Abstract
BACKGROUND AND OBJECTIVES:
Compared with term infants, preterm infants have impaired brain development at term-equivalent age, even in the absence of structural brain injury. However, details regarding the onset and progression of impaired preterm brain development over the third trimester are unknown. Our primary objective was to compare third-trimester brain volumes and brain growth trajectories in ex utero preterm infants without structural brain injury and in healthy in utero fetuses. As a secondary objective, we examined risk factors associated with brain volumes in preterm infants over the third-trimester postconception.
METHODS:
Preterm infants born before 32 weeks of gestational age (GA) and weighing <1500 g with no evidence of structural brain injury on conventional MRI and healthy pregnant women were prospectively recruited. Anatomic T2-weighted brain images of preterm infants and healthy fetuses were parcellated into the following regions: cerebrum, cerebellum, brainstem, and intracranial cavity.
RESULTS:
We studied 205 participants (75 preterm infants and 130 healthy control fetuses) between 27 and 39 weeks’ GA. Third-trimester brain volumes were reduced and brain growth trajectories were slower in the ex utero preterm group compared with the in utero healthy fetuses in the cerebrum, cerebellum, brainstem, and intracranial cavity. Clinical risk factors associated with reduced brain volumes included dexamethasone treatment, the presence of extra-axial blood on brain MRI, confirmed sepsis, and duration of oxygen support.
CONCLUSIONS:
These preterm infants exhibited impaired third-trimester global and regional brain growth in the absence of cerebral/cerebellar parenchymal injury detected by using conventional MRI.
What’s Known on This Subject:
Third-trimester brain development is characterized by critical and energy-dependent biological processes needed to support optimal brain growth. Available evidence points to disturbed brain development in ex-preterm infants at term and beyond compared with their term-born peers.
What This Study Adds:
Third-trimester brain growth is disturbed in preterm infants compared with healthy in utero fetuses in the absence of structural brain injury. Neonatal intensive care likely influences third-trimester brain development in preterm infants.
Despite marked improvements in neonatal intensive care, life-long neurodevelopmental disabilities remain highly prevalent in survivors of prematurity.1 Brain injury is a common complication of preterm birth and is diagnosed by using conventional neuroimaging techniques in about one-third of preterm infants.2 However, the presence of structural brain injury alone does not account for the frequency and scope of neurodevelopmental impairments in surviving preterm infants.3 Brain development can be altered even without evidence of destructive prematurity-related brain injury.4 Multiple risk factors have also been implicated with aberrant preterm brain maturation, including infection, medication administration, and respiratory complications.5 However, the timing, progression, and functional impact of delayed brain maturation in the absence of structural brain injury remain poorly understood.
A growing body of evidence has shown that brain development is impaired in preterm infants by term-equivalent age (TEA) even in the absence of structural brain injury: compared with healthy term newborns, preterm infants at TEA exhibit decreased cerebral and cerebellar volume, altered cortical surface area,6–9 and microstructural organization,10 as well as impaired functional connectivity.11,12 To date, most inferences about third-trimester brain development in preterm infants have been based on comparative quantitative MRI measures versus those of term healthy newborns at a single time point at the end of the third trimester (ie, TEA). However, these cross-sectional studies at the end of the third trimester were not designed to identify the onset and evolution of impaired brain development in the premature infant. Few studies have investigated the third-trimester brain maturation in the absence of brain injury after early exposure to extrauterine life.13–17 Moreover, another substantial obstacle to progress has been the scarcity of in utero normative fetal MRI studies from which to establish and compare departures from normal third-trimester ex utero brain development.
Recently, we and others have successfully applied advanced quantitative MRI techniques to study in utero brain development in healthy fetuses.18–22 These studies have provided major insights into the rate and progression of normal second- and third-trimester global, regional, and tissue-specific brain development in utero. However, no study to date has prospectively compared in utero brain volume in healthy fetuses versus that of ex utero preterm infants. The aim of the present study was to compare third-trimester global and regional brain volumes and brain growth trajectories in very preterm infants with no parenchymal brain injury versus healthy in utero fetuses by using 3-dimensional volumetric MRI measures. As a secondary objective, we sought to examine the relationship between clinical risk factors and brain volumes in preterm infants.
