Abstract
Background
Few studies have examined how antiplatelet therapies are selected during the routine care of acute myocardial infarction patients, particularly relative to the patient's estimated mortality and bleeding risks.
Methods and Results
We examined patients presenting with acute myocardial infarction treated with percutaneous coronary intervention at 233 US hospitals in the TRANSLATE‐ACS observational study from April 2010 to October 2012. We developed a multivariable logistic regression model to identify factors associated with prasugrel selection. Prasugrel use rates and associated 1‐year risk‐adjusted major adverse cardiovascular events and Global Utilization of Streptokinase and t‐PA for Occluded Coronary Arteries (GUSTO) moderate/severe bleeding outcomes were also examined in relation to predicted mortality and bleeding using the validated Acute Coronary Treatment and Intervention Outcomes (ACTION) risk prediction scores. Among 11 969 patients, 3123 (26%) received prasugrel at the time of percutaneous coronary intervention. The strongest factors associated with prasugrel use included cardiogenic shock (odds ratio [OR] 1.68, 95% CI 1.25–2.26), drug‐eluting stent use (OR 1.45, 95% CI 1.31–1.62), and ST‐segment elevation myocardial infarction presentation (OR 1.23, 95% CI 1.12–1.35). Older age (OR 0.57, 95% CI 0.0.53–0.61), dialysis (OR 0.56, 95% CI 0.32–0.96), prior history of stroke/transient ischemic attack (OR 0.52, 95% CI 0.38–0.73), and interhospital transfer (OR 0.50, 95% CI 0.46–0.55) were associated with lowest prasugrel selection. Prasugrel was used less often than clopidogrel in patients at higher predicted bleeding risk (21.9% versus 29.7%, P<0.001). Yet paradoxically, prasugrel was also less likely than clopidogrel to be used in patients with higher predicted mortality risk (21.1% versus 30.2%, P<0.001). Adjusted bleeding and outcomes events were similar among those receiving prasugrel and clopidogrel in the 4 subgroups of patients based on bleeding risk and ischemic benefits.
Conclusions
In community practice, prasugrel use may be driven more by bleeding risk rather than ischemic benefit. This may result in underutilization of higher potency ADP receptor inhibitor among patients more likely to derive ischemic benefit.
Keywords: acute coronary syndrome, clopidogrel, prasugrel, risk prediction
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease
Introduction
Treatment with dual antiplatelet therapy in patients with acute myocardial infarction (MI) is a cornerstone of guideline‐recommended pharmacologic therapy, especially if percutaneous coronary intervention (PCI) is performed.1, 2, 3 Although clopidogrel has been the most widely used ADP receptor inhibitor (ADPri) in the United States, higher potency ADPris have been shown to further reduce the risk of adverse cardiovascular outcomes when compared with clopidogrel treatment among MI patients; however, the risk of bleeding is also higher among patients treated with these higher potency ADPris.4, 5
Few studies have examined how ADPri therapies are initially selected during routine acute MI care. Although several risk prediction models have been developed to estimate the likelihood of mortality or bleeding in patients with acute MI, how higher potency ADPris, such as prasugrel, are used in relation to these predicted risks in routine clinical practice has not been well characterized. Therefore, we utilized data from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE‐ACS) study to determine predictors of initial prasugrel versus clopidogrel selection at the time of PCI, and to characterize outcomes associated with ADPri selection when stratified by predicted mortality and bleeding risks.6, 7, 8, 9
Methods
Study Population
The TRANSLATE‐ACS (ClinicalTrials.gov identifier: NCT01088503) study design has been described previously.10 In brief, TRANSLATE‐ACS was a prospective, multicenter, longitudinal observational study to describe patterns of ADPri use and to evaluate the comparative effectiveness and safety of these agents in contemporary clinical practice in the United States. The study enrolled patients ≥18 years of age presenting with ST‐segment elevation myocardial infarction (STEMI) or non‐ST‐segment myocardial infarction (NSTEMI) revascularized by PCI and treated with ADPri during the index hospitalization. Patients were excluded if unable to provide written informed consent or if participating in another research study directing approved or investigational ADPri selection and use in the 12 months following the index MI. As the study protocol did not direct a treatment intervention, all management decisions were made by treating clinicians in accordance with local standards of care and guideline recommendations. The study was approved by the institutional review boards of all participating hospitals, and written informed consent was provided by all patients.
A total of 12 365 patients were enrolled between April 4, 2010 and October 31, 2012. Among enrolled patients, we excluded 138 patients who were initiated on more than 1 ADPri at the time of PCI. Ticlopidine use was rare, and another higher potency ADPri, ticagrelor, received Food and Drug Administration approval late in the study; therefore, the 238 patients who received ticagrelor or ticlopidine were also excluded from the analysis. These exclusions yielded a final study population of 11 969 patients presenting with acute MI who underwent PCI and were treated initially with either prasugrel or clopidogrel.
