Abstract
Background
Fontan survivors demonstrate diminished vascular function and functional outcomes, but the relationships between these measures have not been established.
Methods and Results
We performed a cross‐sectional study of 60 Fontan survivors (52% male) with a mean age of 13.9±4.1 years and mean Fontan duration of 9.9±4.2 years. Multimodality assessment of endothelial function (reactive hyperemia index and flow‐mediated dilation) and arterial stiffness (augmentation index and baseline pulse amplitude) was performed with peripheral arterial tonometry and brachial flow‐mediated dilation. Aerobic capacity was determined using cardiopulmonary exercise testing; mean peak and percentage of predicted oxygen consumption (VO2) were 27.8±7.6 mL/kg per minute and 71.0±21.2%, respectively. Quality of life and physical activity were assessed using the Pediatric Quality of Life Inventory (PedsQL) and the Physical Activity Questionnaire. Vascular measures served as predictor variables, whereas functional measures served as outcome variables. In all cases, worse vascular measures were associated with worse functional measures. Flow‐mediated dilation–derived reactive hyperemia index (P<0.05) was positively associated with VO2 at anaerobic threshold. Peripheral arterial tonometry–derived baseline pulse amplitude (P<0.05) was negatively associated with the ratio of minute ventilation to carbon dioxide at anaerobic threshold. Flow‐mediated dilation–derived reactive hyperemia index and peripheral arterial tonometry–derived augmentation index (P<0.05) were positively and negatively associated, respectively, with peak VO2. Maximum flow‐mediated dilation (P<0.05) was positively associated with Physical Activity Questionnaire score. Peripheral arterial tonometry–derived augmentation index and baseline pulse amplitude (P<0.05) were negatively associated with parent‐reported PedsQL total and physical heath summary scores.
Conclusions
Increased arterial stiffness and decreased endothelial function are associated with lower aerobic capacity, physical activity, and quality of life in adolescent and young adult Fontan survivors. Understanding the cause–effect relationship between vascular function and functional outcomes is an important next step.
Keywords: arterial stiffness, endothelial function, exercise capacity, Fontan procedure, quality of life
Subject Categories: Congenital Heart Disease, Vascular Biology, Endothelium/Vascular Type/Nitric Oxide, Exercise Testing, Cardiovascular Surgery
Introduction
Survival following staged Fontan palliation for functionally univentricular heart disease has improved dramatically over the past 2 decades.1, 2 Although a majority of Fontan patients survive to adolescence and adulthood, the early onset of symptomatic functional decline is common. Multisystem organ dysfunction, or “failing Fontan physiology,” arises from chronic exposure to low cardiac output and high central venous pressure and results in impairment of critical functional outcomes including exercise capacity and quality of life (QOL).3, 4 Essential to reducing morbidities and improving Fontan outcomes is the identification of novel biomarkers that are predictive of the later development of failing Fontan physiology. The early identification of Fontan patients who are at risk for failing Fontan physiology might allow for interventions to reduce the likelihood or to delay the rate of progression to symptomatic Fontan failure and the need for Fontan revision, conversion, or heart transplantation.
Vascular function is an ideal candidate biomarker in Fontan survivors because of the integral role of vascular biology in the development of heart failure and a low cardiac output state. Reduced endothelial function is common in Fontan survivors, is present in presymptomatic cohorts, and is associated with reduced functional capacity.5, 6, 7, 8 Increased arterial stiffness has also been demonstrated in Fontan patients and associated with increased ventricular diastolic stiffness, reduced systolic function, and aortic dilation.9, 10, 11 Prior investigations of vascular function in Fontan patients, however, have generally used only a single modality of vascular testing and have incompletely addressed the relationship between vascular function and key functional outcomes, including QOL. A broad assessment of vascular function throughout the Fontan arterial tree and association between vascular function and key functional outcomes may provide insight into new or distinct potential therapeutic targets for future interventional trials. The aim of this study was to perform a multimodality comprehensive evaluation of vascular function and key functional measures (exercise capacity, cardiac output, physical activity [PA], and QOL) to assess the relationship between vascular function and functional outcomes in adolescent and young adult Fontan survivors.
Methods
This prospective observational study was approved by the institutional review board at Cincinnati Children's Hospital Medical Center. Patients aged 8 to 25 years with univentricular heart disease palliated with Fontan circulation were identified by query of the institutional cardiology databases and approached for study participation. We excluded Fontan patients who had heart transplantation or Fontan revision or conversion surgery, who were <12 months from Fontan completion, who were found to be in unstable atrial arrhythmia or had a history of malignant arrhythmia within the previous 6 months, who had severe asthma, who had exercise‐associated syncope within the previous 6 months, who had other significant chronic disease (eg, cancer), or who were currently receiving intravenous inotropic support. Patients with a permanent pacemaker were included, provided the device had rate‐responsive pacing. All participants provided informed consent and eligible minors provided assent before enrolling in the study.
Data Collection and Questionnaires
After a 12‐hour fast, participants had questionnaire, anthropometric, and vascular function data collected. Following a snack and 1 hour rest, participants underwent cardiopulmonary exercise testing. Relevant demographic and medical characteristic data were extracted from the medical record. Trained personnel obtained 2 measures of height (in meters) and weight (in kilograms). The average of each was used in analyses. Body surface area was calculated according to the Mosteller formula.12 Body mass index was calculated as kg/m2.
Multimodality Vascular Function Assessment
Vascular testing was performed in a darkened, temperature controlled, and quiet environment. Participants refrained from exposure to tobacco, caffeine, and exercise for >8 hours prior to testing. Brachial flow‐mediated vasodilation (FMD) testing was performed simultaneously with peripheral arterial tonometry (PAT) testing. Participants with sensitivity to latex were excluded from PAT testing because of the presence of latex in the probe. Following reactive hyperemia testing, participants underwent assessment of pulse wave analysis and pulse wave velocity (PWV), followed by heart rate variability and laser flow Doppler. Participants were withdrawn from the study if brachial FMD could not be obtained satisfactorily, as it was the primary outcome measure. Only vascular testing results that met the manufacturers' quality indices were included in analyses.
