Abstract
Background
Several studies have investigated the impact of body mass index (BMI) on the prognosis of atrial fibrillation, but the results remain controversial. We sought to estimate the association of BMI with atrial fibrillation–related outcomes.
Methods and Results
We systematically searched the Cochrane Library, PubMed, and Elsevier databases for all studies reporting associations between BMI and atrial fibrillation–related outcomes. Relative risks (RRs) and 95% CIs were extracted and pooled. Nine studies with 49 364 participants were included. Underweight BMI was associated with an increased risk of stroke or systemic embolism (RR 1.67, 95% CI 1.12–2.49), all‐cause mortaliity (RR 2.61, 95% CI 2.21–3.09), and cardiovascular death (RR 2.49, 95% CI 1.38–4.50). Nevertheless, the pooled RRs of overweight and obese patients were lower than those of normal‐weight patients for stroke or systemic embolism (overweight: RR 0.91, 95% CI 0.80–1.04; obese: RR 0.84, 95% CI 0.72–0.98; grade 1 obesity: RR 0.89, 95% CI 0.71–1.11; grade 2 obesity: RR 0.64, 95% CI 0.45–0.91; grade 3 obesity: RR 0.82, 95% CI 0.54–1.25), all‐cause death (overweight: RR 0.78, 95% CI 0.62–0.96; obese: RR 0.84, 95% CI 0.64–1.10; grade 1 obesity: RR 0.64, 95% CI 0.57–0.73; grade 2 obesity: RR 0.70, 95% CI 0.47–1.03; grade 3 obesity: RR 0.72, 95% CI 0.59–0.88), and cardiovascular death (overweight: RR 0.79, 95% CI 0.58–1.08; obese: RR 0.99, 95% CI 0.79–1.24).
Conclusions
Underweight BMI is associated with an increased risk of stroke or systemic embolism, cardiovascular death, and all‐cause death in Asian patients with atrial fibrillation, whereas in all atrial fibrillation patients, overweight and obese BMI was not associated with increased risks of these outcomes.
Keywords: atrial fibrillation, body mass index, death, prognosis, stroke, systemic embolism
Subject Categories: Arrhythmias, Atrial Fibrillation, Obesity, Risk Factors
Introduction
Atrial fibrillation (AF), the most frequent type of cardiac rhythm disorder in clinical practice, is associated with elevated cardiovascular and cerebrovascular morbidity and mortality. Because of the increased incidence and prevalence of AF, more attention has been placed on the management and prevention of AF.1 It is well known that AF is not only closely associated with stroke or systemic embolism (SSE) but also associated with all‐cause death.2 Although several risk‐scoring systems have shown modest predictive ability for stroke risk, most variables considered in those systems are not modifiable risk factors. To improve the estimation of stroke risk, it is important to explore the modifiable risk factors for clinical outcomes in AF patients.
Obesity is a modifiable cardiovascular risk factor,3 and body mass index (BMI; in kg/m2) is the most commonly used anthropometric measure of the degree of adiposity. Prior studies have reported that obesity is an independent risk factor for the incidence, recurrence, and progression of AF.4, 5, 6, 7 A previous meta‐analysis of 97 studies found that overweight BMI (25 to <30) was significantly associated with lower all‐cause mortality and that moderate obesity (BMI 30 to <35) was not associated with higher mortality.8 Consequently, it seems that overweight and moderately obese patients have more favorable prognosis than those with normal BMI. This counterintuitive phenomenon has been referred to as the “obesity paradox.” To date, the existence of the obesity paradox has been documented in patients with noncardiovascular diseases, including diabetes mellitus,9, 10 chronic kidney disease11 (particularly among hemodialysis patients12), and stroke13 as well as cardiovascular diseases, including coronary artery disease,14 hypertension,15 and heart failure.16 Nevertheless, the data on the impact of obesity on adverse outcomes in AF patients are conflicting. A cross‐sectional study of 480 AF patients showed no association between obesity and risk of thromboembolic events.17 In the prospective Danish Diet, Cancer, and Health study, obesity was associated with a worse prognosis in AF patients.18 In addition, a weight loss intervention program for patients with AF was able to reduce the burden of AF.19, 20 In contrast, an association between obesity and better survival and outcomes in AF patients has also been reported.21, 22 Because of these inconsistencies, the obesity paradox in AF patients is still not fully understood. The aim of this meta‐analysis was to evaluate the association between BMI and clinical outcomes in patients with AF.
Methods
The protocol and reporting of the results of this meta‐analysis were based on the Meta‐Analysis of Observational Studies in Epidemiology guidelines.23 Ethics approval was not provided because we performed this meta‐analysis using data only from published studies.
Literature Search and Study Selection
We systematically searched the Cochrane Library, PubMed, and Elsevier databases through May 2016 for studies published in any language that reported adverse outcomes of AF based on BMI. Three groups of key words were combined using the Boolean operator “and”. The first group of key words was linked to body mass (“body mass index” OR “body weight” OR “obesity” OR “overweight” OR “central obesity”). The second group was linked to the type of diagnosis (“atrial fibrillation” OR “atrial flutter” OR “atrial tachycardia” OR “supraventricular tachycardia” OR “arrhythmia”). The last group of key words was linked to outcomes (“cardiac death” OR “cardiovascular death” OR “mortality” OR “death” OR “stroke” OR “systemic embolism” OR “thromboembolism”). We also reviewed reference lists and conference abstracts to identify other relevant studies.
Clinical studies were included if they fulfilled the following criteria: (1) included nonvalvular AF patients; (2) were designed as randomized controlled trials or observational (prospective or retrospective cohort) studies (post hoc analyses of randomized controlled trials were deemed equivalent to observational studies24); (3) assessed primary outcomes that included SSE, all‐cause death, and cardiovascular death; and (4) assessed BMI as a categorical variable according to the standard World Health Organization (WHO) definition,25 in which “normal weight” is defined as BMI 18.5 to <25, underweight as BMI <18.5, overweight as 25 to <30, and obese as BMI ≥30. Studies with insufficient data were excluded from this meta‐analysis. For multiple publications and reports using the same data, we included the articles with the longest follow‐up or the largest numbers of participants.
