Abstract
Background
Remote ischemic preconditioning (RIPC) is an attractive therapeutic procedure for protecting the heart against ischemia/reperfusion injury. Despite evidence of humoral mediators transported through the circulation playing a critical role, their actual identities so far remain unknown. We sought to identify plasmatic RIPC‐induced metabolites that may play a role.
Methods and Results
Rat plasma samples from RIPC and control groups were analyzed using a targeted metabolomic approach aimed at measuring 188 metabolites. Principal component analysis and orthogonal partial least‐squares discriminant analysis were used to identify the metabolites that discriminated between groups. Plasma samples from 50 patients subjected to RIPC were secondarily explored to confirm the results obtained in rats. Finally, a combination of the metabolites that were significantly increased in both rat and human plasma was injected prior to myocardial ischemia/reperfusion in rats. In the rat samples, 124 molecules were accurately quantified. Six metabolites (ornithine, glycine, kynurenine, spermine, carnosine, and serotonin) were the most significant variables for marked differentiation between the RIPC and control groups. In human plasma, analysis confirmed ornithine decrease and kynurenine and glycine increase following RIPC. Injection of the glycine and kynurenine alone or in combination replicated the protective effects of RIPC seen in rats.
Conclusions
We have hereby reported significant variations in a cocktail of amino acids and biogenic amines after remote ischemic preconditioning in both rat and human plasma.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01390129.
Keywords: amino acids, infarction, ischemia, reperfusion
Subject Categories: Basic Science Research, Ischemia, Biomarkers, Coronary Artery Disease
Introduction
Cardiac remote ischemic preconditioning (RIPC) is a phenomenon whereby transient nonlethal ischemia episodes applied to a tissue remote from the heart protect the myocardium from ischemia–reperfusion (I/R) injury.1, 2, 3 Using transient limb ischemia as a stimulus, RIPC has emerged as an attractive strategy in several clinical settings posing the risk of cardiac I/R damage.4, 5 Despite intensive research, the actual protective mechanisms underlying RIPC remain unknown, yet a well‐accepted theory holds that a neural pathway to the remote organ and blood‐borne factors are involved, which activate the survival signaling pathways in the target organ or tissue.6, 7
Several studies have suggested that endogenous factors are implicated in protective mechanisms, such as adenosine,8 endocannabinoids,9 bradykinin,10 opioids,11 erythropoietin,12 microvesicles,13 apolipoprotein A‐I,14, 15 miRNA 144,16 nitrite,17and stromal‐derived factor 1α.18 However, the humoral factors that mediate RIPC have yet to be identified.
Small metabolites, such as lipids, sugars, carnitines, and amino acids, could be good candidates for the protective responses triggered by RIPC. Indeed, they have not only structural or energetic functions but they could also act on receptors located in the plasmatic membrane or cytoplasm, thereby inducing cellular response.19, 20, 21
Metabolomic approaches enable the identification of metabolic signatures of specific physiological and pathological conditions. We therefore hypothesized that metabolic fingerprints induced by RIPC may enable the identification of RIPC metabolic biomarkers and thus potential protective molecules.
Materials and Methods
The experimental protocol was divided into 4 stages (Figure 1). In the first stage, we analyzed the cardioprotective effects of RIPC in a rat model of I/R injury. The second stage was an exploratory phase to detect which molecules were associated with RIPC by means of a targeted metabolomic approach in the same rat model. The third stage was a confirmatory phase conducted in humans in order to validate the results obtained in rats. In the final stage, we injected rats with the metabolites associated with RIPC found in both rat and human plasma in order to test their ability to protect the heart against I/R injury.
Figure 1.
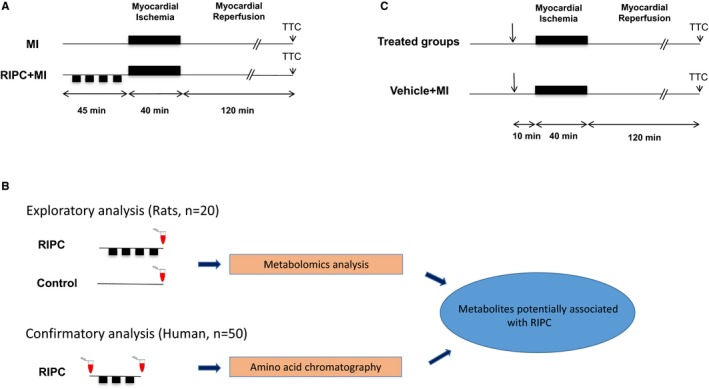
Experimental protocol. A, Cardioprotective effects of remote ischemic preconditioning (RIPC). In the myocardial infarction (MI) group, rats were subjected to 40 minutes of coronary occlusion followed by 120 minutes of reperfusion (n=8). In the RIPC+MI group (n=8), rats were subjected to RIPC achieved by a vascular clamp placed on the upper right femoral artery in order to induce 4 cycles of 5‐minute limb ischemia, interspersed with 5‐minute limb reperfusion before MI. Infarct size was assessed using 2,3,5‐triphenyltetrazolium chloride (TTC) staining. B, Schematic overview of experimental protocols applied for metabolomic analysis. In the exploratory stage, 20 rats were subjected to RIPC or a control procedure (exposure of upper right femoral artery without clamping). Blood was sampled immediately after the RIPC procedure in the RIPC group or 40 minutes after exposure of the femoral artery in the Control group. A targeted metabolomics analysis was conducted in order to find which metabolites potentially participate in the protection conferred by RIPC. In the confirmatory analysis, a set of 50 patients were subjected to RIPC achieved by 3 cycles of 5‐minute inflation to 200 mm Hg and 5‐minute deflation of an automated upper‐arm cuff inflator. Blood was sampled before and after RIPC. Based on the results of the exploratory analysis, amino acid chromatography was conducted on these samples in order to confirm changes of these metabolites in humans. C, Administration of potentially cardioprotective metabolites found in (B). MI was induced in rats as described in (A). In the treated groups (n=39), metabolites alone or in combination were injected intraperitoneally 10 minutes before coronary occlusion. In the Vehicle+MI group, rats received vehicle only intraperitoneally (n=12).