Data Collection
The TRANSLATE‐ACS study captured detailed baseline sociodemographic, clinical, and presentation characteristics, processes of care, and longitudinal outcomes data for each patient. In‐hospital data collection used standardized definitions created by the National Cardiovascular Data Registry, a collection of quality‐improvement registries involving over 2000 hospitals in the United States (http://cvquality.acc.org/NCDR-Home.aspx). Hospital characteristics were obtained by linking sites participating in the TRANSLATE‐ACS study with the American Hospital Association 2008 Survey.
Postdischarge study follow‐up was completed via centralized telephone interview at 6 weeks and 6, 12, and 15 months by trained personnel at the Duke Clinical Research Institute. The outcomes of interest included (1) the composite outcome of major adverse cardiovascular events (MACE) consisting of death, recurrent MI, stroke, or unplanned coronary revascularization and (2) Global Utilization of Streptokinase and t‐PA for Occluded Coronary Arteries (GUSTO) moderate or severe bleeding. These events were independently adjudicated via medical record review by study physicians from the Duke Clinical Research Institute.
Statistical Analysis
Baseline patient and procedure data were compared between patients initially treated with prasugrel or clopidogrel. Data are presented as frequencies and percentages for categorical variables and medians (25th, 75th percentiles) for continuous variables. We compared baseline patient, presentation, and procedural characteristics for the 2 groups using Pearson χ2 tests for categorical variables and Wilcoxon Rank‐Sum tests for continuous variables.
We used a logistic regression model to determine factors associated with the selection of prasugrel as the ADPri at the time of PCI. Candidate variables included all TRANSLATE‐ACS specific variables, listed in Table S1. We utilized a split‐sample methodology wherein we randomly selected 80% of the population and developed a series of univariate logistic models for the prasugrel indicator variable. Each variable with a univariate P<0.10 was entered as a candidate into a forward selection logistic model for prasugrel use. We assessed linearity of all continuous variables, and used P<0.05 as the selection to enter criteria. The model was then fit on a 20% validation sample and finally re‐fit on the 100% sample. We used the C‐index to assess discriminative ability. The model fit among the validation sample had C‐index=0.699, which was close to the C‐index from the final model C=0.704.
As in‐hospital data collection in TRANSLATE‐ACS used standardized definitions based on the National Cardiovascular Data Registry, we calculated the Acute Coronary Treatment and Intervention Outcomes (ACTION) mortality6 and bleeding7 risk scores for each patient. The ACTION mortality risk model is a previously validated risk prediction score that incorporates the following variables: age, peripheral artery disease, heart rate on admission, systolic blood pressure on admission, heart failure±shock on admission, and electrocardiographic changes, baseline troponin ratio, and baseline serum creatinine. The previously validated ACTION bleeding risk model included the following variables: age, weight, sex, diabetes mellitus, previous peripheral artery disease, heart rate on admission, systolic blood pressure on admission, heart failure±shock on admission, and electrocardiographic changes, baseline troponin ratio (times upper limit of normal), baseline serum creatinine, and home warfarin use. We examined the Pearson correlation between the ACTION mortality and bleeding scores, stratified by prasugrel versus clopidogrel treatment groups. To graphically display trends between ACTION mortality and bleeding risk scores, we used a scatterplot with penalized B‐spline curves.
We divided patients into 4 groups based on their predicted mortality and bleeding risk scores above and less than or equal to the population median: (1) predicted mortality and bleeding risks both high, (2) predicted mortality and bleeding risks both low, (3) high predicted mortality but low predicted bleeding risk, and (4) low predicted mortality but high predicted bleeding risk. In each group, we used cumulative incidence curves with log rank testing to compare 1‐year post‐PCI MACE and bleeding outcomes between patients initially treated with prasugrel versus clopidogrel. We used Cox proportional hazards regression modeling with inverse probability weighted propensity adjustment to determine the adjusted MACE and bleeding outcomes associated with initial ADPri selection. To calculate inverse probability weights, we fit a propensity score model using logistic regression for prasugrel (as the outcome). We adjusted for 56 prespecified demographic, clinical, presentation, and procedural variables selected based on clinical expertise (Table S1). Balance of the covariates between treatment groups was assessed using standardized differences. The inverse probability weights are based on a function of the propensity score. To limit the potential influence of large weights related to extreme values for the propensity score, we capped the weights at 10 times the average stabilized weight. Outcomes were assessed in an “as‐treated” fashion with censoring of events occurring more than 7 days after ADPri discontinuation or switch.
