | 0.013 |
| Cardiac index, L/min per m2 | 3.60±1.00 | 3.20±0.99 | 0.016 |
| End‐systolic elastance (Ees), mm Hg/mL | 1.27±0.51 | 1.46±0.74 | 0.083 |
| VA coupling ratio, Ees/Ea | 1.04±0.32 | 1.05±0.37 | 0.88 |
| PRSW, erg·cm−3·103 | 76±16 | 69±17 | 0.014 |
| Peak power index, mm Hg/s | 314±86 | 323±99 | 0.57 |
| Ventricular efficiency, % | 20.1±2.1 | 19.7±2.8 | 0.34 |
| Diastolic function | |||
| DWS | 0.26±0.11 | 0.27±0.12 | 0.41 |
DWS indicates diastolic wall strain; PRSW, preload recruitable stroke work; SVRI, systemic vascular resistance index; VA coupling, ventricular‐arterial coupling.
Table 8.
Ventricular and Arterial Properties in Fontan Patients According to Beta‐Blocker Therapy
| Any Beta‐Blocker (n=25) | No Beta‐Blocker (n=145) | P Value | |
|---|---|---|---|
| Sex, female n (%) | 11 (44) | 64 (44) | 0.98 |
| Age, y | 27.9±9.0 | 26.0±7.8 | 0.27 |
| Body surface area, m2 | 1.75±0.23 | 1.75±0.21 | 0.99 |
| Time post‐Fontan, y | 14.6±7.3 | 12.0±7.8 | 0.12 |
| Single right ventricle, n (%) | 10 (40) | 47 (32) | 0.46 |
| Stable clinical status, n (%) | 17 (68) | 113 (78) | 0.51 |
| Heart rate, beat/min | 71±15 | 75±15 | 0.25 |
| Vascular properties | |||
| Systolic blood pressure, mm Hg | 100±13 | 111±15 | 0.0003 |
| Pulse pressure, mm Hg | 38±12 | 44±12 | 0.021 |
| Aortic compliance, mL/mm Hg | 2.56±1.13 | 1.95±0.85 | 0.0024 |
| SVRI, dyne·s·cm−5·m2 | 1794±495 | 2189±823 | 0.022 |
| Effective arterial elastance (Ea), mm Hg/mL | 1.10±0.35 | 1.40±0.56 | 0.010 |
| Ventricular geometry | |||
| Ventricular end‐diastolic volume index, mL/m2 | 99±22 | 96±36 | 0.61 |
| Ventricular mass index, g/m2 | 83±30 | 93±33 | 0.19 |
| Systolic function, VA coupling and efficiency | |||
| Ejection fraction, % | 51±8 | 50±10 | 0.48 |
| Stroke volume index, mL/m2 | 51±13 | 46±15 | 0.13 |
| Cardiac index, L/min per m2 | 3.54±0.91 | 3.32±1.03 | 0.31 |
| End‐systolic elastance (Ees), mm Hg/mL | 1.22±0.49 | 1.42±0.70 | 0.18 |
| VA coupling ratio (Ees/Ea) | 1.15±0.39 | 1.03±0.34 | 0.12 |
| PRSW, erg·cm−3·103 | 73±14 | 71±18 | 0.67 |
| Peak power index, mm Hg/s | 227±82 | 328±94 | 0.008 |
| Energetic efficiency, % | 20.7±2.6 | 19.7±2.5 | 0.061 |
| Diastolic function | |||
| DWS | 0.27±0.12 | 0.27±0.11 | 0.89 |
DWS indicates diastolic wall strain; PRSW, preload recruitable stroke work; SVRI, systemic vascular resistance index; VA coupling, ventricular‐arterial coupling.
Discussion
As compared to normal controls, adult Fontan patients had similar vascular function and ventricular afterload, but eccentric remodeling and impaired contractility, as assessed by multiple load‐insensitive indices. Systolic performance was impaired even in the presence of preserved EF. Ventricular mechanical and energetic efficiencies were reduced in Fontan patients with heightened sensitivity of both to HR. On average, despite reduced ventricular contractility and impaired VA coupling, CI was higher in Fontan patients, in part, attributed to relative tachycardia and eccentric remodeling. However, in 25% of Fontan patients, the Fontan circulation was unable to maintain CI and these patients were distinguished from more‐successful Fontan circulation patients by increased afterload, smaller ventricles, and worse myocardial diastolic function, rather than worse systolic performance. These data demonstrate the spectrum of ventricular vascular function in generally stable adult Fontan patients. Our findings contribute to our understanding of circulatory physiology in adult Fontan patients and have implications for the study of therapies to prevent progression to HF in this unique at‐risk population.
