Abstract
Background
To clarify the influence of hypertension and blood pressure (BP) control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation, a post hoc analysis of the J‐RHYTHM Registry was performed.
Methods and Results
A consecutive series of outpatients with atrial fibrillation was enrolled from 158 institutions. Of 7937 patients, 7406 with nonvalvular atrial fibrillation (70.8% men, 69.8±10.0 years) were followed for 2 years or until an event occurred. Hypertension was defined as a systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, a history of hypertension, and/or antihypertensive drug use. Hypertension was an independent risk factor for major hemorrhage (hazard ratio 1.52, 95% CI 1.05–2.21, P=0.027) but not for thromboembolism (hazard ratio 1.05, 95% CI 0.73–1.52, P=0.787). When patients were divided into quartiles according to their systolic BP at the time closest to the event or at the end of follow‐up (Q1, <114; Q2, 114–125; Q3, 126–135; and Q4, ≥136 mm Hg), odds ratios for both events were significantly higher in Q4 than in Q1 (thromboembolism, odds ratio 2.88, 95% CI 1.75–4.74, P<0.001; major hemorrhage, odds ratio 1.61, 95% CI 1.02–2.53, P=0.041) after adjustment for components of CHA 2 DS 2‐VASc score, warfarin use, and antiplatelet use. A systolic BP of ≥136 mm Hg was an independent risk factor for thromboembolism and major hemorrhage.
Conclusions
BP control appears to be more important than a history of hypertension and baseline BP values at preventing thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation.
Clinical Trial Registration
URL: http://www.umin.ac.jp/ctr. Unique identifier: UMIN000001569.
Keywords: anticoagulation, atrial fibrillation, blood pressure, hypertension, thromboembolism
Subject Categories: Atrial Fibrillation, Hypertension, High Blood Pressure, Ischemic Stroke, Intracranial Hemorrhage
Introduction
Atrial fibrillation (AF) is a common arrhythmia and a potent risk factor for cardiogenic embolism.1, 2 Hypertension is also a common disease and the most frequent comorbidity in patients with AF.3, 4 Since hypertension is one of the risk factors for thromboembolism and hemorrhagic complications in patients with AF, it is a component of the CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and history of stroke or transient ischemic attack [TIA]),5 the CHA2DS2‐VASc (CHADS2 components, plus vascular disease [coronary artery disease], age 65–74 years, and female sex),6 and the HAS‐BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio [INR], elderly [>65 years], drugs/alcohol concomitantly)7 scores. However, hypertension is not always detected as an independent predictor of thromboembolism or hemorrhagic complications.8, 9, 10, 11, 12 In patients with nonvalvular AF (NVAF) who were not receiving anticoagulation therapy, hypertension was an independent risk factor for ischemic stroke.13 By contrast, the presence of hypertension at baseline was not a significant risk factor for thromboembolism or major hemorrhage in NVAF patients when including those treated with warfarin.14, 15 Differences in patient characteristics may have contributed to these inconsistent results. Another possible explanation for the inconsistent results was that blood pressure (BP) control was not considered when a history of hypertension was selected as an explanatory variable for multivariate analysis. The association between BP values and the event incidence has not been elucidated in patients with NVAF in any previous report of the J‐RHYTHM Registry.14, 15, 16, 17, 18, 19, 20 Therefore, in order to investigate the influence of BP control rather than hypertension on event rates of thromboembolism and major hemorrhage, a post hoc analysis was performed using quartiles of BP values at the time of enrollment and at the time closest to these events in the J‐RHYTHM Registry.
Methods
Study Design of the J‐RHYTHM Registry
The J‐RHYTHM Registry was conducted as a nationwide prospective observational study to investigate the present status of anticoagulation therapy and optimal anticoagulation therapy in Japanese patients with AF.21 The study design and baseline patient characteristics have been reported elsewhere.3, 21 Briefly, the study protocol conformed to the Declaration of Helsinki and was approved by the ethics committee of each participating institution. All participants gave written informed consent at the time of enrollment. A consecutive series of outpatients with AF of any type was enrolled from 158 institutions, regardless of the use of antithrombotic drugs. All drugs, including antihypertensive drugs, and their dosages were selected at the discretion of the treating cardiologists. Patients with valvular AF22 were excluded from this subanalysis. Seated brachial BP was measured in each patient at the time of enrollment (baseline) and at each follow‐up visit by either the auscultatory method or an automated sphygmomanometer, as was appropriate at each institution. Hypertension was defined as a peripheral systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg,23 a prior history of hypertension, and/or the use of antihypertensive drugs.21
Follow‐Up and Definition of End Points
Patients were followed up for 2 years or until an event, whichever occurred first. Thromboembolism included symptomatic ischemic stroke, transient ischemic attack, and systemic embolic events. Major hemorrhage included intracranial hemorrhage (ICH), gastrointestinal hemorrhage, and other hemorrhages requiring hospitalization. If any event occurred during the follow‐up period, BP values at the time prior to and closest to the event were obtained.21 The diagnostic criteria for each event have been described elsewhere.3, 21
Grouping of Patients
Patients were divided into 2 groups based on the presence of hypertension at the time of enrollment (Hypertension and No‐hypertension) and event incidence rates between the 2 groups were compared. In addition, patients were divided into 4 groups according to the quartiles of their systolic BP at baseline (Q1, <116; Q2, 116–125; Q3, 126–135; and Q4, ≥136 mm Hg) and at the time prior to and closest to an event or at the end of follow‐up (Q1, <114; Q2, 114–125; Q3, 126–135; and Q4, ≥136 mm Hg). Patients were also divided into quartiles based on their diastolic BP.
Statistical Analysis
Data are presented as mean±SD. The statistical significance of differences in mean values was analyzed using Student t test or analysis of variance, as appropriate. Frequencies of parameters or events were compared using the χ2 test or Fisher's exact test, as appropriate. Trend among the quartiles was tested by Cochran‐Armitage test for categorical variables or Jonckheere‐Terpstra test for continuous variables, as appropriate. Kaplan–Meier curves between 2 groups were compared with log‐rank tests. A Cox proportional hazards model was used to investigate the influence of hypertension and baseline BP values on events. Odds ratios (ORs) of BP quartiles at the time closest to the event or at the end of the follow‐up period were calculated with multivariate logistic regression analysis. Explanatory variables for multivariate analysis were adopted from well‐known risk factors, i.e., unadjusted model (Model 1) and adjusted models for other components (except hypertension) of CHADS2 score (Model 2), other components (except hypertension) of CHA2DS2‐VASc score (Model 3), and Model 3 plus use of warfarin and antiplatelet (Model 4). The use of antihypertensive drugs was not included as an explanatory variable for multivariate analysis to avoid multicollinearity. Additionally, ORs of BP quartiles for major hemorrhage and ICH were determined after adjustment for other components (except hypertension) of HAS‐BLED score18 in 7015 patients with data available for the calculation of this score. In the present analysis, “H” in the HAS‐BLED score was defined as a systolic BP ≥140 mm Hg at the time of enrollment. “L” and “D” were defined as 1 or more episode(s) of INR ≥3.5 during the follow‐up period and concomitant antiplatelet use, respectively.15, 18 The optimal cutoff BP values for thromboembolic and hemorrhagic events were determined as the points closest to the top‐left corner of the receiver operating characteristic curves (minimum of [1−sensitivity]2+[1−specificity]2). Two‐tailed P‐values of <0.05 were considered to be statistically significant. All statistical analyses were performed with SPSS software version 23.0 (IBM Corporation, Armonk, NY).
Results
Of 7937 patients with AF enrolled in the J‐RHYTHM Registry,3 421 patients were excluded because they had valvular AF.22 Of the remaining 7516 patients with NVAF, 110 (1.5%) patients were lost to follow‐up. Therefore, a total of 7406 patients with NVAF were included in the present analyses.16
Baseline Patient Characteristics and Medications
Baseline patient characteristics and medications of the 2 groups are shown in Table 1. Male sex, cardiomyopathy, and heart failure were less prevalent in the Hypertension group than in the No‐hypertension group. By contrast, mean age, CHADS2 score, and the prevalence of diabetes mellitus, use of warfarin or antiplatelet, coadministration of warfarin and antiplatelet, and use of antihypertensive drugs were all higher in the Hypertension group (Table 1). Mean INR and distribution of INR were comparable between the 2 groups, but time in therapeutic range 24 was slightly, but significantly, better in the Hypertension group than in the No‐hypertension group (Table 1). Although 94.8% of patients were taking antihypertensive drugs, baseline BP was higher in the Hypertension group than in the No‐hypertension group (Table 1). Patient characteristics and medications in the quartiles of systolic BP at the time of enrollment are summarized in Table 2. The prevalence of hypertension was 41.2%, 43.1%, 64.8%, and 78.7% in quartiles 1 to 4, respectively (Table 2). Significant trends among quartiles were observed for baseline frequencies of several comorbidities and medications (Table 2).