Statistical significance was defined as P<0.05. All analyses were performed by the NCDR data analysis center at the Duke Clinical Research Institute using SAS software (version 9.3; SAS Institute Inc, Cary, NC).
Results
Patient and Hospital Characteristics
Among the 11 969 STEMI and NSTEMI patients who underwent PCI in our study population, 3123 (26.1%) patients were treated initially with prasugrel. These patients were younger, less likely female, and more likely to have private insurance status than patients treated with clopidogrel (all P<0.001). There were statistically significant but modest differences in race and Hispanic ethnicity between the 2 groups. Prasugrel‐treated patients also had significantly fewer comorbid conditions than clopidogrel‐treated patients, with lower rates of prior MI, heart failure, revascularization, and stroke (all P<0.001). There were notable but low proportions of patients age ≥75 years (2.8%), weight <60 kg (2.4%), or with a history of prior stroke/transient ischemic attack (1.9%) treated with prasugrel, populations in which prasugrel use was not advised. Both groups had similar low rates of prior gastrointestinal or genitourinary bleeding history; patients treated with prasugrel were less likely to have been on oral anticoagulation therapy prior to admission than clopidogrel‐treated patients. We observed some regional differences in hospitals, and patients receiving prasugrel were less likely to present to teaching hospitals. Prasugrel‐treated patients presented at smaller hospitals as well (Table 1).
Table 1.
Patient and Hospital Characteristicsa
| Prasugrel (n=3123) | Clopidogrel (n=8846) | P‐Value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 57 (50–63) | 61 (53–70) | <0.001 |
| Female, % | 21.5 | 30.2 | <0.001 |
| BMI, kg/m2 | 29.8 (26.5–33.8) | 29.2 (25.8–33.3) | <0.001 |
| Race | <0.001 | ||
| White | 88.1 | 87.9 | |
| Black | 7.6 | 9.3 | |
| Asian | 1.8 | 1.1 | |
| Other | 2.3 | 1.1 | |
| Hispanic | 4.1 | 3.1 | 0.005 |
| Insurance status | <0.001 | ||
| Private | 68.3 | 63.3 | |
| Federal/State | 14.7 | 22.6 | |
| None | 17.0 | 14.1 | |
| Clinical characteristics | |||
| Prior MI, % | 14.6 | 21.3 | <0.001 |
| Prior heart failure, % | 3.0 | 7.0 | <0.001 |
| Prior PCI, % | 17.8 | 23.0 | <0.001 |
| Prior CABG, % | 5.5 | 10.6 | <0.001 |
| Prior stroke/TIA, % | 1.9 | 6.6 | <0.001 |
| Peripheral arterial disease, % | 3.3 | 7.5 | <0.001 |
| Hypertension, % | 61.5 | 68.8 | <0.001 |
| Diabetes mellitus, % | 24.6 | 27.2 | 0.003 |
| Dyslipidemia, % | 62.0 | 66.9 | <0.001 |
| Atrial fibrillation/flutter, % | 2.9 | 5.3 | <0.001 |
| Current/recent smoker | 40.7 | 37.4 | <0.001 |
| Chronic lung disease | 6.6 | 11.0 | <0.001 |
| Dialysis | 0.5 | 1.4 | <0.001 |
| GI/GU bleeding within 6 months | 0.8 | 1.2 | 0.13 |
| Home oral anticoagulation | 1.1 | 3.8 | <0.001 |
| Presentation features | |||
| Transfer in from acute‐care hospital | 26.1 | 43.7 | <0.001 |
| STEMI | 58.6 | 49.3 | <0.001 |
| Cardiac arrest on presentation | 3.6 | 2.7 | 0.02 |
| Heart failure within 2 weeks | 3.6 | 7.7 | <0.001 |
| Cardiogenic shock | 2.5 | 1.9 | 0.06 |
| Heart rate, bpm | 77 (66–90) | 76 (65–88) | 0.02 |
| Systolic blood pressure | 141 (123–160) | 139 (121–158) | 0.002 |
| Creatinine clearance, mL/minb | 78.6 (64.0–95.8) | 70.6 (53.3–89.8) | <0.001 |
| High predicted bleeding risk, % | 38.8 | 48.8 | <0.001 |
| High predicted mortality risk, % | 36.5 | 48.1 | <0.001 |
| Hospital characteristics | |||
| Region (%) | <0.001 | ||
| West | 16.2 | 13.7 | |
| Northeast | 14.6 | 16.0 | |
| Midwest | 34.8 | 39.0 | |
| South | 34.3 | 31.9 | |
| Surgery capability, % | 90.8 | 89.9 | 0.11 |
| Teaching hospital, % | 30.6 | 44.2 | <0.001 |
| Number of hospital beds, median (IQR) | 411 (316–587) | 459 (337–639) | 0.02 |
BMI indicates body mass index; CABG, coronary artery bypass graft surgery; GI, gastrointestinal; GU, genitourinary; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction; TIA, transient ischemic attack.
