Abstract
CONTEXT:
Research shows, conclusively, that perinatal HIV infection has negative effects on cognitive functioning of children and adolescents. However, the extent of these cognitive impairments is unknown. Current literature does not document specific cognitive domains most affected in HIV-infected children and adolescents.
OBJECTIVE:
To systematically review and meta-analyze the degree of cognitive impairment, and the specific cognitive domains affected, in children and adolescents with perinatally acquired HIV infection.
DATA SOURCES:
We systematically searched 5 electronic bibliographic databases, namely: PubMed, PsychINFO, Academic Search Premier, Scopus, and WorldCat, by using a search protocol specifically designed for this study.
STUDY SELECTION:
Studies were selected on the basis of set a priori eligibility criteria. Titles, abstracts, and full texts were assessed by 2 independent reviewers.
DATA EXTRACTION:
Data from included studies were extracted into Microsoft Excel by 2 independent reviewers.
RESULTS:
Twenty-two studies were identified for inclusion in the systematic review and of this, 6 studies were included in the meta-analysis. Results from the meta-analysis indicated that working memory and executive function were the domains most affected by the HIV virus.
LIMITATIONS:
Only 27% of the included studies were suitable to enter into the meta-analysis. There was significant geographic bias in published studies, with only 32% (7/22) of included studies from sub-Saharan Africa.
CONCLUSIONS:
The evidence supports an association between HIV infection in children and adolescents and cognitive impairment in the domains of working memory, executive function and processing speed, with effect size estimates also providing some support for deficits in visual memory and visual-spatial ability.
Perinatally acquired HIV infection has negative effects on cognitive functioning of children and adolescents living with the virus.1 However, the exact degree (ie, which domains are more impaired than others, or how many cognitive domains are impaired and how many are not) of these cognitive impairments is not fully established. Although it is relatively commonly reported that global cognition (with HIV-associated encephalopathy being the most severe manifestation) is affected,2 there is no consensus in the current literature as to which specific cognitive domains are most commonly affected in HIV-infected children and adolescents. The individual domain-specific consequences (ie, impairment within ≥1 cognitive domains) of HIV are likely to be more severe among perinatally infected children and adolescents as compared with those who are behaviorally infected.
It is well established that macrophages and microglial cells are a critical reservoir of HIV in the brain,3 but its role in HIV neurocognitive disorders is not yet fully established. The effects of HIV on cognitive functioning ranges from pervasive to being very subtle, with encephalopathy at the severe end of the spectrum.4,5 Regarding brain structure, investigations of the effects of HIV on the brain by using neuroimaging have discovered that HIV-infected adolescents have significant damage to the neuronal microstructure.6–8 The more recent of these types of investigations found that brain volume for both gray and white matter was lower and that white matter hyperintensities were higher in perinatally HIV-infected children.9 Another recent study showed that neuronal damage was associated with altered neurometabolite levels.10 These studies demonstrate that compromised brain integrity is associated with impaired global cognitive functioning in perinatally HIV-infected children and adolescents.6–10 These studies provide evidence of central nervous system compromise in perinatally HIV-infected children and adolescents and that this has consequences for the child’s cognitive abilities.
Perinatally infected children may present more frequently than adults with central nervous system disease11 due to the vulnerability of the developing brain. The deleterious effects of the virus on the brain may be more severe in children because HIV-related brain degeneration is occurring during a period of rapid brain growth and development.
A recent qualitative review12 of neurodevelopment in perinatally HIV-infected children found that HIV-infected children performed poorly on tests measuring performance within the following specific cognitive domains: executive functioning (most notably with regards to processing speed, working memory, planning/reasoning, and attention), visual-spatial ability and visual memory, and planning/reasoning. Although it is important to have descriptive and qualitative accounts of any public health problem, in this field, there is a lack of quantitative reviews.
Our limited understanding of the domain-specific cognitive impairments associated with perinatal HIV in children and adolescents is a significant barrier to treatment. To date, no systematic review has been published to assess the state of science on cognitive impairment among perinatally HIV-infected children and adolescents. Furthermore, no aggregated quantitative evidence has been published on the cognitive domains that are most affected by perinatal HIV infection. This systematic review and meta-analysis aims to address these gaps in this clinically relevant area.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.13 The PICOS method14 was used to develop the research questions, which are: “What is the extent of cognitive impairment in perinatally HIV-infected children and adolescents compared to HIV-negative controls?” and “Which cognitive domains are the most commonly affected?”.
