Abstract
Background
It is unclear whether ideal cardiovascular health (CVH), and particularly cumulative exposure to ideal CVH (cumCVH), is associated with incident diabetes. We aimed to fill this research gap.
Methods and Results
The Kailuan Study is a prospective cohort of 101 510 adults aged 18 to 98 years recruited in 2006–2007 and who were subsequently followed up at 2‐ (Exam 2), 4‐ (Exam 3), and 6 (Exam 4)‐year intervals after baseline. The main analysis is restricted to those individuals with complete follow‐up at all 4 examinations and who had no history of diabetes until Exam 3. Cumulative exposure to ideal CVH (cumCVH) was calculated as the summed CVH score for each examination multiplied by the time between the 2 examinations (score×year). Logistic regression models were used to assess the association between cumCVH and incident diabetes. In fully adjusted models, compared with the lowest quintile of cumCVH, individuals in the highest quintile had ~68% (95% confidence interval [CI] 60‐75) lower risk for incident diabetes (compared with 61% [95% CI 52‐69] lower risk when using baseline CVH). Every additional year lived with a 1‐unit increase in ideal CVH was associated with a 24% (95% CI 21‐28) reduction in incident diabetes.
Conclusions
Ideal CVH is associated with a reduced incidence of diabetes, but the association is likely to be underestimated if baseline measures of CVH exposure are used. Measures of cumulative exposure to ideal CVH are more likely to reflect lifetime risk of diabetes and possibly other health outcomes.
Clinical Trial Registration
URL: https://www.chictr.org. Unique identifier: ChiCTRTNC‐11001489.
Keywords: cardiovascular disease risk factors, cumulative exposure, diabetes mellitus, epidemiology, health status, ideal cardiovascular health
Subject Categories: Diabetes, Type 2
Introduction
In January 2010 the American Heart Association (AHA) defined the concept of ideal cardiovascular health (CVH) as the simultaneous presence of 4 ideal health behaviors (nonsmoking, normal body mass index [BMI], being physically active, and having a healthy diet) combined with 3 ideal health factors (normal levels of total cholesterol, blood pressure, and fasting blood glucose).1 Evidence from prospective studies have suggested that having ideal CVH is associated with a protective effect against the development of subclinical atherosclerosis,2, 3 metabolic syndrome,4 stroke,5 cardiovascular disease,6, 7 cancer,8 and all‐cause mortality.9, 10, 11
An inherent limitation of previous studies, however, has been the reliance on a single time point by which to assess CVH, which may have occurred several decades prior to the event and is therefore likely to yield biased estimates of the association. Moreover, there has been no consideration of how these health metrics vary within individuals over time and the subsequent impact that this would have on the cumulative exposure to CVH and future risk of disease. To the best of our knowledge, there have been no data published on the association between ideal CVH—in particular, cumulative exposure to ideal CVH (cumCVH)—and new‐onset diabetes, which is an independent predictor of cardiovascular events and all‐cause mortality.12 Hence, the objective of the current study was to explore and quantify the prospective association between cumCVH and incident diabetes in the Chinese population using the Kailuan Study.
Methods
Study Population
The Kailuan Study7 is a prospective cohort study conducted in the Kailuan community in Tangshan city, China. From June 2006 to October 2007, a total of 101 510 participants (81 110 men and 20 400 women, aged 18–98 years) were recruited (Exam 1) and were followed‐up in 3 visits in 2008–2009 (Exam 2), 2010–2011 (Exam 3), and 2012–2013 (Exam 4). The primary analysis is based on a subgroup of 34 323 individuals (25 961 men and 8362 women) for whom complete follow‐up data were available and who did not have a diagnosis of diabetes prior to Exam 4 (Figure 1). The study was approved by the Ethics Committees of Kailuan General Hospital following the guidelines outlined by the Helsinki Declaration. All participants agreed to participate in this study and provided written informed consent.
Figure 1.
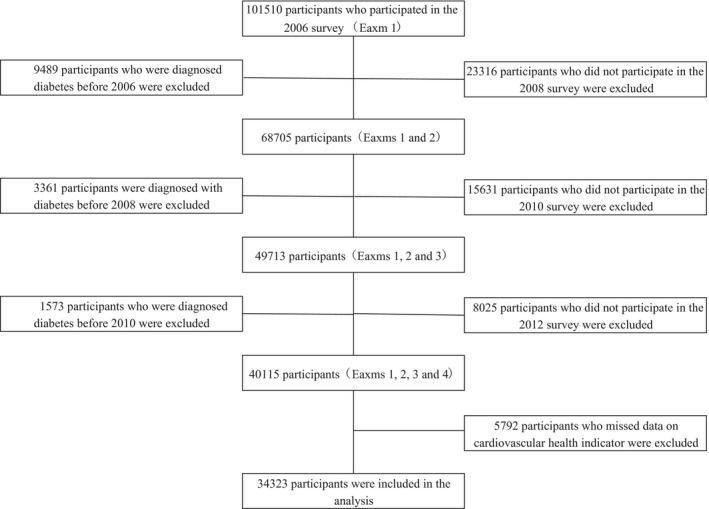
Selection of Kailuan study participants for analysis.