Data are expressed as percentage of patients for categorical variables, median (25th, 75th percentiles) for continuous variables.
Creatinine clearance was calculated by the Cockroft‐Gault equation among patients not receiving dialysis.
Patients treated with prasugrel were significantly less likely to have been transferred in from an acute care hospital compared with patients treated with clopidogrel (26.1% versus 43.7%, P<0.001). They were also more likely to present with STEMI (58.6% versus 49.3%, P<0.001). Upon angiography, patients who received prasugrel at the time of PCI were more likely to have single‐vessel disease but less likely to have significant left main coronary artery disease (Table 2). The proportion of patients with left ventricular ejection fraction ≤40% was similar in both groups (20.6% versus 20.9%, P=0.71).
Table 2.
In‐Hospital Angiographic Findings and Procedures
| Prasugrel (n=3123) | Clopidogrel (n=8846) | P‐Value | |
|---|---|---|---|
| In‐hospital procedures | |||
| Angiographic findings | <0.001 | ||
| 1‐vessel disease | 53.9 | 46.8 | |
| 2‐vessel disease | 30.4 | 31.8 | |
| 3‐vessel disease | 14.2 | 18.8 | |
| Left main >50% stenosis | 1.8 | 3.5 | <0.001 |
| BMS used | 22.7 | 29.2 | <0.001 |
| DES used | 75.7 | 68.7 | <0.001 |
| Procedural success | 92.3 | 91.8 | 0.28 |
Data are expressed as percentage of patients for categorical variables. BMS indicates bare metal stent; DES, drug‐eluting stent.
Factors Associated With Initial Prasugrel Selection
We used a multivariable logistic regression model to identify factors associated with the initial selection of prasugrel versus clopidogrel (Figure 1). Strong predictors of initial prasugrel use were cardiogenic shock (odds ratio [OR] 1.68, 95% CI 1.25–2.26), DES use (OR 1.45, 95% CI 1.31–1.62), and STEMI presentation (OR 1.23, 95% CI 1.12–1.35), whereas the factors associated with lowest initial prasugrel use were older age (70 versus 60: OR 0.57, 95% CI 0.53–0.61), dialysis treatment (OR 0.56, 95% CI 0.32–0.96), a prior history of stroke/transient ischemic attack (OR 0.52, 95% CI 0.38–0.73), home oral anticoagulation therapy (OR 0.52, 95% CI 0.36–0.76), and interhospital transfer (OR 0.50, 95% CI 0.46–0.55). Factors that were notably not associated with initial prasugrel selection included insurance status, history of atrial fibrillation/flutter, or gastrointestinal/genitourinary bleeding in the 6 months prior to presentation.
Figure 1.
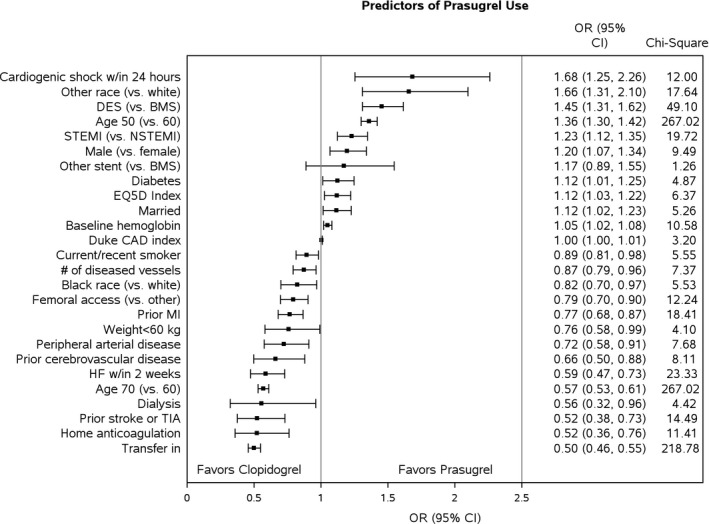
Study population characteristics. Displayed are the factors associated with initial prasugrel selection. BMS indicates bare metal stent; CAD, coronary artery disease; DES, drug‐eluting stent; HF, heart failure; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction; TIA, transient ischemic attack. Other race includes Asian, American Indian/Alaskan Native, and Native Hawaiian/Pacific Islander.