Table 1.
Baseline Patient Characteristics and Medications
| Overall | No‐Hypertension | Hypertension | P‐Valuea | |
|---|---|---|---|---|
| Number of patients | 7406 | 2929 | 4477 | |
| Age, y | 69.8±10.0 | 68.1±10.8 | 70.8±9.2 | <0.001 |
| Sex, male | 5241 (70.8) | 2146 (73.3) | 3095 (69.1) | <0.001 |
| Type of AF | ||||
| Paroxysmal | 2835 (38.3) | 1123 (38.3) | 1712 (38.2) | <0.001 |
| Persistent | 1081 (14.6) | 459 (15.7) | 622 (13.9) | |
| Permanent | 3490 (47.1) | 1347 (46.0) | 2143 (47.9) | |
| Comorbidities | ||||
| Coronary artery disease | 781 (10.5) | 261 (8.9) | 520 (11.6) | 0.115 |
| Cardiomyopathy | 634 (8.6) | 348 (11.9) | 286 (6.4) | <0.001 |
| HCM | 264 (3.6) | 115 (3.9) | 149 (3.6) | 0.196 |
| DCM | 370 (5.0) | 233 (8.0) | 137 (3.3) | <0.001 |
| Congenital heart disease | 96 (1.3) | 56 (1.9) | 40 (0.9) | 0.133 |
| COPD | 131 (1.8) | 53 (1.8) | 78 (1.7) | 0.901 |
| Hyperthyroidism | 131 (1.8) | 61 (2.1) | 70 (1.6) | 0.419 |
| Risk factors for stroke | ||||
| Heart failure | 2055 (27.7) | 850 (29.0) | 1205 (26.9) | <0.001 |
| Hypertension | 4477 (60.5) | 0 (0.0) | 4477 (100.0) | <0.001 |
| Age (≥75 y) | 2565 (34.6) | 880 (30.0) | 1685 (37.6) | 0.002 |
| Diabetes mellitus | 1359 (18.3) | 427 (14.6) | 932 (20.8) | 0.002 |
| Stroke/TIA | 1022 (13.8) | 380 (13.0) | 642 (14.3) | 0.103 |
| CHADS2 score | ||||
| 0 | 1157 (15.6) | 1157 (39.5) | 0 (0.0) | <0.001 |
| 1 | 2512 (33.9) | 967 (33.0) | 1545 (34.5) | |
| ≥2 | 3737 (50.5) | 805 (27.5) | 2932 (65.5) | |
| Mean | 1.7±1.2 | 1.0±1.0 | 2.1±1.1 | <0.001 |
| CHA2DS2‐VASc score | ||||
| 0 | 487 (6.6) | 487 (16.6) | 0 (0.0) | <0.001 |
| 1 | 1147 (15.5) | 671 (22.9) | 476 (10.6) | |
| 2 | 1666 (22.5) | 758 (25.9) | 908 (20.3) | |
| ≥3 | 4106 (55.4) | 1013 (34.6) | 3093 (69.1) | |
| Mean | 2.8±1.6 | 2.0±1.4 | 3.3±1.5 | <0.001 |
| HAS‐BLED score, [n] | [7015] | [2766] | [4249] | |
| 0 | 1117 (15.9) | 603 (21.8) | 514 (12.1) | <0.001 |
| 1 | 2689 (38.3) | 1183 (42.8) | 1506 (35.4) | |
| 2 | 2140 (30.5) | 719 (26.0) | 1421 (33.4) | |
| ≥3 | 1069 (15.2) | 261 (9.4) | 808 (19.0) | |
| Mean | 1.5±1.0 | 1.3±0.9 | 1.6±1.0 | <0.001 |
| Systolic BP, mm Hg | 126.0±16.2 | 120.1±14.8 | 129.8±15.9 | <0.001 |
| Diastolic BP, mm Hg | 73.5±17.0 | 71.1±10.5 | 75.1±19.9 | <0.001 |
| Heart rate/min | 72.5±13.2 | 72.8±12.9 | 72.3±13.4 | 0.111 |
| Medications | ||||
| Warfarin | 6404 (86.5) | 2461 (84.0) | 3943 (88.1) | <0.001 |
| Dosage, mg/day | 2.9±1.2 | 2.9±1.2 | 2.8±1.1 | <0.001 |
| INR | ||||
| <1.6 | 1670 (26.1) | 674 (27.4) | 996 (25.3) | 0.366 |
| 1.6 to 1.99 | 2348 (36.7) | 879 (35.7) | 1469 (37.3) | |
| 2.0 to 2.59 | 1854 (29.0) | 704 (28.6) | 1150 (29.2) | |
| 2.6 to 2.99 | 363 (5.7) | 143 (5.8) | 220 (5.6) | |
| ≥3.0 | 169 (2.6) | 61 (2.5) | 108 (2.7) | |
| Mean | 1.91±0.49 | 1.90±0.51 | 1.91±0.48 | 0.427 |
| TTRb, % | 59.3±29.2 | 56.9±29.8 | 60.8±28.7 | <0.001 |
| [n=6064] | [n=2330] | [n=3734] | ||
| Antiplatelet | 1937 (26.2) | 659 (22.5) | 1278 (28.5) | <0.001 |
| Aspirin | 1675 (22.6) | 580 (19.8) | 1095 (24.5) | <0.001 |
| Others | 433 (5.8) | 129 (4.4) | 304 (6.8) | <0.001 |
| Warfarin+antiplatelet | 1358 (18.3) | 417 (14.2) | 941 (21.0) | <0.001 |
| Antihypertensive drugs | 5354 (72.3) | 1108 (37.8) | 4246 (94.8) | <0.001 |
| ARB/ACE‐I | 3934 (53.1) | 655 (22.4) | 3279 (73.2) | <0.001 |
| Others | 3545 (47.9) | 716 (24.4) | 2829 (63.2) | <0.001 |
Data are number of patients (%) or mean±SD. ACE‐I indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BP, blood pressure; CHADS2, congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, and history of stroke or TIA; CHA2DS2‐VASc, additionally, vascular disease (coronary artery disease), age 65 to 74 y, and female sex; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; HAS‐BLED, hypertension (systolic BP ≥140 mm Hg), abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR (episodes of INR ≥3.5), elderly (age >65 y), drugs (use of antiplatelets)/alcohol concomitantly; HCM, hypertrophic cardiomyopathy; INR, international normalized ratio of prothrombin time; TIA, transient ischemic attack; TTR, time in therapeutic range.
Comparison between 2 groups with and without hypertension.
Target INR was 2.0 to 3.0 (<70 y) or 1.6 to 2.6 (≥70 y).
Table 2.