Eligibility
Titles, abstracts, and full texts were assessed for inclusion by 2 independent reviewers (NP and TA). The following a priori eligibility criteria were applied. The study sample was composed of (1) perinatally/vertically infected children and adolescents between the ages of 6 to 18 years; (2) the study reported continuous data for cognitive outcomes measured by means of a standardized neuropsychological test measure/s; (3) the study included a healthy control group of HIV-unexposed and uninfected individuals; and (4) the study was published in English. Review articles and case study reports were excluded. No other explicit exclusion criteria were applied. The decision to only include data for HIV-unexposed and uninfected controls is based on emerging literature that reports subtle deficits in overall cognition, motor coordination, and language in this cohort.15,16
Search Strategy
We conducted a systematic search using a search protocol specifically designed for this study (Supplemental Information) in 5 electronic databases: PubMed, PsychINFO, Academic Search Premier, Scopus, and WorldCat. The initial screening process involved 2 independent reviewers (NP and TA) who assessed the titles and abstracts yielded from the systematic search and classified studies as either “include” or “exclude” based on the eligibility criteria as set out above. Full-text articles were sought for the included studies and for those whose abstract did not provide enough information to appropriately assess whether they met criteria for inclusion in this review. Finally, the full-text articles were assessed once again by 2 reviewers (NP and TA) to make a final decision regarding inclusion in the review according to the eligibility criteria. Disagreements between the 2 reviewers regarding the inclusion or exclusion of particular studies were settled by consultation with a third reviewer (DS).
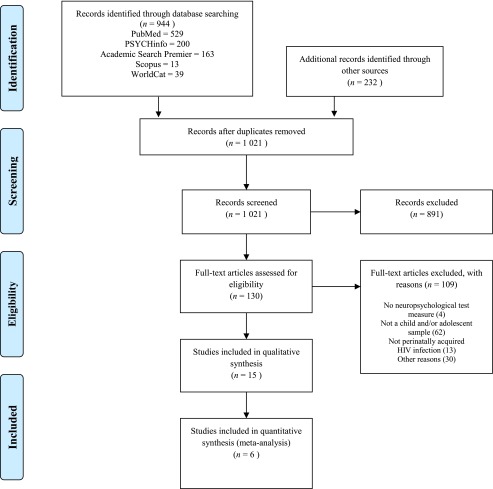
The initial systematic search produced 1177 studies (945 database studies + 232 gray literature studies). After the removal of duplicates, 1022 studies were assessed for possible inclusion. Of the 1022 studies, after screening of the titles and full text, 891 and 109, respectively, were excluded. An additional 101 studies were excluded because they did not meet the eligibility criteria. Thus, 22 studies were included in the final review (see Fig 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram detailing the identification of eligible studies.
Data Extraction
Data from the included studies were extracted by 2 independent reviewers (NP and TA) into Microsoft Excel. We extracted descriptive (eg, country, sample demographics, measures used, etc) and continuous outcomes data (ie, means and SDs for neuropsychological test measures) for each of the included studies.
For studies that reported only means and confidence intervals (CIs), we calculated SDs by using the formula: = ((SQRT(N))*((upper limit − lower limit)/3.92)). For studies that reported data for >1 patient and/or control groups (eg, highly active antiretroviral therapy [HAART] naive), we calculated the weighted means and SDs using the following formulae: = SUMPRODUCT (range means, range N)/SUM (range N) and = SQRT (SUMPRODUCT (range SD, range N)/(SUM (range N)−1)), respectively, to create 2 sets of data (ie, HIV-infected and HIV-uninfected controls) for pairwise meta-analysis.
Meta-analysis
Data were analyzed using RevMan version 5.3 software.17 The main comparison for the meta-analysis was cognitive performance in HIV-infected individuals versus HIV-uninfected control subjects who were not exposed in utero to the virus. Separate analyses were conducted for each of the cognitive domains of interest. A random effects model was used to determine the effect of the HIV virus on domain-specific cognitive performance. Effect size estimates (ESEs) of 0.2 were considered small, ESEs of 0.4 to 0.6 were considered moderate, and ESEs of 0.8 were considered large.17 Heterogeneity was assessed by means of the I2 statistic, with higher percentage scores representing a greater proportion of variability across the ESEs that could not be accounted for by chance alone.18 In comparisons where studies used the same tests, the mean difference was used to calculate the effect size, but for studies using different tests, the standard mean difference was used. Random effects were used for all comparisons because there was heterogeneity between all included studies.