Assessment of Cardiovascular Health Metrics
Information on smoking, physical activity, and salt intake (as a proxy for diet) was collected via questionnaires at baseline and during each of the 3 follow‐up visits. Smoking status was based on self‐report and classified as “never” (ideal health behavior), “former” (intermediate health behavior), or “current” (poor health behavior). Information on physical activity level (minutes of moderate or vigorous activity per week) was obtained from questionnaires and categorized as follows: ≥80 (ideal); 1 to 79 (intermediate) and; 0 (poor) minutes of moderate or vigorous activity per week.5 Because information on dietary pattern was not available, the amount of salt used during cooking was used as a surrogate marker, as studies have shown that high intakes of salt are correlated with poor dietary patterns.13 We collected 24‐hour dietary salt intake for this study. A standard spoon was used for participants to recall how much salt they ate in the last 24 hours. Self‐reported use of salt was classified as “low” (<6 g/day, representing “ideal”), “medium” (6–10 g/day; “intermediate”), or “high” (>10 g/day; “poor”). In a random sample of 1000 participants, 24‐hour natriuresis was measured to determine the correlation with self‐reported use of salt. The correlation was high (r=0.78), indicating that self‐reported use of salt was associated with actual salt intake in this study.
Height was measured to an accuracy of 0.1 cm using a tape measure, and weight was measured to the nearest 0.1 kg with calibrated platform scales. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m2). Using the AHA definitions,1 BMI was classified as ideal (<25 kg/m2), intermediate (25–29.9 kg/m2), or poor (≥30 kg/m2).5, 7, 11, 14 Blood pressure (BP) was measured by a mercury sphygmomanometer. Three readings of systolic and diastolic blood pressure (SBP and DBP) were taken at 5‐minute intervals after participants had rested in a chair for at least 5 minutes. Blood pressure was classified as ideal (SBP <120 mm Hg and DBP <80 mm Hg and untreated with BP‐lowering medications), intermediate (120 mm Hg ≤ SBP ≤139 mm Hg, 80 mm Hg ≤ DBP ≤89 mm Hg, or treated to SBP/DBP <120/80 mm Hg), or poor (SBP ≥140 mm Hg, DBP ≥90 mm Hg, or treated to SBP/DBP >120/80 mm Hg).
Blood samples were collected from the antecubital vein after overnight fasting. Fasting blood glucose (FBG) was measured using the hexokinase/glucose‐6‐phosphate‐dehydrogenase method.15 FBG was classified as ideal (<5.6 mmol/L and untreated), intermediate (5.6–6.9 mmol/L or treated to <5.6 mmol/L), or poor (≥7.0 mmol/L or treated to ≥5.6 mmol/L). Total cholesterol (TC) and triglycerides were measured enzymatically. Total cholesterol status was classified as ideal (<200 mg/dL and untreated), intermediate (200–239 mg/dL or treated to <200 mg/dL), or poor (≥240 mg/dL or treated to ≥200 mg/dL), respectively.
Assessment of Potential Covariates
A 10‐second 12‐lead electrocardiogram was used to measure the resting heart rate (RHR) after the individual had rested in the supine position for 5 minutes. The number of R‐R intervals (number of QRS complexes–1) was divided by the time difference between the first and last beat, and the results were converted to beats per minute (bpm).16 High‐density lipoprotein cholesterol (HDL‐C) and LDL‐C levels were measured using a direct test method17 (interassay coefficient of variation <10%; Mind Bioengineering Co, Ltd, Shanghai, China). “High‐sensitivity” C‐reactive protein (hs‐CRP) was measured by a high‐sensitivity nephelometry assay (Cias Latex CRP‐H, Kanto Chemical, Tokyo, Japan). Serum uric acid (UA) concentrations were measured using an oxidase method. All biochemical variables were measured at the central laboratory of Kailuan General Hospital with use of a Hitachi autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan).
Information on demographic and clinical characteristics (age, sex, alcohol use, personal monthly income, education, and history of diseases) was collected via questionnaire at study baseline. Participants were classified into 3 categories: <40, 40 to 59, and ≥60 years according to baseline age. Previous history of disease, including myocardial infarction, stroke, and cancer, was collected by self‐report. The use of antihypertensive, cholesterol‐lowering, and glucose‐lowering medications within the past 2 weeks before the baseline interview was also self‐reported. The average monthly income was categorized as “<¥600,” “¥600 to ¥800,” or “≥¥800.” The educational attainment was categorized as “illiteracy or primary,” “middle school,” and “high school or above.”
Cumulative Exposure to Ideal Cardiovascular Health
To examine the association between cumulative exposure to CVH metrics (except the FBG metric), a dichotomized variable for each component of the health metrics was created: “ideal”=2; “intermediate”=1; and “poor”=0. The total ideal CVH score of each individual was the sum score of the 6 ideal CVH metrics and ranged from 0 to 12. CumCVH was defined as the summed CVH score for each examination multiplied by the time between the two consecutive visits in years: CVH1×time1–2+CVH2×time2–3+CVH3×time3–4, where CVH1, CVH2, and CVH3 indicate CVH at examinations 1 (baseline), 2, and 3, and time1–2, time2–3, time3–4, indicate the participant‐specific time intervals between consecutive Exams 1 to 3, in years. Participants were categorized into quintiles of cumCVH point score: Quintile 1 <39 points; Quintile 2 39 to 43 points; Quintile 3 44 to 48 points; Quintile 4 49 to 54 points; and Quintile 5 ≥55 points.
Assessment of New‐Onset Diabetes
In line with the ADA guidelines, participants were diagnosed with diabetes mellitus at Exam 4 (2012–2013) if they were currently treated with insulin or oral hypoglycaemic agents or had a FBG concentration ≥7.0 mmol/L.18
Statistical Analyses
Continuous variables were described as mean±standard deviation (SD) and were compared by ANOVA or the Kruskal‐Wallis test. Categorical variables were described as percentages and were compared using χ2 tests. A logistic regression model was used to estimate the risk of diabetes associated with cumCVH metrics (primary analysis) and with CVH at study baseline (secondary analysis). Odds ratios (ORs) and 95% CIs were calculated. We fitted 3 multivariate models. Model 1 adjusted for age and sex. Model 2 additionally adjusted for education level, income level, and alcohol consumption. Model 3 further adjusted for hs‐CRP, UA, RHR in 2006, and medication usage before 2012. The interactions of cumCVH with sex and age on risk of diabetes were assessed. Because there were 11 hospitals that conducted the laboratory test assays, we used a random effect for each hospital to account for the potential measurement bias.