Methods
Participants
We studied preterm infants and in utero healthy control fetuses recruited prospectively from longitudinal observational studies performed at 2 medical centers: Children’s Boston Hospital (Boston, MA) and Children’s National Health System (Washington, DC). The design of the study was cross-sectional, and it included only a single observation for each participant.
Preterm Cohort
Very preterm infants born before 32 weeks’ gestational age (GA) weighing <1500 g were prospectively enrolled. We specifically excluded any preterm infants with known or suspected chromosomal anomalies, congenital malformations, central nervous system infection, and metabolic disorders. Also excluded were preterm infants with any evidence of parenchymal cerebral or cerebellar injury (ie, white matter injury, grade III–IV intraventricular hemorrhage [IVH], cerebellar hemorrhage) according to conventional MRI.
Fetal Cohort
Healthy pregnant women with normal fetal ultrasounds were recruited as normal control subjects in a prospective study comparing brain development in fetuses with congenital heart disease.23–25 We excluded multiple pregnancies, chromosomal abnormalities, and congenital infection.
The studies were approved by the 2 institutional review boards, and written informed consent was obtained from each participant.
MRI Acquisition
Preterm Cohort
Preterm newborns were scanned under natural sleep by using either a 1.5-T MRI scanner Signa Excite, a 1.5-T Discovery MR450 scanner, or a 3-T Discovery MR750 (all: GE Healthcare, Milwaukee, MI). Preterm infants requiring temperature monitoring underwent scanning by using a Nomag MRI-compatible incubator (LMT Medical Systems GmbH, Luebeck, Germany).
Fetal Cohort
Fetal MRI studies were performed at 1.5 T either on an Achieva scanner (Philips Medical System, Best, Netherlands) or on a Discovery MR450 scanner (GE Healthcare).
For both cohorts, comparable sagittal, axial, and coronal T2-weighted images were collected by using single-shot fast spin echo sequences (2-mm slice thickness, 0-mm gap). An experienced pediatric neuroradiologist at each site reviewed all the MRI studies and noted the presence or absence of extra-axial blood.
Volumetric Analyses
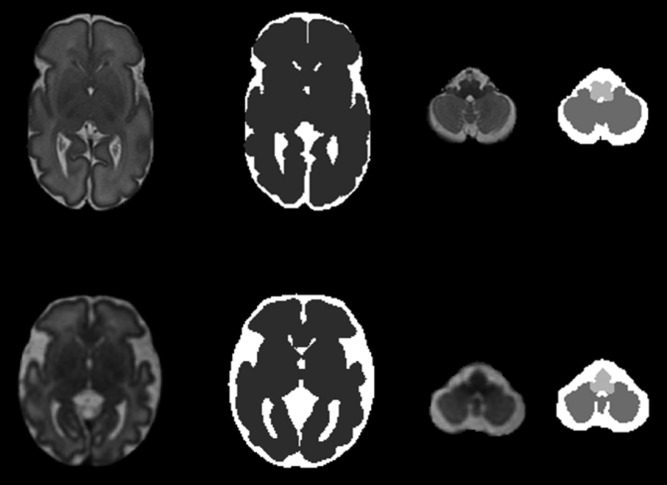
After visual quality inspection of the anatomic images acquired, images without motion artifacts were selected and corrected for intensity nonhomogeneity.26 For both cohorts, we reconstructed 3-dimensional images of the brain from the 2-dimensional images acquired27 and used a parcellation pipeline18 to delineate the brain regions of interest. The cerebrum, cerebellum, and brainstem were then manually corrected when necessary by using ITK-SNAP software.28 Figure 1 provides an example of the brain parcellation performed in preterm infants and in utero fetuses. Volumes were computed by multiplying the number of voxels included in each brain region by the volume of each voxel and are expressed in cubic centimeters (milliliters). Total brain volume (TBV) was defined as the sum of cerebral, cerebellar, and brainstem volumes; the intracranial cavity volume (ICV) was the sum of TBV and cerebrospinal fluid.