Quality Assessment
The Downs and Black checklist19 was used to assess the methodological quality of the included studies. This 27-item checklist assesses the quality of randomized controlled trial (RCT) and non-RCT studies on the following subscales: (1) reporting, (2) external validity, (3) bias, (4) confounding, and (5) power. The Downs and Black checklist has been identified as one of the 2 most useful tools to assess quality for non-RCT type studies.18 Because this checklist was designed for use for both RCT and non-RCT studies, only a subset of 16 items were applicable to the observational studies included in this review.
Assessing the methodological quality of studies is important for identifying the strengths and weaknesses of a particular study. Though the Downs and Black checklist has been identified as one of the 2 most useful tools to assess quality for non-RCT studies,18 it is important to acknowledge the fact that any quality assessment also reflects the standard of reporting of a particular study. With this in mind, the quality assessments of the studies were not included in the inferential analyses, but rather were used to evaluate the methodological rigor of studies included in this review and to provide commentary and interpretation of the generalizability of the findings. See Supplemental Table 3 for the findings of the quality assessment.
Results
Description of Search Results
The search strategy yielded a total of 1022 records (after duplicates were removed) from both database and gray literature sources. After screening for eligibility, 22 studies were included. Only 6 of the included studies included data for an HIV-uninfected control group who were not prenatally exposed to the virus, and only these studies were then entered into the meta-analysis. The characteristics of the final 22 included studies are presented in Table 1.
TABLE 1.
Characteristics of Included Studies
| Study ID | Study Design | Country | Domains Assessed | nHIV+ | tHIV+ | eHIV+ | eHIV– | uHIV– | HIV-Infected, N | Controls, N | Total, N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angelini (20) | NR | Italy | GIQ, visual-spatial ability, visuomotor coordination | 1 | 1 | 1 | 0 | 0 | 62 | 0 | 62 |
| Boivin (21) | NR | Uganda | Learning, psychomotor speed, working memory, attention | 1 | 1 | 0 | 0 | 0 | 28 | 0 | 60 |
| Boivin 2010 (22) | NR | Uganda | Memory, visual-spatial processing, immediate and delayed memory, executive function | 1 | 0 | 0 | 0 | 0 | 102 | 0 | 102 |
| Cohen (23)a | Cross-sectional | Netherlands | GIQ (VIQ + PIQ), processing speed, attention, working memory, executive function | 1 | 1 | 1 | 0 | 1 | 35 | 37 | 72 |
| Franklin (24) | Longitudinal | NR | GIQ | 0 | 1 | 0 | 0 | 0 | 39 | 0 | 39 |
| Fundaro (25) | NR | Italy | Memory, visual-spatial ability, language, learning, spatial organization | 0 | 1 | 0 | 1 | 0 | 8 | 8 | 16 |
| Hoare (7) | Cross-sectional | South Africa | Information processing speed, attention, working memory, visual attention, executive function | 0 | 1 | 0 | 0 | 0 | 50 | 0 | 50 |
| Hoare (6)a | Cross-sectional | South Africa | GIQ, motor coordination, information processing speed, attention, working memory, visual-spatial ability, visual memory, executive function | 1 | 0 | 0 | 0 | 1 | 12 | 12 | 24 |
| Kandawasvika (26)a | Cross-sectional | Zimbabwe | GIQ, information processing, numeracy, memory, motor ability | 1 | 1 | 0 | 1 | 1 | 32 | 274 | 306 |
| Keller (27)a | NR | US | Visual-spatial ability, visual memory, language, learning, motor coordination | 1 | 1 | 1 | 0 | 0 | 20 | 13 | 33 |
| Koekkoek (28) | NR | Netherlands | GIQ, working memory, attention, information processing, executive function, visual-spatial perception | 1 | 1 | 0 | 0 | 1 | 22 | 0 | 22 |
| Llorente (29) | Longitudinal | US | Executive function, attention, working memory, visual-spatial ability | 0 | 1 | 1 | 1 | 0 | 76 | 85 | 161 |
| Llorente (30) | NR | US | Language, information processing speed | 0 | 1 | 0 | 0 | 0 | 22 | 0 | 22 |