Brachial FMD
Participants underwent high‐resolution B‐mode ultrasound imaging (GE Vivid) of the brachial artery proximal to the antecubital fossa in the supine position. A pneumatic blood pressure tourniquet was applied to the forearm below the antecubital fossa. Images were obtained at baseline. The blood pressure cuff was inflated to 75 mm Hg above systolic blood pressure (≥200 mm Hg); after 5 minutes, the cuff was rapidly deflated. Brachial artery diameters were obtained immediately, at 60, 90, and 120 seconds after deflation. End‐diastolic measures of diameter were made from digital images. Brachial FMD is the percentage change in diameter. Doppler flow velocity was also measured at baseline and immediately after deflation to assess reactive hyperemia index (RHI).13 FMD‐derived RHI (FMD‐RHI) is the percentage change in Doppler velocity at each time point after deflation.
Peripheral Arterial Tonometry
The EndoPAT 2000 (Itamar Medical) probes were placed on 1 finger of each hand (typically the index finger). The plethysmography probes record pulsatile volume signals. A 5‐minute baseline recording was acquired and continued throughout cuff occlusion and the 5‐minute period following cuff deflation. PAT‐derived RHI (PAT‐RHI) is the ratio of the average amplitude of the signal for the second minute after cuff deflation divided by the average amplitude of the PAT signal for 3.5 minutes of baseline, normalized to the control arm.14 PAT has been shown to be feasible and reproducible in adolescent patients.15 Log‐transformed PAT‐RHI and Framingham Heart Study modification of RHI are additional validated PAT measures.14 In addition to generating peripheral reactive hyperemia data, PAT also provides the peripheral augmentation index (AI; raw [PAT‐AI] and adjusted for heart rate [PAT‐AI75]), a measure of wave reflection related to arterial stiffness.
Pulse Wave Analysis and PWV
Pulse wave analysis and PWV were measured with a SphygmoCor CPV System (Atcor Medical), which has been validated in children.16 The distances from the carotid artery to the distal artery of interest (femoral, radial, dorsalis pedis) were measured. A tonometer was used to collect proximal and distal arterial waveforms gated by the R wave on a simultaneously recorded ECG. PWV was calculated as the carotid‐to‐distal path length divided by the time delay measured between the feet of the 2 waveforms.17 Three recordings of PWV were obtained for each proximal‐to‐distal path and averaged. Repeated measures in our laboratory showed a coefficient of variation of <7%.16 Augmentation pressure (represented as ΔP) was defined as the difference between late and early peak in blood pressure; the central AI (represented as AIx) was defined as the ratio between augmentation pressure and pulse pressure (represented as PP), according to the formula AIx=(ΔP/PP)×100.17 Central AI was collected by placing the tonometer over the right radial artery. The device analyzes pulse waves using a validated generalized transfer function to calculate a central aortic pressure wave.18 Central AI was derived from the central pressure waveform by calculating the difference between the main outgoing wave and the reflected wave of the central arterial waveform, expressed as a percentage of the central pulse pressure. Because central AI is affected by heart rate, values were adjusted to a standard of 75 beats per minute. Reproducibility studies in our laboratory demonstrated an intraclass correlation coefficient of 0.7.16
Heart Rate Variability
Following PWV testing, an assessment of heart rate variability was performed using the SphygmoCor CPV System. ECG leads were attached to provide input for lead II. Once a stable ECG signal was observed, 10 minutes of resting ECG data were captured. Next, a Valsalva maneuver was prompted, followed by 1 minute of data capture. Finally, a standing maneuver was prompted, followed by 1 minute of data capture. Major variables analyzed included percentage of consecutive R‐R intervals differing by >50 ms, root mean square of the difference of successive R‐R intervals, and mean of the standard deviations for each R‐R interval.
Laser Flow Doppler
Postheating reactive hyperemia was measured with the Periflux System 5000 (Perimed AB). Using a forearm probe, the software recorded a baseline waveform for 5 minutes (measured in perfusion units), after which the probe was heated to 44°C for 15 minutes. Postheating reactive hyperemia, or the change from baseline to heating (percentage change from baseline), was calculated offline.
Cardiopulmonary Exercise Testing
Maximal exercise stress testing was performed with a calibrated and electronically braked cycle ergometer (Corival Lode Cycle 400; Lode) using a ramp protocol. Following pedaling in an unloaded state for 3 minutes, workload was then increased continuously with a slope chosen to achieve each participant's predicted maximal work rate after 10 to 12 minutes of cycling.19 Expired gases were measured continuously by breath‐by‐breath gas analysis throughout the study duration using a metabolic cart (TrueMax 2400; Parvo Medics). Peak oxygen consumption (VO2) was defined as the highest VO2 achieved during the test. Ventilatory anaerobic threshold was measured by the V‐slope method. Maximal exercise was defined by achievement of a respiratory exchange ratio of ≥1.1. Values for VO2 were indexed to body weight and expressed as a percentage of predicted values for healthy age‐ and sex‐matched participants undergoing a similar protocol.20 The ventilatory equivalents of carbon dioxide (VE/VCO2) were measured at anaerobic threshold. The oxygen pulse was measured at peak exercise and indexed to body surface area. Cardiac output was measured at rest and at 2 minutes into each stage of exercise (Innocor) using the foreign gas rebreathing technique, previously described in the Fontan population, and reported as adjusted for body surface area (cardiac index).21, 22 Heart rate and a 6‐lead rhythm strip were recorded at prespecified intervals throughout exercise (GE Marquette Case 8000; GE Healthcare). Oxygen saturation was measured continuously during exercise using a forehead probe signal extraction pulse oximeter (Masimo Corp).