We conducted several sensitivity analyses to test the robustness of our findings: first, individuals with a prior history of cardiovascular diseases (CVD) at Exam 4 were excluded; second, because duration of follow‐up is likely to have influenced an individual's exposure to cumCVH, a time‐weighted cumCVH model was used to assess the association between CVH and incident diabetes. The time‐weighted cumCVH was calculated by (CVH1×time1–2+CVH2×time2–3+CVH3×time3–4)/(time1–2+time2–3+time3–4). Third, to check whether exclusion of missing data (~60%) influenced the main findings, we examined the relationship between CVH at baseline (Exam 1) and incident diabetes (at Exam 2). Finally, the individual influence that each of the 6 CVH metrics had on risk of incident diabetes was examined after excluding each of the 7 metrics from the cumCVH score in turn. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). All statistical tests were 2‐sided, and the significance level was set at P<0.05.
Results
Baseline participant characteristics stratified by quintile of cumCVH exposure are shown in Table 1. In general, participants in the lowest quintile for cumCVH were younger. In addition, participants in the lowest quintile for cumCVH were predominantly male, were less educated, and had lower monthly incomes than those in higher quintiles. The distribution of CVH indices across quintiles of cumCVH differed by sex most notably for smoking (very few women were current or former smokers) and BMI (more overweight and obese women than men irrespective of quintile) (Table S1).
Table 1.
Characteristics of Studied Participants in 2006 According to Cumulative Exposure of CVH
| Group of Cumulative Exposure of CVH | P Value | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Cardiovascular Health scores | 5 (5–6) | 7 (6–8) | 7 (7–8) | 8 (8–9) | 9 (9–10) | <0.001 |
| No. of participants, n | 6864 | 6865 | 6864 | 6866 | 6864 | |
| Age, y | 46.00±9.91 | 47.34±11.10 | 47.50±11.46 | 47.93±12.28 | 48.35±12.98 | <0.001 |
| Men, n (%) | 6439 (93.81) | 5944 (86.58) | 5491 (80.00) | 4663 (67.91) | 3424 (49.88) | <0.001 |
| Education, n (%) | <0.001 | |||||
| Illiteracy/primary school | 468 (6.82) | 473 (6.89) | 428 (6.24) | 422 (6.15) | 405 (5.90) | |
| Middle school | 4822 (70.31) | 4935 (71.90) | 4959 (72.30) | 4791 (69.80) | 4456 (64.95) | |
| High school or above | 1568 (22.86) | 1456 (21.21) | 1472 (21.46) | 1651 (24.05) | 2000 (29.15) | |
| Income, ¥/month, n (%) | <0.001 | |||||
| <¥600 | 2582 (37.64) | 2036 (29.67) | 1896 (27.63) | 1805 (26.30) | 1540 (22.46) | |
| ¥600 to ¥800 | 3176 (46.30) | 3811 (55.55) | 4024 (58.65) | 4086 (59.54) | 4207 (61.34) | |
| ≥¥800 | 1102 (16.06) | 1014 (14.78) | 941 (13.72) | 972 (14.16) | 1111 (16.20) | |
| Alcohol drinking, n (%) | <0.001 | |||||
| Never | 2305 (33.61) | 3486 (50.82) | 4149 (60.50) | 4725 (68.84) | 5248 (76.50) | |
| Past | 250 (3.64) | 230 (3.35) | 181 (2.64) | 150 (2.19) | 127 (1.85) | |
| Current, <1 times/day | 2045 (29.81) | 1670 (24.34) | 1501 (21.89) | 1278 (18.62) | 1024 (14.93) | |
| Current, 1+times/day | 2259 (32.93) | 1474 (21.49) | 1027 (14.98) | 711 (10.36) | 461 (6.72) | |
| Smoking, n (%) | <0.001 | |||||
| Poor | 4293 (62.54) | 2763 (40.25) | 1884 (27.45) | 1076 (15.67) | 422 (6.15) | |
| Intermediate | 652 (9.50) | 725 (10.56) | 690 (10.05) | 556 (8.10) | 370 (5.39) | |
| Ideal | 1919 (27.96) | 3377 (49.19) | 4290 (62.50) | 5234 (76.23) | 6072 (88.46) | |
| Physical activity, n (%) | <0.001 | |||||
| Poor | 1126 (16.40) | 752 (10.95) | 581 (8.46) | 417 (6.07 | 243 (3.54) | |
| Intermediate | 5140 (74.88) | 5394 (78.59) | 5471 (79.71) | 5431 (79.10) | 5158 (75.15) | |
| Ideal | 598 (8.71) | 718 (10.46) | 812 (11.83) | 1018 (14.83) | 1463 (21.31) | |
| Salt intake, n (%) | <0.001 | |||||
| Poor | 1485 (21.63) | 812 (11.83) | 568 (8.28) | 416 (6.06) | 285 (4.15) | |
| Intermediate | 4970 (72.41) | 5551 (80.86) | 5704 (83.10) | 5801 (84.49) | 5603 (81.63) | |
| Ideal | 409 (5.96) | 502 (7.31) | 592 (8.62) | 649 (9.45) | 976 (14.22) | |
| BMI, kg/m2 | 27.12±3.41 | 25.74±3.30 | 24.84±3.12 | 24.03±2.87 | 22.72±2.60 | <0.001 |
| Systolic blood pressure, mm Hg | 135.38±19.61 | 130.67±18.99 | 127.44±18.43 | 124.04±18.02 | 117.53±16.47 | <0.001 |
| Diastolic blood pressure, mm Hg | 87.93±11.67 | 84.43±10.84 | 82.44±10.54 | 80.09±10.05 | 76.07±9.36 | <0.001 |
| Fasting blood glucose concentration, mmol/L | 5.13±0.67 | 5.06±0.66 | 5.02±0.64 | 4.98±0.64 | 4.91±0.60 | <0.001 |
| Total cholesterol concentration, mmol/L | 5.41±1.22 | 4.97±1.17 | 4.77±1.09 | 4.68±1.04 | 4.53±0.88 | <0.001 |
| Resting heart rate, bpm | 74.54±9.86 | 73.45±9.44 | 73.01±9.63 | 72.72±9.63 | 72.00±9.45 | <0.001 |
| Uric acid, μmol/L | 314.82±85.49 | 292.27±80.27 | 280.16±78.18 | 270.92±77.70 | 260.99±74.80 | <0.001 |
| High‐sensitivity C‐reactive protein, mg/L | 0.90 (0.40–2.29) | 0.75 (0.30–2.08) | 0.68 (0.24–1.77) | 0.60 (0.22–1.66) | 0.45 (0.19–1.30) | <0.001 |
Education level (elementary school, high school, or college or above), income level (income ≥ ¥800/month, ¥600 to ¥800/month, and <¥600/month), drinking (never, past, current <1 time/day, or current, 1+times/day). BMI indicates body mass index; CVH, ideal cardiovascular health; Q1, quintile 1; Q2, quintile 2; Q3, quintile 3; Q4, quintile 4; Q5, quintile 5.