Animal Studies
All the experiments were approved by our regional ethics committee governing animal experimentation in the Loire region: “Comité Régional d'Ethique pour l'Expérimentation Animale‐Pays de la Loire” (CEEA.2012.50). The myocardial I/R injury and RIPC methods used have been described in a previous publication.15
Male Wistar rats, 8‐ to 10‐weeks old, were randomly assigned to 1 of the following groups (Figure 1): MI group, where rats were subjected to 40 minutes of myocardial ischemia (MI) followed by 2 hours (H) of reperfusion without any further intervention (n=8); RIPC+MI group, rats were subjected to RIPC immediately before MI, with RIPC achieved using a vascular clamp placed on the upper right femoral artery in order to induce 4 cycles of 5‐minute limb ischemia interspersed with 5‐minute limb reperfusion (n=8); RIPC group, consisting of RIPC only (n=10); Control group, where the upper right femoral artery was exposed but not clamped (n=10); Metabolites+MI group, with intraperitoneal injection of a cocktail of metabolites (glycine and kynurenin) 10 minutes before MI (n=13); Kyn+MI and Gly+MI groups, with intraperitoneal injection of kynurenin (n=13) or glycine (n=13), respectively, 10 minutes before MI; and Vehicle+MI group, with intraperitoneal injection of the vehicle 10 minutes before MI (n=12).
Area at risk (AAR) was expressed as a percentage of the area of the entire left ventricle (LV) and infarct size was calculated as a percentage of the AAR.
EDTA‐treated plasma samples were collected immediately after the RIPC procedure in the RIPC group and 40 minutes after exposition of the femoral artery in the Control group, then stored at −80°C until commencing metabolomic analysis.
Human Study
The Angers University Hospital ethics committee approved the protocol and the study was conducted in accordance with the Helsinki Declaration and French law. All participants provided written informed consent before being included in the study. The study was registered at ClinicalTrials.gov (Identifier: NCT01390129).
A total of 50 patients scheduled for elective aortic valve replacement because of aortic valve stenosis underwent RIPC under anesthesia prior to cardiac surgery. The exclusion criteria were as follows: combined surgery with another valve or coronary artery bypass graft, emergency surgery, coronary stenosis >70%, left ventricular ejection fraction <35%, history of MI, coronary revascularization, and preoperative treatment with nicorandil, metformin, or sulfonylurea within 8 days before surgery. RIPC was achieved by 3 cycles of 5‐minute inflation to 200 mm Hg and 5‐minute deflation of an automated upper‐arm cuff inflator. EDTA plasma samples were collected in every patient before and immediately after RIPC and stored at −80°C until analysis (Figure 1).
LC‐MS‐/MS‐Targeted Metabolomic Analysis
We applied a targeted quantitative metabolomic approach to analyze the rat plasma samples using the Biocrates AbsoluteIDQ ® p180 kit (Biocrates Life Sciences AG, Innsbruck, Austria). This kit covers endogenous metabolites playing pleiotropic roles in bioenergetics metabolism, cell signaling, and cell structures and previously involved in numerous diseases including cardiovascular ones.22, 23
This kit was used in combination with a QTRAP 5500 (AB SCIEX, Toronto, Canada) mass spectrometer, enabling the quantification of up to 188 different endogenous molecules, including acylcarnitines, amino acids, biogenic amines, glycerophospholipids, sphingolipids, and sugars (http://www.biocrates.com/products/research-products/absoluteidq-p180-kit). Flow‐injection analysis (flow‐injection analysis–mass spectrometry (MS)/MS) was used for quantifying acylcarnitines, glycerophospholipids, sphingolipids, and sugar, while liquid chromatography (LC) was employed to separate out amino acids and biogenic amines prior to MS detection (LC‐MS/MS). This kit enables the quantification of a large amount of metabolites using only a small sample volume and exhibits good analytical performance that can be monitored on each run by 3 levels of quality controls.
The samples were prepared according to the kit user manual. Briefly, 10 μL of plasma for each sample was added to the center of the filter placed on the upper wall of the well in a 96‐well plate. Metabolites were then extracted in a methanol solution using ammonium acetate after drying the filter spot under nitrogen flow and derivatization with phenylisothiocyanate for amino acids and biogenic amine quantification. Finally, the extracts were diluted with MS running solvent prior to flow‐injection analysis and LC‐MS/MS analysis.
Amino Acid Chromatography
Amino acid chromatography was performed on the human plasma samples using an UptiSphere BP2 chromatography column. A total of 49 amino acids and amino acid––derived molecules can be detected and quantified by means of an API 3000 Triple Quadrupole Mass Spectrometer (AB Sciex®).
Statistical Analysis
The bilateral Student t test was used to compare metabolite concentrations and abundances. When more than 2 group means were compared, an ANOVA was used after verifying homoscedasticity and Gaussian distribution of the concerned variable. When the ANOVA null hypothesis was rejected, post‐hoc least‐square difference test was used in pairwise mean comparison. For comparison of data obtained from the same sample, the paired Student t test was employed. In paired data, the mean difference for each metabolite represents the mean of the difference (after RIPC−before RIPC) calculated over all the subjects. When the normal distribution or ratios between 2normally distributed variables could not be reasonably assessed, the nonparametric Mann–Whitney test or its version for paired data were used for means comparison, respectively. A P<0.05 was considered statistically significant, unless otherwise mentioned. Univariate analyses were conducted using R software, Version 3.1.1 (R Core Team, Vienna, Austria).