PA and QOL
PA was assessed using the Physical Activity Questionnaire for older children and for adolescents.23 QOL was assessed using the Pediatric Quality of Life Inventory (PedsQL) version 4.0 Core scales (Child scale: age 8–12 years; Teen scale: age 13–18 years; Young Adult scale: age 19–25 years).24 Parents of participants aged ≤18 years completed the appropriate PedsQL Child or Teen parent‐proxy report.
Statistical Analysis
Analyses were performed in SAS 9.3 (SAS Institute). Values were summarized as frequency (categorical variables), mean±SD (normally distributed variables), or median and interquartile range (nonnormally distributed variables). Pearson and Spearman correlations were calculated between vascular variables and functional measures. Multivariable general linear models were constructed for exercise capacity, PA, and QOL using vascular variables identified in correlation analysis and other risk factors including age, race, sex, weight, body mass index z score or waist circumference, systolic and diastolic blood pressure, body surface area, heart rate, and ventricular morphology. No imputation was used for missing values. A stepwise regression procedure was used in model building. Predictor variables with P≤0.15 were allowed to enter and stay in the model. The R 2 value reported is adjusted in all models, based on the number of variables retained in each model. Collinearity was assessed with a variance inflation factor >0.5 used as a criterion of collinearity. Collinear variables were modeled separately; the model with the smaller Akaike information criterion was chosen. For all statistical comparisons, a P value <0.05 was considered significant.
Results
Fontan Patients
Sixty patients with Fontan circulation completed the protocol. Baseline characteristics of the study population are described in Table 1. The mean age was 13.9±4.1 years, and the cohort was 52% male. The mean duration of Fontan circulation was 9.9±4.2 years. Median resting oxygen saturation was 97% (interquartile range 95–98%). Morphology of the dominant single ventricle was most commonly left (65%).
Table 1.
Demographic and Medical Characteristics of the Study Population
| Characteristic | Cohort (n=60) |
|---|---|
| Age, y | |
| At Fontan operation | 4.0±1.2 |
| At study enrollment | 13.9±4.1 |
| Male, n (%) | 31 (52) |
| Race, n (%) | |
| White | 54 (90) |
| Black | 5 (8) |
| Other | 1 (2) |
| Height, cm | 150.0±16.1 |
| Weight, kg | 43.5 (31.4, 58.3) |
| Body surface area, m2 | 1.3 (1.1, 1.7) |
| Body mass index, kg/m2 | 18.7 (16.1, 22.6) |
| Fontan type, n (%) | |
| Lateral tunnel | 13 (22) |
| Extracardiac conduit | 47 (78) |
| Cardiac anatomic diagnosis | |
| Tricuspid atresia | 22 (37%) |
| Hypoplastic left heart syndrome | 15 (25%) |
| Double inlet left ventricle | 9 (15%) |
| Pulmonary atresia, intact ventricular septum | 3 (5%) |
| Atrioventricular septal defect, hypoplastic left ventricle | 2 (3%) |
| Transposition of the great arteries, hypoplastic right ventricle | 2 (3%) |
| Other | 6 (10%) |
| Ventricular morphology, n (%) | |
| Left | 39 (65) |
| Right | 18 (30) |
| Mixed | 3 (5) |
| Pacemaker present, n (%) | 6 (10) |
| On ACEI, n (%) | 41 (68) |
Data are presented as mean±SD, median (interquartile range), or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor.
Vascular Function Assessment
Results of comprehensive vascular function assessment are displayed in Table 2. All 60 enrolled participants completed brachial FMD assessment with analyzable data. PAT testing was completed with high‐quality data in 57 (95%) Fontan patients. PAT study quality was inadequate in 3 patients (5%) primarily because of the presence of substantial artifact (a manifestation of patient noncompliance). PWV testing was completed with analyzable data in 46 participants (77%), and laser flow Doppler testing was successfully completed in 47 participants (78%). Heart rate variability could be assessed in 31 participants (52%). Most testing failures were due to the presence of abnormal QRS axis, low voltage QRS, and the presence of frequent ectopic beats. Pulse wave analysis was incomplete in the majority of patients (only 18 [30%] completed testing with analyzable data), largely due to low‐amplitude pulse waves, irregular cardiac rhythm, and frequent ectopic beats; therefore, pulse wave analysis data were not included in the final analysis. Minor adverse events included pain during the laser flow Doppler heating protocol in 1 patient; no major adverse events occurred during vascular testing.
Table 2.
Endothelial Function and Arterial Stiffness Measures
| Variable | n | Value |
|---|---|---|
| Brachial FMD | ||
| Percentage of FMD | 60 | 10.2±5.5 |
| FMD‐RHI | 60 | 73.3±29.7 |
| PAT | ||
| PAT‐RHI | 57 | 1.2 (0.2–4.8) |
| Natural log of RHI | 57 | 0.24±0.40 |
| Framingham modified RHI | 57 | 0.17±0.44 |
| PAT‐BPA | 57 | 271.7 (177.0–419.5) |
| AI | 57 | −1.42±12.67 |
| AI at 75 beats/min | 57 | −4.29±12.15 |
| Laser flow Doppler | ||
| Percentage change of mean | 47 | 1539 (1119–2213) |
| PWV | ||
| PWV leg, m/s | 45 | 7.47 (6.81–8.43) |
| PWV arm, m/s | 43 | 6.47 (5.5–7.27) |
| PWV trunk, m/s | 46 | 3.8 (3.23–4.63) |
| Pulse wave analysis | ||
| Augmentation index | 18 | 10.9±12.8 |
| Central pulse pressure | 18 | 22.9±4.2 |
| Heart rate variability | ||
| Mean of SD for R‐R intervals, ms | 31 | 41.2 (29.4–52.0) |
| Root mean square difference of R‐R intervals | 31 | 29.5 (20.9–48.4) |
Data are presented as mean±SD or median (interquartile range). AI indicates augmentation index; BPA, baseline pulse amplitude; FMD, flow‐mediated dilation; PAT, peripheral arterial tonometry; PWV, pulse wave velocity; RHI, reactive hyperemia index.