Data Extraction and Quality Assessment
Two researchers (W.G.Z. and R.W.) screened the titles and abstracts of the studies independently. The full texts of the selected titles or abstracts were reviewed for more detail using the aforementioned inclusion criteria. Any disagreements were resolved through discussion or by a third researcher (K.H.) if needed. For each study, the following data were recorded: first author, year of publication, geographic location, participants (sex, age, and sample size), follow‐up duration in years, outcomes, BMI categories, maximum adjusted covariates, and relative risks (RRs) with 95% CIs. If both unadjusted and adjusted RRs existed in 1 study, the most completely adjusted RRs were extracted. The Newcastle–Ottawa Scale (NOS) items, with a total score of 9 stars, were used to evaluate the quality of the observational studies.26 We defined studies with a NOS score ≥6 stars as moderate to high quality and studies with a NOS score <6 stars as low quality.
Statistical Analyses
All statistical analyses were performed using Review Manager (RevMan) version 5.30 software (Nordic Cochrane Center; http://ims.cochrane.org/revman). The RRs were used as the common risk estimates and then were pooled by the software. To examine the influence of individual studies on the pooled results, a sensitivity analysis was performed by removing each study. Consistency was evaluated using the Cochrane Q test as well as the I2 statistic. I2 values of ≤25%, 50%, and ≥75% represented low, moderate, and high inconsistency, respectively. To estimate the pooled RRs more conservatively, we used random rather than fixed‐effects models because the former is better able to explain the between‐study heterogeneity. A subgroup analysis and sensitivity analysis were performed to explore the sources of heterogeneity if appropriate. A funnel plot was drawn to assess the degree of possible publication bias. A visually significant asymmetry in the funnel plots indicated major publication bias. A P<0.05 indicated statistical significance.
Results
Study Selection
As shown in Figure 1, we initially identified 2140 studies in the Cochrane Library (n=124), PubMed (n=990), and Elsevier (n=1026) databases. No additional studies were identified through manual searches. We excluded 1526 studies based on screening the title or abstract, and the full text of the remaining 614 studies was reviewed. Of those, 590 studies were not related to obesity and the outcomes of AF and so were excluded. The 24 remaining studies were then reviewed in more detail, and 15 of the 24 were excluded for the following reasons: (1) cross‐sectional design (n=2)17, 21; (2) publication type (2 reviews27, 28 and 1 comment29); (3) BMI not used as a categorical variable (n=3)30, 31, 32 or standard BMI classification not reported (n=1)33; (4) study evaluated the effect of BMI on the progression or recurrence of AF (n=2)6, 7; (5) “overweight” was used as the reference (n=1)22; (6) study evaluated the effect of BMI on a composite end point of SSE, all‐cause death, or cardiovascular death (n=2)34, 35; and (7) duplicate data were used (n=1).36 Finally, 9 studies (1 retrospective cohort study, 5 prospective cohort studies, and 3 post hoc analyses of randomized controlled trials) comprising 49 364 participants were included in this meta‐analysis.18, 37, 38, 39, 40, 41, 42, 43, 44
Figure 1.
Overview of the research strategy. AF indicates atrial fibrillation; BMI, body mass index.
The detailed characteristics of the included studies are provided in Table 1. All studies grouped their patients using the standard WHO BMI categories. In the study by Kwon et al,39 which included 2 cohorts, the Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS) cohorts were studied separately. Two studies further categorized obesity into grade 1 (BMI 30 to <35), grade 2 (BMI 35 to <40), and grade 3 (BMI ≥40).37, 41 There was a linear increase in age from the obese patients (67.3±1.6 years) to the underweight patients (80.4±1.8 years). As shown in Table 2, the reporting quality of the included studies was globally acceptable because all studies had a NOS score of ≥6 stars.
Table 1.
Basic Characteristics of the 9 Studies Included in this Meta‐Analysis
| Study (First Author, Year) | Region | Design | Sex, Age | Follow‐up | Participants, N | Outcomes | Categories of BMIa | Maximum Adjusted Covariates | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SSE | All‐Cause Death | Cardiovascular Death | ||||||||
| Sandhu, 201637 | Asia/Pacific, Europe, Latin America, North America | Prospective cohort | Both, 69.0±9.6 y | Median 1.8 y | 17 913 | Yes | Yes | No | Normal weight, overweight, obese | Age, sex, geographic region, SBP, HR, previous SSE/TIA, DM, HF, hypertension, MI, peripheral artery disease/CABG/PCI, eGFR, alcohol consumption, smoking status, AF type, and baseline medications |
| Proietti, 201638 | Europe, Australia, New Zealand, Asia, North America | Post hoc analysis of RCT | Both, median 72 y | Mean 1.6 y | 3630 | Yes | Yes | No | Normal weight, overweight, obese | Age, sex, thromboembolic risk |
| Inoue, 201644 | Japan | Post hoc analysis of RCT | Both, 70±10 y | Mean 2.0 y | 6379 | No | Yes | Yes | Underweight, normal weight, overweight, obese | Age, warfarin, HF, CAD, stroke/TIA, antiplatelets, permanent AF |
| Kwon, 201639 | United States | Prospective cohort | Both, 63.4±6.2 y (ARIC) and 79.1±6.2 y (CHS) | Median 6.9 y (ARIC) and 5.7 y (CHS) | 1222 (ARIC) and 756 (CHS) | Yes | No | Yes | Normal weight, overweight, obese | Age, race, sex, CHA2DS2‐VASc score |
| Wang, 201540 | China | Retrospective cohort | Both, 74.5±13.9 y | Median 2.1 y | 1286 | Yes | Yes | Yes | Underweight, normal weight, overweight, obese | Congestive HF, hypertension, DM, prior stroke/TIA, peripheral vascular disease, previous TE other than stroke/TIA, age ≥75 y, smoking, paroxysmal AF, renal dysfunction, anticoagulation therapy, and sex category |
| Pandey, 201641 | United States | Prospective cohort | Both, 60–85 y | Median 2.0 y | 9606 | Yes | Yes | No | Normal weight, overweight, obese | CHA2DS2‐VASc score, education level, cognitive impairment, renal function, left atrial size, functional status, COPD, sleep apnea, CAD, cancer, HR, conduction abnormalities, frailty, height, hematocrit, smoking, cardiac rhythm management, and BMI categories |
| Hamatani, 201542 | Japan | Prospective cohort | Both, 73.9±10.7 y | Median 2.0 y | 2945 | Yes | Yes | No | Underweight, normal weight | Congestive HF, hypertension, age ≥75 y, DM, history of stroke, OAC prescription, vascular disease, sex, renal dysfunction |
| Overvad, 201318 | Denmark | Prospective cohort | Both, 59.3–74.4 y | Median 4.9 y | 3135 | Yes | Yes | No | Normal weight, overweight, obese | VKA treatment, CHADS2 and CHA2DS2‐VASc scores |
| Ardestani, 201043 | United States | Post hoc analysis of RCT | Both, 69.6±8 y | Mean 3.5 y | 2492 | No | Yes | Yes | Normal weight, overweight, obese | Unclearb |
CHA2DS2‐VASc score is composed of congestive heart failure or left ventricular ejection fraction ≤40%; hypertension; age ≥75 years; DM; stroke, TIA, or thromboembolism history; vascular disease; age 65–74 years, and sex (female). CHADS2 score is composed of congestive heart failure, hypertension, age ≥75 years, DM, and prior stroke or TIA. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHS, Cardiovascular Health Study; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; MI, myocardial infarction; NA, not available; OAC, oral anticoagulant; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; SBP, systolic blood pressure; SSE, stroke or systemic embolism; TE, thromboembolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
BMI was categorized according to the World Health Organization/National Institutes of Health classification scheme, in which normal BMI is defined as 18.5 to <25, underweight as BMI <18.5, overweight as 25 to <30, and obese as BMI ≥30.