FIGURE 1.
Parcellation of preterm (top row) and fetal (lower row) brain. Anatomic T2-weighted images and corresponding parcellation maps in a preterm infant at 31 4/7 weeks GA (top row) and a fetus at 32 2/7 weeks’ GA (lower row). Dark gray: cerebrum; intermediate gray: cerebellum; light gray: brainstem; and white: cerebrospinal fluid.
Prenatal and Postnatal Clinical Data Collection
Prenatal information, mode of delivery, birth weight, GA at birth, Apgar score, and sex were collected for both cohorts. Additional clinical risk factors abstracted in the preterm cohort included length of mechanical ventilation and oxygen support, need for patent ductus arteriosus (PDA) ligation, sepsis (ie, confirmed sepsis by positive blood culture), postnatal dexamethasone treatment, and the presence of extra-axial blood on conventional MRI.
Statistical Analysis
Descriptive statistics were summarized by using median, range, means, and SDs for continuous variables and proportions for categorical factors. Linear regression models were developed by using Stata 13 (Stata Corp, College Station, TX) to evaluate the relationship between global and regional measurements of brain volume according to GA at MRI in healthy fetuses and preterm newborns. During model development, we evaluated the need for cross-product terms to allow for differing growth trajectories in the 2 groups and for higher order effects to introduce curvilinearity if necessary to improve model fit.
Within the preterm cohort, linear regression analysis was first used to evaluate the relationship between the presence/level of each clinical risk factor individually (ie, chorioamnionitis, cesarean delivery, PDA ligation, sepsis, dexamethasone treatment, number of days ventilated /on oxygen support, presence of extra-axial blood) and brain volume controlling for GA at birth, day of life at MRI, and sex. We chose to control for GA at birth rather than birth weight in the models because the 2 factors were highly correlated (r = 0.74) and because GA was judged less likely to interfere with risk factor identification. Results were expressed as regression (slope) coefficients with 95% confidence intervals (CIs). We then performed a multiple regression analysis that evaluated the combined main and interactive effects of each of the clinical risk factors associated with differences in global or regional brain volumes. Only those 2- and 3-way interactions that achieved at least a borderline level of statistical significance (P < .12) were retained in final models; the decision on which of the alternative models to use to represent the combined impact of risk factors on brain growth was aided by the use of Akaike information criterion statistics.29,30 We consider these analyses hypothesis-generating and thus did not correct for multiple comparisons, and we were more lenient in choosing terms to include in the model to avoid missing the identification of potentially important risk factors.
Results
Clinical Characteristics of the Cohort
A total of 205 subjects were studied: 75 very preterm infants (birth GA: 22–32 weeks) with a mean corrected GA at MRI of 34 ± 2.5 weeks (range: 27–39 weeks) and 130 healthy fetuses with a mean GA at MRI of 32 ± 3.3 weeks (range: 27–39 weeks). All MRI scans in the preterm and fetal cohorts were structurally normal (ie, had no parenchymal brain injury/malformations). The clinical characteristics of the cohort are summarized in Table 1.
TABLE 1.