| Martin (31) | Cross-sectional | US | GIQ (VIQ + PIQ) | 0 | 1 | 0 | 0 | 0 | 41 | 0 | 41 |
| Nichols (32) | Cross-sectional | US | Visual and verbal memory and learning | 0 | 1 | 1 | 1 | 0 | 173 | 85 | 258 |
| Nozyce (33) | RCT | US | GIQ (VIQ + PIQ) | 0 | 1 | 0 | 0 | 0 | 274 | 0 | 274 |
| Puthanakit (34) | RCT | Thailand | GIQ, memory, motor coordination | 1 | 1 | 0 | 1 | 1 | 284 | 319 | 603 |
| Ravindrin (35)a | NR | India | Attention, language, visual memory, verbal learning, verbal memory, visual perceptual/spatial ability, visuomotor function, fine motor skills, executive function | 0 | 1 | 0 | 0 | 1 | 20 | 20 | 40 |
| Rice (36) | Prospective cohort | US | Language | 0 | 1 | 0 | 1 | 1 | 284 | 153 | 437 |
| Ruel (37)a | NR | Uganda | Attention, GIQ, motor proficiency | 1 | 0 | 0 | 0 | 1 | 161 | 106 | 267 |
| Smith (4) | NR | US | GIQ, verbal comprehension, perceptual reasoning, working memory, processing speed | 0 | 1 | 1 | 1 | 0 | 358 | 200 | 558 |
| Walker (38) | Case-control | Jamaica | GIQ, memory, attention, fine motor coordination | 1 | 1 | 1 | 0 | 0 | 287 | 0 | 287 |
1, yes; 0, no; eHIV+, encephalopathy HIV-infected; eHIV–, exposed HIV-uninfected; GIQ, general IQ/functioning; nHIV+, HAART naive HIV-infected; NR, not reported; PIQ, performance IQ; tHIV+, HAART-treated HIV-infected; uHIV–, unexposed HIV-uninfected; VIQ, verbal IQ.
Denotes the studies included in the meta-analysis.
Participants
A total of 3734 participants were included across the 22 included studies, and of these, 2390 were HIV infected. Of the 1312 controls, 807 were HIV exposed but uninfected and 505 were HIV unexposed. According to the quality assessments, the majority of the studies (19/22) included participants who are representative of the patient populations they are meant to represent. The average mean age reported was 9.53 years with an SD of 2.19 years for the studies who reported these data (7/22). The age range was 2 months to 17 years across the studies who reported these data (20/22).
Types of Neuropsychological Measures
Most of the included studies (18/22) made use of psychometrically sound neuropsychological test measures. These measures are standardized for use in children and adolescents and are widely used for assessing cognitive ability in this group. Given that standardized test measures were used, data were comparable between studies, although different tests may have been used. While extracting the data, every effort was made to analyze tests that were as similar as possible (ie, we selected WASI data for all studies that reported WASI data, etc). If this was not possible, then the data were not entered into the meta-analysis.
Findings From the Meta-analysis
Ten separate between-group comparisons were carried out for the meta-analysis, and these 10 comparisons consisted of the following cognitive domains: attention, executive function, general intellectual functioning, language, motor coordination, processing speed, verbal memory, visual memory, visual-spatial ability, and working memory. Each comparison sought to determine the effect of HIV on cognitive performance within that particular domain.
Table 2 presents the results of the between-group comparisons for each cognitive domain. Statistically significant effects were found for 2 out of 10 domains, with executive function and processing speed showing significant group differences. When ranked according to the effect size, the top 3 domains were (ranked from largest effect size): working memory (mean difference = 16.46; 95% CI = –14.22 to 47.13; number of studies = 2; P = .12), processing speed (standard mean difference = 9.36; 95% CI = 3.73 to 14.98; number of studies = 2; P = .00) and executive function (standard mean difference = 3.68; 95% CI = 1.35 to 6.02; number of studies = 4; P = .00). Looking at the ESEs, it appears that working memory, processing speed, and executive function are the domains with the largest between-group differences.
TABLE 2.