Multivariable Cox models were performed, but the adjustments could not be determined.
Table 2.
Quality Assessment of the 9 Included Studies
| Study (First Author, Year) | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed Cohort | Nonexposed Cohort | Ascertainment of Exposure | Outcome of Interest | Assessment of Outcome | Length of Follow‐up | Adequacy of Follow‐up | |||
| Sandhu, 201637 | * | * | * | * | ** | * | * | * | 9 |
| Proietti, 201638 | * | * | * | * | ** | * | * | * | 9 |
| Inoue, 201644 | * | * | * | * | ** | * | * | * | 9 |
| Kwon, 201639 | * | * | * | * | ** | * | * | * | 9 |
| Wang, 201540 | * | * | * | * | ** | * | * | * | 9 |
| Pandey, 201641 | * | * | * | * | ** | * | * | * | 9 |
| Hamatani, 201542 | * | * | * | * | ** | * | * | * | 9 |
| Overvad, 201318 | * | * | * | * | ** | * | * | * | 9 |
| Ardestani, 201043 | * | * | * | * | ** | * | * | * | 9 |
Asterisks represent stars used in the Newcastle–Ottawa Scale.
Meta‐Analysis
BMI and SSE
As shown in Figure 2 and Table 3, underweight BMI was significantly associated with an increased risk of SSE (RR 1.67, 95% CI 1.12–2.49; P=0.01). In contrast, overweight BMI was not associated with an increased risk of SSE (RR 0.91, 95% CI 0.80–1.04; P=0.18). The obese group (RR 0.84, 95% CI 0.72–0.98; P=0.02) had a lower risk of SSE than the normal‐weight group. In addition, the summary RRs were 0.89 (95% CI 0.71–1.11), 0.64 (95% CI 0.45–0.91), and 0.82 (95% CI 0.54–1.25) for grade 1, 2, and 3 obesity, respectively (Table 3).
Figure 2.
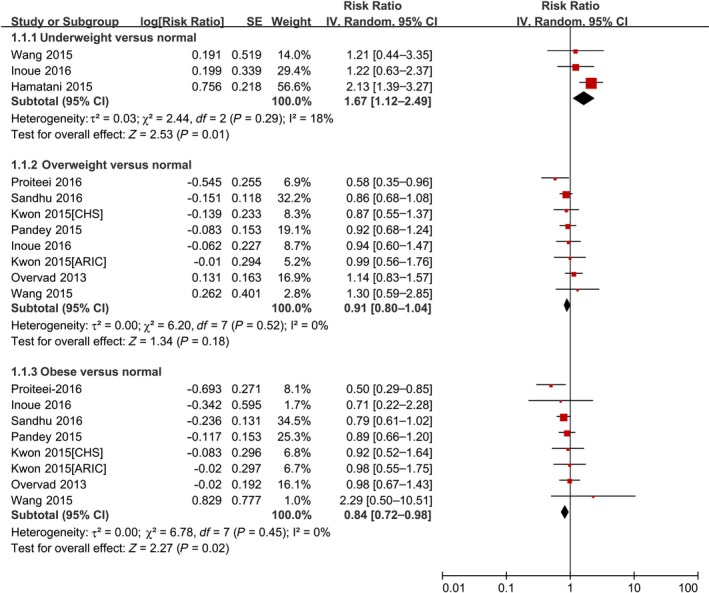
Forest plot of the association between body mass index and stroke or systemic embolism in patients with AF. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; IV, inverse variance; SE, standard error.
Table 3.
Summary of the Random‐Effects RRs of the Associations Between BMI and Adverse Outcomes Among Patients With AF
| BMI Categories | SSE | All‐Cause Death | Cardiovascular Death | |||
|---|---|---|---|---|---|---|
| No. of RRs | Summary RR (95% CI) | No. of RRs | Summary RR (95% CI) | No. of RRs | Summary RR (95% CI) | |
| Underweight (BMI <18.5) | 3 | 1.67 (1.12–2.49)a | 3 | 2.61 (2.21–3.09)a | 2 | 2.49 (1.38–4.50)a |
| Overweight (25 to <30) | 8 | 0.91 (0.80–1.04) | 7 | 0.78 (0.62–0.96)a | 5 | 0.79 (0.58–1.08) |
| Obese (BMI ≥30) | 8 | 0.84 (0.72–0.98)a | 7 | 0.80 (0.64–1.10) | 5 | 0.99 (0.79–1.24) |
| Grade 1 obesity (30 to <35) | 2 | 0.89 (0.71–1.11) | 2 | 0.64 (0.57–0.73)a | NA | NA |
| Grade 2 obesity (35 to <40) | 2 | 0.64 (0.45–0.91)a | 2 | 0.70 (0.47–1.03) | NA | NA |
| Grade 3 obesity (BMI ≥40) | 2 | 0.82 (0.54–1.25) | 2 | 0.72 (0.59–0.88)a | NA | NA |
AF indicates atrial fibrillation; BMI, body mass index; NA, not available; RR, relative risk; SSE, stroke or systemic embolism.