During a mean±SD follow‐up of 6.3±0.50 years there were 1301 (21% women) cases of incident diabetes (Exam 4). Table 2 shows the adjusted odds ratios (ORs) of incident diabetes associated with quintiles of cumCVH exposure. The incidence of new‐onset diabetes ranged from 6.16% in the lowest quintile of cumCVH to 2.19% in the highest. In the fully adjusted model, compared with participants in the lowest quintile, those in the highest quintile for cumCVH exposure had ~68% lower risk of developing diabetes (OR 0.32; 95%, CI 0.25–0.40). For every unit increase in cumCVH, the risk of diabetes decreased by ~4% (OR 0.96, 95% CI 0.95–0.96). The effect was consistent across sex and age groups (Table 2) and did not materially differ following exclusion of the individual risk factors. Exclusion of individuals with a prior history of CVD did not materially affect the results (Table 2). The ideal health behaviors (smoking, diet, exercise, and BMI) also reduced the odds of diabetes (Figure 2).
Table 2.
Odds Ratios and 95% Confidence Intervals of Diabetes in Relation to Quintile and Unit Increase in Cumulative Exposure of CVH
| Group of Cumulative Exposure of CVH | 1 Unit Increase | P for Trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Total, n | 6864 | 6865 | 6864 | 6866 | 6864 | ||
| Case number, n (%) | 423 (6.16) | 295 (4.30) | 266 (3.88) | 167 (2.43) | 150 (2.19) | ||
| Model 1a | 1.00 | 0.64 (0.55–0.74) | 0.56 (0.48–0.66) | 0.33 (0.28–0.40) | 0.28 (0.23–0.35) | 0.95 (0.95–0.96) | <0.001 |
| Model 2b | 1.00 | 0.63 (0.54–0.74) | 0.55 (0.47–0.65) | 0.32 (0.27–0.39) | 0.28 (0.23–0.34) | 0.95 (0.95–0.96) | <0.001 |
| Model 3c | 1.00 | 0.69 (0.59–0.81) | 0.62 (0.52–0.73) | 0.35 (0.28–0.43) | 0.32 (0.25–0.40) | 0.96 (0.95–0.96) | <0.001 |
| Sex | |||||||
| Women | 42 (9.88) | 47 (5.10) | 63 (4.59) | 56 (2.54) | 67 (1.95) | ||
| Model 3c | 1.00 | 0.57 (0.36–0.92) | 0.65 (0.41–1.01) | 0.39 (0.24–0.62) | 0.30 (0.18–0.47) | 0.95 (0.94–0.97) | <0.001 |
| Men | 381 (5.92) | 248 (4.17) | 203 (3.70) | 111 (2.38) | 83 (2.42) | ||
| Model 3c | 1.00 | 0.71 (0.60–0.84) | 0.61 (0.50–0.73) | 0.33 (0.26–0.42) | 0.35 (0.27–0.46) | 0.96 (0.95–0.97) | <0.001 |
| P‐interaction | 0.311 | 0.885 | 0.958 | 0.218 | |||
| Age, y | |||||||
| <40 | 65 (3.86) | 39 (2.42) | 24 (1.44) | 13 (0.73) | 12 (0.64) | ||
| Model 3c | 1.00 | 0.75 (0.49–1.14) | 0.46 (0.28–0.76) | 0.22 (0.12–0.43) | 0.23 (0.11–0.47) | 0.95 (0.93–0.96) | <0.001 |
| 40 to 59 | 304 (6.51) | 210 (4.75) | 187 (4.37) | 109 (2.72) | 97 (2.58) | ||
| Model 3c | 1.00 | 0.75 (0.62–0.91) | 0.69 (0.56–0.84) | 0.40 (0.31–0.51) | 0.37 (0.28–0.49) | 0.96 (0.95–0.97) | <0.001 |
| P‐interaction | 0.572 | 0.027 | 0.017 | 0.021 | |||
| ≥60 | 54 (10.59) | 46 (5.51) | 55 (6.00) | 45 (4.16) | 41 (3.33) | ||
| Model 3c | 1.00 | 0.49 (0.32–0.75) | 0.57 (0.37–0.86) | 0.33 (0.21–0.53) | 0.30 (0.18–0.47) | 0.96 (0.94–0.98) | <0.001 |
| P‐interaction | 0.345 | 0.209 | 0.101 | 0.143 | |||
| Sensitivity analysis | |||||||
| Model 4d | 1.00 | 0.69 (0.59–0.82) | 0.63 (0.53–0.74) | 0.35 (0.28–0.43) | 0.32 (0.26–0.40) | 0.96 (0.95–0.97) | <0.001 |
CVH indicates ideal cardiovascular health; Q1, quintile 1; Q2, quintile 2; Q3, quintile 3; Q4, quintile 4; Q5, quintile 5.