Results From the Meta-analysis
| Comparison | Studies | Participants | Statistical Method | ESE | P | I2 (%) |
|---|---|---|---|---|---|---|
| Working memory | 2 | 95 | Mean difference (IV, random, 95% CI) | 16.46 (–14.22 to 47.13) | .12 | 100 |
| Processing speed | 2 | 96 | Mean difference (IV, random, 95% CI) | 9.36 (3.73 to 14.98) | .00* | 0 |
| Executive function | 4 | 400 | Standard mean difference (IV, random, 95% CI) | 3.68 (1.35 to 6.02) | .00* | 98 |
| Visual memory | 4 | 361 | Standard mean difference (IV, random, 95% CI) | 2.71 (–2.31 to 7.74) | .29 | 99 |
| Visual-spatial ability | 5 | 433 | Standard mean difference (IV, random, 95% CI) | 0.20 (–1.56 to 1.97) | .82 | 98 |
| Attention | 4 | 402 | Standard mean difference (IV, random, 95% CI) | −0.51 (–1.15 to 0.13) | .12 | 84 |
| Language | 2 | 70 | Standard mean difference (IV, random, 95% CI) | −0.85 (–2.18 to 0.48) | .21 | 85 |
| General intellectual functioning | 4 | 548 | Standard mean difference (IV, random, 95% CI) | −1.22 (–2.53 to 0.10) | .07 | 97 |
| Motor coordination | 4 | 361 | Standard mean difference (IV, random, 95% CI) | −1.99 (–4.50 to 0.52) | .12 | 98 |
| Verbal memory | 2 | 112 | Standard mean difference (IV, random, 95% CI) | −6.80 (–19.43 to 5.83) | .29 | 98 |
Domains ranked in order of largest to smallest effect size. IV, inverse variance.
P = .05.
Findings From the Systematic Review
The 16 studies that were not entered into the meta-analysis were looked at qualitatively. With regards to significant differences between perinatally HIV-infected children and adolescents and HIV-uninfected controls (both exposed and unexposed), 3 of 15 studies explicitly reported differences in the domain of executive function4,20,29 and 1 of the 15 studies explicitly reported a group difference for working memory.4 A few studies reported findings on components of executive function and working memory (eg, semantic fluency or attention),21,28 or on measures that encompass executive function as part of the scales total scoring system (eg, the Kaufman Assessment Battery for Children, 2nd edition (KABC-II)). There were a few studies that reported findings for processing speed (4/15)4,21,28,30 and visual memory (3/15).25,28,32 The remainder of the studies only reported findings for overall or global cognition.22,24,31,33,34,38 See Supplemental Table 4 for additional details on the qualitative analysis for all of the included studies.
Discussion
This systematic review assessed the current state of scientific evidence on cognitive impairment in perinatally HIV-infected children and adolescents. In addition, we used meta-analytic techniques to rank the most important cognitive domains affected by perinatal HIV infection, with the aim of informing clinical practice and guiding the development of future interventions to improve the cognitive well-being of this important population throughout their life trajectory.
Results from the meta-analysis revealed that children and adolescents with perinatally acquired HIV demonstrated significant impairments in executive function and processing speed relative to HIV-uninfected and -unexposed controls. There is an increasing recognition in the research community that statistical significance only tells part of the story, and that ESEs can be a bit more informative, particularly with respect to underpowered studies with small samples. In medical research and in traditional meta-analytical approaches, the size of the effect is equal to the magnitude of the difference between the 2 groups of interest39 and can “be said to be the true measure of the significance of the difference” between the 2 groups.40 In recognition of the literature, and both the small sample sizes and number of studies, we decided before conducting this meta-analysis that it would be useful to estimate the strength of the association between HIV infection and impairment in particular cognitive domains by using the mean or standard mean difference ESEs.
Based on the results of this meta-analysis, large effects (ESE ≥0.8) of HIV status suggesting greater impairment in HIV-infected children and adolescents were observed for the following domains (ranked from largest to smallest effect size): working memory, processing speed, executive function, and visual memory. Small ESEs were observed for visual-spatial ability, attention, language, general intellectual functioning, motor coordination, and verbal memory. The difference between the upper and lower limits of the CI were greater than the actual effect estimate for all cognitive domains assessed. This lack of precision in the effect estimates reflects the small number of studies providing data for most of the domains. It could also potentially reflect methodological differences between the studies included in the meta-analysis, as suggested by the fact that substantial heterogeneity was observed for most of the domains in the effects reported by individual studies.