The Adult Fontan Population
Adult Fontan patients are increasingly referred for consideration of HF therapies.1, 21 The Alliance for Adult Research in Congenital Cardiology identified research priorities to advance the care of adults with CHD.22 Among the top priorities for Fontan patients were causes of Fontan failure and optimal drug therapies to preserve ventricular function. The current study addresses these priorities by examining ventricular vascular function and their coupling using relatively load‐independent indices.11, 12 Furthermore, given that one index of a successful Fontan circulation is the maintenance of normal resting systemic perfusion, we examined the ventricular and vascular properties in Fontan patients with or without normal CI.
Vascular Function
Multiple factors, including hypoxia, collateral vessels, aortic procedures, liver disease, and renin angiotensin system (RAS) activation, could alter arterial function in Fontan patients.1, 23 Whereas elevated afterload has been described in pediatric Fontan patients and in its theoretical models,3, 24, 25 overall, vascular resistance, arterial compliance, and the sensitivity of afterload to HR3, 8 were similar in adult Fontan patients and controls. Of note, Ea sensitivity to increasing HR in Fontan patients was virtually the same as controls, suggesting a minor contribution of the pulmonary circulation as described in the lump parameter simulation model.25 Use of RAS antagonists and beta‐blockers were associated with more‐favorable vascular function, but causality cannot be assessed in this cross‐sectional study.
Ventricular Geometry
In adult Fontan patients, on average, EDV was increased relative to controls, with the degree of eccentric remodeling being related to severity of systolic dysfunction. A cardiac magnetic resonance imaging study showed widely varying EDV index and EF in Fontan patients relative to controls, but did not address the relationship between remodeling and ventricular function.26 The relationship between systolic dysfunction and eccentric remodeling is consistent with typical findings in non‐CHD HF.11 LV wall thickness in Fontan patients was, on average, within normal limits, but significantly higher than in control patients. Fontan patients are exposed to cyanosis for years, a known stimulus for ventricular hypertrophy,27 and indeed a previous small study reported higher ventricular mass in Fontan patients as compared to healthy controls.28
Systolic Function, VA Coupling, and Ventricular Efficiency
In the normal circulation, arterial and end‐systolic ventricular elastances are coupled to optimize cardiovascular performance and efficiency.29 In adult Fontan patients, this normal coupling was perturbed owing to disproportionate impairment in systolic function. Impairment in contractile function was worse in SRV than SLV. Contractile dysfunction and ventricular inefficiency were apparent even in Fontan patients with preserved (>50%) EF.
A previous study of VA coupling in a small number (n=17) of clinically stable pediatric Fontan patients suggested that increased afterload, rather than reduced contractility, impaired VA coupling relative to controls.3 The other coupling studies in Fontan patients used similar methods as ours or even much simplified, less‐rigorous methods, but did not include similarly studied non‐Fontan patients to determine whether VA parameters were abnormal relative to persons without Fontan circulation.30, 31 A mathematical simulation study and an animal study of acute Fontan physiology indicated that immediately after creation of a Fontan circulation, Ees is mildly reduced whereas Ea is increased.24, 32 In contrast, chronically, in adult Fontan patients, we found that contractility was significantly decreased as assessed by multiple load‐insensitive measures of ventricular contractility (Ees, PRSW, and PPI) whereas Ea was similar to age, sex, and body size matched controls without CHD. Consistent with our data, the multicenter pediatric Fontan study showed that EF tended to decrease with increasing age and time post‐Fontan.2 Whereas the severity of ventricular remodeling and systolic dysfunction in the current cohort of clinically stable adult Fontan patients was less severe than that observed in similarly studied older adults with overt non‐CHD systolic HF,11 the impact of even mild systolic dysfunction may be significant in the Fontan circulation, particularly during exercise and may progress over time. Whether treatment with standard HF therapies used in non‐CHD HF patients with reduced EF would improve long‐term outcomes in adult Fontan patients remains an area of active investigation.1
Ventricular efficiency varied inversely with HR in Fontan patients, but much less so in control patients consistent with previous studies in experimental systolic dysfunction33 and with modeling studies of the Fontan circulation.25 HR dependence of both Ees and ventricular efficiency in Fontan patients provide insight into the adverse impact of tachycardia on clinical status in Fontan patients. The marked reduction in ventricular efficiency shown in adult Fontan patients may suggest a novel therapeutic target. A number of agents with the potential to modify myocardial energetics are currently under study in non‐CHD HF and may hold promise in the Fontan population.34
Although most measures of ventricular performance were significantly more impaired in SRV than SLV, these differences were not striking. These findings underscore the differences in adult versus pediatric Fontan cohorts, where SRV morphology is associated with worse EF and survival. Relatively preserved SRV function in adult Fontan patients may represent a form of survivorship bias. Notably, ventricular performance indices were similar in SLV patients with or without tricuspid atresia.