All data were unit variance–scaled prior to multivariate statistical analysis. Principal component analysis score plot was conducted to detect grouping of similar animals and outliers according to metabolite concentration (X matrix). An orthogonal partial least‐squares discriminant analysis, a supervised pattern‐recognition method, was also performed to maximize the variation between groups and determine the most significant variables contributing to this variation. The quality of models was validated by determining R² (goodness‐of‐fit parameter) and Q² (goodness‐of‐prediction parameter) values. Models with poor predictive capabilities (Q²<0.5) were not retained. The model with the best predictive capabilities, ie, the highest Q² value, was selected based on the variable importance for the projection (VIP) and coefficient values. VIP values summarize the importance of each variable for the orthogonal partial least‐squares discriminant analysis model, while coefficient values summarize the relationship between the Y (RIPC/Control) and X (matrix of measured metabolites) variables. Coefficient values are very similar to coefficients obtained from multiple regression analysis. By plotting VIP versus coefficient values (“volcano” plot), the significant variables of orthogonal partial least‐squares discriminant analysis models can be detected and selected. The multivariate data analysis was conducted using SIMCA‐P software 13.0 (Umetrics, Umeả, Sweden).
Results
RIPC‐Induced Cardioprotection
While the AAR was not significantly different between the MI (n=8) and RIPC+MI (n=8) groups (30.6±1.7% and 32.8±1.5%, respectively, P=0.34), infarct size was significantly smaller in the RIPC+MI group compared to the MI group (34.5±2.3% and 42.5±2.4%, respectively, P=0.03) (Figure 2).
Figure 2.
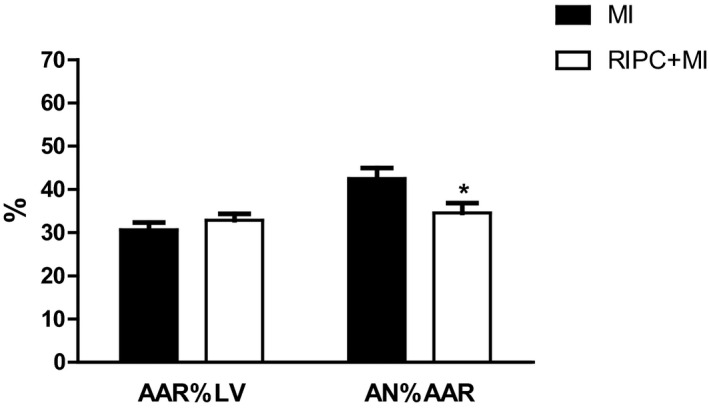
Cardioprotective effects of remote ischemic preconditioning (RIPC) in rats. Bar graph showing infarct size, expressed as area of necrosis (AN) percentage of area at risk (AAR), and AAR as a percentage of left ventricle (LV) area. Results are given as mean±SEM (*P<0.05).
LC‐MS/MS Targeted Metabolomic Analysis
Plasma samples from both the RIPC (n=10) and control (n=10) groups were analyzed using the AbsoluteIDQ ® p180 kit.
All 6 groups of molecules measured in the kit, namely acylcarnitines, amino acids, biogenic amines, glycerophospholipids, sphingolipids, and sugars, were examined. The difference between the mean concentrations of each group of rats was not statistically significant for any of the 6 families. Similarly, RIPC/Control ratios were close to 1 for the 6 groups of molecules. Principal component analysis score plot revealed no outliers, yet could not distinguish RIPC from Control samples (Figure 3A). However, a model obtained using the supervised orthogonal partial least‐squares discriminant analysis method fitted our data well (R 2X=0.66, R 2Y=0.995). This model differentiated RIPC samples from Control with good predictive capabilities (Q²=0.61) (Figure 3B). The most discriminatory metabolites in group separation, based on the VIP and coefficient values or volcano plot, have been presented in Figure 4A. Ornithine, kynurenine, spermine, glycine, carnosine, and serotonin all exhibited the highest VIP values (2.04, 2.00, 1.94, 1.93, 1.86, and 1.80, respectively). The correlation between ornithine and RIPC was negative (coefficient value: −0.17), whereas kynurenine, spermine, glycine, carnosine, and serotonin correlated positively with RIPC (coefficient values: 0.22, 0.18, 0.23, 0.17, and 0.14, respectively).
Figure 3.

Principal component analysis (PCA) (A) and orthogonal projection to latent structures discriminant analysis (OPLS‐DA) (B) of the rat cohort. On the PCA, the first principal plan, defined by the first 2 principal components (PC 1 and 2), did not show a clear separation between the remote ischemic preconditioning (RIPC) (closed circles, •) and control (open circles, ○) groups. There were no outliers, according to PCA. However, the OPLS‐DA model was able to make a clear distinction between both groups (R 2Y=0.995, Q²=0.61). In the OPLS‐DA model, the first latent variable takes into account intergroup variability.
Figure 4.