Cardiopulmonary Exercise Testing
Fifty‐four (92%) of the 59 participants who attempted cardiopulmonary exercise testing achieved maximal exercise (Table 3). One participant was unable to attempt exercise testing because of physical limitation. The exercise capacity of this Fontan cohort was typical, with a mean peak VO2 of 27.8±7.6 mL/kg per minute and percentage of predicted peak VO2 of 71±21.1%. VO2 and percentage of predicted VO2 at anaerobic threshold were 21.8±5.8% and 90±24.5%, respectively. The VE/VCO2 was 33.8±6.6. Innocor‐derived measures of cardiac output and stroke volume could be obtained at peak exercise in 32 patients (59%). No major adverse events occurred during exercise stress testing.
Table 3.
Resting, Submaximal, and Maximal Cardiopulmonary Exercise Measures
| Variable | n | Value |
|---|---|---|
| Rest | ||
| Heart rate, beats/min | 59 | 89±17 |
| Systolic blood pressure, mm Hg | 59 | 114±10 |
| Diastolic blood pressure, mm Hg | 59 | 69±7 |
| O2 saturation (%) | 59 | 96 (94, 98) |
| Cardiac index, L/min/m2 | 56 | 2.61±0.7 |
| Anaerobic threshold | ||
| VO2, mL/kg/min | 57 | 21.1±5.8 |
| Percentagea of predicted peak VO2 | 57 | 90.2±24.5 |
| VE/VCO2 | 57 | 33.8±6.6 |
| Peak exercise | ||
| VO2, mL/kg/min | 54 | 27.8±7.6 |
| Percentagea of predicted peak VO2 | 54 | 71.0±21.2 |
| Work, W | 50 | 2067 (1623, 3355) |
| Heart rate, beats/min | 56 | 166±24 |
| Respiratory rate, breaths/min | 54 | 51±11 |
| Respiratory exchange ratio | 54 | 1.12±0.09 |
| O2 pulse, mL/beat/BSA | 54 | 5.47±1.41 |
| O2 saturation (%) | 56 | 94 (90, 96) |
| Cardiac index, L/min/m2 | 32 | 5.07±1.07 |
| Stroke volume, mL | 32 | 47.6±14.5 |
Data are presented as mean±SD or median (interquartile range). BSA, body surface area; VE/VCO2, ventilatory equivalents of carbon dioxide; VO2, oxygen consumption.
Percentage predicted for age and sex.
PA and QOL
The Physical Activity Questionnaire was completed by all 60 participants. All 60 participants completed the appropriate PedsQL Core scale. Parents of participants aged <18 years completed the appropriate PedsQL parent‐proxy report (Table 4).
Table 4.
Quality of Life and Physical Activity Scores
| Variable | n | Score |
|---|---|---|
| PedsQL, self‐report | ||
| Total score | 59 | 75.±15 |
| Physical health summary score | 60 | 76±16 |
| Psychological health summary score | 59 | 75±16 |
| PedsQL, parent‐proxy report | ||
| Total score | 56 | 70±16 |
| Physical health summary score | 56 | 72±19 |
| Psychological health summary score | 56 | 69±16 |
| PAQ score (adolescent and child scales combined) | 60 | 2.4±0.8 |
Data are presented as mean±SD. PAQ indicates Physical Activity Questionnaire; PedsQL, Pediatric Quality of Life Inventory.
Univariate Associations Between Vascular Function and Functional Outcomes
Clinically meaningful correlations between measures of vascular function and exercise performance are shown in Figure 1. PAT‐derived baseline pulse amplitude (PAT‐BPA; a measure of arterial flow and waveform reflection prior to cuff occlusion; higher values indicated stiffer vessels) was negatively correlated with VE/VCO2 at anaerobic threshold (higher values indicated less efficiency). This functional measure was the only one studied that demonstrated a potential benefit associated with increased arterial stiffness. FMD‐RHI (higher values indicated better endothelial function) was positively correlated with peak VO2. In contrast, PAT‐AI75 (higher values indicated stiffer vessels) was negatively correlated with peak VO2.
Figure 1.
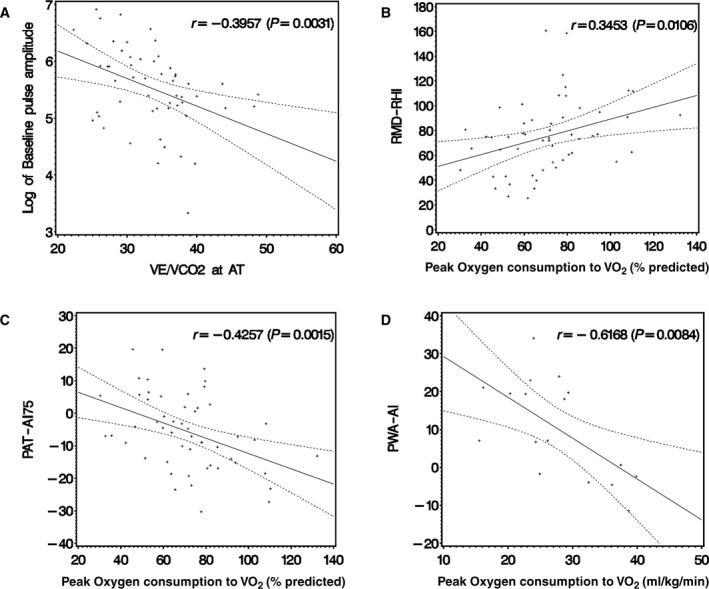
Scatterplots of vascular function parameters vs measures of exercise performance. A, Peripheral arterial tonometry–derived baseline pulse amplitude (PAT‐BPA) vs ventilatory equivalents of carbon dioxide (VE/VCO 2) at anaerobic threshold (AT). B, Flow mediated vasodilation–derived reactive hyperemia index (FMD‐RHI) vs percentage of predicted peak oxygen consumption (VO 2). C, PAT‐derived augmentation index at 75 beats per minute (PAT‐AI75) vs percentage of predicted peak VO 2. D, Pulse wave analysis–derived augmentation index (PWA‐AI) vs peak VO 2. In each case, there was a significant correlation between vascular and exercise measures. Regression line and 95% CI are depicted.