P<0.05 indicates statistical significance.
BMI and all‐cause death
As shown in Figure 3 and Table 3, underweight BMI was significantly associated with an increased risk of all‐cause death (RR 2.61, 95% CI 2.21–3.09; P<0.00001). In contrast, overweight BMI was associated with a decreased risk of all‐cause death (RR 0.78, 95% CI 0.62–0.96; P=0.02), and obesity was not associated with an increased risk of all‐cause death (RR 0.84, 95% CI 0.64–1.10; P=0.21). In addition, the summary RRs were 0.64 (95% CI 0.57–0.73), 0.70 (95% CI 0.47–1.03), and 0.72 (95% CI 0.59–0.88) for grade 1, 2, and 3 obesity, respectively (Table 3).
Figure 3.
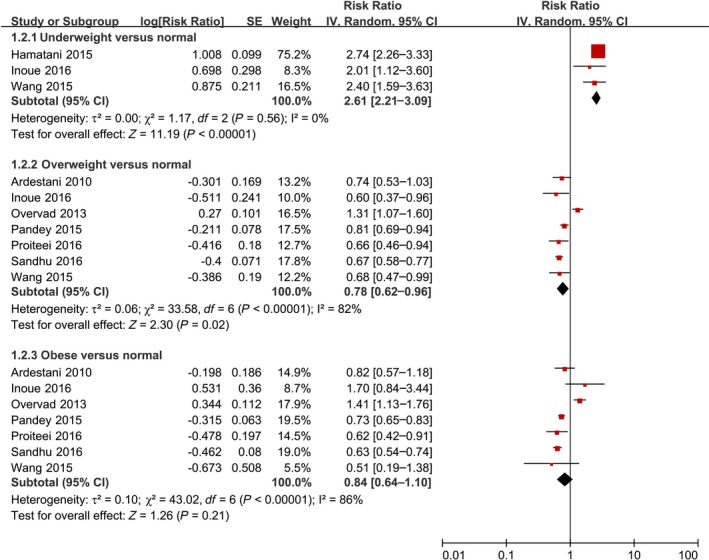
Forest plot of the association between body mass index and all‐cause death in patients with AF. AF indicates atrial fibrillation; IV, inverse variance; SE, standard error.
BMI and cardiovascular death
As shown in Figure 4 and Table 3, underweight BMI was significantly associated with an increased risk of cardiovascular death (RR 2.49, 95% CI 1.38–4.50; P=0.003). Overweight BMI (RR 0.79, 95% CI 0.58–1.08; P=0.14) and obesity (RR 0.99, 95% CI 0.79–1.24; P=0.93) were not associated with an increased risk of cardiovascular death. None of the studies further categorized obesity into grades 1 to 3 when considering the relationship between BMI and cardiovascular death.
Figure 4.
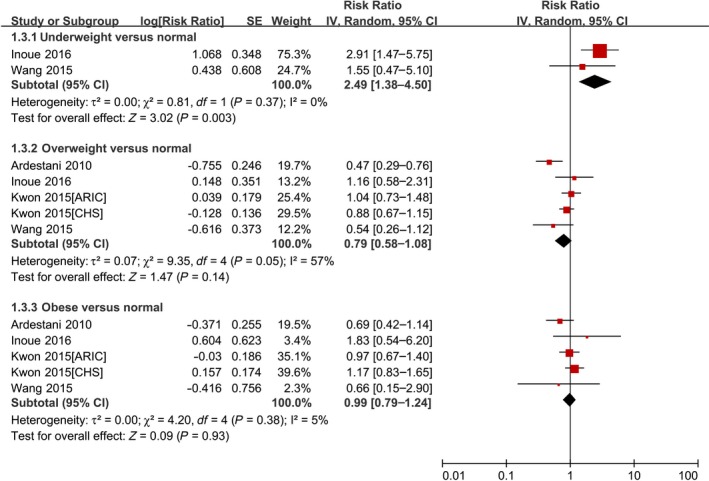
Forest plot of the association between body mass index and cardiovascular death in patients with AF. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; IV, inverse variance; SE, standard error.
The pooled RRs of SSE, all‐cause death, and cardiovascular death from all studies are presented in the Figure 5. In the sensitivity analysis, after omitting 1 study at a time, none of the aforementioned RR values changed substantially. Specifically, after we removed the retrospective study by Wang et al,40 the results also remained stable. The RR values mentioned above changed only slightly when we redid these analyses with fixed‐effects models.
Figure 5.
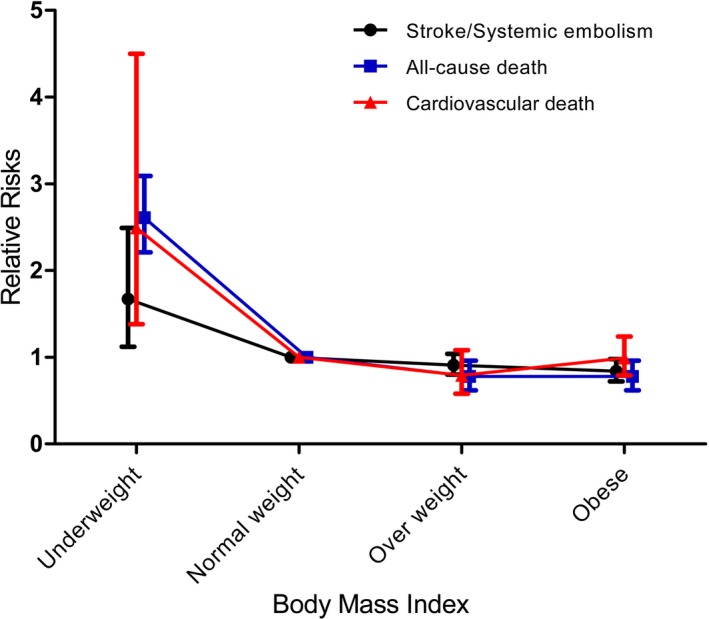
The pooled relative risks of stroke or systemic embolism, all‐cause death, and cardiovascular death from all studies.