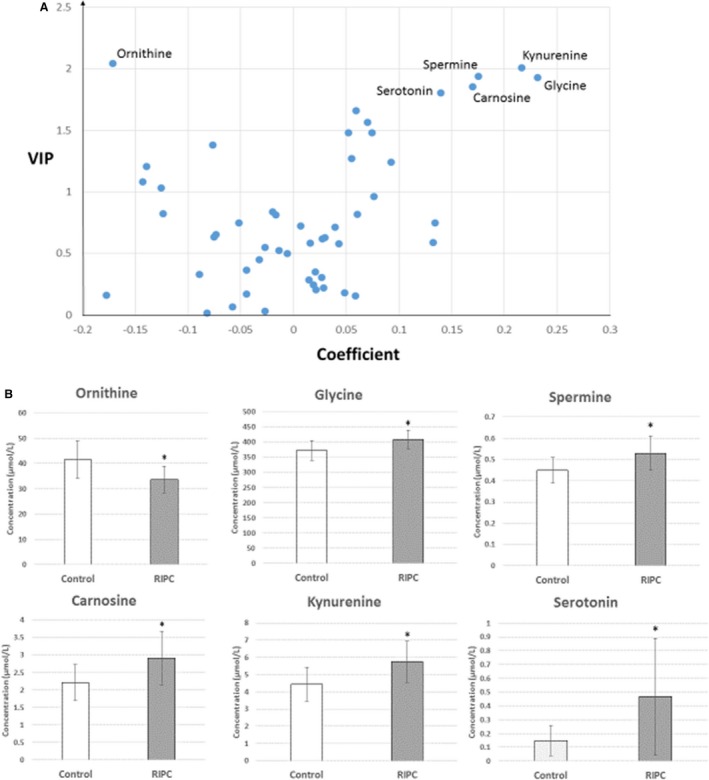
“Volcano” plot of the orthogonal projection to latent structures discriminant analysis OPLS‐DA model (A) and bar graph comparing the mean concentration (±SEM) of the Control (n=10) and remote ischemic preconditioning (RIPC) (n=10) groups for the top 6 discriminating molecules (B). A, In the volcano plot, the x‐axis represents the coefficient of the regression between the X (metabolites) and Y (response) matrices, while the variable importance for the projection (VIP) is represented on the y‐axis. The VIP summarizes the importance of each variable in accounting for both the X matrix and the correlation between X and Y. Variables with a VIP value >1 were considered pertinent for the model. A group of 6 molecules (ornithine on the top left and glycine, kynurenine, spermine, carnosine, and serotonin on the top right) show the largest VIP and coefficient values, which were retained as the most important variables discriminating between RIPC and Control groups. B, The mean concentration values (μmol/L) were significantly higher in the RIPC group, except for ornithine, for which the mean concentration was significantly lower in the RIPC group (*P<0.05). The ratio of the mean concentration values for RIPC and Control groups (RIPC/Control) was larger than 3 for serotonin, while the other molecules exhibited a RIPC‐to‐Control ratio lower than 1.5 (glycine, kynurenine, spermine, and carnosine) or larger than 0.5 (ornithine).
The mean ornithine concentration was 24% lower in the RIPC group compared to the control group (P=0.013). The concentrations of the other 5 molecules were significantly increased in the RIPC group compared to the control group (serotonin, P=0.042; glycine, P=0.013; carnosine, P=0.035; kynurenine, P=0.021; spermine, P=0.027). However, while the mean concentration of serotonin was higher by more than 300%, the other 4 molecules exhibited relative increases of only 50% or less (Figure 4B).
Amino Acid Chromatography
On the basis of multivariate analysis results, we performed amino acid chromatography on human samples in order to confirm these exploratory‐phase findings. Plasma samples were extracted prior to and post‐RIPC for all 50 patients, as previously described. All patient clinical characteristics can be found in Table 1.
Table 1.
Characteristics of the Study Population
| Characteristics | RIPC (n=50) |
|---|---|
| Age, y | 75.8±9.5 |
| Sex (male/female) | 27/23 |
| Body mass index, kg/m2 | 29.2±6.4 |
| Diabetes mellitus, n (%) | 6 (12) |
| Dyslipidemia, n (%) | 25 (50) |
| Hypertension, n (%) | 37 (74) |
| Currently smoking, n (%) | 4 (8) |
| GFR, mL/min per 1.73 m2 | 89.6±23.9 |
| COPD, n (%) | 5 (10) |
| NYHA class | 2.3±0.6 |
| LV ejection fraction, % | 65.7±10.3 |
| Medication at inclusion | |
| Platelet inhibitors, n (%) | 8 (16) |
| β‐Blockers, n (%) | 14 (28) |
| ACE‐inhibitors or ARBs, n (%) | 11 (22) |
| Statins, n (%) | 19 (38) |
| Diuretics, n (%) | 21 (42) |
| Calcium channel blockers, n (%) | 9 (18) |
| Insulin, n (%) | 0 |
Data are presented as mean±SD or n (%). ACE indicates angiotensin‐converting enzyme; ARBs, angiotensin‐II‐receptor blockers; COPD, chronic obstructive pulmonary disease; GFR, estimated glomerular filtration rate; LV, left ventricular; NYHA, New York Heart Association; RIPC, remote ischemic preconditioning.
We successfully quantified 33 metabolites (19 amino acids and 14 amino acid–derived molecules) in the patient plasma samples. The metabolites that exhibited significant alterations in concentration following RIPC are reported in Table 2. Among these molecules, we confirmed the results found in rats, with increased kynurenine (P<0.01) and glycine (P<0.08) concentrations and decreased ornithine (P<0.01) concentrations observed following RIPC. In addition, tryptophan concentrations were found to be diminished (P<0.01) following RIPC in the human plasma.
Table 2.
Amino Acid Chromatography of Human Plasma
| Molecules | Relative Change (%) |
|---|---|
| Concentration increased after RIPC | |
| 3‐Methylhistidine*** | 7.8 |
| Cystine*** | 6.0 |
| Kynurenine*** | 5.9 |
| Arginine*** | 5.3 |
| Serine*** | 3.1 |
| AABU*** | 2.5 |
| 1‐Methylhistidine** | 8.4 |
| Glutamate* | 5.2 |
| Glycine* | 2.3 |
| Concentration decreased after RIPC | |
| Aspartate*** | −9.2 |
| Ornithine*** | −4.7 |
| Tyrosine*** | −3.7 |
| Phenylalanine*** | −3.4 |
| Tryptophan*** | −3.4 |
| Citrulline*** | −3.2 |
| Isoleucine** | −1.9 |
| Sarcosine* | −25.8 |
| Valine* | −1.2 |
AABU indicates α‐aminobutyric acid.
Only metabolite concentrations that varied significantly after remote ischemic preconditioning (RIPC), according to Student t test for paired samples, are displayed. The relative changes (in %) were calculated as 100×(Concentration after RIPC−Concentration before RIPC)/Concentration before RIPC (***P<0.01, **P<0.05, *P<0.08).
Arginine is hydrolyzed to l‐ornithine under the effects of arginase, a key reaction of the urea cycle. Arginine concentration was significantly increased (P<0.01) and l‐ornithine significantly decreased (P<0.01) in the human samples following RIPC. Accordingly, the arginine‐to‐ornithine ratio, an indicator of arginase activity, was increased by 8.9% following RIPC in humans (P<0.001). Interestingly, the concentration of citrulline, an amino acid derived from ornithine in the urea cycle, was also found to be diminished following preconditioning (P<0.01) (Table 2).