Correlations among measures of vascular function, PA, and QOL are shown in Figure 2. Percentage of FMD (higher values indicated better endothelial function) was positively correlated with Physical Activity Questionnaire score, whereas PAT‐BPA was negatively correlated with Physical Activity Questionnaire score. PAT‐BPA was negatively correlated with both total and physical health summary parent‐proxy PedsQL scores.
Figure 2.
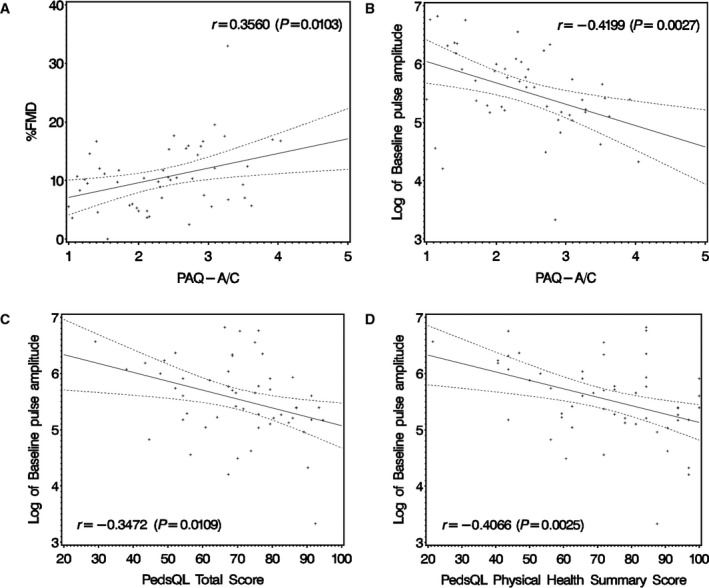
Scatterplots of vascular function parameters vs measures of physical activity and quality of life. A, Percentage of flow‐mediated vasodilation (%FMD) vs Physical Activity Questionnaire for Adolescents and for Older Children (PAQ–A/C) score. B, Peripheral arterial tonometry–derived baseline pulse amplitude (PAT‐BPA) vs PAQ score. C, PAT‐BPA vs Pediatric Quality of Life Inventory (PedsQL) score (parent‐proxy report). D, PAT‐BPA vs PedsQL physical health summary score (parent‐proxy report). In each case, there was a significant correlation between vascular and physical activity or quality of life measures. Regression line and 95% CI are depicted.
Multivariable Associations Between Vascular Function and Functional Outcomes
Multivariable associations with exercise capacity and noninvasively derived cardiac output are shown in Table 5. Sex, height, waist circumference, PAT‐AI, and FMD‐RHI were the most consistent variables to demonstrate association with measures of exercise performance, with systolic blood pressure and heart rate also playing roles in cardiac output and PAT‐BPA influencing VE/VCO2 at anaerobic threshold (R 2 range 0.22–0.56). FMD‐RHI, a classic measure of endothelial function, was the most common vascular association identified across exercise measures. Although PAT‐derived measures of arterial stiffness were also found to be associated with exercise performance measures, PAT‐derived measures of endothelial function (eg, PAT‐RHI) were not. Age, weight, body mass index z score, and duration of Fontan circulation were not associated with exercise performance. Although cardiac output was not assessed in all patients, the model explains much of the variability (R 2=0.56) in this clinically relevant noninvasive measure of cardiac output.
Table 5.
Factors Associated With Exercise Capacity and Cardiac Output
| Variable | VO2 at AT, mL/kg/min (n=56) | VE/VCO2 at AT (n=54) | Max VO2, mL/kg/min (n=52) | Max VO2, % predicted (n=53) | Max O2 Pulse, mL/beat/BSA (n=54) | Max cardiac index, L/min/m2 (n=32) |
|---|---|---|---|---|---|---|
| Intercept | 34.4 | 62.4 | 41.3 | 0.42 | 0.32 | 7.47 |
| Female | −4.35 | 0.10 | −0.71 | 2.67 | ||
| Height | −0.18 | 0.03 | ||||
| Waist Circ | −0.24 | −0.27 | ||||
| HR | −0.11 | |||||
| SBP | −0.05 | |||||
| PAT‐AI75 | −0.28 | −0.008 | ||||
| FMD‐RHI | 0.051 | 0.09 | 0.003 | 0.013 | 0.05 | |
| PAT‐BPA | −0.007 | |||||
| Adjusted R 2 | 0.30 | 0.38 | 0.38 | 0.36 | 0.22 | 0.56 |
Values are β coefficients for significant covariates left in the model after stepwise regression. All variables P≤0.05. AI75 indicates augmentation index standardized to 75 beats/min; AT, anaerobic threshold; BPA, baseline pulse amplitude; BSA, body surface area; Circ, waist circumference; FMD, flow mediated dilation; HR, heart rate; Max, maximum; PAT, peripheral arterial tonometry; RHI, reactive hyperemia index; SBP, systolic blood pressure; VE/VCO2, ventilatory equivalents of carbon dioxide; VO2, oxygen consumption.
Multivariable associations with PA and QOL are shown in Table 6. PAT‐AI (higher values indicated stiffer vessels) and PAT‐BPA were the most consistent variables to demonstrate an association with PA and QOL, with FMD‐RHI and age influencing Physical Activity Questionnaire and weight for height affecting the QOL physical health summary score (R 2 range 0.01–0.32). Weight, body mass index z score, and duration of Fontan circulation were not associated with PA or QOL. PAT‐BPA was the most common vascular association identified across PA and QOL measures. Identified associations were significantly related to parent‐proxy reported QOL (R 2 range 0.28–0.32) but not to self‐reported QOL measures (R 2 range 0.01–0.04).