Publication Bias
For the meta‐analysis of the reported adverse outcomes of AF based on BMI, a possible lack of publication bias was observed by inspecting the funnel plot (Figure 6).
Figure 6.
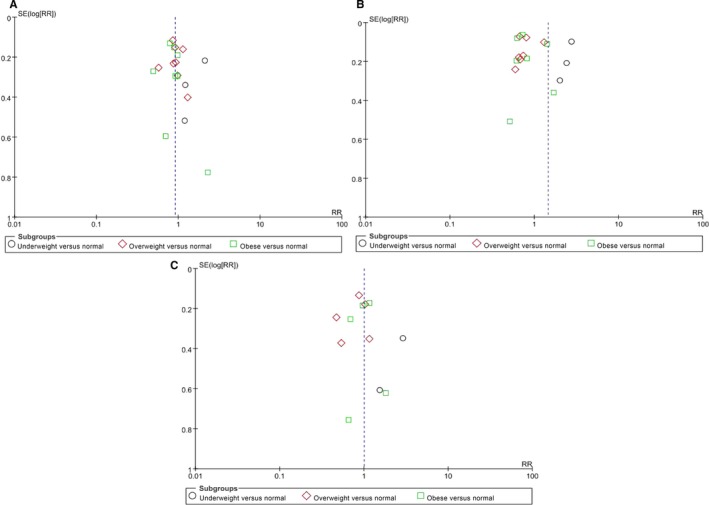
Funnel plot of the reported adverse outcomes of AF based on body mass index: (A) stroke or systemic embolism; (B) all‐cause death; (C) cardiovascular death. AF indicates atrial fibrillation; RR, relative risk; SE, standard error.
Discussion
In the present study, underweight BMI was associated with increased risks of SSE, cardiovascular death, and all‐cause death, whereas both overweight and obesity were associated with decreased risks of these outcomes. All included studies adjusted for cardiovascular risk factors and other potential confounders; therefore, the observed associations were less likely to be related to confounding factors. In addition, these results were stable in the sensitivity analyses. All studies classified their patients using the standard WHO BMI categories. To the best of our knowledge, our meta‐analysis is the first to quantify the association between BMI and adverse outcomes in AF patients.
The present results indicate that underweight BMI was significantly associated with increased risks of poor AF‐related outcomes including SSE, cardiovascular death, and all‐cause death. In addition, underweight BMI was significantly associated with increased risk of the composite end point of SSE, all‐cause death, or cardiovascular death.35, 42 The detailed mechanisms behind the increased risk of poor outcomes owing to low body weight remain unclear, but there are several possible explanations. Patients with low body weight are more susceptible to becoming ill as a result of poor nutritional status.45 In addition, low body weight is correlated with poor endothelial function and worse systemic inflammation, both of which contribute to platelet aggregation and adhesion and, ultimately, SSE and death.46, 47 Moreover, the renin–angiotensin system is activated in patients with low body weight, leading to advanced atrial fibrosis.48 Finally, adipokines such as leptin, adiponectin, and resistin, which are correlated with BMI, have been reported to be involved in the development of AF.49, 50 As shown by Haynes et al, low body weight is associated with abnormal circulating adipokine levels,51 which may contribute to eating disorders and poor nutritional status.52 Accordingly, it is suggested that abnormal adipokine levels could contribute to the poor outcomes in AF patients. All 3 of the relevant included studies assessed Asian patients with AF, and thus our results regarding the association between underweight and adverse outcomes may not be generalizable to AF populations in different countries.
As in patients with coronary artery disease,14 hypertension,15 and heart failure,16 overweight and obese patients with AF had lower risks of SSE, cardiovascular death, and all‐cause death than AF patients with normal BMI. The pooled data from 2 studies37, 41 further found that extreme obesity (BMI ≥40) was still associated with a reduced risk of poor AF‐related outcomes. Given the small number of studies included in this analysis, the association between extreme obesity and prognosis of AF requires further confirmation. Consistent with this finding, several studies have also demonstrated a decreased risk of AF‐related outcomes for each 1‐U increase in BMI.21, 30, 31, 36, 41 Most of these studies were conducted in Western populations; however, the obesity standards in Asia differ from those of Western populations, and thus differences by race may exist. Nonetheless, overweight AF patients had a higher survival rate compared with underweight or normal‐weight patients in a Chinese study.22
Among Japanese AF patients, neither overweight status nor obesity was associated with increased death.44 In 2 prospective studies including a mixed population,37, 38 both overweight status and obesity were associated with decreased risks of SSE and all‐cause death. Although our meta‐analysis did not include a subgroup analysis based on race because of the limited data, we have reason to believe that an inverse association between BMI and AF‐related outcomes exists in other countries. In addition, considering the composite end points of SSE, all‐cause death, cardiovascular death, and other factors,18, 34, 36, 37, 39, 41 the overweight and obese groups had lower RRs of 0.87 (95% CI 0.67–1.15) and 0.82 (95% CI 0.59–1.14), respectively, than the normal‐weight group (data not shown). Furthermore, the prevalence of left atrial appendage thrombus was not increased in overweight and obese patients.53 Consequently, an inverse association between BMI and composite end points also occurred, even in patients receiving anticoagulants.34
A previous meta‐analysis of 16 studies showed a positive association between obesity and AF,54 and the prevalence of AF has been shown to be increased in obesity.55 For the first time, however, our cumulative meta‐analysis documented the existence of the obesity paradox in AF patients. The obesity paradox is a phenomenon in which overweight and obese patients with established AF seem to have a more favorable prognosis than those with normal BMI. The underlying mechanisms of the observed obesity paradox in AF patients are not well understood. A possible explanation of this phenomenon is the use of more aggressive pharmacological interventions in overweight and obese patients, who represent a population with a high prevalence of comorbidities and who require closer management of cardiovascular risks.36 Furthermore, as reported, plasma renin and angiotensin levels, both of which lead to poor outcomes, are not as high in overweight and obese patients under stress.56 In addition, Davos et al57 found that higher BMI could prevent death in AF patients by providing greater metabolic reserve, namely, a better ability to withstand the increased catabolic stress of disease development. Furthermore, in adipose tissue, increased expression of tumor necrosis factor α receptor has been found, and this could help disperse inflammation and arrhythmogenic substrates that are activated in cardiomyocytes.58 Finally, natriuretic peptide levels, which have been reported to predict stroke and mortality in AF patients, are lower in obesity.59, 60 Interestingly, Piepoli et al61 demonstrated that exercise tolerance mediated the relationship between BMI and survival.