Prasugrel Use and Outcomes Stratified by Predicted Bleeding and Mortality Risks
The median ACTION mortality risk score was 30 (25th, 75th percentiles: 25, 35), and the median ACTION bleeding risk score was 25 (25th, 75th percentiles: 21, 29). Prasugrel was used less frequently among patients with high predicted bleeding risk (21.9% versus 29.7%, P<0.001). However, prasugrel was also used less frequently among patients with high predicted mortality risk (21.1% versus 30.2%, P<0.001). When stratified into 4 bleeding–ischemic risk groups (Figure 2), prasugrel was used most frequently among patients at low predicted risk of both bleeding and mortality and least frequently among patients at high predicted risk of both bleeding and mortality. It was used similarly among discordant‐risk patients, ie, those with low mortality and high bleeding risk and those with high mortality and low bleeding risk (26.2% versus 24.1%, P=0.14).
Figure 2.
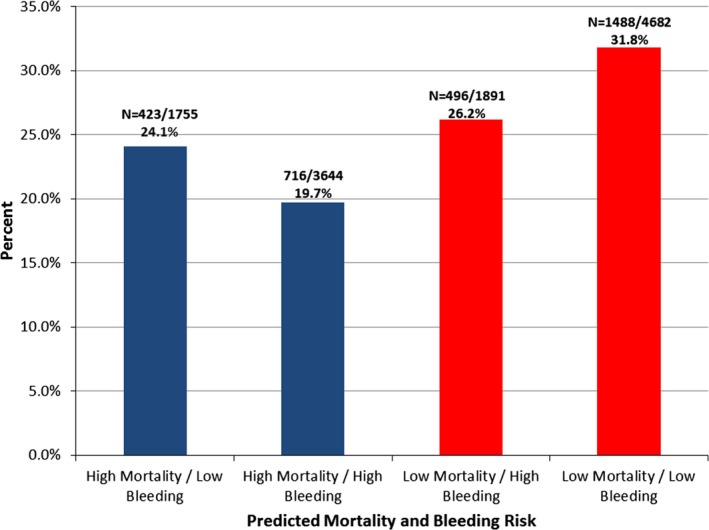
Prasugrel selection by predicted mortality and bleeding risks. Each bar represents the percentage of patients initially receiving prasugrel across strata or mortality and bleeding risk. High‐risk patients include those with ACTION mortality or bleeding scores above the population median. ACTION indicates Acute Coronary Treatment and Intervention Outcomes.
Prasugrel‐treated patients had lower ACTION mortality and bleeding risk scores compared with clopidogrel‐treated patients. ACTION mortality and bleeding scores were moderately correlated (Pearson correlation coefficient 0.61, P<0.001), with modest differences between patients treated with prasugrel (Pearson correlation coefficient=0.59) and those treated with clopidogrel (Pearson correlation coefficient=0.62) (Figure 3). Notably, 3641/5396 (67.5%) of patients at higher predicted mortality also had higher predicted bleeding risk.
Figure 3.
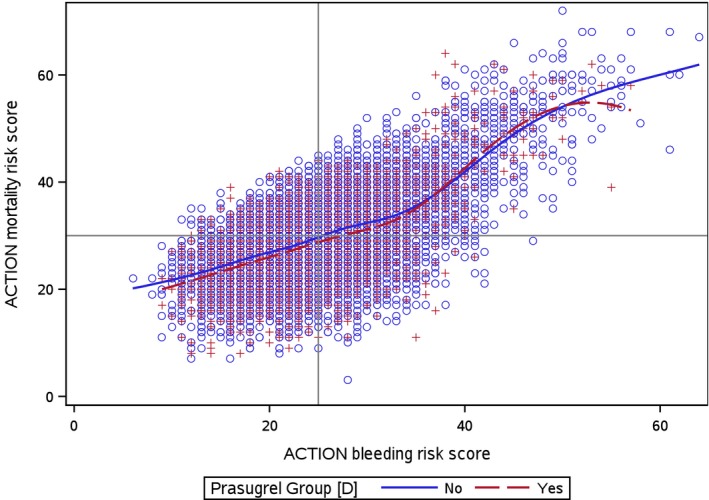
Correlation of ACTION mortality and bleeding risk scores, stratified by ADPri selection. This figure represents a scatter plot of all patients by ACTION mortality and bleeding risk score stratified by initial ADPri selection (red=prasugrel, blue=clopidogrel). The horizontal line and vertical line represent the median ACTION mortality and bleeding risk scores, respectively. Penalized B‐splines curves were created for clopidogrel and prasugrel. ADPri indicates ADP receptor inhibitor. ACTION indicates Acute Coronary Treatment and Intervention Outcomes.