Baseline Patient Characteristics and Medications of Each Quartile
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P‐Value for Trend | |
|---|---|---|---|---|---|
| Range of Systolic BP, mm Hg | <116 | 116 to 125 | 126 to 135 | ≥136 | |
| Number of patients | 1844 | 1820 | 1765 | 1977 | |
| Age, y | 68.9±10.6 | 68.5±10.3 | 69.8±9.8 | 70.8±9.1 | <0.001 |
| Sex, male | 1365 (74.0) | 1301 (71.5) | 1237 (70.1) | 1338 (67.7) | <0.001 |
| Type of AF | |||||
| Paroxysmal | 616 (33.4) | 713 (39.2) | 705 (39.9) | 801 (40.5) | <0.001 |
| Persistent | 286 (15.5) | 268 (14.7) | 264 (15.0) | 263 (13.3) | |
| Permanent | 942 (51.1) | 839 (46.1) | 796 (45.1) | 913 (46.2) | |
| Comorbidities | |||||
| Coronary artery disease | 197 (10.7) | 198 (10.9) | 204 (11.6) | 182 (9.2) | 0.208 |
| Cardiomyopathy | 253 (13.7) | 154 (8.5) | 116 (6.6) | 111 (5.6) | <0.001 |
| HCM | 84 (4.6) | 63 (3.5) | 58 (3.3) | 59 (3.0) | 0.011 |
| DCM | 169 (9.2) | 91 (5.0) | 58 (3.3) | 52 (2.6) | <0.001 |
| Congenital heart disease | 37 (2.0) | 19 (1.0) | 13 (0.7) | 23 (1.2) | 0.015 |
| COPD | 46 (2.5) | 21 (1.2) | 31 (1.8) | 33 (1.7) | 0.172 |
| Hyperthyroidism | 40 (2.2) | 31 (1.7) | 29 (1.6) | 31 (1.6) | 0.172 |
| Risk factors for stroke | |||||
| Heart failure | 683 (37.0) | 495 (27.2) | 445 (25.2) | 432 (21.9) | <0.001 |
| Hypertension | 759 (41.2) | 785 (43.1) | 1144 (64.8) | 1556 (78.7) | <0.001 |
| Age (≥75 y) | 587 (31.8) | 630 (34.6) | 603 (34.2) | 745 (37.7) | <0.001 |
| Diabetes mellitus | 291 (15.8) | 324 (17.8) | 357 (20.2) | 387 (19.6) | <0.001 |
| Stroke/TIA | 262 (14.2) | 250 (13.7) | 253 (14.3) | 257 (13.0) | 0.376 |
| CHADS2 score | |||||
| 0 | 389 (21.1) | 344 (18.9) | 247 (14.0) | 177 (9.0) | 0.135 |
| 1 | 609 (33.0) | 586 (32.2) | 621 (35.2) | 696 (35.2) | |
| ≥2 | 846 (45.9) | 890 (48.9) | 897 (50.8) | 1104 (55.8) | |
| Mean | 1.5±1.2 | 1.6±1.3 | 1.7±1.2 | 1.8±1.2 | 0.367 |
| CHA2DS2‐VASc score | |||||
| 0 | 170 (9.2) | 148 (8.1) | 106 (6.0) | 63 (3.2) | <0.001 |
| 1 | 329 (17.8) | 293 (16.1) | 269 (15.2) | 256 (12.9) | |
| 2 | 437 (23.7) | 409 (22.5) | 384 (21.8) | 436 (22.1) | |
| ≥3 | 908 (49.2) | 970 (53.3) | 1006 (57.0) | 1222 (61.8) | |
| Mean | 2.6±1.6 | 2.7±1.6 | 2.9±1.6 | 3.0±1.5 | <0.001 |
| HAS‐BLED score [n] | [1764] | [1708] | [1664] | [1879] | |
| 0 | 360 (20.4) | 360 (21.1) | 311 (18.7) | 86 (4.6) | <0.001 |
| 1 | 794 (45.0) | 738 (43.2) | 734 (44.1) | 423 (22.5) | |
| 2 | 457 (25.9) | 441 (25.8) | 465 (27.9) | 777 (23.3) | |
| ≥3 | 153 (8.7) | 169 (9.9) | 154 (9.3) | 593 (31.6) | |
| Mean | 1.2±0.9 | 1.3±0.9 | 1.3±0.9 | 2.1±1.0 | <0.001 |
| Systolic BP, mm Hg | 105.8±7.2 | 120.7±2.6 | 130.1±2.7 | 146.0±9.7 | <0.001 |
| Diastolic BP, mm Hg | 65.2±19.4 | 71.5±9.0 | 76.4±21.3 | 80.6±11.1 | <0.001 |
| Heart rate/min | 72.3±13.1 | 72.4±13.4 | 72.1±12.7 | 73.0±13.5 | 0.127 |
| Medications | |||||
| Warfarin | 1624 (88.1) | 1562 (85.8) | 1517 (85.9) | 1701 (86.0) | 0.094 |
| Dosage, mg/day | 2.8±1.2 | 2.9±1.2 | 2.9±1.2 | 2.9±1.2 | 0.004 |
| INR | |||||
| <1.6 | 397 (24.4) | 401 (25.7) | 394 (26.0) | 478 (28.1) | 0.002 |
| 1.6 to 1.99 | 589 (36.3) | 571 (36.6) | 544 (35.9) | 644 (37.9) | |
| 2.0 to 2.59 | 487 (30.0) | 478 (30.6) | 441 (29.1) | 448 (26.3) | |
| 2.6 to 2.99 | 111 (6.8) | 74 (4.7) | 92 (6.1) | 86 (5.1) | |
| ≥3.0 | 40 (2.5) | 38 (2.4) | 46 (3.0) | 45 (2.6) | |
| Mean | 1.89±0.52 | 1.91±0.48 | 1.91±0.49 | 1.88±0.49 | 0.001 |
| TTRa, % | 58.3±28.7 | 59.0±29.5 | 60.2±29.5 | 59.9±29.1 | 0.042 |
| [n=1544] | [n=1488] | [n=1439] | [n=1593] | ||
| Antiplatelet | 432 (23.4) | 485 (26.6) | 472 (26.7) | 548 (27.7) | 0.004 |
| Aspirin | 376 (20.9) | 431 (23.7) | 409 (23.2) | 449 (22.7) | 0.273 |
| Others | 76 (4.1) | 106 (5.8) | 118 (6.7) | 138 (7.0) | <0.001 |
| Warfarin+antiplatelet | 297 (16.1) | 298 (16.4) | 331 (18.8) | 385 (19.5) | 0.001 |
| Antihypertensive drugs | 1239 (67.2) | 1237 (68.0) | 1303 (73.8) | 1575 (79.7) | <0.001 |
| ARB/ACE‐I | 867 (47.0) | 882 (48.5) | 958 (54.3) | 1227 (62.1) | <0.001 |
| Others | 819 (44.4) | 544 (46.4) | 866 (49.1) | 1016 (51.4) | <0.001 |
Data are number of patients (%) or mean±SD. ACE‐I indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BP, blood pressure; CHADS2, congestive heart failure, hypertension, age ≥75 y, diabetes mellitus, and history of stroke or TIA; CHA2DS2‐VASc, additionally, vascular disease (coronary artery disease), age 65 to 74 y, and female sex; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; HAS‐BLED, hypertension (systolic BP ≥140 mm Hg), abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR (episodes of INR ≥3.5), elderly (age >65 y), drugs (use of antiplatelets)/alcohol concomitantly; HCM, hypertrophic cardiomyopathy; INR, international normalized ratio of prothrombin time; TIA, transient ischemic attack; TTR, time in therapeutic range.
Target INR was 2.0 to 3.0 (<70 y) or 1.6 to 2.6 (≥70 y).
Event Rates and Hypertension
During the 2‐year follow‐up period, thromboembolic events occurred in 126 patients (1.7%) and major hemorrhagic events occurred in 140 patients (1.9%), including ICH in 50 patients (0.7%). The unadjusted 2‐year incidence rate of thromboembolism was similar between the 2 groups (1.8% in the Hypertension group versus 1.6% in the No‐hypertension group, P=0.540), whereas the rate of major hemorrhage was higher in the Hypertension group than in the No‐hypertension group (2.3% versus 1.3%, P=0.006) (Table 3). The Kaplan–Meier curves for thromboembolism and major hemorrhage are shown in Figure 1. There was no significant difference in the event‐free rate of thromboembolism between the 2 groups (P=0.478 by log‐rank test) (Figure 1A), whereas event‐free rate of major hemorrhage was significantly lower in the Hypertension group than in the No‐hypertension group (P=0.004 by log‐rank test) (Figure 1B). The hazard ratio (HR) of hypertension for thromboembolism was not significantly high in any model (Table 4). By contrast, the HRs of hypertension for major hemorrhage were significantly high in the crude model (Model 1) and in all adjusted models (Models 2, 3, and 4) (Table 4).
Table 3.