Moreover, cardiorespiratory fitness mitigates the obesity paradox observed in patients with heart failure61, 62, 63 and coronary heart disease.64 Patients with relatively good preserved cardiorespiratory fitness, for example, have shown favorable prognosis regardless of their adiposity levels, and thus no obesity paradox has been observed among fit coronary heart disease patients.64 Similarly, in AF, we hypothesized that BMI‐related cardiorespiratory fitness would greatly modify the occurrence of the obesity paradox. Physical activity is associated with small reductions in AF risk, even in overweight patients.65, 66 Our previous meta‐analysis found that a higher level of physical fitness was associated with a lower risk of AF.67 Specifically, Pathak et al68 studied obese patients with symptomatic AF who were divided into 3 groups based on their baseline cardiorespiratory fitness. After 4‐year follow‐up, the rate of AF recurrence was 88%, 65%, and 34% in the low‐, adequate‐, and high‐fitness groups, respectively. In addition, the rate of AF recurrence was 82% in the group with <2 metabolic equivalent gain but only 39% in the group with >2 metabolic equivalent gain. Whether cardiorespiratory fitness is involved in the obesity paradox in AF patients requires further investigation. In addition, genetic factors may be involved.28 Lean patients who develop cardiovascular disease have gene variants that make them more susceptible to these illnesses and that place them at high risk when they become ill. Lean patients with cardiovascular disease may exhibit completely different etiology and genetic disposition than obese patients with the same disease, and this difference may be associated with worse clinical prognosis. Another possibility is that when chronic disease develops in lean patients, the body becomes catabolic and requires greater energy and caloric reserves than usual. If these patients lack sufficient nutritional status, they may become malnourished despite their normal body mass.
The obesity paradox may be partly explained by the presence of selection bias for patients with diabetes mellitus69 or cardiovascular disease.70 Moreover, selection bias may be a possible explanation of the paradoxical effects of obesity on adverse outcomes in AF patients. In the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, for example, normal‐weight patients with AF were more likely to have unmeasured confounding factors (eg, older age, abnormal renal function) other than obesity that increased their risk of AF, and this may have introduced a selection bias. If these unmeasured factors were also strong risk factors for adverse outcomes, normal‐weight patients with AF would have had a higher risk of these outcomes than obese AF patients. In this meta‐analysis, obese AF patients with higher BMI were younger than the normal‐weight patients. We speculate that obese AF patients have a higher prevalence of obesity‐related diseases (eg, coronary artery disease, cardiovascular risk factors, diabetes mellitus) that may lead to early onset of AF. Age is also a strong risk factor for stroke and death; therefore, nonobese AF patients would have had more risk factors than obese AF patients, leading to poor prognosis. Although most of the included studies adjusted for age in the multivariate analyses, it might not be possible to fully account for the differences in age between BMI groups. When analyzing observational studies on this topic in the future, we should ensure that the start of follow‐up and patient exposures coincide.69
Despite the obesity paradox, purposeful weight reduction still results in several potentially beneficial effects in AF patients. Recent studies have indicated that lifestyle modifications (eg, weight management) may help prevent and treat AF.71 In the Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation (ARREST‐AF), aggressive risk factor modifications (ie, weight management) improved the long‐term success of AF ablation.72 Abed et al19 indicated that weight reduction with intensive risk factor modification resulted in a decreased burden of AF. Weight loss alone is also associated with dose‐dependent, long‐term effects on reducing AF burden20, 71 and is associated with greater AF‐free survival.68 In this meta‐analysis, however, data on weight reduction in obese patients could not be collected; therefore, the impact of targeted weight reduction was not evaluated.
Limitations
Our study had several potential limitations. First, the association between BMI and outcomes in AF patients may be modified by sex.18 Future studies should address the role of sex differences in the obesity paradox. Second, the primary safety outcome of bleeding was not included in this study; the relationship between BMI and bleeding risk in AF patients remains controversial. Although 4 studies explored this relationship,37, 38, 40, 42 a pooled analysis could not be performed owing to the limited data. Third, BMI is used mainly to measure overall body size and may not assess true body adiposity. Waist circumference is also associated with decreased risks of all‐cause death and SSE in AF patients.37 Further studies should expand the findings on the obesity paradox from BMI to other body composition parameters used to estimate the degree of adiposity. Fourth, significant heterogeneity among studies existed and may have resulted from the differences in study design, sample size, analytic strategies, and participant characteristics; however, a subgroup analysis based on these factors could not be performed owing to the limited data. Finally, although most of the included studies adjusted for a range of confounding variables, we could not exclude the effects of residual confounding, which may have partly explained the obesity paradox.
Conclusions
In summary, the published literature demonstrates that underweight BMI is associated with increased risks of SSE, cardiovascular death, and all‐cause death in Asian patients with AF. Whether these findings are generalizable to all AF populations requires further confirmation; however, neither overweight BMI nor obesity was associated with increased risk of SSE, cardiovascular death, and all‐cause death in AF patients. Future studies should ensure that the start of follow‐up and patient exposures are consistent between groups.