Clinical Characteristics of the Cohort
| Characteristic | Preterm Cohort (n = 75) | Fetal Cohort (n = 130) | P |
|---|---|---|---|
| GA at MRI, wk | .007 | ||
| Mean ± SD | 33.95 ± 2.53 | 32.1 ± 3.27 | |
| Range | 27–39 | 27–39 | |
| Female, n (%) | 39 (52) | 64 (50) | .74 |
| Maternal age, y | .93 | ||
| Mean ± SD | 29.4 ± 6.3 | 29.5 ± 5.8 | |
| Range | 15–41 | 18–43 | |
| Maternal highest level of education, n (%) | .02 | ||
| Less than seventh grade | 1 (1) | 0 | |
| Junior high school | 2 (3) | 0 | |
| Partial junior high school | 7 (9) | 7 (5) | |
| High school | 14 (19) | 30 (23) | |
| Partial college or specialized training | 12 (16) | 28 (22) | |
| Standard college | 19 (25) | 26 (20) | |
| Graduate professional training | 8 (11) | 39 (30) | |
| Not collected | 12 (16) | 0 | |
| GA at birth, wk | <.001 | ||
| Mean ± SD | 27.2 ± 2.36 | 39.3 ± 1.2a | |
| Range | 22–32 | 36–41.3 | |
| Birth weight, g | <.001 | ||
| Mean ± SD | 947 ± 283 | 3362 ± 432a | |
| Range | 400–1490 | 1953–4310 | |
| Small for GA | 10 (13) | 7 (5)a | .14 |
| Vaginal delivery, n (%) | 24 (32) | 78 (68)a | <.001 |
| Apgar score at 5 min, median (range) | 8 (0–9) | 9 (6–10)a | <.001 |
| Ethnicity, n (%) | .003 | ||
| Hispanic | 14 (19) | 13 (10) | |
| White | 30 (40) | 35 (27) | |
| Asian | 0 | 13 (10) | |
| Black | 30 (40) | 61 (47) | |
| Multiethnic | 1 (1) | 8 (6) |
Birth data were not available in 10 participants of the fetal cohort.
Comparisons of Brain Volumes by Site
Table 2 summarizes the brain volumes of the preterm and fetal cohorts by site; the ratio of preterm infants to fetuses was similar at each site. Notably, the few significant differences that were present in the preterm and fetal cohorts at a young age (<33 weeks’ GA) disappeared after controlling for GA at MRI.
TABLE 2.
Brain Volumes of the Preterm and Fetal Cohorts According to Site and Gestational Age
| Variable | Preterm Infants (n = 75) | Fetuses (n = 130) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boston (n = 37 [50%]) | Boston (n = 55 [42%]) | |||||||||||
| Washington DC (n = 38 [50%]) | Washington DC (n = 75 [58%]) | |||||||||||