Two‐Year Incidence Rates of Events
| Presence of Hypertension | No‐Hypertension | Hypertension | P‐Valuea |
|---|---|---|---|
| Number of patients | 2929 | 4477 | |
| Thromboembolism | 46 (1.6%) | 80 (1.8%) | 0.540 |
| Cerebral infarction | 38 | 66 | |
| TIA | 4 | 5 | |
| Systemic embolism | 4 | 9 | |
| Major hemorrhage | 39 (1.3%) | 101 (2.3%) | 0.006 |
| Intracranial | 12 | 38 | |
| Gastrointestinal | 10 | 37 | |
| Others | 17 | 26 |
| Quartiles of SBP at the Time of Enrollment, mm Hg | Quartile 1 (<116) | Quartile 2 (116–125) | Quartile 3 (126–135) | Quartile 4 (≥136) | P‐Value for Trend |
|---|---|---|---|---|---|
| Number of patients | 1844 | 1820 | 1765 | 1977 | |
| Thromboembolism | 32 (1.7%) | 32 (1.8%) | 27 (1.5%) | 35 (1.8%) | 0.941 |
| Cerebral infarction | 23 | 26 | 26 | 29 | |
| TIA | 5 | 3 | 0 | 1 | |
| Systemic embolism | 4 | 3 | 1 | 5 | |
| Major hemorrhage | 32 (1.7%) | 30 (1.6%) | 33 (1.9%) | 45 (2.3%) | 0.181 |
| Intracranial | 13 | 13 | 9 | 15 | |
| Gastrointestinal | 10 | 7 | 16 | 14 | |
| Others | 9 | 10 | 8 | 16 |
| Quartiles of SBP at the Time Closest to Event, mm Hg | Quartile 1 (<114) | Quartile 2 (114–125) | Quartile 3 (126–135) | Quartile 4 (≥136) | P‐Value for Trend |
|---|---|---|---|---|---|
| Number of patientsb | 1680 | 1869 | 1711 | 1843 | |
| Thromboembolism | 22 (1.3%) | 17 (0.9%) | 12 (0.7%) | 66 (3.6%) | <0.001 |
| Cerebral infarction | 16 | 14 | 7 | 60 | |
| TIA | 4 | 1 | 2 | 1 | |
| Systemic embolism | 2 | 2 | 3 | 5 | |
| Major hemorrhage | 32 (1.9%) | 25 (1.3%) | 23 (1.3%) | 52 (2.8%) | 0.041 |
| Intracranial | 6 | 4 | 10 | 29 | |
| Gastrointestinal | 15 | 9 | 9 | 11 | |
| Others | 11 | 12 | 4 | 12 |
Data are number of patients (%). SBP indicates systolic blood pressure; TIA, transient ischemic attack.
Comparison between 2 groups.
No blood pressure data at the time closest to event or at the end of follow‐up in 303 patients.
Figure 1.
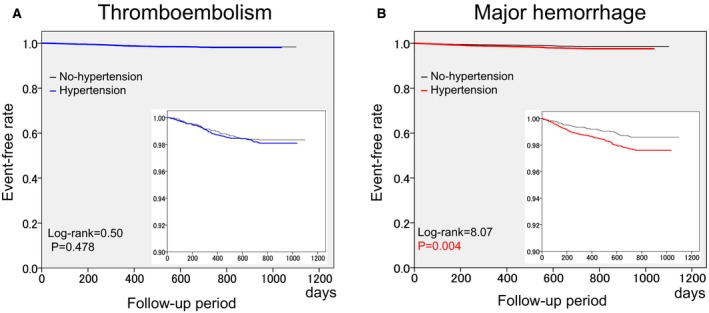
Kaplan–Meier curves of thromboembolism (A) and major hemorrhage (B). Additional boxes show the magnified event‐free curves with 10‐fold scale. P‐values: comparison between No‐hypertension and Hypertension groups by log‐rank test.
Table 4.
Influence of Hypertension on Thromboembolism and Major Hemorrhage (Cox Proportional Hazards Model)
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐Value | HR (95% CI) | P‐Value | HR (95% CI) | P‐Value | HR (95% CI) | P‐Value | |
| Thromboembolism | ||||||||
| Hypertension | 1.14 (0.79–1.64) | 0.479 | 1.03 (0.72–1.49) | 0.862 | 1.03 (0.72–1.49) | 0.864 | 1.05 (0.73–1.52) | 0.787 |
| Heart failure | — | — | 1.02 (0.70–1.50) | 0.914 | 1.03 (0.70–1.52) | 0.878 | 1.10 (0.75–1.63) | 0.621 |
| Age (≥75 y) | — | — | 2.28 (1.60–3.29) | <0.001 | 2.69 (1.63–4.45) | <0.001 | 2.80 (1.69–4.64) | <0.001 |
| Diabetes mellitus | — | — | 1.27 (0.83–1.94) | 0.271 | 1.25 (0.81–1.91) | 0.313 | 1.27 (0.83–1.95) | 0.275 |
| Stroke/TIA | — | — | 1.75 (1.16–2.66) | 0.008 | 1.72 (1.13–2.61) | 0.011 | 1.82 (1.19–2.79) | 0.006 |
| Coronary artery disease | — | — | — | — | 0.96 (0.55–1.67) | 0.889 | 0.92 (0.52–1.63) | 0.771 |
| Age (65–74 y) | — | — | — | — | 1.24 (0.72–2.14) | 0.435 | 1.31 (0.76–2.26) | 0.329 |
| Sex, female | — | — | — | — | 0.78 (0.52–1.17) | 0.228 | 0.77 (0.52–1.16) | 0.210 |
| Warfarin use | — | — | — | — | — | — | 0.44 (0.28–0.68) | <0.001 |
| Antiplatelet use | — | — | — | — | — | — | 1.08 (0.71–1.64) | 0.720 |
| Major hemorrhage | ||||||||
| Hypertension | 1.70 (1.17–2.46) | 0.005 | 1.58 (1.09–2.30) | 0.015 | 1.56 (1.08–2.27) | 0.019 | 1.52 (1.05–2.21) | 0.027 |
| Heart failure | — | — | 1.68 (1.20–2.37) | 0.003 | 1.66 (1.18–2.32) | 0.004 | 1.60 (1.14–2.26) | 0.007 |
| Age (≥75 y) | — | — | 1.88 (1.34–2.63) | <0.001 | 2.61 (1.59–4.29) | <0.001 | 2.52 (1.53–4.14) | <0.001 |
| Diabetes mellitus | — | — | 1.26 (0.85–1.86) | 0.256 | 1.17 (0.79–1.74) | 0.441 | 1.14 (0.76–1.70) | 0.525 |
| Stroke/TIA | — | — | 1.67 (1.12–2.49) | 0.012 | 1.58 (1.06–2.37) | 0.025 | 1.49 (0.99–2.23) | 0.055 |
| Coronary artery disease | — | — | — | — | 1.31 (0.83–2.06) | 0.240 | 1.16 (0.71–1.90) | 0.556 |
| Age (65–74 y) | — | — | — | — | 1.52 (0.90–2.55) | 0.116 | 1.46 (0.87–2.46) | 0.154 |
| Sex, female | — | — | — | — | 0.60 (0.40–0.90) | 0.013 | 0.61 (0.40–0.91) | 0.016 |
| Warfarin use | — | — | — | — | — | — | 2.31 (1.11–4.82) | 0.025 |
| Antiplatelet use | — | — | — | — | — | — | 1.32 (0.89–1.96) | 0.167 |
Model 1: Unadjusted (crude); Model 2: Adjusted for other components of CHADS2 score (congestive heart failure, age ≥75 y, diabetes mellitus, and history of stroke or TIA); Model 3: Adjusted for other components of CHA2DS2‐VASc score (additionally, vascular disease [coronary artery disease], age 65–74 y, and female sex); Model 4: Adjusted for other components of CHA2DS2‐VASc score, warfarin use, and antiplatelet use. HR indicates hazard ratio; TIA, transient ischemic attack.
Event Rates and BP at the Time of Enrollment
Systolic BP at the time of enrollment in patients who were subsequently complicated with thromboembolism or major hemorrhage (127±20 or 128±17 mm Hg) was not different from that in event‐free patients (126±16 mm Hg). Diastolic BP at the time of enrollment in patients complicated with these events during the follow‐up period (74±13 and 73±11 mm Hg) was also similar to that in event‐free patients (74±17 mm Hg).
When patients were divided into quartiles of systolic BP at the time of enrollment, HRs for thromboembolism or major hemorrhage did not differ among the quartiles in any model; this was also true for quartiles of diastolic BP at the time of enrollment (Table 5). When baseline BP values were used as a continuous variable, adjusted risk (by Model 4) of thromboembolism and major hemorrhage tended to increase slightly for every 10‐mm Hg increase in baseline systolic BP (HR 1.02, 95% CI 0.92–1.14, P=0.724 for thromboembolism and HR 1.09, 95% CI 0.98–1.21, P=0.098 for major hemorrhage), although not statistically significant either.
Table 5.