Sources of Funding
The authors wish to acknowledge support from the National Natural Science Foundation of China (81370288; 8153000545; 81530013) and the National Basic Research Program of China (973 Program: 2013CB531103).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004006 doi: 10.1161/JAHA.116.004006)
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 2. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 3. Karasoy D, Bo JT, Hansen ML, Schmiegelow M, Lamberts M, Gislason GH, Hansen J, Torp‐Pedersen C, Olesen JB. Obesity is a risk factor for atrial fibrillation among fertile young women: a nationwide cohort study. Europace. 2013;15:781–786. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Parise H, Levy D, D'Agostino RS, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 5. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol. 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guglin M, Maradia K, Chen R, Curtis AB. Relation of obesity to recurrence rate and burden of atrial fibrillation. Am J Cardiol. 2011;107:579–582. [DOI] [PubMed] [Google Scholar]
- 7. Tsang TSM, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA. 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carnethon MR, Rasmussen‐Torvik LJ, Palaniappan L. The obesity paradox in diabetes. Curr Cardiol Rep. 2014;16:446. [DOI] [PubMed] [Google Scholar]
- 10. Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell‐Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohebi R, Simforoosh A, Tohidi M, Azizi F, Hadaegh F. Obesity paradox and risk of mortality events in chronic kidney disease patients: a decade of follow‐up in Tehran lipid and glucose study. J Ren Nutr. 2015;25:345–350. [DOI] [PubMed] [Google Scholar]
- 12. Rhee CM, Ahmadi SF, Kalantar‐Zadeh K. The dual roles of obesity in chronic kidney disease: a review of the current literature. Curr Opin Nephrol Hypertens. 2016;25:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10:99–104. [DOI] [PubMed] [Google Scholar]
- 14. Romero‐Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez‐Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. [DOI] [PubMed] [Google Scholar]
- 15. Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper‐Dehoff RM, Zhou Q, Pepine CJ. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. [DOI] [PubMed] [Google Scholar]
- 16. Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez‐Jimenez F, Arbab‐Zadeh A, Mukherjee D, Lazar JM. Meta‐analysis of the relation of body mass index to all‐cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–1434. [DOI] [PubMed] [Google Scholar]
- 17. Novo G, Mansueto P, La Franca ML, Di Leo R, Di Rosa S, Fazio G, Mansueto S, Ferrara F, Novo S. Risk factors, atrial fibrillation and thromboembolic events. Int Angiol. 2008;27:433–438. [PubMed] [Google Scholar]
- 18. Overvad TF, Rasmussen LH, Skjoth F, Overvad K, Lip GY, Larsen TB. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med. 2013;126:640–649. [DOI] [PubMed] [Google Scholar]
- 19. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 20. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long‐term effect of goal‐directed weight management in an atrial fibrillation cohort: a long‐term follow‐up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 21. Yanagisawa S, Inden Y, Yoshida N, Kato H, Miyoshi‐Fujii A, Mizutani Y, Ito T, Kamikubo Y, Kanzaki Y, Hirai M, Murohara T. Body mass index is associated with prognosis in Japanese elderly patients with atrial fibrillation: an observational study from the outpatient clinic. Heart Vessels. 2016;31:1553–1561. [DOI] [PubMed] [Google Scholar]
- 22. Wang J, Yang Y, Zhu J, Zhang H, Shao X, Tian L, Huang B, Yu L, Gao X, Wang M. Overweight is associated with improved survival and outcomes in patients with atrial fibrillation. Clin Res Cardiol. 2014;103:533–542. [DOI] [PubMed] [Google Scholar]
- 23. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 24. Gu WJ, Wang F, Tang L, Liu JC. Single‐dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta‐analysis of randomized controlled trials and observational studies. Chest. 2015;147:335–346. [DOI] [PubMed] [Google Scholar]
- 25. Obesity: preventing and managing the global epidemic. Report of a WHO consultation (WHO Technical Report Series 894). World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 26. Wells GA, Shea B, O Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta‐analyses. 2014. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 30, 2016.
- 27. Goel K, Lopez‐Jimenez F, De Schutter A, Coutinho T, Lavie CJ. Obesity paradox in different populations: evidence and controversies. Future Cardiol. 2014;10:81–91. [DOI] [PubMed] [Google Scholar]
- 28. Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and prevalence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. [DOI] [PubMed] [Google Scholar]
- 29. Badheka AO, Rathod A, Bharadwaj A, Afonso L, Jacob S. Obesity paradox in outcomes of atrial fibrillation. Am J Cardiol. 2011;108:474. [DOI] [PubMed] [Google Scholar]
- 30. Chu C, Lee W, Hsu P, Lee M, Lee H, Chiu C, Lin T, Lee C, Yen H, Voon W, Lai W, Sheu S, Su H. Association of increased epicardial adipose tissue thickness with adverse cardiovascular outcomes in patients with atrial fibrillation. Medicine. 2016;95:e2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Proietti M, Raparelli V, Basili S, Olshansky B, Lip GY. Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: the AFFIRM Trial. Int J Cardiol. 2016;207:258–263. [DOI] [PubMed] [Google Scholar]
- 32. Lee WH, Hsu PC, Chu CY, Lee HH, Lee MK, Lee CS, Yen HW, Lin TH, Voon WC, Lai WT, Sheu SH, Su HM. Anemia as an independent predictor of adverse cardiac outcomes in patients with atrial fibrillation. Int J Med Sci. 2015;12:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2‐VASc Risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66:1851–1859. [DOI] [PubMed] [Google Scholar]
- 34. Senoo K, Lip GYH. Body mass index and adverse outcomes in elderly patients with atrial fibrillation: The AMADEUS Trial. Stroke. 2016;47:523–526. [DOI] [PubMed] [Google Scholar]
- 35. Hamatani Y, Yamashita Y, Esato M, Chun Y, Tsuji H, Wada H, Hasegawa K, Abe M, Lip GYH, Akao M. Predictors for stroke and death in non‐anticoagulated Asian patients with atrial fibrillation: the Fushimi AF Registry. PLoS One. 2015;10:e142394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Badheka AO, Rathod A, Kizilbash MA, Garg N, Mohamad T, Afonso L, Jacob S. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123:646–651. [DOI] [PubMed] [Google Scholar]
- 37. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, Wallentin L. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. 2016. Available at: http://eurheartj.oxfordjournals.org/content/early/2016/04/11/eurheartj.ehw124. Accessed September 2, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Proietti M, Lane DA, Lip GYH. Relation of non‐valvular atrial fibrillation to body mass index (from the SPORTIF Trials). Am J Cardiol. 2016;118:72–78. [DOI] [PubMed] [Google Scholar]