Cardioprotective Effects of Glycine and Kynurenine
On the basis of the results demonstrating kynurenine and glycine increase following RIPC in both rat and human plasma, we tested the cardioprotective effects of an intraperitoneal injection of glycine (500 mg/kg body weight) and kynurenine (300 mg/kg body weight) alone (Gly+MI and Kyn+MI, respectively) or in combination (Metabolites+MI) prior to myocardial ischemia in the rat MI model. The doses and routes of administration were chosen according to the literature.24, 25, 26
While the AAR was not significantly different between all groups, infarct size area was significantly smaller in the Metabolites+MI group compared to Vehicle+MI group (27.1±4.5% of AAR versus 41.8±3.3%, respectively; P=0.003) (Figure 5). Similarly, infarct size was significantly smaller after kynurenine or glycine injection alone (Kyn+MI=27.7±2.5% and Gly+MI=32.2±2.2% of AAR, P=0.004 and 0.045, respectively versus Vehicle+MI group).
Figure 5.

Cardioprotective effects of glycine (GLY) and kynurenine (KYN) in rats. Bar graph showing infarct size expressed as area of necrosis percentage of area at risk. Results are given as mean± SEM; *P<0.05 vs Vehicle+MI group. MI indicates myocardial ischemia.
Discussion
Despite evidence indicating that humoral mediators transported through the circulation play a critical role in the RIPC‐induced protective mechanism, their actual identities remain unknown. We have hereby reported a metabolomic signature of RIPC involving a cocktail of amino acids and biogenic amines. In our analyses, we found that ornithine concentrations were diminished while glycine, kynurenine, spermine, carnosine, and serotonin concentrations were increased in rat plasma following RIPC. These results were partially confirmed in human plasma, with diminished ornithine and increased kynurenine and glycine plasma concentrations following RIPC. Interestingly, glycine and kynurenine injected prior to MI in the rat MI model replicated the protective effect of RIPC.
Glycine is a protein‐forming amino acid serving as a substrate in the synthesis of a variety of compounds from different pathways, such as serine, methylenetetrahydrofolate, purines, and glutathione, among others. Glycine has exhibited cardioprotective properties following myocardial I/R injury. Ruiz‐Meana et al27 described an enhanced vulnerability of cultured cardiomyocytes to necrotic cell death as a result of glycine depletion during simulated ischemia. Adding glycine to the culture during re‐energization even prevented cell death altogether. By using an in vivo MI model, Zhong et al24 observed a significant reduction in infarct size following I/R in rats pretreated with a single dose of glycine. Plasma creatine‐kinase levels were significantly lower, while ventricular ejection fraction and fractional shortening were significantly higher in the glycine‐pretreated group.
Carnosine is a dipeptide formed from β‐alanine and l‐histidine by carnosine synthase and catabolized back to its precursors by carnosinase.28, 29 Carnosine, like other histidine‐containing dipeptides, has well‐known antioxidant properties via metal ion chelation, scavenging reactive oxygen species, and peroxyl radicals.29, 30 Baba et al31 reported a significant reduction in infarct size in mice overexpressing local carnosine synthase compared to their wild‐type littermates, suggesting that treatment with carnosine may constitute a potential therapy for decreasing myocardial I/R injury. We found increased carnosine concentration following RIPC in rat samples, though we failed to confirm this finding in human plasma. Under physiological conditions, high human serum carnosinase activity in fact renders circulating carnosine undetectable.29 However, we were able to measure carnosine in rat plasma since plasma of nonprimate mammals lacks carnosinase activity.
As concerns the kynurenine pathway, indoleamine 2,3‐dioxygenase catalyzes the oxidation of tryptophan to formylkynurenine, which is the first and key reaction of this pathway.32 Indoleamine 2,3‐dioxygenase is present in numerous cells and tissues, including the endothelial layer of microvessels present throughout the myocardium.33 Indoleamine 2,3‐dioxygenase expression is principally upregulated by interferon γ, and kynurenine pathway activation in response to inflammatory stimuli can be detected based on an increased kynurenine‐to‐tryptophan ratio.32 Wang et al34 have demonstrated that both tryptophan and kynurenine were found to dilate preconstricted porcine coronary arteries in a dose‐dependent manner. The vasodilation induced by tryptophan was found to require the contribution of indoleamine 2,3‐dioxygenase, along with an intact endothelium, while kynurenine was able to dilate coronary vessels independently of the endothelium. These findings suggest that kynurenine itself is the vasoactive metabolite of tryptophan, which could play a role in RIPC‐associated cardioprotective effects. Recently, Olenchock et al21 reported that cardioprotection against I/R injury could be induced remotely by skeletal muscle inhibition of the oxygen sensor EGLN1. They showed that EGLN1 loss causes accumulation of circulating α‐ketoglutarate, which stimulates hepatic production and secretion of kynurenic acid that is necessary and sufficient to mediate RIPC‐induced protection.
Serotonin (5‐HT), another tryptophan‐derived molecule, plays a dual role in myocardial ischemia. It is well known that serotonin participates in vascular spasm and platelet aggregation during MI via 5‐HT2A receptors.35 However, the beneficial effects of serotonin have also been observed by Takano et al36 in an animal model of myocardial I/R injury. In isolated rat hearts, serotonin (0.3–1 μmol/L) increased coronary blood flow and cardiac mechanical performances. These hemodynamic changes were accompanied by an increase in NO levels in the coronary effluent and were eradicated by an inhibitor of NO synthase. The authors hypothesized that serotonin released by mast cells and sympathetic nerve endings could be responsible for deleterious effects resulting from 5‐HT2A receptor activation during myocardial ischemia, whereas extracardiac serotonin, on the contrary, could elicit protective vasodilatation in the I/R injury context. Naumenko et al37 reported peak serotonin levels in the myocardial interstitium immediately after preconditioning in a rat model. Serotonin interstitial concentrations increased during ischemia and reperfusion in both preconditioned and control rats, with a more pronounced increase observed in the control group. Because of the specifically preanalytical protocol, we were not able to measure serotonin levels in human plasma. Most patients participating in this study likely suffered from endothelial dysfunction in association with degenerative aortic valve disease.38 The protective effects of serotonin might have been annihilated in this pro‐inflammatory setting with decreased NO bioavailability and increased tumor necrosis factor‐α concentrations.39 Still, as is the case for kynurenine, the role of this tryptophan‐derived molecule in the cardioprotective effects of RIPC is as yet unclear and will require further investigation.