Adjusted for age (years), sex.
Adjusted for as Model 1 plus education level (elementary school, high school, or college or above), income level (income ≥ ¥800/month, ¥600 to ¥800/month, and < ¥600/month), and drinking (never, past, current <1 times/day or current 1+times/day).
Adjusted for as model 2 plus high‐sensitivity C‐reactive protein, uric acid, resting heart rate at Exam 1 and medication usage before Exam 4.
Adjusted for Model 3 and further excluded individuals with cardiovascular disease (myocardial infarction) before Exam 4.
Figure 2.
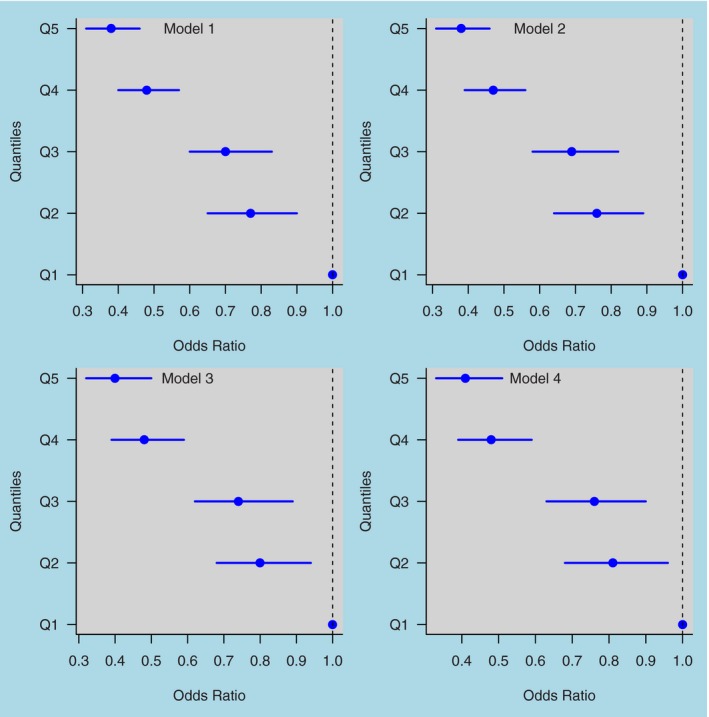
Odds ratios (and 95% confidence intervals) of diabetes in relation to quintile increase in cumulative exposure of ideal cardiovascular health behaviors (smoking, diet, exercise, and BMI). Q1=quintile 1, Q2=quintile 2, Q3=quintile 3, Q4=quintile 4, Q5=quintile 5. Model 1: adjusted for age (years), sex. Model 2: adjusted as for Model 1 plus education level (elementary school, high school, or college or above), income level (income ≥ ¥800/month, ¥600 to ¥800, and income < ¥600/month), and drinking (never, past, current, <1 times/day or current, 1+times/day). Model 3: adjusted as for Model 2 plus high‐sensitivity C‐reactive protein, uric acid, resting heart rate at Exam 1, and medication usage before Exam 4. Model 4: adjusted as for Model 3 and further excluded individuals with cardiovascular disease (myocardial infarction) before Exam 4. BMI indicates body mass index.
The results using the time‐weighted cumulative exposure to CVH were highly comparable to the unweighted model (Table S2). In the fully adjusted model every additional year lived with a 1‐unit increase in ideal CVH was associated with a 24% (95% CI 21–28) reduction in incident diabetes. Furthermore, when we excluded each of the 6 metrics from the cumCVH score in turn, the association was unaffected following exclusion of individual risk factors (Figure 3).
Figure 3.
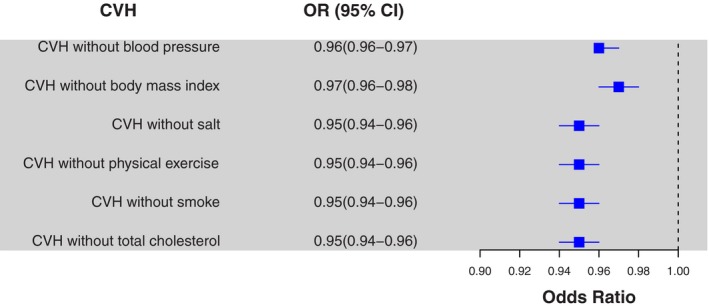
Odds ratios and 95% confidence intervals of diabetes in relation to a 1‐unit increase in cumulative exposure of ideal cardiovascular health, following individual exclusion of cardiovascular health metrics. The models adjusted for age, sex, education level, income level, drinking, high‐sensitivity C‐reactive protein, uric acid, resting heart rate at Exam 1, and medication usage before Exam 4.