- 39. Kwon Y, Norby FL, Jensen PN, Agarwal SK, Soliman EZ, Lip GY, Longstreth WJ, Alonso A, Heckbert SR, Chen LY. Association of smoking, alcohol, and obesity with cardiovascular death and ischemic stroke in atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) Study and Cardiovascular Health Study (CHS). PLoS One. 2016;11:e147065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H, Si Q, Shan Z, Guo Y, Lin K, Zhao X, Wang Y. Effects of body mass index on risks for ischemic stroke, thromboembolism, and mortality in Chinese atrial fibrillation patients: a single‐center experience. PLoS One. 2015;10:e123516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandey A, Gersh BJ, McGuire DK, Shrader P, Thomas L, Kowey PR, Mahaffey KW, Hylek E, Sun S, Burton P, Piccini J, Peterson E, Fonarow GC. Association of body mass index with care and outcomes in patients with atrial fibrillation: results from the ORBIT‐AF Registry. JACC Clin Electrophysiol. 2016;2:355–363. [DOI] [PubMed] [Google Scholar]
- 42. Hamatani Y, Ogawa H, Uozumi R, Iguchi M, Yamashita Y, Esato M, Chun Y, Tsuji H, Wada H, Hasegawa K, Abe M, Morita S, Akao M. Low body weight is associated with the incidence of stroke in atrial fibrillation patients—insight from the Fushimi AF Registry. Circ J. 2015;79:1009–1017. [DOI] [PubMed] [Google Scholar]
- 43. Ardestani A, Hoffman HJ, Cooper HA. Obesity and outcomes among patients with established atrial fibrillation. Am J Cardiol. 2010;106:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Inoue H, Kodani E, Atarashi H, Okumura K, Yamashita T, Origasa H. Impact of body mass index on the prognosis of Japanese patients with non‐valvular atrial fibrillation. Am J Cardiol. 2016;118:215–221. [DOI] [PubMed] [Google Scholar]
- 45. Park DW, Kim YH, Yun SC, Ahn JM, Lee JY, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, Park SJ. Association of body mass index with major cardiovascular events and with mortality after percutaneous coronary intervention. Circ Cardiovasc Interv. 2013;6:146–153. [DOI] [PubMed] [Google Scholar]
- 46. Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Sasaki S, Hara K, Matsuura H, Goto C, Oshima T, Chayama K, Yoshizumi M. Low body mass index is a risk factor for impaired endothelium‐dependent vasodilation in humans: role of nitric oxide and oxidative stress. J Am Coll Cardiol. 2003;42:256–263. [DOI] [PubMed] [Google Scholar]
- 47. Nakajima K, Yamaoka H, Morita K, Ebata M, Eguchi S, Muneyuki T, Munakata H. Elderly people with low body weight may have subtle low‐grade inflammation. Obesity (Silver Spring). 2009;17:803–808. [DOI] [PubMed] [Google Scholar]
- 48. Novo G, Guttilla D, Fazio G, Cooper D, Novo S. The role of the renin‐angiotensin system in atrial fibrillation and the therapeutic effects of ACE‐Is and ARBS. Br J Clin Pharmacol. 2008;66:345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ermakov S, Azarbal F, Stefanick ML, LaMonte MJ, Li W, Tharp KM, Martin LW, Nassir R, Salmoirago‐Blotcher E, Albert CM, Manson JE, Assimes TL, Hlatky MA, Larson JC, Perez MV. The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart. 2016;102:1354–1362. [DOI] [PubMed] [Google Scholar]
- 50. Li G, Liu T. The therapeutic strategies of enhancing adiponectin and lowering leptin may be benefit to controlling atrial fibrillation. Med Hypotheses. 2009;73:122. [DOI] [PubMed] [Google Scholar]
- 51. Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor‐mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franceschini R, Tenconi GL, Zoppoli F, Barreca T. Endocrine abnormalities and outcome of ischaemic stroke. Biomed Pharmacother. 2001;55:458–465. [DOI] [PubMed] [Google Scholar]
- 53. Cohoon KP, McBane RD, Ammash N, Slusser JP, Grill DE, Wysokinski WE. Relationship between body mass index and left atrial appendage thrombus in nonvalvular atrial fibrillation. J Thromb Thrombolysis. 2016;41:613–618. [DOI] [PubMed] [Google Scholar]
- 54. Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta‐analysis. Am Heart J. 2008;155:310–315. [DOI] [PubMed] [Google Scholar]
- 55. Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2016;37:1565–1572. [DOI] [PubMed] [Google Scholar]
- 56. Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37:169–174. [DOI] [PubMed] [Google Scholar]
- 57. Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, Clark AL, Anker SD. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. [DOI] [PubMed] [Google Scholar]
- 58. Lee SH, Chen YC, Chen YJ, Chang SL, Tai CT, Wongcharoen W, Yeh HI, Lin CI, Chen SA. Tumor necrosis factor‐alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80:1806–1815. [DOI] [PubMed] [Google Scholar]
- 59. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 60. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 61. Piepoli MF, Corra U, Veglia F, Bonomi A, Salvioni E, Cattadori G, Metra M, Lombardi C, Sinagra G, Limongelli G, Raimondo R, Re F, Magri D, Belardinelli R, Parati G, Mina C, Scardovi AB, Guazzi M, Cicoira M, Scrutinio D, Di Lenarda A, Bussotti M, Frigerio M, Correale M, Villani GQ, Paolillo S, Passino C, Agostoni P. Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI Score Research Group. Eur J Heart Fail. 2016;18:545–553. [DOI] [PubMed] [Google Scholar]
- 62. Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, Arena R. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. Am J Cardiol. 2015;115:209–213. [DOI] [PubMed] [Google Scholar]
- 64. McAuley PA, Artero EG, Sui X, Lee DC, Church TS, Lavie CJ, Myers JN, Espana‐Romero V, Blair SN. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol. 2014;7:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grundvold I, Skretteberg PT, Liestol K, Gjesdal K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Importance of physical fitness on predictive effect of body mass index and weight gain on incident atrial fibrillation in healthy middle‐age men. Am J Cardiol. 2012;110:425–432. [DOI] [PubMed] [Google Scholar]
- 67. Zhu W, Shen Y, Zhou Q, Xu Z, Huang L, Chen Q, Hong K. Association of physical fitness with the risk of atrial fibrillation: a systematic review and meta‐analysis. Clin Cardiol. 2016;39:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO‐FIT study. J Am Coll Cardiol. 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 69. Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2015;128:334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. [DOI] [PubMed] [Google Scholar]
- 71. Menezes AR, Lavie CJ, De Schutter A, Milani RV, O'Keefe J, DiNicolantonio JJ, Morin DP, Abi‐Samra FM. Lifestyle modification in the prevention and treatment of atrial fibrillation. Prog Cardiovasc Dis. 2015;58:117–125. [DOI] [PubMed] [Google Scholar]
- 72. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]