l‐ornithine and spermine are closely related to the polyamine pathway and urea cycle. Under the action of arginase, arginine is hydrolyzed to l‐ornithine, which participates in the synthesis of polyamine or, alternatively, can be combined in the liver to carbamoyl phosphate to form citrulline in the urea cycle. Interestingly, diminished citrulline and aspartate concentrations combined with increased arginine concentration in the human cohort (Table 2) suggest a modification of the so‐called citrulline–NO cycle induced by RIPC with an increased activity of argininosuccinate synthetase 1. Argininosuccinate synthetase 1 catalyzes the ATP‐dependent condensation reaction between citrulline and aspartate, generating argininosuccinate, which is used in the subsequent reaction catalyzed by argininosuccinate lyase to form arginine.40 The arginine produced in this way can be directed to the synthesis of NO, a molecule involved in the cardioprotection conferred by RIPC.41 In both rats and humans, the arginine‐to‐ornithine ratio, an indicator of arginase activity, was increased following RIPC, suggesting a decrease in the activity of this enzyme. l‐Ornithine concentration decrease could be a result of both an inhibition of arginase activity combined with an increased activity of ornithine decarboxylase in the polyamine pathway. l‐Ornithine is decarboxylated by ornithine decarboxylase to form putrescine, the precursor of the “higher” polyamines spermidine and spermine.42, 43 Spermine concentration was increased in the rat plasma samples after RIPC, and there is evidence that polyamines, particularly spermine, play beneficial roles in calcium homeostasis in I/R injury settings, scavenging free radicals and reducing lipid peroxidation.44, 45 It has been shown that both MI and I/R injury elicit the polyamine stress response characterized by increased ornithine decarboxylase and spermidine/spermine N‐acetyltransferase activities, which lead to a depletion in spermine and spermidine.46, 47, 48 Supplying exogenous spermine prior to I/R injury has been found to restore intracellular polyamine pools and confer cardioprotective effects.48 These results underline a potential implication of the polyamine pathway in RIPC.
Limits
It should be noted that RIPC did not exhibit significant cardiac (72 hours troponin and creatine kinase‐MB area under curve) or kidney (incidence of acute kidney injury) protective effects in the patients undergoing blood sampling.49 These findings may be accounted for by different factors, such as the use of concomitant drugs including statins and propofol anesthesia,50 increased age,51 sex,52 and comorbidities frequently associated with patients requiring cardiac surgery such as LV hypertrophy, hypertension, and diabetes.53, 54 In the patients undergoing blood sampling, all received propofol anesthesia. Although all these factors may impair cardioprotection through altered survival signaling pathways,1 regulation of circulating mediators involved in RIPC may not be affected, but rather these factors may not be effective in these specific clinical conditions.
Different protocols were used in rats and humans, yet not with exactly the same goals. In the first setting, we applied a widely targeted approach using the Biocrates p180 kit. We thereby considered that animal experiences were made in a controlled framework, therefore being less subject to interindividual variations. These conditions were considered as the most accurate for a first screening approach by targeted metabolomics. We then used the human study with patients receiving RIPC to confirm the variations in metabolite concentrations observed in rats. The fact that kynurenine and glycine concentrations were found to be elevated in both species, namely humans and rats, after remote conditioning even when using different protocols and analytical procedures, reinforces our results. However, these methodological‐related differences may be the reason for the detected species‐specific results.
RIPC is a multifactorial phenomenon involving several molecules from the neural and humoral pathways55, 56 such as bradykinin,10 adenosine,8 opioids,11 apolipoprotein A‐I,14, 15 endocannabinoids,9 erythropoietin,12 microvesicles,13 and microRNA16 among others. Our study highlights the role of metabolites, especially kynurenine and glycine, as molecules that are potentially involved in the protection conferred by RIPC. However, the protection triggered by RIPC seems much more complex. It is accounted for by the combined effects of numerous factors acting in an additive or even synergistic manner. Accordingly, our metabolomic approach did not cover the entire set of compounds participating with RIPC, nor the whole set of metabolites, given that we conducted a targeted approach, with other essential metabolites probably not included. Furthermore, we tested the cardioprotective effects of intraperitoneal injections of glycine (0.5 mg/g body weight) and kynurenine (0.3 mg/g body weight), at high dose levels already proven to be neuro‐ or cardioprotective in the scientific literature.24, 25, 26 Because of the use of these high dose levels, the potential implication of these metabolites in RIPC should be considered with caution.
Certain biogenic amines such as serotonin, spermine, and carnosine were not measured in the human plasma samples. We used an already‐existing cohort of human plasma samples, and the particular preanalytical protocols needed for dosing serotonin and spermine were not applied when the samples were taken. Serotonin dosage, for example, requires a particular centrifugation protocol in order to obtain a platelet‐rich plasma, while spermine is measured in red blood cell samples as these are the primary source of spermine and most analytical techniques are not sensitive enough to quantify spermine in the plasma.
Conclusions
RIPC was associated with a plasmatic decrease in ornithine and increase in kynurenine and glycine concentrations in both rat and human samples. Further studies are needed to elucidate the complex protective mechanisms of RIPC involving numerous mediators.
Sources of Funding
This work was supported by a grant from the Fédération Française de Cardiologie. Bakhta and Kalakech were supported by fellowships from the French Ministry of Education and Research. This work was realized in the context of the PREMMI (Pôle de Recherche et d'Enseignement en Médecine Mitochondriale) project supported by the University of Angers, the University Hospital of Angers, the Région Pays de la Loire, and Angers Loire Métropole.