Table 6.
Factors Associated With Physical Activity and Quality of Life
| Variable | PAQ (n=51) | Total QOL Self‐R (n=56) | Phys QOL Self‐R (n=59) | Total QOL Parent‐R (n=52) | Phys QOL Parent‐R (n=52) |
|---|---|---|---|---|---|
| Intercept | 3.4 | 80.5 | 79.62 | 77.74 | 128.93 |
| Age | −0.109 | ||||
| Weight‐for‐height | −103.4 | ||||
| PAT‐AI | −0.32 | −0.438 | |||
| FMD‐RHI | 0.039 | ||||
| PAT‐BPA | −0.016 | −0.011 | −0.02 | −0.024 | |
| Adjusted R 2 | 0.30 | 0.04 | 0.01 | 0.28 | 0.32 |
Values are β coefficients for significant covariates left in the model after stepwise regression. All variables P≤0.05. AI indicates augmentation index; BPA, baseline pulse amplitude; FMD, flow‐mediated dilation; PAQ, Physical Activity Questionnaire; Parent‐R, parent‐proxy reported; PAT, peripheral arterial tonometry; Phys, physical health summary score; QOL, quality of life; RHI, reactive hyperemia index; Self‐R, self‐reported.
A subanalysis stratified by use of angiotensin‐converting enzyme inhibitor therapy did not yield significant differences from the primary analysis reported earlier.
Discussion
In this report, we demonstrated that measures of endothelial function and wave reflection (related to arterial stiffness) are associated with exercise performance, PA, and QOL in adolescent and young adults with Fontan circulation. Lower indices of endothelial function and stiffer arteries were each associated with lower submaximal and maximal exercise capacity, cardiac output, PA, and worse QOL. These results suggest that vascular function parameters could be important biomarkers of decreased functional performance. Furthermore, although these data cannot demonstrate a causal relationship, it is possible that abnormal vascular function is itself a determinant of functional performance and thus a potential therapeutic target to modify the inevitable functional decline observed in adolescent and young adult Fontan survivors.
Prior investigations of vascular health in Fontan cohorts have demonstrated an association between endothelial function and peak exercise performance.5, 6, 7, 8, 25 No prior studies have established a relationship between vascular function and PA or QOL. Moreover, previous Fontan vascular studies have been affected by a number of important limitations. First, prior investigations have been constrained in analysis to assessment of simple correlation between vascular and functional measures.5, 6, 7, 8 Second, many vascular function and exercise performance measures are influenced by differences in sex and anthropometric values; therefore, these prior unadjusted analyses were at risk of bias based on cohort composition. Third, arterial stiffness, which has previously been shown to be abnormal in Fontan patients,26 has not been studied in relationship to functional performance. Fourth, no prior study of vascular function in a Fontan population has used a multimodality approach to assessment of vascular function. In this study, we addressed these concerns and limitations within our study design. We generated multivariable linear models when evaluating data for associations with functional outcome measures, thereby accounting for potential influence of sex, race, body mass index, and other anthropometric variables. Moreover, we used a broad portfolio of indices to assess the Fontan vascular tree with inclusion of multiple measures of endothelial function, wave reflection, and arterial stiffness in the central, conduit, and peripheral arteries. This novel approach facilitated the assessment of each index in the overall predictive models, which potentially improved the coefficients of determination. The selection of vascular indices ultimately included in the final multivariable models could not have been predicted a priori and may reflect real differences in vascular biology and pathology throughout the Fontan vascular tree.
An assessment of the Fontan cohort in this study, relative to recent Fontan cohorts evaluated in large‐scale trials, is important to determine generalizability of the findings presented. Exercise performance in the present cohort closely resembled that reported in a large Pediatric Heart Network cross‐sectional study of exercise performance in adolescent Fontan patients (peak VO2 27.8±7.6 versus 27.2±6.3).19 Moreover, QOL scores were similarly representative.27 The present cohort demonstrated substantially higher percentages of FMD than those obtained in prior studies of Fontan patients (10.2±5.5% versus 4.2% or 6.5±2.4%), more closely resembling the control populations in those studies.5, 8 We speculated that improvements in perioperative management and other “era effects” might have contributed to this improved measure of endothelial function in our cohort. PAT has also been studied in a single‐center cohort of Fontan patients in the more recent era. PAT‐RHI (1.2 [range 0.2–4.8] versus 1.37 [range 1.2–1.6]) and Framingham Heart Study modification of RHI (0.17±0.44 versus 0.17 [range −0.04 to 0.44]) were similar between the cohorts.7 AI has been reported in Fontan patients in a previous study in which Fontan patients demonstrated greater stiffness (delineated by a higher positive value) than healthy controls (4.9±2.4 versus −9.8±2.3); our cohort falls somewhere in between (−1.4±12.7).26 Other measures of endothelial function and measures of arterial stiffness have not been adequately studied in Fontan patients previously to facilitate comparison.