| Boston (n = 10) | DC (n = 17) | P | Boston (n = 27) | DC (n = 21) | P | Boston (n = 40) | DC (n = 35) | P | Boston (n = 15) | DC (n = 40) | P | |
| GA at MRI, wk | 30.47 ± 1.8 [27–30.9] | 31.92 ± 0.8 [30.6–32.9] | .04 | 35.61 ± 1.5 [33.1–39] | 35.4 ± 1.3 [33.3–37.7] | .61 | 28.96 ± 1.4 [27–32.42] | 30.44 ± 1.7 [27–32.86] | .001 | 34.73 ± 1 [33–36.57] | 35.56 ± 1.7 [33–39] | .03 |
| Cerebrum, mL | 160.3 ± 27 [123–205] | 163.2 ± 20 [113–199] | .76 | 203.4 ± 40 [124–276] | 195.5 ± 45 [113–289] | .83 | 138.1 ± 23 [100–290] | 156.3 ± 35 [88–231] | .01* | 248.4 ± 22 [206–280] | 237.6 ± 33 [170–315] | .25 |
| Cerebellum, mL | 7.3 ± 1.8 [4–9.4] | 7.77 ± 1.6 [5–11] | .48 | 12.61 ± 2.2 [8–16] | 11.91 ± 3.2 [7–18] | .38 | 6.94 ± 1.6 [4–11] | 8.13 ± 2.2 [4–12] | .01* | 14.17 ± 1.6 [4–18] | 13.91 ± 2.7 [8–20] | .66 |
| Brainstem, mL | 3.5 ± 0.4 [3–4] | 3.2 ± 0.4 [3–4] | .04* | 4.3 ± 0.5 [3–6] | 4.4 ± 0.8 [3–6] | .7 | 2.9 ± 0.4 [2–4] | 3.1 ± 0.6 [2–5] | .12 | 4.7 ± 0.5 [4–6] | 4.54 ± 0.7 [4–6] | .33 |
| Total brain volume, mL | 171.1 ± 29 [131–218] | 174.16 ± 22 [122–214] | .76 | 236.2 ± 34 [168–296] | 237.9 ± 47 [169–311] | .88 | 148 ± 24 [106–205] | 167.6 ± 37 [93–247] | .01* | 267.3 ± 23 [222–299] | 256.1 ± 36 [183–341] | .27 |
| Intracranial cavity volume, mL | 225.8 ± 37 [175–283] | 241.8 ± 31 [179–294] | .24 | 315.37 ± 48 [228–413] | 324.64 ± 56 [238–441] | .55 | 262.7 ± 43 [190–372] | 284.1 ± 56 [179–398] | .067 | 418.2 ± 32 [360–460] | 394.8 ± 47 [303–495] | .08 |
Differences are not significant when controlling for gestational age at MRI.
Comparisons Between Fetal and Preterm Brain Volumes
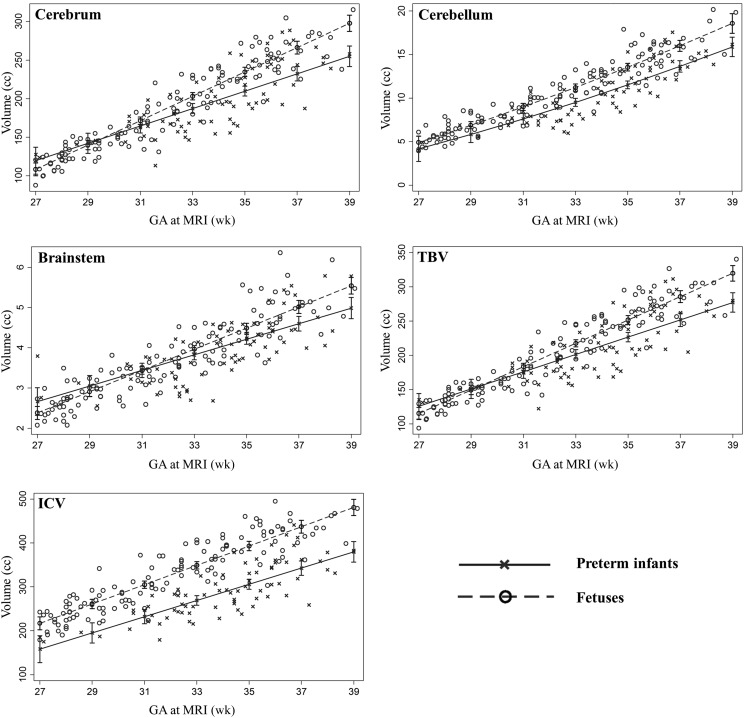
Controlling for GA at MRI and sex, brain volumes were lower in preterm infants compared with in utero fetuses for all the brain regions examined (P < .05) (Fig 2). Third-trimester brain growth trajectories by GA at MRI were slower in the ex utero preterm cohort compared with the fetal cohort for the cerebrum (P = .001), the cerebellum (P = .05), the brainstem (P = .06), TBV (P = .002), and ICV (P = .03).
FIGURE 2.
Brain volumes plotted against GA at MRI in the preterm and fetal cohorts (controlling for sex).