Disclosures
None.
Acknowledgments
The authors would like to thank Pierre Legras and Jerome Roux from the “Service Commun d'Animalerie Hospitalo‐Universitaire,” the University Hospital Joint Animal Care Service, for taking care of the animals.
(J Am Heart Assoc. 2016;5:e003891 doi: 10.1161/JAHA.116.003891)
References
- 1. Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8:619–629. [DOI] [PubMed] [Google Scholar]
- 2. Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. [DOI] [PubMed] [Google Scholar]
- 3. Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Page S, Bejan‐Angoulvant T, Angoulvant D, Prunier F. Remote ischemic conditioning and cardioprotection: a systematic review and meta‐analysis of randomized clinical trials. Basic Res Cardiol. 2015;110:11. [DOI] [PubMed] [Google Scholar]
- 5. Le Page S, Prunier F. Remote ischemic conditioning: current clinical perspectives. J Cardiol. 2015;66:91–96. [DOI] [PubMed] [Google Scholar]
- 6. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre‐, post‐, and remote conditioning. Circ Res. 2015;116:674–699. [DOI] [PubMed] [Google Scholar]
- 7. Pickard JM, Botker HE, Crimi G, Davidson B, Davidson SM, Dutka D, Ferdinandy P, Ganske R, Garcia‐Dorado D, Giricz Z, Gourine AV, Heusch G, Kharbanda R, Kleinbongard P, MacAllister R, McIntyre C, Meybohm P, Prunier F, Redington A, Robertson NJ, Suleiman MS, Vanezis A, Walsh S, Yellon DM, Hausenloy DJ. Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol. 2015;110:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten‐Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. [DOI] [PubMed] [Google Scholar]
- 9. Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar‐Maralani P, Sadeghipour H, Ebrahimi F, Dehpour AR. Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol. 2008;579:246–252. [DOI] [PubMed] [Google Scholar]
- 10. Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKCepsilon‐isoform. Cardiovasc Res. 2002;55:583–589. [DOI] [PubMed] [Google Scholar]
- 11. Weinbrenner C, Schulze F, Sarvary L, Strasser RH. Remote preconditioning by infrarenal aortic occlusion is operative via delta1‐opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res. 2004;61:591–599. [DOI] [PubMed] [Google Scholar]
- 12. Diwan V, Jaggi AS, Singh M, Singh N, Singh D. Possible involvement of erythropoietin in remote renal preconditioning‐induced cardioprotection in rats. J Cardiovasc Pharmacol. 2008;51:126–130. [DOI] [PubMed] [Google Scholar]
- 13. Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas EI, Ferdinandy P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–78. [DOI] [PubMed] [Google Scholar]
- 14. Hibert P, Prunier‐Mirebeau D, Beseme O, Chwastyniak M, Tamareille S, Lamon D, Furber A, Pinet F, Prunier F. Apolipoprotein A‐I is a potential mediator of remote ischemic preconditioning. PLoS One. 2013;8:e77211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalakech H, Hibert P, Prunier‐Mirebeau D, Tamareille S, Letournel F, Macchi L, Pinet F, Furber A, Prunier F. RISK and SAFE signaling pathway involvement in apolipoprotein A‐I‐induced cardioprotection. PLoS One. 2014;9:e107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, Wei C, Hu P, Kharbanda RK, Redington AN. MicroRNA‐144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. [DOI] [PubMed] [Google Scholar]
- 17. Rassaf T, Totzeck M, Hendgen‐Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. [DOI] [PubMed] [Google Scholar]
- 18. Davidson SM, Selvaraj P, He D, Boi‐Doku C, Yellon RL, Vicencio JM, Yellon DM. Remote ischaemic preconditioning involves signalling through the SDF‐1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. [DOI] [PubMed] [Google Scholar]
- 19. Tonack S, Tang C, Offermanns S. Endogenous metabolites as ligands for G protein‐coupled receptors modulating risk factors for metabolic and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304:H501–H513. [DOI] [PubMed] [Google Scholar]
- 20. Wellen KE, Thompson CB. A two‐way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012;13:270–276. [DOI] [PubMed] [Google Scholar]
- 21. Olenchock BA, Moslehi J, Baik AH, Davidson SM, Williams J, Gibson WJ, Pierce KA, Miller CM, Hanse EA, Kelekar A, Sullivan LB, Wagers AJ, Clish CB, Vander Heiden MG, Kaelin WG Jr. EGLN1 inhibition and rerouting of alpha‐ketoglutarate suffice for remote ischemic protection. Cell. 2016;164:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu T, Holzapfel C, Dong X, Bader E, Yu Z, Prehn C, Perstorfer K, Jaremek M, Roemisch‐Margl W, Rathmann W, Li Y, Wichmann HE, Wallaschofski H, Ladwig KH, Theis F, Suhre K, Adamski J, Illig T, Peters A, Wang‐Sattler R. Effects of smoking and smoking cessation on human serum metabolite profile: results from the KORA cohort study. BMC Med. 2013;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeller T, Hughes M, Tuovinen T, Schillert A, Conrads‐Frank A, Ruijter H, Schnabel RB, Kee F, Salomaa V, Siebert U, Thorand B, Ziegler A, Breek H, Pasterkamp G, Kuulasmaa K, Koenig W, Blankenberg S. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol. 2014;29:777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhong X, Li X, Qian L, Xu Y, Lu Y, Zhang J, Li N, Zhu X, Ben J, Yang Q, Chen Q. Glycine attenuates myocardial ischemia‐reperfusion injury by inhibiting myocardial apoptosis in rats. J Biomed Res. 2012;26:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gigler G, Szenasi G, Simo A, Levay G, Harsing LG Jr, Sas K, Vecsei L, Toldi J. Neuroprotective effect of L‐kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur J Pharmacol. 2007;564:116–122. [DOI] [PubMed] [Google Scholar]
- 26. Nozaki K, Beal MF. Neuroprotective effects of L‐kynurenine on hypoxia‐ischemia and NMDA lesions in neonatal rats. J Cereb Blood Flow Metab. 1992;12:400–407. [DOI] [PubMed] [Google Scholar]