We grouped patients based on predicted mortality and bleeding risks and compared outcomes of 1‐year MACE and GUSTO moderate/severe bleeding between those treated with prasugrel versus clopidogrel (Table 3). Among patients at high mortality risk, 1‐year MACE rates were lower among prasugrel‐treated patients (14.4% versus 18.8%, P=0.02) when compared with those who received clopidogrel; this difference was no longer significant after risk adjustment (hazard ratio [HR]adj 1.16, 95% CI 0.89–1.50). In this high predicted mortality risk population, rates of GUSTO moderate/severe bleeding at 1 year were not significantly different between prasugrel‐ and clopidogrel‐treated patients (3.8% versus 5.0%, P=0.14; HRadj=1.20, 95% CI 0.70–2.08). Among patients with high predicted risk of bleeding, MACE rates did not differ significantly between prasugrel‐ and clopidogrel‐treated patients before and after adjustment (17.8% versus 20.8%, P=0.05; HRadj=1.18, 95% CI 0.94–1.48). Bleeding rates did not differ significantly between prasugrel‐ and clopidogrel‐treated patients in both the unadjusted and inverse probability weights adjusted analyses (4.3% versus 5.5%, P=0.22; HRadj 1.47, 95% CI 0.91–2.38). Although the adjusted results were not significant, a change in the directionality of the trend was observed in some of the categories (Table 3).
Table 3.
Major Adverse Cardiovascular Events and GUSTO Moderate/Severe Bleeding Rates Stratified by Mortality and Bleeding Risks
| Predicted Risk | MACE (Pras vs Clop) | Moderate/Severe Bleeding (Pras vs Clop) | ||||
|---|---|---|---|---|---|---|
| Unadjusted (%) | Adj. HR | 95% CI | Unadjusted (%) | Adj. HR | 95% CI | |
| High mortality N=5396 | 14.8 vs 18.8a | 1.16 | 0.89 to 1.50 | 3.8 vs 5.0 | 1.20 | 0.70 to 2.08 |
| Low mortality N=6573 | 12.2 vs 15.5a | 0.94 | 0.77 to 1.15 | 2.0 vs 2.4 | 1.55 | 0.89 to 2.70 |
| High bleeding N=5532 | 17.8 vs 20.8a | 1.18 | 0.94 to 1.48 | 4.3 vs 5.5 | 1.47 | 0.91 to 2.38 |
| Low bleeding N=6437 | 10.4 vs 13.6a | 0.88 | 0.70 to 1.10 | 1.6 vs 1.9 | 0.99 | 0.60 to 1.63 |
| High mortality | ||||||
| Low bleeding N=1755 | 10.6 vs 13.9 | 1.03 | 0.61 to 1.76 | 2.4 vs 3.1 | 0.74 | 0.34 to 1.61 |
| High bleeding N=3641 | 17.4 vs 21.0 | 1.21 | 0.91 to 1.62 | 4.6 vs 5.9 | 1.34 | 0.72 to 2.48 |
| Low mortality | ||||||
| High bleeding N=1891 | 18.3 vs 20.3 | 1.13 | 0.80 to 1.62 | 3.9 vs 4.7 | 1.83 | 0.85 to 3.95 |
| Low bleeding N=4682 | 10.3 vs 13.5a | 0.83 | 0.67 to 1.03 | 1.4 vs 1.4 | 1.20 | 0.63 to 2.29 |
Clop indicates clopidogrel; GUSTO, Global Utilization of Streptokinase and t‐PA for Occluded Coronary Arteries; HR, hazard ratio; MACE, major adverse cardiovascular events; Pras, prasugrel.
P≤0.05.
We then stratified by both predicted mortality and bleeding risk. Among patients whose predicted mortality and bleeding risks were both low, prasugrel use was associated with the lowest observed rate of GUSTO moderate/severe bleeding (1.4%), as well as lower adjusted MACE risk when compared with clopidogrel treatment, though this did not meet statistical significance (HRadj 0.83, 95% CI 0.67–1.03). However, among patients with high predicted mortality and low predicted bleeding risk, we did not observe lower MACE risk associated with prasugrel use (HRadj 1.03, 95% CI 0.61–1.76). Among patients who were predicted to have both high mortality and bleeding risks, prasugrel selection was not associated with any difference in either MACE or GUSTO moderate/severe bleeding (Table 3).
Discussion
In this community‐based cohort of acute MI patients treated with PCI, approximately a quarter of patients were treated with prasugrel. The strongest factors associated with prasugrel selection relative to clopidogrel included cardiogenic shock, STEMI presentation, and DES use, whereas the strongest predictors of prasugrel avoidance were female sex, older age, dialysis treatment, prior stroke/transient ischemic attack, home oral anticoagulation treatment, and transfer‐in status. In general, factors associated with increased bleeding risk, more than mortality risk, appeared to be the dominant consideration affecting clinician treatment choice.