Diastolic Function
DWS is reflective of resistance to myocardial deformation during diastole and thus myocardial diastolic stiffness.16, 17 Impairment in diastolic function has been suggested in the pediatric Fontan population as well2 and may be related to the impact of the pre‐Fontan arterial hypoxia and volume overload, surgical procedures, renin‐angiotensin‐aldosterone system activation, inflammation, and other end‐organ dysfunction.1, 2, 35, 36 Here, diastolic, but not systolic, function was more impaired in patients with reduced CI, suggesting that ventricular diastolic function is a critical factor influencing circulatory success in Fontan patients.
Cardiac Output
The maintained, indeed augmented, CI in adult Fontan patients relative to controls may relate to eccentric remodeling, reduced afterload, or collateral circulations.37 Indeed, other studies assessing aortic blood flow by aortic phase‐contrast method have shown preserved CI in adult Fontan patients.38, 39 Average values for CI have varied widely in previous small studies of predominantly pediatric Fontan patients using a variety of volumetric or aortic or caval flow techniques, with studies showing normal or reduced CI in different cohorts.40, 41 Compared to Fontan patients with preserved CI, those with reduced CI had higher afterload and ventricular systolic elastance with similar VA coupling and EF, but less ventricular eccentric remodeling and worse myocardial diastolic function. These data may support consideration of invasive hemodynamic assessment in adult Fontan patients with reduced resting CI at echocardiography, even in the absence of clinical HF. Invasive assessment would exclude underfilling attributed to pulmonary vascular disease, quantify the severity of ventricular diastolic dysfunction, and guide therapeutic interventions or suitability for clinical trials investigating therapeutic strategies to prevent progression to HF.
Limitations
The retrospective nature of this study may introduce bias as patients returning to a tertiary referral surgical center may be more or less well than those who do not return. The Fontan patients spanned several operative eras, but are representative of adult Fontan survivors in the current era. Limitations of individual echo parameters over time and in Fontan patients have been discussed in the corresponding methods. Whereas our analyses suggest that these limitations do not have a critical impact on our findings and conclusions, the current study should provide a framework for future studies using alternate imaging modalities to confirm or refute our findings. Repeated invasive analyses in otherwise stable Fontan patients are difficult to justify and invasive assessment in only clinically deteriorating patients may provide misleading data concerning Fontan physiology. Thus, the current study, though large and using best available data and validated methodologies, should encourage further similar or improved noninvasive imaging derived analyses to provide confirmation.
Conclusion
Adult Fontan patients have contractile and diastolic dysfunction with normal afterload, impaired VA coupling, and reduced ventricular efficiency with heightened sensitivity to HR. The impact of ventricular morphology is minimal in adult Fontan survivors. Maintenance of CI is dependent on reduced afterload, eccentric remodeling, and preservation of diastolic function. These data underscore fundamental role of HR management in preserving cardiac function and contribute to our understanding of circulatory physiology in adult Fontan patients. This study also has implications for the study of therapies to prevent progression to HF in this unique at‐risk population.