Influence of Blood Pressure at the Time of Enrollment
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐Value | HR (95% CI) | P‐Value | HR (95% CI) | P‐Value | HR (95% CI) | P‐Value | |
| Thromboembolism | ||||||||
| Systolic BP | ||||||||
| Quartile 1 (<116 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (116–125 mm Hg) | 1.00 (0.61–1.64) | 0.990 | 0.97 (0.59–1.59) | 0.905 | 0.98 (0.60–1.60) | 0.922 | 0.96 (0.59–1.58) | 0.881 |
| Quartile 3 (126–135 mm Hg) | 0.86 (0.52–1.44) | 0.565 | 0.83 (0.49–1.38) | 0.465 | 0.83 (0.50–1.39) | 0.480 | 0.84 (0.50–1.41) | 0.417 |
| Quartile 4 (≥136 mm Hg) | 1.01 (0.63–1.63) | 0.968 | 0.96 (0.59–1.55) | 0.853 | 0.96 (0.59–1.57) | 0.878 | 0.86 (0.59–1.57) | 0.801 |
| Diastolic BP | ||||||||
| Quartile 1 (<66 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (66–72 mm Hg) | 1.04 (0.63–1.70) | 0.885 | 1.11 (0.68–1.83) | 0.668 | 1.12 (0.68–1.83) | 0.657 | 1.11 (0.68–1.82) | 0.681 |
| Quartile 3 (73–79 mm Hg) | 0.96 (0.55–1.68) | 0.875 | 1.08 (0.61–1.90) | 0.795 | 1.09 (0.62–1.91) | 0.774 | 1.07 (0.61–1.88) | 0.821 |
| Quartile 4 (≥80 mm Hg) | 0.96 (0.60–1.55) | 0.870 | 1.15 (0.71–1.87) | 0.551 | 1.16 (0.72–1.88) | 0.545 | 1.16 (0.71–1.88) | 0.559 |
| Major hemorrhage | ||||||||
| Systolic BP | ||||||||
| Quartile 1 (<116 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (116–125 mm Hg) | 0.94 (0.57–1.55) | 0.810 | 0.97 (0.59–1.59) | 0.893 | 0.97 (0.59–1.61) | 0.915 | 0.97 (0.59–1.60) | 0.905 |
| Quartile 3 (126–135 mm Hg) | 1.05 (0.65–1.71) | 0.836 | 1.09 (0.67–1.77) | 0.741 | 1.09 (0.67–1.78) | 0.726 | 1.10 (0.67–1.80) | 0.702 |
| Quartile 4 (≥136 mm Hg) | 1.30 (0.83–2.05) | 0.256 | 1.36 (0.86–2.15) | 0.192 | 1.38 (0.87–2.19) | 0.167 | 1.37 (0.87–2.18) | 0.175 |
| Diastolic BP | ||||||||
| Quartile 1 (<65 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (66–72 mm Hg) | 1.33 (0.84–2.13) | 0.227 | 1.48 (0.92–2.36) | 0.103 | 1.50 (0.94–2.39) | 0.091 | 1.50 (0.94–2.40) | 0.089 |
| Quartile 3 (73–79 mm Hg) | 0.87 (0.49–1.54) | 0.626 | 1.03 (0.58–1.84) | 0.926 | 1.05 (0.59–1.88) | 0.869 | 1.06 (0.59–1.89) | 0.857 |
| Quartile 4 (≥80 mm Hg) | 1.10 (0.69–1.75) | 0.680 | 1.39 (0.87–2.23) | 0.167 | 1.43 (0.89–2.28) | 0.140 | 1.42 (0.88–2.27) | 0.148 |
Model 1: Unadjusted (crude); Model 2: Adjusted for other components of CHADS2 score (congestive heart failure, age ≥75 y, diabetes mellitus, and history of stroke or TIA); Model 3: Adjusted for other components of CHA2DS2‐VASc score (additionally, vascular disease [coronary artery disease], age 65–74 y, and female sex); Model 4: Adjusted for other components of CHA2DS2‐VASc score, warfarin use, and antiplatelet use. BP indicates blood pressure; HR, hazard ratio; TIA, transient ischemic attack.
Event Rates and BP at the Time Closest to the Event
Systolic BP at the time prior to and closest to the event (mean, 10 days) in patients who were complicated with thromboembolism or major hemorrhage (140±29 or 132±30 mm Hg, respectively) was significantly higher than that at the end of follow‐up in event‐free patients (126±17 mm Hg, P<0.001 for each). Diastolic BP was significantly higher in patients with thromboembolism (78±15 mm Hg) than in event‐free patients (73±11 mm Hg, P<0.001), but did not differ between patients with major hemorrhage (75±17 mm Hg) and event‐free patients (P=0.108).
When patients were divided into quartiles of systolic BP at the time closest to the event or at the end of follow‐up, the rate of thromboembolism was significantly lower and that of major hemorrhage was significantly higher in patients receiving warfarin of the highest quartile (Q4) (Figure 2). The ORs for thromboembolism in Q4 of systolic BP were significantly higher than those in the lowest quartile (Q1) in all models (Models 1–4); this was also true for quartiles of diastolic BP (Table 6). The OR for major hemorrhage in Q4 of systolic BP was also significantly higher than that in Q1 in adjusted models (Models 2, 3, and 4) (Table 6). No significant difference in adjusted OR for major hemorrhage was observed for diastolic BP (Table 6).
Figure 2.
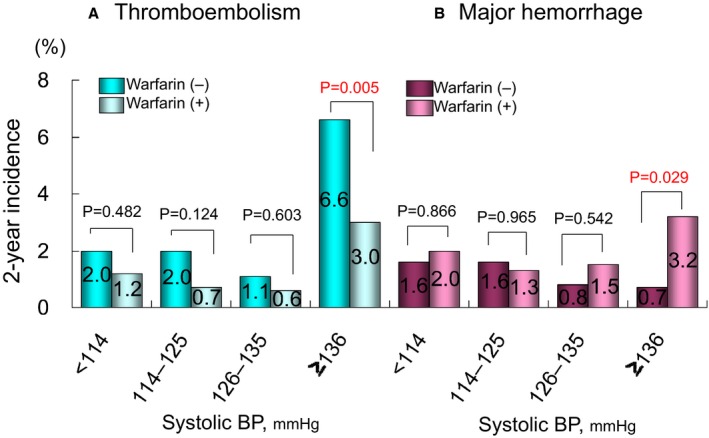
Two‐year incidence of thromboembolism (A) and major hemorrhage (B) in each quartile of blood pressure. P‐values: comparison between patients with and without warfarin in each quartile of blood pressure. BP, blood pressure at the time closest to the event or at the end of follow‐up.
Table 6.