Some of the factors associated with prasugrel selection were consistent with prior studies. For example, patients at high ischemic risk, such as those presenting with STEMI, receiving DES therapy, or developing cardiogenic shock, were more likely to be treated with prasugrel, a finding consistent with prior studies demonstrating benefit in these groups compared with clopidogrel. A prespecified analysis from the TRITON‐TIMI 38 study demonstrated a 32% reduction in a composite ischemic end point among STEMI patients treated with prasugrel compared with clopidogrel,11 and a recent multicenter registry reported similar findings.12 DES is associated with higher rates of late stent thrombosis compared with bare metal stent,13 and the primary TRITON‐TIMI 38 analysis revealed significant reduction in stent thrombosis in prasugrel‐treated patients compared with clopidogrel‐treated patients (1.1% versus 2.4%, HR 0.48, 95% CI 0.36–0.64).4 Although data are limited, 1 small observational study suggests that prasugrel use in patients presenting with acute MI and cardiogenic shock is associated with lower mortality compared with clopidogrel use.14
We also found that prasugrel was avoided in patients at high risk of bleeding, including older patients, women, and patients undergoing dialysis or being treated with oral anticoagulant therapy. Additionally, we found that prasugrel was avoided among patients with prior stroke/transient ischemic attack, concordant with guidance from the Food and Drug Administration. We speculate that high clopidogrel selection among patients transferred‐in from another acute‐care hospital may be reflective of continuing therapy with the initial ADPri selected at the presenting hospital rather than switching.
Our study also highlights the ambiguity that providers face when attempting to weigh the potential benefits of more potent antithrombotic effect against the risks of increased bleeding with prasugrel. We found lower prasugrel use among patients at high predicted risk for bleeding; paradoxically, we found lower prasugrel selection among patients at high predicted mortality risk. This suggests that many clinicians may be prioritizing their concern for bleeding when selecting ADPri therapy. Our analysis underscores that many of the factors associated with increased mortality in validated risk models also are associated with increased risk of bleeding. In our study population, ≈70% of patients had concordant predicted bleeding and mortality risks. However, among the 14% of patients at high mortality risk but low bleeding risk, initial prasugrel selection was lower than in the overall population.
The TRANSLATE‐ACS study observed no significant difference in outcomes associated with prasugrel versus clopidogrel among overall MI patients treated with PCI in routine US clinical practice. This analysis extended these findings by examining outcomes stratified by patients’ predicted risk. We found that adjusted outcomes were not significantly different between prasugrel‐ and clopidogrel‐treated patients, regardless of bleeding and mortality risk. The most optimal risk–benefit ratio may be among patients who have both low predicted risk of bleeding and low predicted mortality risk, as prasugrel‐treated patients in this group trend toward lower adjusted MACE risk compared with clopidogrel, and had the lowest observed rate of bleeding. These results suggest the utility of an integrated risk stratification model, especially with respect to suspected bleeding risk, when selecting the appropriate ADPri. Given the overlap of bleeding and mortality risk predictors, however, there may be underutilization of prasugrel among patients who may be more likely to derive ischemic benefit. Our analysis underscores the need for more discriminatory risk prediction to identify patients at high ischemic risk who may benefit from more potent antithrombotic therapy while balancing the risk for potentially significant bleeding. The ACTION mortality model has been validated to predict mortality, but does not incorporate other factors that may be important in initial prasugrel selection, such as angiographic lesion severity, stent thrombosis, or pharmacogenomic characteristics.
Our study has a number of important limitations that warrant consideration. First, TRANSLATE‐ACS did not capture provider‐reported rationale for ADPri selection. While participating hospital formularies permitted both prasugrel and clopidogrel treatment options, there may be provider treatment preferences, institutional care pathways, or order templates that influenced ADPri selection and were not accounted for in this study. Next, although the ACTION mortality and bleeding risk models have been extensively validated, we observed significant overlap in predicted risk in our study population, which underscores the limited capacity of these models to accurately discriminate patients at high predicted mortality risk and low predicted bleeding risk or vice versa. Additionally, the hospitals that participated in the TRANSLATE‐ACS study may not be broadly representative of hospitals across the country. Finally, despite robust multivariable adjustment, as this is an observational analysis, we are unable to draw causal inferences from these results, and the possibility of unmeasured confounding may exist.
Conclusions
Among contemporary patients presenting with acute MI, the strongest factors associated with initial prasugrel selection were age, DES placement, STEMI presentation, and cardiogenic shock, whereas prasugrel was avoided in patients with risk factors for bleeding. Given the overlap of bleeding and mortality risk predictors, this may result in underutilization of higher potency ADPri among patients more likely to derive ischemic benefit from more potent antithrombotic therapy. Our study highlights the importance of more granular risk stratification when initially selecting ADPri to identify patients for whom the benefit of more potent ADPri may offset increased bleeding risk.