Table 3 shows the adjusted ORs of incident diabetes associated with quintiles of baseline CVH exposure. In the fully adjusted model, compared with participants in the lowest quintile, those in the highest quintile for CVH exposure were at 61% lower risk of developing diabetes (OR 0.39, 95% CI 0.31–0.48). For every unit increase in CVH, the risk of diabetes decreased by ≈17% (OR 0.83, 95% CI 0.80–0.86). The effect was consistent across sex and age groups (Table 3). Similar results were observed when the analysis was conducted in participants (n=65 185) who attended only the first follow‐up examination (Exam 2) (Table S3).
Table 3.
Odds Ratios and 95% Confidence Intervals of Diabetes (Exam 4) in Relation to Quintile Increase of Baseline CVH
| Group of Baseline CVH | 1 Unit Increase | P for Trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Total, n | 4877 | 5078 | 7177 | 7634 | 9557 | ||
| Case number, n (%) | 298 (6.11) | 244 (4.81) | 321 (4.47) | 226 (2.96) | 212 (2.22) | ||
| Model 1a | 1.00 | 0.75 (0.63–0.90) | 0.68 (0.58–0.81) | 0.45 (0.37–0.54) | 0.35 (0.29–0.42) | 0.81 (0.78–0.83) | <0.001 |
| Model 2b | 1.00 | 0.74 (0.62–0.88) | 0.66 (0.56–0.78) | 0.42 (0.35–0.51) | 0.33 (0.27–0.40) | 0.80 (0.77–0.83) | <0.001 |
| Model 3c | 1.00 | 0.78 (0.65–0.94) | 0.73 (0.61–0.87) | 0.50 (0.41–0.60) | 0.39 (0.31–0.48) | 0.83 (0.80–0.86) | <0.001 |
| Sex | |||||||
| Women | 18 (7.29) | 42 (7.07) | 70 (5.43) | 63 (3.28) | 82 (1.90) | ||
| Model 3c | 1.00 | 1.00 (0.54–1.86) | 0.91 (0.51–1.64) | 0.63 (0.35–1.15) | 0.44 (0.24–0.81) | 0.81 (0.73–0.89) | <0.001 |
| Men | 280 (6.05) | 202 (4.50) | 251 (4.26) | 163 (2.85) | 130 (2.48) | ||
| Model 3c | 1.00 | 0.75 (0.62–0.91) | 0.71 (0.59–0.85) | 0.48 (0.39–0.60) | 0.41 (0.32–0.52) | 0.84 (0.80–0.87) | <0.001 |
| P‐interaction | 0.376 | 0.547 | 0.614 | 0.757 | |||
| Age, y | |||||||
| <40 | 42 (4.13) | 33 (3.04) | 29 (1.87) | 21 (1.13) | 28 (0.90) | ||
| Model 3c | 1.00 | 0.82 (0.51–1.33) | 0.54 (0.33–0.90) | 0.35 (0.20–0.61) | 0.32 (0.18–0.56) | 0.82 (0.74–0.91) | <0.001 |
| 40 to 59 | 215 (6.43) | 173 (5.23) | 222 (4.91) | 161 (3.48) | 136 (2.55) | ||
| Model 3c | 1.00 | 0.79 (0.64–0.98) | 0.78 (0.63–0.96) | 0.55 (0.44–0.70) | 0.40 (0.31–0.52) | 0.84 (0.80–0.88) | <0.001 |
| P‐interaction | 0.875 | 0.053 | 0.021 | 0.035 | |||
| ≥60 | 41 (7.95) | 38 (5.53) | 70 (6.35) | 44 (3.85) | 48 (4.26) | ||
| Model 3c | 1.00 | 0.74 (0.46–1.20) | 0.80 (0.51–1.23) | 0.50 (0.31–0.82) | 0.56 (0.34–0.92) | 0.87 (0.79–0.95) | <0.001 |
| P‐interaction | 0.976 | 0.110 | 0.112 | 0.008 | |||
CVH indicates ideal cardiovascular health; Q1, quintile 1; Q2, quintile 2; Q3, quintile 3; Q4, quintile 4; Q5, quintile 5.
Adjusted for age (years), sex.
Adjusted for as Model 1 plus education level (elementary school, high school, or college or above), income level (income ≥ ¥800/month, ¥600 to ¥800/month, and income < ¥600/month), and drinking (never, past, current <1 time/day, or current 1+times/day).
Adjusted for as Model 2 plus high‐sensitivity C‐reactive protein, uric acid, resting heart rate at Exam 1, and medication usage before Exam 4.
Table S4 shows comparison of demographic and other characteristics of participants and nonparticipants. The individuals included in the present study were significantly younger (47.43±11.62 years) than excluded participants (53.85±12.97 years); had a higher level of education (23.75% vs 18.80%; P<0.001); and had lower levels of systolic blood pressure, diastolic blood pressure, fasting blood glucose, total cholesterol, high‐sensitivity C‐reactive protein concentration, uric acid concentration, and resting heart rate (Table S4).
Discussion
The concept of “ideal cardiovascular health” recognizes that vascular risk factors—such as high blood pressure, diabetes, cigarette smoking, and poor diet—frequently cluster and that an aggregate measure of these risk factors is likely to be a truer reflection of an individual's level of vascular risk than any single risk factor in isolation. Findings from the current study extend previous work2, 3, 4, 6, 8, 9, 19 (Table S5) by demonstrating for the first time that ideal CVH is associated with a substantially lower risk of incident diabetes compared with those with poor CVH when measured either at baseline or cumulatively: specifically, every additional year lived with a 1‐unit increase in ideal CVH was associated with a 24% reduction in incident diabetes.