The Laughton et al study12 found that HIV-infected children performed poorly in the cognitive domains of executive functioning (most notably with regards to processing speed, memory, and attention), visual-spatial ability, visual memory, processing speed, and planning/reasoning. Cohen et al23 found that HIV-positive children performed more poorly than controls in all cognitive domains, but most notably on general intellectual functioning, processing speed, attention, and working memory. A study conducted in South Africa found that even asymptomatic HIV-positive children performed more poorly on cognitive measures than controls and, once again, most notably on tests of general intellectual functioning, visual-spatial ability, visual memory, and semantic fluency (ie, executive functioning).6
The findings from these studies seem to consistently implicate working memory, executive function, processing speed, and perceptual deficits in HIV-infected children and adolescents. Executive functioning emerges as one of the more prominent domains to be affected in this population, which is consistent with the finding in this review of significant differences in this domain. The vulnerability of executive function and processing speed to the effects of perinatal HIV infection was confirmed by the results of this meta-analysis, in which these domains were determined to be relatively impaired in HIV-infected children and adolescents, with significant group differences observed in these cases. Impairments in working memory is also supported by this review, with this domain obtaining the largest effect size. Although not ranked in the top 3 domains, this review also found that visual memory yielded relatively large effect sizes (ESE >0.8), as compared with other domains.
However, the results of this meta-analysis (with regards specifically to the ESEs) differ from previous findings in 2 important ways: (1) most studies found that general intellectual functioning was severely impaired and this review did not, and (2) a large number of studies found that motor coordination was severely impaired and this review did not. In fact, the findings from this review and meta-analysis showed that general intellectual functioning and motor coordination were ranked eighth and ninth out of a total of 10 domains with regards to effect size. Reasons for the differences could be related to methodological differences and/or differences in focus areas with each study’s research questions (which in turn will determine the methodology used). A more thorough comparison of the current findings to each of the included studies is provided in Supplemental Table 4. Individual studies may only be able to provide weak to moderate evidence, whereas this meta-analysis provides much stronger evidence because the results are pooled from 6 meta-analyzed studies.
When judging the generalizability of the results of this review to populations from sub-Saharan Africa (SSA), the quality of the included studies must be taken into account along with the demographic information of the participants included in the studies. Each of the included studies exhibited some methodological flaws as rated on the Down’s and Black quality rating scale. Furthermore, the geographical disparity in the scientific evidence base is notable. The majority of the 22 included studies (53%) occurred in developed countries. Few studies (32%) occurred in SSA, where the burden of HIV disease is most prevalent. Overall, this review identified that the scientific evidence in this field needs further development both with regards to depth of knowledge as well as rigor of methodological design.
Our understanding of child and adolescent HIV-associated cognitive impairment is limited by multiple factors: (1) the absence of appropriate control data in many studies, (2) the failure of many studies to focus specifically on perinatally acquired HIV, (3) the inclusion in some studies of seroreverters, (4) the fact that not all studies use standardized neuropsychological test measures, (5) the practice in some studies to include/collapse data from treatment naive, treated, and encephalopathy patients (ideally these groups should be separated for analysis purposes), and (6) the incomplete and inconsistent reporting of neuropsychological test result data or outcomes of statistical tests. Nevertheless, the strength of this review is that we were able to overcome many of these limitations by applying strict study inclusion criteria, by extracting as much data as possible from included studies, by using the data presented to calculate means and SDs when they were not presented, and by excluding studies without appropriate control data from the meta-analysis.
Implications
This review revealed that significant cognitive differences between HIV-infected and HIV-uninfected children and adolescents are seen in the domains of executive function and processing speed. This review also revealed that the domains with the largest effect sizes were working memory, processing speed, and executive function. This is in line with previous findings and is despite a paucity of methodologically sound studies in the field of child and adolescent HIV-associated cognitive impairment. With this in mind, future studies or interventions ought to primarily focus on these domains.
Strengths and Limitations
This review is the first systematic review and meta-analysis of child and adolescent HIV-associated cognitive impairment. This review successfully meta-analyzed 6 studies in an attempt to rank cognitive domains from most impaired to least impaired. Given some of the methodological flaws identified in the included studies, and based on the fact that 53% of the included studies were from developed countries and only 32% were from SSA, in which the burden of HIV disease is most prevalent, one should exercise caution when generalizing these results to SSA. However, and importantly, what this review emphasizes is that this particular research field is very underdeveloped and that more methodologically rigorous studies need to be conducted in this area, particularly in areas of the world where HIV is most prevalent, such as SSA.