Influence of Blood Pressure at the Time Closest to the Event or at the End of Follow‐Up
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P‐Value | OR (95% CI) | P‐Value | OR (95% CI) | P‐Value | OR (95% CI) | P‐Value | |
| Thromboembolism | ||||||||
| Systolic BP | ||||||||
| Quartile 1 (<114 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (114–125 mm Hg) | 0.69 (0.37–1.31) | 0.257 | 0.72 (0.38–1.37) | 0.319 | 0.72 (0.38–1.37) | 0.315 | 0.75 (0.40–1.43) | 0.384 |
| Quartile 3 (126–135 mm Hg) | 0.53 (0.26–1.08) | 0.081 | 0.56 (0.27–1.14) | 0.108 | 0.55 (0.27–1.13) | 0.103 | 0.56 (0.28–1.15) | 0.113 |
| Quartile 4 (≥136 mm Hg) | 2.80 (1.72–4.56) | <0.001 | 2.87 (1.75–4.70) | <0.001 | 2.86 (1.74–4.69) | <0.001 | 2.88 (1.75–4.74) | <0.001 |
| Diastolic BP | ||||||||
| Quartile 1 (<65 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (65–71 mm Hg) | 0.66 (0.36–1.20) | 0.175 | 0.75 (0.41–1.37) | 0.348 | 0.75 (0.41–1.37) | 0.342 | 0.76 (0.42–1.42) | 0.411 |
| Quartile 3 (72–79 mm Hg) | 0.70 (0.38–1.29) | 0.252 | 0.84 (0.46–1.56) | 0.589 | 0.84 (0.46–1.56) | 0.588 | 0.86 (0.46–1.60) | 0.638 |
| Quartile 4 (≥80 mm Hg) | 1.65 (1.01–2.56) | 0.046 | 2.04 (1.27–3.29) | 0.003 | 2.05 (1.27–3.31) | 0.003 | 2.09 (1.29–3.38) | 0.003 |
| Major hemorrhage | ||||||||
| Systolic BP | ||||||||
| Quartile 1 (<114 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (114–125 mm Hg) | 0.70 (0.41–1.18) | 0.182 | 0.76 (0.45–1.29) | 0.304 | 0.75 (0.44–1.28) | 0.291 | 0.74 (0.43–1.26) | 0.262 |
| Quartile 3 (126–135 mm Hg) | 0.70 (0.41–1.20) | 0.199 | 0.77 (0.44–1.32) | 0.336 | 0.76 (0.44–1.31) | 0.325 | 0.75 (0.44–1.30) | 0.307 |
| Quartile 4 (≥136 mm Hg) | 1.50 (0.96–2.33) | 0.077 | 1.62 (1.03–2.54) | 0.038 | 1.62 (1.03–2.56) | 0.036 | 1.61 (1.02–2.53) | 0.041 |
| Diastolic BP | ||||||||
| Quartile 1 (<65 mm Hg) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Quartile 2 (65–71 mm Hg) | 0.66 (0.36–1.20) | 0.175 | 1.00 (0.61–1.64) | 0.989 | 1.00 (0.61–1.63) | 0.990 | 0.97 (0.60–1.59) | 0.916 |
| Quartile 3 (72–79 mm Hg) | 0.70 (0.38–1.29) | 0.252 | 0.95 (0.57–1.61) | 0.861 | 0.96 (0.57–1.62) | 0.879 | 0.95 (0.56–1.61) | 0.851 |
| Quartile 4 (≥80 mm Hg) | 1.61 (1.01–2.56) | 0.046 | 1.10 (0.69–1.77) | 0.681 | 1.11 (0.69–1.78) | 0.660 | 1.10 (0.68–1.76) | 0.707 |
Model 1: Unadjusted (crude); Model 2: Adjusted for other components of CHADS2 score (congestive heart failure, age ≥75 y, diabetes mellitus, and history of stroke or TIA); Model 3: Adjusted for other components of CHA2DS2‐VASc score (additionally, vascular disease [coronary artery disease], age 65–74 y, and female sex); Model 4: Adjusted for other components of CHA2DS2‐VASc score, warfarin use, and antiplatelet use. BP indicates blood pressure; OR, odds ratio; TIA, transient ischemic attack.
After adjustment for other components of HAS‐BLED score, the OR for major hemorrhage in Q4 of systolic BP tended to be higher than that in Q1 (OR 1.44, 95% CI 0.91–2.27, P=0.124). By contrast, the OR for ICH in Q4 was markedly higher than that in Q1 (OR 4.55, 95% CI 1.89–10.96, P=0.001) (Figure 3).
Figure 3.
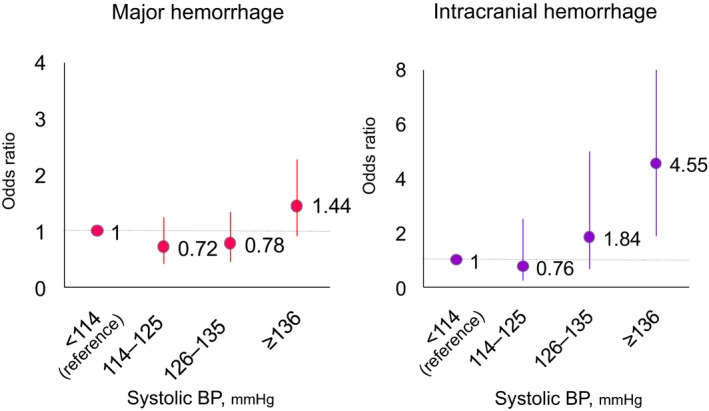
Odds ratios for major hemorrhage (A) and intracranial hemorrhage (B) in each quartile of blood pressure. Odds ratios were adjusted for other components (except hypertension) of HAS‐BLED score. See text for details. BP, blood pressure at the time closest to the event or at the end of follow‐up.
Cutoff BP Values for Events
Areas under the curve of the receiver operating characteristic analysis for thromboembolism, major hemorrhage, and ICH were 0.663 (95% CI 0.60–0.73, P<0.001), 0.556 (95% CI 0.50–0.62, P=0.027), and 0.708 (95% CI 0.63–0.79, P<0.001), respectively. Cutoff BP values were determined to be 137/79 mm Hg (with a sensitivity of 55.6% and a specificity of 77.0% for systolic BP), 130/73 mm Hg (53.0% and 58.8%), and 138/78 mm Hg (57.7% and 77.6%), for thromboembolism, major hemorrhage, and ICH, respectively.
Discussion
Although our previous reports partially revealed the influence of hypertension on event incidence,14, 15, 16, 17, 18, 19, 20 we confirmed in the present study that hypertension was not an independent risk factor for thromboembolism but a risk for major hemorrhage mostly in anticoagulated patients. Second, BP values at baseline were not related to the incidence of thromboembolism or major hemorrhage. By contrast, patients in the highest quartile of systolic BP (≥136 mm Hg) at the time closest to an event were characterized as high risk, with significantly higher ORs for thromboembolism, major hemorrhage, and ICH, even after adjustment for multiple confounders. Third, both the efficacy of stroke prevention and the undesirable increase in hemorrhage with warfarin were evident in the highest quartile of systolic BP of ≥136 mm Hg. Fourth, the cutoff BP values for thromboembolism, major hemorrhage, and ICH were determined to be 137/79, 130/73, and 138/78 mm Hg, respectively.
Hypertension and Thromboembolism
Hypertension is a well‐known risk factor for stroke. In the INTERSTROKE study, a large‐scale case–control study from 22 countries, self‐reported history of hypertension was the strongest risk factor for stroke of all types (OR 2.64, 99% CI 2.26–3.08). History of hypertension also had the highest OR for ischemic stroke (OR 2.37, 99% CI 2.00–2.79) compared with any other risk factors except for AF.25 Increasing BP is also related to increasing stroke risk.26 The influence of BP values on the event rates in anticoagulated patients with AF was investigated in the combined data set of the Stroke Prevention using an ORal Thrombin Inhibitor in AF (SPORTIF) III and V trials.27 The HR of the highest quartile of systolic BP for stroke/systemic embolic events was 1.83 (95% CI 1.22–2.74), and the event rate for stroke/systemic embolic events increased markedly at a mean systolic BP of >140 mm Hg.27 In previous reports in patients with NVAF who were not receiving anticoagulation therapy, hypertension was an independent risk factor for ischemic stroke,13 whereas it was not in patients treated with warfarin.14, 15 Since these analyses were performed post hoc on data from prospective clinical studies, patient characteristics varied among studies.13, 14, 15, 27 In addition, since the definition of hypertension in CHADS2 and CHA2DS2‐VASc scores included a history of hypertension and antihypertensive drug use, measured BP values were not considered. In the present study, neither hypertension (defined as above) nor the BP value at the time of enrollment was an independent risk factor for thromboembolism. By contrast, the highest quartile of systolic BP (≥136 mm Hg) at the time closest to the event was detected as a significant risk factor for thromboembolism. Since 94.8% of patients received antihypertensive drugs and the mean BP was controlled <130 mm Hg at the time of enrollment, even in the Hypertension group, these results appear reasonable.11, 12
Hypertension and Major Hemorrhage
Hypertension is a clear risk factor for hemorrhagic stroke in the general population.28 In the INTERSTROKE study, self‐reported history of hypertension was the strongest risk factor for hemorrhagic stroke (OR 3.80, 99% CI 2.96–4.78) compared with other risk factors.25 The effects of hypertension and BP control on event rates during antithrombotic therapy were investigated in the Bleeding with Antithrombotic Therapy (BAT) Study in Japan.29 An increase in BP levels during antithrombotic treatment was positively associated with the development of ICH, suggesting that adequate BP control was important for avoiding ICH.29 In addition, the optimal cutoff BP level to predict impending risk of ICH was determined to be ≥130/81 mm Hg.29 In 2 of our previous reports in patients with NVAF, hypertension was not an independent risk factor for major hemorrhage.14, 15 In the former study, which aimed to determine the impact of sex on prognosis in patients with NVAF,14 the adjusted OR of hypertension for major hemorrhage was comparably high to that in the present study (OR 1.48, 95% CI 0.93–2.36, P=0.100). However, it was not statistically significant when factors with P<0.25 in the antecedent univariate analysis were included as explanatory variables in the multivariate analysis. Consequently, the adopted explanatory variables differed from those in the present study. In the latter study, which aimed to assess the significance of each risk factor comprising the HAS‐BLED score in NVAF patients treated with warfarin,15 the adjusted HR of hypertension for major hemorrhage was not significantly high (HR 1.15, 95% CI 0.73–1.74, P=0.54), when the definition of hypertension was a systolic BP ≥140 mm Hg at the time of enrollment. By contrast, hypertension was detected as a significant risk factor for major hemorrhage in the present study, even after adjustment for other components of CHA2DS2‐VASc score, warfarin use, and antiplatelet use (Table 4). Hypertension, when including a history of hypertension in the definition, may have a larger contribution to the incidence of hemorrhagic events than to thromboembolism. By contrast, BP value at the time of enrollment was not a significant risk factor for major hemorrhage; however, systolic BP value at the time prior to and closest to the event was an independent risk factor for both major hemorrhage and thromboembolism, even in multiple adjusted models (Table 4). BP values at the time closest to the event would be more important for predicting both events than BP values at the time of enrollment.