Sources of Funding
Dr Saiki's time was funded, in part, by the International Exchange Aid, Fukuda Foundation for Medical Technology, Japan. Dr Redfield's time was funded, in part, by the National Institutes of Health (U10 HL 110262, P01 HL 76611 and R01 HL 105418).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e003887 doi: 10.1161/JAHA.116.003887)
References
- 1. Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL. Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation. 2016;133:770–801. [DOI] [PubMed] [Google Scholar]
- 2. Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ III. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Senzaki H, Masutani S, Ishido H, Taketazu M, Kobayashi T, Sasaki N, Asano H, Katogi T, Kyo S, Yokote Y. Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol. 2006;47:2528–2535. [DOI] [PubMed] [Google Scholar]
- 4. Sanders SP, Yeager S, Williams RG. Measurement of systemic and pulmonary blood flow and QP/QS ratio using Doppler and two‐dimensional echocardiography. Am J Cardiol. 1983;51:952–956. [DOI] [PubMed] [Google Scholar]
- 5. Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. [DOI] [PubMed] [Google Scholar]
- 6. Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. [DOI] [PubMed] [Google Scholar]
- 7. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. [DOI] [PubMed] [Google Scholar]
- 8. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation. 2005;112:2254–2262. [DOI] [PubMed] [Google Scholar]
- 9. Senzaki H, Chen CH, Kass DA. Single‐beat estimation of end‐systolic pressure‐volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–2506. [DOI] [PubMed] [Google Scholar]
- 10. Saiki H, Kuwata S, Kurishima C, Masutani S, Senzaki H. Potential of the single beat estimation for end systolic pressure‐volume relation in patients with single ventricular heart (abstract). Circulation. 2015;132:A12735. [Google Scholar]
- 11. Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, St John Sutton M, Cappola TP. Ventricular‐arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular‐vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol. 1986;250:R1021–R1027. [DOI] [PubMed] [Google Scholar]
- 14. Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single‐beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284:H744–H750. [DOI] [PubMed] [Google Scholar]
- 15. Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta‐adrenergic‐stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. [DOI] [PubMed] [Google Scholar]
- 16. Takeda Y, Sakata Y, Higashimori M, Mano T, Nishio M, Ohtani T, Hori M, Masuyama T, Kaneko M, Yamamoto K. Noninvasive assessment of wall distensibility with the evaluation of diastolic epicardial movement. J Card Fail. 2009;15:68–77. [DOI] [PubMed] [Google Scholar]
- 17. Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL, Redfield MM. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hart JP, Cabreriza SE, Gallup CG, Hsu D, Spotnitzt HM. Validation of left ventricular end‐diastolic volume from stroke volume and ejection fraction. ASAIO J. 2002;48:654–657. [DOI] [PubMed] [Google Scholar]
- 19. Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. [DOI] [PubMed] [Google Scholar]
- 20. Pombo JF, Troy BL, Russell RO Jr. Left ventricular volumes and ejection fraction by echocardiography. Circulation. 1971;43:480–490. [DOI] [PubMed] [Google Scholar]
- 21. Griffiths ER, Kaza AK, Wyler von Ballmoos MC, Loyola H, Valente AM, Blume ED, del Nido P. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg. 2009;88:558–563; discussion 563‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cotts T, Khairy P, Opotowsky AR, John AS, Valente AM, Zaidi AN, Cook SC, Aboulhosn J, Ting JG, Gurvitz M, Landzberg MJ, Verstappen A, Kay J, Earing M, Franklin W, Kogon B, Broberg CS. Clinical research priorities in adult congenital heart disease. Int J Cardiol. 2014;171:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hebson CL, McCabe NM, Elder RW, Mahle WT, McConnell M, Kogon BE, Veledar E, Jokhadar M, Vincent RN, Sahu A, Book WM. Hemodynamic phenotype of the failing Fontan in an adult population. Am J Cardiol. 2013;112:1943–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nogaki M, Senzaki H, Masutani S, Kobayashi J, Kobayashi T, Sasaki N, Asano H, Kyo S, Yokote Y. Ventricular energetics in Fontan circulation: evaluation with a theoretical model. Pediatr Int. 2000;42:651–657. [DOI] [PubMed] [Google Scholar]