Sources of Funding
The TRANSLATE‐ACS study is sponsored by Daiichi Sankyo and Lilly USA and. The Duke Clinical Research Institute is the coordinating center for this study, which represents a collaborative effort with the American College of Cardiology.
Disclosures
Dr Vora was funded by NIH T‐32 training grant T32 HL069749 and L30 HL124592. However, no relationships exist related to the analysis presented. Dr Peterson reports research funding for the American College of Cardiology, American Heart Association, Eli Lilly & Company, Janssen Pharmaceuticals, and Society of Thoracic Surgeons (all significant); consulting (including CME) for Merck & Co. (modest), Boehringer Ingelheim, Genentech, Janssen Pharmaceuticals, and Sanofi‐Aventis (all significant). Dr Effron reports being an employee and shareholder of Eli Lilly & Company. Dr Faries reports being a full‐time employee of Eli Lilly and Company; minor shareholder in Eli Lilly and Company. Dr Zettler reports being an employee of Eli Lilly & Company at the time this analysis was completed. Dr Fonarow reports being a consultant to Eli Lilly & Company (modest) and Janssen (modest). Dr Baker reports being an employee of Daiichi Sankyo, Inc. Dr Wang reports research funding from AstraZeneca, Gilead, Lilly, The Medicines Company, and Canyon Pharmaceuticals (all significant); educational activities or lectures (generates money for Duke) for AstraZeneca (modest); consulting (including CME) for Medco (modest) and American College of Cardiology (significant). The remaining authors have no disclosures to report.
Supporting information
Table S1. TRANSLATE‐ACS Model Covariate List
(J Am Heart Assoc. 2016;5:e003946 doi: 10.1161/JAHA.116.003946)
References
- 1. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; American College of C, American Heart Association Task Force on Practice G, Society for Cardiovascular A, Interventions, Society of Thoracic Surgeons and American Association for Clinical C . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 2. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 3. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 5. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 6. Chin CT, Chen AY, Wang TY, Alexander KP, Mathews R, Rumsfeld JS, Cannon CP, Fonarow GC, Peterson ED, Roe MT. Risk adjustment for in‐hospital mortality of contemporary patients with acute myocardial infarction: the acute coronary treatment and intervention outcomes network (ACTION) registry‐get with the guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113–122.e2. [DOI] [PubMed] [Google Scholar]
- 7. Mathews R, Peterson ED, Chen AY, Wang TY, Chin CT, Fonarow GC, Cannon CP, Rumsfeld JS, Roe MT, Alexander KP. In‐hospital major bleeding during ST‐elevation and non–ST‐elevation myocardial infarction care: derivation and validation of a model from the ACTION Registry®‐GWTG™ . Am J Cardiol. 2011;107:1136–1143. [DOI] [PubMed] [Google Scholar]
- 8. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. [DOI] [PubMed] [Google Scholar]
- 9. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 10. Chin CT, Wang TY, Anstrom KJ, Zhu B, Maa JF, Messenger JC, Ryan KA, Davidson‐Ray L, Zettler M, Effron MB, Mark DB, Peterson ED. Treatment with adenosine diphosphate receptor inhibitors—longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE‐ACS) study design: expanding the paradigm of longitudinal observational research. Am Heart J. 2011;162:844–851. [DOI] [PubMed] [Google Scholar]
- 11. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, Antman EM. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST‐elevation myocardial infarction (TRITON‐TIMI 38): double‐blind, randomised controlled trial. Lancet. 2009;373:723–731. [DOI] [PubMed] [Google Scholar]
- 12. Zeymer U, Hochadel M, Lauer B, Kaul N, Wohrle J, Andresen D, Schwimmbeck P, Solzbach U, Thiele H, Gitt A, Diller F, Zahn R. Use, efficacy and safety of prasugrel in patients with ST segment elevation myocardial infarction scheduled for primary percutaneous coronary intervention in clinical practice. Results of the prospective ATACS‐registry. Int J Cardiol. 2015;184:122–127. [DOI] [PubMed] [Google Scholar]
- 13. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus‐eluting and paclitaxel‐eluting stents in routine clinical practice: data from a large two‐institutional cohort study. Lancet. 2007;369:667–678. [DOI] [PubMed] [Google Scholar]
- 14. Orban M, Mayer K, Morath T, Bernlochner I, Hadamitzky M, Braun S, Schulz S, Hoppmann P, Hausleiter J, Tiroch K, Mehilli J, Schunkert H, Massberg S, Laugwitz KL, Sibbing D, Kastrati A. Prasugrel vs clopidogrel in cardiogenic shock patients undergoing primary PCI for acute myocardial infarction. Results of the ISAR‐SHOCK registry. Thromb Haemost. 2014;112:1190–1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. TRANSLATE‐ACS Model Covariate List