Conclusions
Based on the findings of this review, when considering which cognitive domains are most affected by the HIV virus, preference should be given to (in order of most to least important): working memory, processing speed, and executive function. However, caution should be exercised when generalizing these findings to SSA (which bears the largest burden of HIV disease) because only 32% of included studies were from SSA and the size of the CIs were relatively large for some of these findings.
Acknowledgment
We thank the Health Sciences Library at the University of Cape Town for assistance in obtaining full-text articles.
Glossary
- CI
confidence interval
- ESE
effect size estimate
- HAART
highly active antiretroviral therapy
- RCT
randomized controlled trial
- SSA
sub-Saharan Africa
Footnotes
Ms Phillips was responsible for developing the concept for the paper, conducting the ground work regarding the systematic search (ie, developing the search terms and strategies, conducting the actual systematic search, maintaining documentation regarding the systematic search process and results), assessing titles and/or abstracts for inclusion, assessing full text articles for inclusion, data extraction, performing the meta-analysis, interpretation of the results for the meta-analysis, and write-up of the final manuscript; Ms Amos was responsible for assessing titles and/or abstracts for inclusion, assessing full-text articles for inclusion, data extraction, assisting Ms Phillips with the meta-analysis, and she reviewed the manuscript and suggested/made relevant changes before submission; Prof Kuo helped to develop the search terms used to conduct the systematic search, provided guidance of the proper process and documentation of systematic searching, continually checked the search data for quality control, reviewed the manuscript, and suggested/made relevant changes before submission; Prof Hoare provided clinical input during the entire process (eg, input into which age categories to assess, division of HIV groups, and clinical relevance of the data presented), reviewed the manuscript, and suggested/made relevant changes before submission; Dr Ipser provided consultation regarding the data extraction and meta-analytic procedures, as well as guidance on formulae for calculating weighted means and SDs, reviewed the manuscript, and suggested/made relevant changes before submission; Prof Thomas provided input regarding the data to be extracted in each specific cognitive domain, reviewed the manuscript, and suggested/made relevant changes before submission; Prof Stein assisted with the concept for the paper, provided supervision of this work throughout the entire process, critically reviewed the manuscript, and suggested/made relevant changes before submission; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: Writing of this paper was supported for Dr Kuo by the National Institute of Mental Health of the National Institutes of Health under K01 MH096646 and L30 MH098313; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This research was not directly funded. Ms Phillips receives funding from the National Health Scholarships Programme from the South African Medical Research Council toward her PhD studies for 2015 to 2016. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Wachsler-Felder JL, Golden CJ. Neuropsychological consequences of HIV in children: a review of current literature. Clin Psychol Rev. 2002;22(3):443–464 [DOI] [PubMed] [Google Scholar]
- 2.Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11(1):1–9 [DOI] [PubMed] [Google Scholar]
- 3.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS. 2013;8(3):165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith R, Chernoff M, Williams PL, et al. ; Pediatric HIV/AIDS Cohort Study (PHACS) Team . Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31(6):592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donald KA, Hoare J, Eley B, Wilmshurst JM. Neurologic complications of pediatric human immunodeficiency virus: implications for clinical practice and management challenges in the African setting. Semin Pediatr Nerol. 2014;21(1):3–11 [DOI] [PubMed] [Google Scholar]
- 6.Hoare J, Fouche J-P, Spottiswoode B, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naïve “slow progressors”. J Neurovirol. 2012;18(3):205–212 [DOI] [PubMed] [Google Scholar]
- 7.Hoare J, Fouche JP, Phillips N, et al. Clinical associations of white matter damage in cART-treated HIV-positive children in South Africa. J Neurovirol. 2015;21(2):120–128 [DOI] [PubMed] [Google Scholar]
- 8.Blokhuis C, Kootstra N, Caan M, Pajkrt D. Neurodevelopmental delay in pediatric HIV/AIDS: current perspectives. Neurobehav HIV Med. 2016;7:1–13 [Google Scholar]
- 9.Cohen S, Caan MW, Mutsaerts H-J, et al. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86(1):19–27 [DOI] [PubMed] [Google Scholar]