To assess the potential import of associations between vascular measures and functional capacity reported, it is relevant to understand how vascular measures in this Fontan cohort compared with those of adolescents and young adults without Fontan circulation or congenital heart disease who underwent evaluation of vascular health. Interestingly, percentage of FMD was higher in this Fontan cohort (10.2±5.5%) than in many other disease states, including familial hypercholesterolemia (1.2±0.4%), hypertension (4.5±4.0%), and obesity (6.6±2.3%), whereas PAT‐RHI (1.2 [range 0.2–4.8]) and Framingham Heart Study modification of RHI (0.17±0.44) were lower compared with participants with type 2 diabetes mellitus (T2DM; 1.6±0.5), obesity (1.5±0.4), and sickle cell disease (1.6).28, 29, 30, 31, 32, 33 It is reasonable to infer from these comparisons that endothelial function might be better preserved in conduit arteries than in the more distal peripheral arteries of Fontan patients. Laser flow Doppler was low in Fontan patients (1539 [range 1119–2213]) compared with both adolescent and young adult controls (2539±1255) and those with T2DM (1870±945).34 This further supports the conclusion that peripheral or tissue‐level endothelial function is significantly diminished in Fontan circulation. PWV in this Fontan cohort was lower compared with adolescent and young adult participants with T2DM or obesity and with lean controls when assessed in the leg (7.47 [range 6.8–8.4] versus 8.0±1.2 in controls and 8.3±1.6 in participants with T2DM), in the arm (6.47 [range 5.5–7.3] versus 7.4±1.1 in controls and 7.6±1.1 in participants with T2DM) and in the trunk (3.8 [range 3.2–4.6] versus 5.4±0.7 in controls and 6.7±1.2 in participants with T2DM).16 To the extent that these velocity data suggest that Fontan patients have substantially less stiff vessels (across the upper extremities and thoracoabdominal aorta) than healthy controls, despite numerous known physiological insults and invasive data to the contrary, it is reasonable to question the validity of these stiffness data.10, 11 Indeed, noninvasive measures of waveform reflection and arterial stiffness may be less accurate in the setting of anatomically abnormal arteries (either congenital or acquired), which is common in the Fontan population. Moreover, low cardiac output might serve to normalize the velocity of blood flow through stiff arteries, thereby contributing to false‐negative results from PWV testing. Invasive validation studies have never been performed in a Fontan cohort to evaluate these potential concerns.
The link between vascular dysfunction and atherosclerotic cardiovascular risk has been well established in populations without congenital heart disease. Large cohort studies have repeatedly demonstrated that endothelial dysfunction (including assessment with both FMD and PAT) is an independent risk factor for major adverse cardiovascular events, including stroke and myocardial infarction.35, 36 Although the present study contributes to a growing body of work focused on better understanding the role of vascular health and the implications of vascular dysfunction on outcomes in Fontan‐palliated univentricular congenital heart disease patients, risk factors for the development of vascular dysfunction have not been established. Moreover, the rates of change (eg, progression) in key measures of endothelial function and arterial stiffness—important for incorporation of these variables as outcome measures in a therapeutic clinical trial—have not been defined. In addition, it is necessary to determine whether or not lower endothelial function and stiffer arteries are independently associated with adverse cardiovascular outcomes, such as symptomatic Fontan failure, cardiac transplantation, and death. Future work will need to link vascular dysfunction with these adverse cardiovascular outcomes to demonstrate the clinical utility of vascular function measures to serve as biomarkers in the Fontan population. Finally, determination of the cause–effect relationship between vasculopathy and clinical deterioration is necessary prior to consideration of large‐scale intervention trials targeting vascular dysfunction as a primary outcome measure.
Several study limitations not mentioned previously are worth noting. First, this study was performed at a single center, and center‐based biases in surgical or medical management may limit generalizability of the findings. Second, this cross‐sectional study did not include a control group of healthy non‐Fontan participants; therefore, this analysis does not provide direct insight into the relationship between vascular and functional measures in a healthy control population. We have made efforts to relate vascular measures in this Fontan cohort to data from previously studied Fontan cohorts and to normative data from patients without congenital heart disease and from healthy control populations. Third, this study did not include an assessment of ventricular size and function, limiting potential analyses using these relevant measures. Fourth, the analyses of factors associated with exercise, PA, and QOL measures were necessarily limited to those parameters assessed in this study, which was focused on vascular function. Other potentially important associated factors may exist, may add to our understanding of the physiological mechanisms of vascular limitations, and may influence the models presented. Last, a sizable percentage of participants in this sample regularly used cardiovascular medications. Medications were not held on the day of testing to avoid interfering with ongoing care; certain drugs may have had differential effects on vasodilatory function and affected the observed findings. Despite this concern, an analysis of patients stratified by use of angiotensin‐converting enzyme inhibitor therapy did not demonstrate differences in vasodilator response.
In conclusion, in adolescent and young adult patients with Fontan circulation, there is an association between greater arterial stiffness, lower endothelial function and worse exercise performance, cardiac output, PA and QOL. These associations require further investigation to better understand the cause–effect nature of the relationships. Ongoing efforts should assess risk factors for, and the rate of progression of, vascular dysfunction and determine whether or not vascular measures predict future adverse outcomes such as death or cardiac transplantation.