- 27. Ruiz‐Meana M, Pina P, Garcia‐Dorado D, Rodriguez‐Sinovas A, Barba I, Miro‐Casas E, Mirabet M, Soler‐Soler J. Glycine protects cardiomyocytes against lethal reoxygenation injury by inhibiting mitochondrial permeability transition. J Physiol. 2004;558:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellia F, Vecchio G, Rizzarelli E. Carnosinases, their substrates and diseases. Molecules. 2014;19:2299–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93:1803–1845. [DOI] [PubMed] [Google Scholar]
- 30. Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM. Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol. 1999;31:113–121. [DOI] [PubMed] [Google Scholar]
- 31. Baba S, Zhang D, Hoetker D, Guo Y, Bhatnagar A. Cardiospecific Overexpression of Carnosine Synthase Attenuates Myocardial Ischemia Reperfusion Injury. Circulation. 2014;130:A11928. [Google Scholar]
- 32. Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Changsiri D, Wang Y, Dos Remedios C, Celermajer D, Stocker R. Indoleamine 2, 3‐ dioxygenase is induced in myocardium during inflammation. Heart Lung Circ. 2010;19S:S102. [Google Scholar]
- 34. Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF Jr, Hunt NH, Stocker R. Kynurenine is an endothelium‐derived relaxing factor produced during inflammation. Nat Med. 2010;16:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Golino P, Piscione F, Willerson JT, Cappelli‐Bigazzi M, Focaccio A, Villari B, Indolfi C, Russolillo E, Condorelli M, Chiariello M. Divergent effects of serotonin on coronary‐artery dimensions and blood flow in patients with coronary atherosclerosis and control patients. N Engl J Med. 1991;324:641–648. [DOI] [PubMed] [Google Scholar]
- 36. Takano S, Hoshino Y, Li L, Matsuoka I, Ono T, Kimura J. Dual roles of 5‐hydroxytryptamine in ischemia‐reperfusion injury in isolated rat hearts. J Cardiovasc Pharmacol Ther. 2004;9:43–50. [DOI] [PubMed] [Google Scholar]
- 37. Naumenko SE, Latysheva TV, Gilinskii MA. Myocardial serotonin during ischemia under conditions of ischemic preconditioning. Bull Exp Biol Med. 2014;157:459–461. [DOI] [PubMed] [Google Scholar]
- 38. Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini LA, Picano E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J Am Coll Cardiol. 2003;41:136–141. [DOI] [PubMed] [Google Scholar]
- 39. Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, Elter‐Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108:344–352. [DOI] [PubMed] [Google Scholar]
- 40. Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol. 2011;2:8–23. [PMC free article] [PubMed] [Google Scholar]
- 41. Tota B, Quintieri AM, Angelone T. The emerging role of nitrite as an endogenous modulator and therapeutic agent of cardiovascular function. Curr Med Chem. 2010;17:1915–1925. [DOI] [PubMed] [Google Scholar]
- 42. Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. [DOI] [PubMed] [Google Scholar]
- 43. Persson L. Polyamine homoeostasis. Essays Biochem. 2009;46:11–24. [DOI] [PubMed] [Google Scholar]
- 44. Nilsson BO, Gomez MF, Sward K, Hellstrand P. Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle–implications for cellular growth and contractility. Acta Physiol Scand. 2002;176:33–41. [DOI] [PubMed] [Google Scholar]
- 45. Marzabadi MR, Llvaas E. Spermine prevent iron accumulation and depress lipofuscin accumulation in cultured myocardial cells. Free Radic Biol Med. 1996;21:375–381. [DOI] [PubMed] [Google Scholar]
- 46. Han L, Xu C, Guo Y, Li H, Jiang C, Zhao Y. Polyamine metabolism in rat myocardial ischemia‐reperfusion injury. Int J Cardiol. 2009;132:142–144. [DOI] [PubMed] [Google Scholar]
- 47. Wang W, Zhang H, Xue G, Zhang L, Zhang W, Wang L, Lu F, Li H, Bai S, Lin Y, Lou Y, Xu C, Zhao Y. Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool. Oxid Med Cell Longev. 2014;2014:457429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao YJ, Xu CQ, Zhang WH, Zhang L, Bian SL, Huang Q, Sun HL, Li QF, Zhang YQ, Tian Y, Wang R, Yang BF, Li WM. Role of polyamines in myocardial ischemia/reperfusion injury and their interactions with nitric oxide. Eur J Pharmacol. 2007;562:236–246. [DOI] [PubMed] [Google Scholar]
- 49. Pinaud F, Corbeau JJ, Baufreton C, Binuani JP, De Brux JL, Fouquet O, Angoulvant D, Furber A, Prunier F. Remote ischemic preconditioning in aortic valve surgery: results of a randomized controlled study. J Cardiol. 2015;67:36–41. [DOI] [PubMed] [Google Scholar]
- 50. Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. [DOI] [PubMed] [Google Scholar]
- 51. Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. [DOI] [PubMed] [Google Scholar]
- 52. Murphy E, Steenbergen C. Gender‐based differences in mechanisms of protection in myocardial ischemia‐reperfusion injury. Cardiovasc Res. 2007;75:478–486. [DOI] [PubMed] [Google Scholar]
- 53. Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sorensen HT, Botker HE; Investigators C . Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open. 2015;5:e006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kleinbongard P, Neuhauser M, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G. Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. Cardiology. 2016;133:128–133. [DOI] [PubMed] [Google Scholar]
- 55. Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, Zhangdong H, Schmidt M, Downey GP, Redington AN. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158:155–161. [DOI] [PubMed] [Google Scholar]
- 56. Redington KL, Disenhouse T, Strantzas SC, Gladstone R, Wei C, Tropak MB, Dai X, Manlhiot C, Li J, Redington AN. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:241. [DOI] [PubMed] [Google Scholar]