- 10.Van Dalen YW, Blokhuis C, Cohen S, et al. Neurometabolite alterations associated with cognitive performance in perinatally HIV-infected children. Medicine (Baltimore). 2016;95(12):e3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux M-J; French Pediatric HIV Infection Study and the SEROCO Group . HIV-1-related encephalopathy in infants compared with children and adults. Neurology. 2000;54(5):1089–1095 [DOI] [PubMed] [Google Scholar]
- 12.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16(1):18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(4):b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006;2006:359–363 [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122(1). Available at: www.pediatrics.org/cgi/content/full/122/1/e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr SJ, Puthanakit T, Vibol U, et al. ; SEARCH 012 Study Team . Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014;26(11):1327–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 The Cochrane Collaboration; 2011. Available at: http://handbook.cochrane.org [Google Scholar]
- 18.Deeks JJ, Dinnes J, D’Amico R, et al. ; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group . Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x, 1–173 [DOI] [PubMed] [Google Scholar]
- 19.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelini L, Zibordi F, Triulzi F, et al. Age-dependent neurologic manifestations of HIV infection in childhood. Neurol Sci. 2000;21(3):135–142 [DOI] [PubMed] [Google Scholar]
- 21.Boivin MJ, Busman RA, Parikh SM, et al. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24(5):667–673 [DOI] [PubMed] [Google Scholar]
- 22.Boivin MJ, Ruel TD, Boal HE, et al. HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS. 2010;24(8):1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S, Ter Stege JA, Geurtsen GJ, et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin Infect Dis. 2015;60(7):1111–1119 [DOI] [PubMed] [Google Scholar]
- 24.Franklin S, Lim HJ, Rennie KM, Eastwood D, Cuene B, Havens PL. Longitudinal intellectual assessment of children with HIV infection. J Clin Psychol Med Settings. 2005;12(4):367–376 [Google Scholar]
- 25.Fundarò C, Miccinesi N, Baldieri NF, Genovese O, Rendeli C, Segni G. Cognitive impairment in school-age children with asymptomatic HIV infection. AIDS Patient Care STDS. 1998;12(2):135–140 [DOI] [PubMed] [Google Scholar]
- 26.Kandawasvika GQ, Kuona P, Chandiwana P, et al. The burden and predictors of cognitive impairment among 6- to 8-year-old children infected and uninfected with HIV from Harare, Zimbabwe: a cross-sectional study. Child Neuropsychol. 2015;21(1):106–120 [DOI] [PubMed] [Google Scholar]
- 27.Keller MA, Venkatraman TN, Thomas A, et al. Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology. 2004;62(10):1810–1817 [DOI] [PubMed] [Google Scholar]
- 28.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12(4):290–297 [DOI] [PubMed] [Google Scholar]
- 29.Llorente AM, Brouwers P, Leighty R, et al. An analysis of select emerging executive skills in perinatally HIV-1-infected children. Appl Neuropsychol Child. 2014;3(1):10–25 [DOI] [PubMed] [Google Scholar]
- 30.Llorente AM, Turcich M, Lawrence KA. Differences in neuropsychological performance associated with ethnicity in children with HIV-1 infection: preliminary findings. Appl Neuropsychol. 2004;11(1):47–53 [DOI] [PubMed] [Google Scholar]
- 31.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART). Dev Neuropsychol. 2006;30(2):633–657 [DOI] [PubMed] [Google Scholar]
- 32.Nichols SL, Chernoff MC, Malee K, et al. ; Memory and Executive Functioning Substudy of the Pediatric HIVAIDS Cohort Study . Learning and memory in children and adolescents with perinatal HIV infection and perinatal HIV exposure. Pediatr Infect Dis J. 2016;35(6):649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nozyce ML, Lee SS, Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117(3):763–770 [DOI] [PubMed] [Google Scholar]
- 34.Puthanakit T, Ananworanich J, Vonthanak S, et al. ; PREDICT Study Group . Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. Pediatr Infect Dis J. 2013;32(5):501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravindran OS, Rani MP, Priya G. Cognitive deficits in HIV infected children. Indian J Psychol Med. 2014;36(3):255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice ML, Buchanan AL, Siberry GK, et al. Language impairment in children perinatally infected with HIV compared to children who were HIV-exposed and uninfected. J Dev Behav Pediatr. 2012;33(2):112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54(7):1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker SY, Pierre RB, Christie CD, Chang SM. Neurocognitive function in HIV-positive children in a developing country. Int J Infect Dis. 2013;17(10):e862–e867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coe R. It’s the effect size, stupid: what effect size is and why it is important. Paper presented at: British Educational Research Association Annual Conference; September 12–14, 2002; Exeter, United Kingdom [Google Scholar]