The associations were broadly similar in subgroups stratified by sex and age and after adjusting for potential confounders. In addition, participants in the lowest quintile for cumCVH were younger. This might be caused by the fact that younger people have to spend more time working and do not pay attention to their health care. The young have more living pressure because of their low income. Moreover, our results show that the relationship between CVH and incident diabetes (and possibly other vascular outcomes) is likely to be underestimated when a single measure of exposure at study baseline is used as opposed to a cumulative measure. These findings thus provide the first evidence that mid‐ to long‐term exposure to CVH is not only strongly associated with future risk of diabetes but that it is likely to be a more accurate indicator of the true magnitude of risk as compared with a single measure of CVH done several years before the onset of diabetes.
Over the last 2 decades the prevalence of diabetes in China has more than quadrupled from 2.5% in 1994 to an estimated 11.6% in 2010, paralleling the rapid increase in the prevalence of overweight and obesity in the population that has occurred.20 By 2030 it is estimated that the prevalence of diabetes in China will increase further to 42.3 million people living with diabetes.21 Although much of the estimated increase in diabetes prevalence is due to China's aging population,22, 23 our findings suggest that widespread adoption of public health interventions that target the prevention or prompt reversal of adverse health behaviors such as smoking, high salt intake, and physical inactivity may have a beneficial impact on reducing the incidence of diabetes across the life course. This proposition is supported by intervention and observational studies that have shown that diabetes is amenable to lifestyle interventions.24, 25, 26, 27, 28, 29 For example, in the Finnish Diabetes Prevention Study, Tuomilehto et al reported a lower incidence of diabetes among middle‐aged men who adopted a healthier lifestyle that included weight loss, improved dietary habits, and increased physical activity relative to men who did not alter their behavior.25 Smoking cessation has also been associated with a reduced risk of incident diabetes,27 whereas cumulative exposure to obesity (ie, duration and degree of overweight) is positively associated with diabetes risk.30, 31, 32 In our study cumulative exposure to healthy behaviors (not smoking, diet, exercise, and weight loss) is also associated with a reduced diabetes incidence.
The current study is unique in that the measure of cumulative CVH exposure included 5 (smoking, BMI, physical activity, blood pressure, and total cholesterol) out of the 7 indices that define the AHA's ideal CVH index (with salt use as a proxy of diet). Furthermore, each of these individual risk factors appeared to confer similar risks of diabetes, as the association between cumCVH exposure and incident diabetes was attenuated to a comparable extent following their individual removal from the model (Figure 3). These findings imply that preventative efforts to reduce diabetes incidence that encompass strategies that promote a more holistic approach to optimal vascular health (eg, smoking cessation, weight loss, increased physical activity, low‐fat diets) may yield greater benefits than would be expected by targeting individual health behaviors alone.
The biological mechanisms underpinning the association between low ideal CVH and incident diabetes remain speculative but are likely to be driven by increased insulin resistance and inflammation. Obesity, cigarette smoking, physical inactivity, and poor diet have each been reported to increase insulin resistance,33, 34, 35, 36 which in turn is associated with increased plasma concentrations of free fatty acids,37 elevated plasma concentrations of proinflammatory cytokines,38, 39 and increased oxidative stress.40, 41
Strengths and Limitations
Our study has several strengths: it is the first prospective study to address the association between cumulative exposure to CVH and incident diabetes in a well‐characterized population. Other strengths of our study include its large sample size and information on a broad spectrum of biological and behavioral covariates. However, as with all observational studies, there are some inherent limitations. First, we had no information on dietary habits and therefore used self‐reported salt intake as a surrogate indicator of dietary behavior. Previous studies have documented the correlation between the “healthfulness” of different dietary patterns with salt intake and showed that the least optimal diets were those containing the most salt. In the current study the correlation between self‐reported salt intake and 24‐hour natriuresis among 1000 randomly selected participants was high (r=0.78), indicating good agreement between self‐reported salt intake and salt excretion. Second, as all participants were recruited from Tangshan city (an industrial city located in northern China), the cohort is not nationally representative, and thus the findings pertaining to the prevalence of CVH are not generalizable to other parts of China. In contrast, the primary outcome was the estimate of the relative risk of diabetes associated with cumCVH exposure that should be generalizable to the Chinese population (which is supported by the robustness of the findings across age and sex groups).Third, the diagnosis of diabetes was based on a single measure of FBG at Exam 4 without using the oral glucose tolerance test (OGTT), which is due to lack of availability of oral glucose tolerance test data for such a large cohort. Finally, a substantial portion of the participants were dropped from subsequent exams, which might underestimate the benefits of CVH on diabetes. When we compared the characteristics of the final participants and the excluded participants, the results showed that the excluded participants potentially have a higher risk of diabetes compared with the included participants.
In summary, the current findings demonstrate the importance of reducing chronic exposure to adverse health behaviors and risk factors in order to minimize the future risk of diabetes. The study also highlights the need to take into consideration cumulative exposure when estimating risk rather than relying on a single measure of exposure that can often precede the outcome by several decades. In China, public health campaigns that promote, encourage, and support individuals to maintain or adopt a healthier lifestyle early in life or during midlife could have significant beneficial effects in stemming the growing prevalence of diabetes.
Sources of Funding
Guo is supported by Career Development Fellowship of Australian National Health and Medical Research Council (#APP1107107).
Disclosures
None.
Supporting information
Table S1. The Percentages of Each of the 7 Cardiovascular Health Indices According to Cumulative Exposure of CVH
Table S2. Odds Ratios and 95% Confidence Intervals of Diabetes According to the Time‐Weighted Cumulative Exposure of CVH
Table S3. Odds Ratios and 95% Confidence Intervals of Diabetes (Exam 2) in Relation to Quintile Increase of Baseline Exposure of CVH (Exam 1)
Table S4. Comparison of Demographic and Other Characteristics of Participants and Nonparticipants
Table S5. Previous Analyses of the Relationship Between Ideal Cardiovascular Health Metrics and Risk of Outcomes
(J Am Heart Assoc. 2016;5:e004132 doi: 10.1161/JAHA.116.004132)
Contributor Information
Yuming Guo, Email: y.guo1@uq.edu.au.