Preterm Infants: Clinical Risk Factors and Impaired Brain Volume
The clinical risk factors were similar in the 2 preterm cohorts except for length of ventilation (Table 3). The statistically significant relationships between individual clinical risk factors and regional brain volume when controlling for GA at birth, day of life at MRI, and sex are summarized in Table 4. Dexamethasone treatment, the presence of extra-axial blood, sepsis, and length of oxygen support were associated with reduced brain volumes. We then included the aforementioned risk factors in a multiple regression model analysis (Table 5). The 3-way interaction effect among dexamethasone treatment, extra-axial blood, and length of oxygen support explained >75% of the reduction in cerebral/cerebellar volumes, TBV, and ICV. In addition, sepsis was significantly associated with reductions in cerebral volume, TBV, and ICV.
TABLE 3.
Clinical Risk Factors
| Variable | All Preterm Infants (n = 75) | Preterm Cohort From Boston (n = 37) | Preterm Cohort From Washington, DC (n = 38) | P |
|---|---|---|---|---|
| Chorioamnionitis | 15 (20) | 4 (11) | 11 (28) | .05 |
| Cesarean delivery | 51 (68) | 25 (49) | 26 (51) | .94 |
| Dexamethasone | 12 (16) | 5 (14) | 7 (18) | .56 |
| PDA ligation | 14 (19) | 8 (22) | 6 (16) | .52 |
| Sepsis | 11 (15) | 8 (22) | 3 (8) | .09 |
| Extra-axial blood | 16 (21) | 9 (24) | 7 (18) | .53 |
| Length of mechanical ventilation, d | 19.87 ± 23.7 [0–91] | 28.1 ± 24.9 [1–81] | 12 ± 19.5 [0–80] | .002 |
| Length of oxygen support, d | 55.9 ± 35.3 [2–144] | 60.5 ± 33.2 [3–122] | 51.4 ± 37.2 [2–142] | .27 |
Data are presented as n (%) or mean ± SD [range].
TABLE 4.
Relationship Between Brain Volumes and Individual Clinical Risk Factors in the Preterm Cohort (n = 75)
| Variable | Cerebrum | Cerebellum | Brainstem | TBV | ICV |
|---|---|---|---|---|---|
| Chorioamnionitis | — | — | — | — | — |
| Cesarean delivery | −0.07 | — | — | −0.07 | — |
| [–0.1; –9 × 10−3] | [–0.1; –6 ×10−3] | ||||
| (.03) | (.03) | ||||
| Dexamethasone | −0.128 | −0.167 | −0.66 | −0.13 | −38.87 |
| [–0.22; –0.04] | [–0.3; –0.05] | [–1; –0.3] | [–0.2;–0.05] | [–60; –18] | |
| (.004) | (.008) | (<.001) | (.003) | (<.001) | |
| PDA ligation | — | — | — | — | — |
| Sepsis | −0.075 | — | — | −0.072 | −29.25 |
| [–0.15; –3 × 10−3] | [–0.15; –0.001] | [–52;–6.5] | |||
| (.04) | (.05) | (.01) | |||
| Extra-axial blood | −0.093 | −0.188 | — | −0.95 | −27.3 |
| [–0.15; –0.04] | [–0.29; –0.09] | [–0.15; –0.04] | [–44.5; –10.1] | ||
| (.002) | (<.001) | (.002) | (.002) | ||
| Length of mechanical ventilation, d | — | — | — | — | — |
| Length of oxygen support, d | −0.002 | −0.002 | — | −0.001 | — |
| [–3 × 10−3; –3 x 10−4] | [–4 × 10−3; –7 × 105] | [–3 × 10−3; –4 × 104] | |||
| (.02) | (.04) | (.02) |
Regression coefficient [95% CI] in milliliters and corresponding (P value) of the clinical risk factor of interest controlling for GA at birth, age at MRI (days), and sex. Em dashes indicate the absence of a significant relationship between a given risk factor and brain volume.
TABLE 5.