Sources of Funding
This study was funded in part by an American Heart Association Beginning Grant‐in‐Aid (Goldstein) #14BGIA18740061.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e004258 doi: 10.1161/JAHA.116.004258)
References
- 1. Khairy P, Fernandes SM, Mayer JE Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long‐term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. [DOI] [PubMed] [Google Scholar]
- 2. Anderson PA, Sleeper LA, Mahony L, Colan SD, Atz AM, Breitbart RE, Gersony WM, Gallagher D, Geva T, Margossian R, McCrindle BW, Paridon S, Schwartz M, Stylianou M, Williams RV, Clark BJ III. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol. 2008;52:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein BH, Connor CE, Gooding L, Rocchini AP. Relation of systemic venous return, pulmonary vascular resistance, and diastolic dysfunction to exercise capacity in patients with single ventricle receiving Fontan palliation. Am J Cardiol. 2010;105:1169–1175. [DOI] [PubMed] [Google Scholar]
- 4. McCrindle BW, Zak V, Pemberton VL, Lambert LM, Vetter VL, Lai WW, Uzark K, Margossian R, Atz AM, Cook A, Newburger JW; Pediatric Heart Network I . Functional health status in children and adolescents after Fontan: comparison of generic and disease‐specific assessments. Cardiol Young. 2014;24:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jin SM, Noh CI, Bae EJ, Choi JY, Yun YS. Impaired vascular function in patients with Fontan circulation. Int J Cardiol. 2007;120:221–226. [DOI] [PubMed] [Google Scholar]
- 6. Inai K, Saita Y, Takeda S, Nakazawa M, Kimura H. Skeletal muscle hemodynamics and endothelial function in patients after Fontan operation. Am J Cardiol. 2004;93:792–797. [DOI] [PubMed] [Google Scholar]
- 7. Goldstein BH, Golbus JR, Sandelin AM, Warnke N, Gooding L, King KK, Donohue JE, Gurney JG, Goldberg CS, Rocchini AP, Charpie JR. Usefulness of peripheral vascular function to predict functional health status in patients with Fontan circulation. Am J Cardiol. 2011;108:428–434. [DOI] [PubMed] [Google Scholar]
- 8. Mahle WT, Todd K, Fyfe DA. Endothelial function following the Fontan operation. Am J Cardiol. 2003;91:1286–1288. [DOI] [PubMed] [Google Scholar]
- 9. Sarkola T, Jaeggi E, Slorach C, Hui W, Bradley T, Redington AN. Assessment of vascular remodeling after the Fontan procedure using a novel very high resolution ultrasound method: arterial wall thinning and venous thickening in late follow‐up. Heart Vessels. 2013;28:66–75. [DOI] [PubMed] [Google Scholar]
- 10. Schlangen J, Fischer G, Petko C, Hansen JH, Voges I, Rickers C, Kramer HH, Uebing A. Arterial elastance and its impact on intrinsic right ventricular function in palliated hypoplastic left heart syndrome. Int J Cardiol. 2013;168:5385–5389. [DOI] [PubMed] [Google Scholar]
- 11. Biglino G, Schievano S, Steeden JA, Ntsinjana H, Baker C, Khambadkone S, de Leval MR, Hsia TY, Taylor AM, Giardini A. Reduced ascending aorta distensibility relates to adverse ventricular mechanics in patients with hypoplastic left heart syndrome: noninvasive study using wave intensity analysis. J Thorac Cardiovasc Surg. 2012;144:1307–1313; discussion 1313‐4. [DOI] [PubMed] [Google Scholar]
- 12. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 13. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 14. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross‐sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahmud FH, Van Uum S, Kanji N, Thiessen‐Philbrook H, Clarson CL, Mahmud FH, Van Uum S, Kanji N, Thiessen‐Philbrook H, Clarson CL. Impaired endothelial function in adolescents with type 1 diabetes mellitus. J Pediatr. 2008;152:557–562. [DOI] [PubMed] [Google Scholar]
- 16. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity‐related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H; European Network for Non‐invasive Investigation of Large A . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 18. O'Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14:S147–S157. [PubMed] [Google Scholar]
- 19. Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross‐sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. [DOI] [PubMed] [Google Scholar]
- 20. Cooper DM, Weiler‐Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. [DOI] [PubMed] [Google Scholar]
- 21. Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J. 2008;29:1681–1687. [DOI] [PubMed] [Google Scholar]
- 22. Shafer KM, Garcia JA, Babb TG, Fixler DE, Ayers CR, Levine BD. The importance of the muscle and ventilatory blood pumps during exercise in patients without a subpulmonary ventricle (Fontan operation). J Am Coll Cardiol. 2012;60:2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29:1344–1349. [DOI] [PubMed] [Google Scholar]
- 24. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. [DOI] [PubMed] [Google Scholar]
- 25. Binotto MA, Maeda NY, Lopes AA. Altered endothelial function following the Fontan procedure. Cardiol Young. 2008;18:70–74. [DOI] [PubMed] [Google Scholar]
- 26. Lambert E, d'Udekem Y, Cheung M, Sari CI, Inman J, Ahimastos A, Eikelis N, Pathak A, King I, Grigg L, Schlaich M, Lambert G. Sympathetic and vascular dysfunction in adult patients with Fontan circulation. Int J Cardiol. 2013;167:1333–1338. [DOI] [PubMed] [Google Scholar]
- 27. Uzark K, Zak V, Shrader P, McCrindle BW, Radojewski E, Varni JW, Daniels K, Handisides J, Hill KD, Lambert LM, Margossian R, Pemberton VL, Lai WW, Atz AM; Pediatric Heart Network I . Assessment of quality of life in young patients with single ventricle after the Fontan operation. J Pediatr. 2016;170:166–172.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium‐dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aggoun Y, Farpour‐Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008;29:792–799. [DOI] [PubMed] [Google Scholar]
- 30. Mahmud FH, Hill DJ, Cuerden MS, Clarson CL. Impaired vascular function in obese adolescents with insulin resistance. J Pediatr. 2009;155:678–682. [DOI] [PubMed] [Google Scholar]
- 31. Haller MJ, Stein J, Shuster J, Theriaque D, Silverstein J, Schatz DA, Earing MG, Lerman A, Mahmud FH. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2007;8:193–198. [DOI] [PubMed] [Google Scholar]
- 32. Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, Lam CW, Metreweli C, Celermajer DS. Overweight in children is associated with arterial endothelial dysfunction and intima‐media thickening. Int J Obes Relat Metab Disord. 2004;28:852–857. [DOI] [PubMed] [Google Scholar]
- 33. Sivamurthy KM, Dampier C, MacDermott M, Maureen M, Cahill M, Hsu LL. Peripheral arterial tonometry in assessing endothelial dysfunction in pediatric sickle cell disease. Pediatr Hematol Oncol. 2009;26:589–596. [DOI] [PubMed] [Google Scholar]
- 34. Shah AS, Gao Z, Dolan LM, Dabelea D, D'Agostino RB Jr, Urbina EM. Assessing endothelial dysfunction in adolescents and young adults with type 1 diabetes mellitus using a non‐invasive heat stimulus. Pediatr Diabetes. 2015;16:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. [DOI] [PubMed] [Google Scholar]
- 36. Suzuki T, Hirata K, Elkind MS, Jin Z, Rundek T, Miyake Y, Boden‐Albala B, Di Tullio MR, Sacco R, Homma S. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS). Am Heart J. 2008;156:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]