In addition, we assessed the ORs of BP quartiles for major hemorrhage and ICH after adjustment for other components of the HAS‐BLED score.15, 18 This score contains warfarin and antiplatelet use, both of which are included in Model 4 of the present study. Although the OR for major hemorrhage in the highest quartile of systolic BP was not significant, the OR for ICH in the highest quartile of systolic BP at the time closest to the event was markedly higher compared with that in the lowest quartile, even after adjustment for the component of HAS‐BLED score (Figure 3). A systolic BP of ≥136 mm Hg would be a strong risk factor for ICH.
Optimal Cutoff BP Values for Events
The present study has provided the cutoff BP values to predict impending risk of events. The accuracy of the receiver operating characteristic curves for thromboembolism and major hemorrhage based on areas under the curve was fair; the cutoff systolic BP values for these events (137 and 130 mm Hg, respectively) in the present analyses were comparable to those in previous recommendations.29, 30 The cutoff systolic BP for ICH in patients with NVAF in the present study (138 mm Hg) was higher than that in the BAT study (130 mm Hg).29 Patients in the BAT study were taking oral antithrombotic agents for the secondary prevention of cardiovascular or cerebrovascular diseases, regardless of AF,29 and therefore would be at higher risk for ICH than those in the present study.
Limitations
The present study had several limitations. First, this study was a post hoc analysis of data from the J‐RHYTHM Registry3, 16 and was therefore hypothesis‐generating in nature. Second, the registry was established in only 158 selected institutions in Japan and most of the participating physicians specialized in cardiology and in the management of cardiac arrhythmias. Therefore, these results may not be generalizable to the overall Japanese population with NVAF. Third, the method of BP measurement was not standardized. BP values were obtained by the auscultatory method or an automated sphygmomanometer, as appropriate for daily clinical practice in each institution. Although visit‐to‐visit variability in BP is a known risk factor for stroke,31 it was not considered in the present analysis. Instead, BP values at the time of enrollment and the time prior to and closest to the event were selected for the analysis. Changes in antihypertensive drugs and dosages during the follow‐up period were not considered in the analysis. Fourth, the HAS‐BLED score was missing in 391 patients with NVAF; these patients were excluded from Figure 3. In these 391 patients, thromboembolism occurred in 6 and major hemorrhage occurred in 9 patients, but no ICH events were observed.
Conclusions
A systolic BP of ≥136 mm Hg at the time closest to the event was an independent risk for thromboembolism and major hemorrhage. BP control appeared to be more important than a history of hypertension and baseline BP values at preventing thromboembolism and major hemorrhage in patients with NVAF.
Appendix
The following persons participated in the J‐RHYTHM Registry: Executive Committee: H. Inoue, K. Okumura, H. Atarashi, and T. Yamashita. Local Executive Committee: M. Sakurai, Y. Kawamura (Hokkaido); K. Okumura, I. Kubota (Tohoku); Y. Kaneko, K. Matsumoto (North Kanto); S. Ogawa, H. Atarashi, T. Yamashita (South Kanto); H. Inoue, Y. Aizawa (Hokuetsu); I. Kodama, E. Watanabe (Chubu); Y. Koretsune, Y. Okuyama (Kansai); A. Shimizu, O. Igawa (Chugoku); S. Bando, M. Fukatani (Shikoku); T. Saikawa, A. Chishaki (Kyushu). Statistical Advisor: H. Origasa. Participating Investigators: N. Kato, K. Kanda, J. Kato, H. Obata, M. Aoki, H. Honda (Hokkaido); Y. Konta, T. Hatayama, Y. Abe, K. Terata, T. Yagi, A. Ishida, T. Komatsu, H. Tachibana, H. Suzuki, Y. Kamiyama, T. Watanabe, M. Oguma, M. Itoh, O. Hirono, Y. Tsunoda, K. Ikeda, T. Kanaya, K. Sakurai, H. Sukekawa, S. Nakada (Tohoku); T. Itoh, S. Tange, M. Manita, M. Ohta, H. Eguma, R. Kato, Y. Endo, T. Ogino, M. Yamazaki, H. Kanki, M. Uchida, S. Miyanaga, K. Shibayama, N. Toratani, T. Kojima, M. Ichikawa, M. Saito, Y. Umeda, T. Sawanobori, H. Sohara, S. Okubo, T. Okubo, T. Tokunaga, O. Kuboyama, H. Ito, Y. Kitahara (North Kanto); K. Sagara, T. Satoh, E. Kodani, K. Sugi, Y. Kobayashi, Y. Higashi, T. Katoh, Y. Hirayama, N. Matsumoto, M. Takano, T. Ikeda, S. Yusu, S. Niwano, Y. Nakazato, Y. Kawano, M. Sumiyoshi, N. Hagiwara, K. Murasaki, H. Mitamura, S. Nakagawa, K. Okishige, K. Azegami, H. Aoyagi, K. Sugiyama, M. Nishizaki, N. Yamawake, I. Watanabe, K. Ohkubo, H. Sakurada, S. Fukamizu, M. Suzuki, W. Nagahori, T. Nakamura, Y. Murakawa, N. Hayami, K. Yoshioka, M. Amino, K. Hirao, A. Yagishita, K. Ajiki, K. Fujiu, Y. Imai, A. Yamashina, T. Ishiyama (South Kanto); M. Sakabe, K. Nishida, H. Asanoi, H. Ueno, J. D. Lee, Y. Mitsuke, H. Furushima, K. Ebe, M. Tagawa, M. Sato, M. Morikawa (Hokuetsu); K. Yamashiro, K. Takami, T. Ozawa, M. Watarai, M. Yamauchi, H. Kamiya, H. Hirayama, Y. Yoshida, T. Murohara, Y. Inden, H. Osanai, N. Ohte, T. Goto, I. Morishima, T. Yamamoto, E. Fujii, M. Senga, H. Hayashi, T. Urushida, Y. Takada, R. Kato, N. Tsuboi, T. Noda, T. Hirose, T. Onodera, S. Kageyama, T. Osaka, T. Tomita, K. Shimada, M. Nomura, H. Izawa, A. Sugiura, T. Arakawa, K. Kimura (Chubu); T. Mine, T. Makita, H. Mizuno, A. Kobori, T. Haruna, M. Takagi, T. Watanabe, N. Tanaka, H. Shimizu, T. Kurita, K. Motoki, N. Takeda, Y. Kijima, M. Ito, A. Nakata, Y. Ueda, A. Hirata, S. Kamakura, K. Satomi, T. Noda, Y. Yamada (Kansai); Y. Yoshiga, H. Ogawa, M. Kimura, T. Hayano, T. Kinbara, H. Tatsuno, M. Harada, K. F. Kusano, M. Adachi, A. Yano, M. Sawaguchi, J. Yamasaki, T. Matsuura, Y. Tanaka, H. Moritani, T. Maki, S. Okada, M. Takechi, T. Hamada (Chugoku); A. Nishikado, Y. Takagi, I. Matsumoto, T. Yamamoto, T. Soeki, Y. Doi, M. Okawa, H. Seo, S. Kitamura, K. Yamamoto, M. Akizawa, N. Kaname (Shikoku); S. Ando, S. Narita, T. Nakamura, T. Inou, Y. Fukuizumi, K. Saku, M. Ogawa, Y. Urabe, M. Ikeuchi, S. Harada, H. Yamabe, Y. Imamura, Y. Yamanouchi, K. Sadamatsu, K. Yoshida, T. Kubota, N. Takahashi, N. Makino, Y. Higuchi, T. Ooie, T. Iwao, K. Kitamura, T. Imamura, K. Maemura, N. Komiya, M. Hayano, H. Yoshida, K. Yamashiro, K. Kumagai (Kyushu).