Shouling Wu, Email: drwusl@163.com.
References
- 1. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2. Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circulation. 2014;130:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aatola H, Hutri‐Kahonen N, Juonala M, Laitinen TT, Pahkala K, Mikkila V, Telama R, Koivistoinen T, Lehtimaki T, Viikari JS, Raitakari OT, Kahonen M. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 2014;3:e000532 doi: 10.1161/JAHA.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkila V, Jokinen E, Hutri‐Kahonen N, Laitinen T, Kahonen M, Lehtimaki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125:1971–1978. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, Wu S, Zhao X. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. [DOI] [PubMed] [Google Scholar]
- 6. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4‐year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5:487–493. [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Artero EG, Espana‐Romero V, Lee DC, Sui X, Church TS, Lavie CJ, Blair SN. Ideal cardiovascular health and mortality: Aerobics Center Longitudinal Study. Mayo Clin Proc. 2012;87:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Chi HJ, Cui LF, Yang XC, Wu YT, Huang Z, Zhao HY, Gao JS, Wu SL, Cai J. The ideal cardiovascular health metrics associated inversely with mortality from all causes and from cardiovascular diseases among adults in a northern Chinese industrial city. PLoS One. 2014;9:e89161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Q, Zhang S, Wang C, Gao X, Zhou Y, Zhou H, Wang A, Wu J, Bian L, Wu S, Zhao X. Ideal cardiovascular health metrics on the prevalence of asymptomatic intracranial artery stenosis: a cross‐sectional study. PLoS One. 2013;8:e58923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang L, Cui L, Wang Y, Vaidya A, Chen S, Zhang C, Zhu Y, Li D, Hu FB, Wu S, Gao X. Resting heart rate and the risk of developing impaired fasting glucose and diabetes: the Kailuan prospective study. Int J Epidemiol. 2015;44:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang A, Liu X, Guo X, Dong Y, Wu Y, Huang Z, Xing A, Luo Y, Jonas JB, Wu S. Resting heart rate and risk of hypertension: results of the Kailuan cohort study. J Hypertens. 2014;32:1600–1605; discussion 1605. [DOI] [PubMed] [Google Scholar]
- 17. Bachorik PS, Ross JW. National Cholesterol Education Program recommendations for measurement of low‐density lipoprotein cholesterol: executive summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995;41:1414–1420. [PubMed] [Google Scholar]
- 18. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 19. Alman AC, Maahs DM, Rewers MJ, Snell‐Bergeon JK. Ideal cardiovascular health and the prevalence and progression of coronary artery calcification in adults with and without type 1 diabetes. Diabetes Care. 2014;37:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. [DOI] [PubMed] [Google Scholar]
- 21. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 22. Li G, Hu Y, Pan X. Prevalence and incidence of NIDDM in Daqing City. Chin Med J. 1996;109:599–602. [PubMed] [Google Scholar]
- 23. Yang G, Kong L, Zhao W, Wan X, Zhai Y, Chen LC, Koplan JP. Emergence of chronic non‐communicable diseases in China. Lancet. 2008;372:1697–1705. [DOI] [PubMed] [Google Scholar]
- 24. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. [DOI] [PubMed] [Google Scholar]
- 25. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, Keinanen‐Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 26. Knowler WC, Barrett‐Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindstrom J, Ilanne‐Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen‐Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow‐up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. [DOI] [PubMed] [Google Scholar]
- 28. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown‐Friday JO, Goldberg R, Venditti E, Nathan DM. 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all‐cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23‐year follow‐up study. Lancet Diabetes Endocrinol. 2014;2:474–480. [DOI] [PubMed] [Google Scholar]
- 30. Everhart JE, Pettitt DJ, Bennett PH, Knowler WC. Duration of obesity increases the incidence of NIDDM. Diabetes. 1992;41:235–240. [DOI] [PubMed] [Google Scholar]
- 31. Sakurai Y, Teruya K, Shimada N, Umeda T, Tanaka H, Muto T, Kondo T, Nakamura K, Yoshizawa N. Association between duration of obesity and risk of non‐insulin‐dependent diabetes mellitus. The Sotetsu Study. Am J Epidemiol. 1999;149:256–260. [DOI] [PubMed] [Google Scholar]
- 32. Abdullah A, Stoelwinder J, Shortreed S, Wolfe R, Stevenson C, Walls H, de Courten M, Peeters A. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14:119–126. [DOI] [PubMed] [Google Scholar]
- 33. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non‐insulin‐dependent diabetes mellitus. N Engl J Med. 1991;325:147–152. [DOI] [PubMed] [Google Scholar]
- 35. Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. [DOI] [PubMed] [Google Scholar]
- 36. Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha‐induced skeletal muscle insulin resistance involves suppression of AMP‐kinase signaling. Cell Metab. 2006;4:465–474. [DOI] [PubMed] [Google Scholar]
- 40. Styskal J, Van Remmen H, Richardson A, Salmon AB. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 2012;52:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The Percentages of Each of the 7 Cardiovascular Health Indices According to Cumulative Exposure of CVH
Table S2. Odds Ratios and 95% Confidence Intervals of Diabetes According to the Time‐Weighted Cumulative Exposure of CVH
Table S3. Odds Ratios and 95% Confidence Intervals of Diabetes (Exam 2) in Relation to Quintile Increase of Baseline Exposure of CVH (Exam 1)
Table S4. Comparison of Demographic and Other Characteristics of Participants and Nonparticipants
Table S5. Previous Analyses of the Relationship Between Ideal Cardiovascular Health Metrics and Risk of Outcomes