- 25. Sundareswaran KS, Pekkan K, Dasi LP, Whitehead K, Sharma S, Kanter KR, Fogel MA, Yoganathan AP. The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise. Am J Physiol Heart Circ Physiol. 2008;295:H2427–H2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eicken A, Fratz S, Gutfried C, Balling G, Schwaiger M, Lange R, Busch R, Hess J, Stern H. Hearts late after Fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. J Am Coll Cardiol. 2003;42:1061–1065. [DOI] [PubMed] [Google Scholar]
- 27. Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114:565–571. [DOI] [PubMed] [Google Scholar]
- 28. Vitarelli A, Conde Y, Cimino E, D'Angeli I, D'Orazio S, Ventriglia F, Bosco G, Colloridi V. Quantitative assessment of systolic and diastolic ventricular function with tissue Doppler imaging after Fontan type of operation. Int J Cardiol. 2005;102:61–69. [DOI] [PubMed] [Google Scholar]
- 29. Kubota T, Alexander J Jr, Itaya R, Todaka K, Sugimachi M, Sunagawa K, Nose Y, Takeshita A. Dynamic effects of carotid sinus baroreflex on ventriculoarterial coupling studied in anesthetized dogs. Circ Res. 1992;70:1044–1053. [DOI] [PubMed] [Google Scholar]
- 30. Ozawa H, Ueno T, Iwai S, Kawata H, Nishigaki K, Kishimoto H, Sawa Y. Contractility‐afterload mismatch in patients with protein‐losing enteropathy after the Fontan operation. Pediatr Cardiol. 2014;35:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shabanian R, Shahbaznejad L, Razaghian A, Kiani A, Rahimzadeh M, Seifirad S, Kocharian A, Gilani JS, Navabi MA. Sildenafil and ventriculo‐arterial coupling in Fontan‐palliated patients: a noninvasive echocardiographic assessment. Pediatr Cardiol. 2013;34:129–134. [DOI] [PubMed] [Google Scholar]
- 32. Szabo G, Buhmann V, Graf A, Melnitschuk S, Bahrle S, Vahl CF, Hagl S. Ventricular energetics after the Fontan operation: contractility‐afterload mismatch. J Thorac Cardiovasc Surg. 2003;125:1061–1069. [DOI] [PubMed] [Google Scholar]
- 33. Ohte N, Cheng CP, Little WC. Tachycardia exacerbates abnormal left ventricular‐arterial coupling in heart failure. Heart Vessels. 2003;18:136–141. [DOI] [PubMed] [Google Scholar]
- 34. Ormerod JO, Ashrafian H, Frenneaux MP. Impaired energetics in heart failure—a new therapeutic target. Pharmacol Ther. 2008;119:264–274. [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto M, Saiki H, Tamai A, Seki M, Inuzuka R, Masutani S, Senzaki H. Ventricular fibrogenesis activity assessed by serum levels of procollagen type III N‐terminal amino peptide during the staged Fontan procedure. J Thorac Cardiovasc Surg. 2016;151:1518–1526. [DOI] [PubMed] [Google Scholar]
- 36. Saiki H, Kuwata S, Kurishima C, Iwamoto Y, Ishido H, Masutani S, Senzaki H. Aldosterone‐cortisol imbalance immediately after Fontan operation with implications for abnormal fluid homeostasis. Am J Cardiol. 2014;114:1578–1583. [DOI] [PubMed] [Google Scholar]
- 37. Valverde I, Nordmeyer S, Uribe S, Greil G, Berger F, Kuehne T, Beerbaum P. Systemic‐to‐pulmonary collateral flow in patients with palliated univentricular heart physiology: measurement using cardiovascular magnetic resonance 4D velocity acquisition. J Cardiovasc Magn Reson. 2012;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rathod RH, Prakash A, Kim YY, Germanakis IE, Powell AJ, Gauvreau K, Geva T. Cardiac magnetic resonance parameters predict transplantation‐free survival in patients with Fontan circulation. Circ Cardiovasc Imaging. 2014;7:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van De Bruaene A, La Gerche A, Claessen G, De Meester P, Devroe S, Gillijns H, Bogaert J, Claus P, Heidbuchel H, Gewillig M, Budts W. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging. 2014;7:265–273. [DOI] [PubMed] [Google Scholar]
- 40. Ovroutski S, Nordmeyer S, Miera O, Ewert P, Klimes K, Kuhne T, Berger F. Caval flow reflects Fontan hemodynamics: quantification by magnetic resonance imaging. Clin Res Cardiol. 2012;101:133–138. [DOI] [PubMed] [Google Scholar]
- 41. Latus H, Gerstner B, Kerst G, Moysich A, Gummel K, Apitz C, Bauer J, Schranz D. Effect of inhaled nitric oxide on blood flow dynamics in patients after the Fontan procedure using cardiovascular magnetic resonance flow measurements. Pediatr Cardiol. 2016;37:504–511. [DOI] [PubMed] [Google Scholar]