Sources of Funding
The J‐RHYTHM Registry is registered at University hospital Medicine Information Network (UMIN) Clinical Trials Registry (UMIN000001569) and was supported by a grant from the Japan Heart Foundation (12080025). This research was partially supported by the Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus from Japan Agency for Medical Research and Development (AMED) (15656344).
Disclosures
Dr Atarashi received research funding from Boehringer Ingelheim, and remuneration from Bayer Healthcare, Boehringer Ingelheim, and Daiichi‐Sankyo; Dr Inoue received remuneration from Daiichi‐Sankyo, Bayer Healthcare, and Bristol‐Myers Squibb; Dr Okumura received research funding from Boehringer Ingelheim and Daiichi‐Sankyo and remuneration from Boehringer Ingelheim, Bayer Healthcare, Daiichi‐Sankyo, and Pfizer; Dr Yamashita received research funding from Daiichi‐Sankyo, Bayer Healthcare, Tanabe‐Mitsubishi, Ono Pharmaceutical, and Bristol‐Myers Squibb and remuneration from Daiichi‐Sankyo, Pfizer, Bayer Healthcare, Bristol‐Myers Squibb, Boehringer Ingelheim, Eisai, and Ono Pharmaceutical; Dr Origasa received remuneration from Daiichi‐Sankyo and Bayer Healthcare.
Acknowledgments
We would like to thank all investigators of the J‐RHYTHM Registry listed in the Appendix and references.3, 17, 21
(J Am Heart Assoc. 2016;5:e004075 doi: 10.1161/JAHA.116.004075)
This work was presented in part at the 38th Annual Scientific Meeting of the Japanese Society of Hypertension on October 10, 2015 in Matsuyama, Japan.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation, analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 3. Atarashi H, Inoue H, Okumura K, Yamashita T, Kumagai N, Origasa H. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation—A report from the J‐RHYTHM Registry. Circ J. 2011;75:1328–1333. [DOI] [PubMed] [Google Scholar]
- 4. Akao M, Chun YH, Esato M, Abe M, Tsuji H, Wada H, Hasegawa K. Inappropriate use of oral anticoagulants for patients with atrial fibrillation. Circ J. 2014;78:2166–2172. [DOI] [PubMed] [Google Scholar]
- 5. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 7. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 8. The Stroke Prevention in Atrial Fibrillation Investigators . Bleeding during antithrombotic therapy in patients with atrial fibrillation. Arch Intern Med. 1996;156:409–416. [PubMed] [Google Scholar]
- 9. Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53:1319–1327. [DOI] [PubMed] [Google Scholar]
- 10. Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. [DOI] [PubMed] [Google Scholar]
- 11. Nagarakanti R, Wallentin L, Noack H, Brueckmann M, Reilly P, Clemens A, Connolly SJ, Yusuf S, Ezekowitz MD. Comparison of characteristics and outcomes of dabigatran versus warfarin in hypertensive patients with atrial fibrillation (from the RE‐LY Trial). Am J Cardiol. 2015;116:1204–1209. [DOI] [PubMed] [Google Scholar]
- 12. Rao MP, Halvorsen S, Wojdyla D, Thomas L, Alexander JH, Hylek EM, Hanna M, Bahit MC, Lopes RD, De Caterina R, Erol C, Goto S, Lanas F, Lewis BS, Husted S, Gersh BJ, Wallentin L, Granger CB. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) Trial. J Am Heart Assoc. 2015;4:e002015. doi:10.1161/JAHA.115.002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki S, Yamashita T, Okumura K, Atarashi H, Akao M, Ogawa H, Inoue H. Incidence of ischemic stroke in Japanese patients with atrial fibrillation not receiving anticoagulation therapy—pooled analysis of the Shinken Database, J‐RHYTHM Registry, and Fushimi AF Registry. Circ J. 2015;79:432–438. [DOI] [PubMed] [Google Scholar]
- 14. Inoue H, Atarashi H, Okumura K, Yamashita T, Origasa H, Kumagai N, Sakurai M, Kawamura Y, Kubota I, Matsumoto K, Kaneko Y, Ogawa S, Aizawa Y, Chinushi M, Kodama I, Watanabe E, Koretsune Y, Okuyama Y, Shimizu A, Igawa O, Bando S, Fukatani M, Saikawa T, Chishaki A. Impact of gender on the prognosis of patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014;113:957–962. [DOI] [PubMed] [Google Scholar]
- 15. Tomita H, Okumura K, Inoue H, Atarashi H, Yamashita T, Origasa H. Assessment of risk factors for bleeding in Japanese patients with non‐valvular atrial fibrillation receiving warfarin treatment: a subanalysis of the J‐RHYTHM Registry. Int J Cardiol. 2015;201:308–310. [DOI] [PubMed] [Google Scholar]
- 16. Inoue H, Okumura K, Atarashi H, Yamashita T, Origasa H, Kumagai N, Sakurai M, Kawamura Y, Kubota I, Matsumoto K, Kaneko Y, Ogawa S, Aizawa Y, Chinushi M, Kodama I, Watanabe E, Koretsune Y, Okuyama Y, Shimizu A, Igawa O, Bando S, Fukatani M, Saikawa T, Chishaki A. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non‐valvular atrial fibrillation: results of the J‐RHYTHM Registry. Circ J. 2013;77:2264–2270. [DOI] [PubMed] [Google Scholar]
- 17. J‐RHYTHM Registry Investigators . Determinants of warfarin use and international normalized ratio levels in atrial fibrillation patients in Japan—Subanalysis of the J‐RHYTHM Registry. Circ J. 2011;75:2357–2362. [DOI] [PubMed] [Google Scholar]
- 18. Okumura K, Inoue H, Atarashi H, Yamashita T, Tomita H, Origasa H. Validation of CHA2DS2‐VASc and HAS‐BLED scores in Japanese patients with nonvalvular atrial fibrillation: an analysis of the J‐RHYTHM Registry. Circ J. 2014;78:1593–1599. [DOI] [PubMed] [Google Scholar]
- 19. Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Origasa H. Use of warfarin in elderly patients with non‐valvular atrial fibrillation—Subanalysis of the J‐RHYTHM Registry. Circ J. 2015;79:2345–2352. [DOI] [PubMed] [Google Scholar]
- 20. Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Origasa H. Secondary prevention of stroke with warfarin in patients with non‐valvular atrial fibrillation: subanalysis of the J‐RHYTHM Registry. J Stroke Cerebrovasc Dis. 2016;25:585. [DOI] [PubMed] [Google Scholar]
- 21. Atarashi H, Inoue H, Okumura K, Yamashita T, Origasa H. Investigation of optimal anticoagulation strategy for stroke prevention in Japanese patients with atrial fibrillation—The J‐RHYTHM Registry study design. J Cardiol. 2011;57:95–99. [DOI] [PubMed] [Google Scholar]
- 22. Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T. Target intensity of anticoagulation with warfarin in Japanese patients with valvular atrial fibrillation: subanalysis of the J‐RHYTHM Registry. Circ J. 2015;79:325–330. [DOI] [PubMed] [Google Scholar]
- 23. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 24. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 25. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 26. Wang JG, Staessen JA, Franklin SS, Fagard R, Gueyffier F. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension. 2005;45:907–913. [DOI] [PubMed] [Google Scholar]
- 27. Lip GY, Frison L, Grind M. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J. 2007;28:752–759. [DOI] [PubMed] [Google Scholar]
- 28. Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 29. Toyoda K, Yasaka M, Uchiyama S, Nagao T, Gotoh J, Nagata K, Koretsune Y, Sakamoto T, Iwade K, Yamamoto M, Takahashi JC, Minematsu K. Blood pressure levels and bleeding events during antithrombotic therapy: the Bleeding with Antithrombotic Therapy (BAT) Study. Stroke. 2010;41:1440–1444. [DOI] [PubMed] [Google Scholar]
- 30. Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim‐Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 31. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]