Relationship Between Brain Volumes and Combined Clinical Risk Factors in the Preterm Cohort (n = 75)
| Brain Areas | Modela | Percentage of Variance Explained |
|---|---|---|
| Cerebrum | Dexamethasone | Extra-axial blood | Length of oxygen support | Sepsis | 78 |
| Cerebellum | Dexamethasone | Extra-axial blood | Length of oxygen support | 85 |
| Brainstem | Dexamethasone | 60 |
| Total brain volume | Dexamethasone | Extra-axial blood | Length of oxygen support | Sepsis | 79 |
| Intracranial cavity volume | Dexamethasone | Extra-axial blood | Length of oxygen support | Sepsis | 77 |
The estimate of variance explained includes the main effects as well as 2- and 3-way interactions (not shown); all models had a P value < .001.
Discussion
To our knowledge, our study reports for the first time decreased third-trimester global and regional brain growth in very preterm infants without evidence of cerebral or cerebellar parenchymal injury compared with healthy in utero control fetuses. Using volumetric MRI, we found differences in brain growth trajectories between preterm infants who experience their “third trimester” of development ex utero and in utero healthy fetuses. Our findings suggest that even in the absence of structural brain injury, the developmental trajectory of the preterm brain is altered over the third trimester. Evidence of clinical risk factors associated with reduced brain volumes in preterm infants included individual and synergistic effects of dexamethasone treatment, the presence of extra-axial blood, the length of oxygen support, and confirmed sepsis.
Previous studies suggest that brain volumes in preterm survivors are altered by TEA compared with healthy term newborns and are characterized by decreased parenchymal volumes.9,31–35 Alterations in brain volumes at TEA in the preterm infants have also been correlated with worse neurocognitive outcomes.36–38 Moreover, prematurely born children and adults exhibit altered regional volumetric brain growth compared with their term-born peers39,40 suggesting long-lasting consequences of early-life disturbances in brain growth.41 However, to date, few studies have investigated third-trimester volumetric brain development in preterm infants and healthy in utero fetuses. Only 1 recent study (by Lefèvre et al42) used a retrospective cross-sectional design to compare 27 preterm infants (birth GA: 25–35 weeks) and 14 fetuses; the investigators found that brain volumes were similar, whereas cortical folding trajectories were altered in preterm infants compared with healthy control fetuses. Our data showed reductions in brain volumes across the third trimester of ex utero life in preterm infants compared with the in utero healthy control fetuses. Our larger sample size (N = 205) suggest that we were better-powered to detect subtle but important differences in regional brain growth. The present results extend earlier findings of altered brain growth in preterm infants by TEA9,32 and demonstrate a progressive fall-off/dysmaturation of the brain after preterm birth over the third trimester.
The third trimester of gestation is a critical period of prolific brain development. Multiple biological processes are actively taking place, including the beginning of myelination, neuronal organization, spinogenesis, and synaptogenesis.43 It is well established that there is a fourfold increase in brain size during the third trimester of gestation18 accompanied by a dramatic increase in brain surface area with the formation of tertiary sulci and gyri.44 This well-orchestrated and precisely timed development of the neural circuitry may be vulnerable to insults associated with preterm birth. Longitudinal studies have explored the relationship between clinical risk factors and neurodevelopmental outcomes in preterm born infants, including postnatal infections,45–47 medications,48,49 and bronchopulmonary dysplasia.50 At TEA, several clinical risk factors have been associated with decreased brain volume, including white matter brain injury,31,34,35 respiratory illness,31,34 small birth weight for GA,34,35 increasing prematurity,9 and low-grade IVH.9,51 Our study extends these observations and shows that among the clinical risk factors examined, postnatal dexamethasone treatment, the presence of extra-axial blood, respiratory illness, and sepsis were inversely associated with brain growth in preterm infants during the third trimester.
We report an association between dexamethasone treatment and impaired cerebral, cerebellar, and brainstem growth during the third trimester. Our findings are in keeping with previously published research suggesting a detrimental effect of dexamethasone treatment on preterm brain volumes at TEA52,53 and during adolescence.54 Animal models have shown that dexamethasone administration during the critical developmental period is associated with abnormal apoptosis and alterations in synaptic function.55,56 Respiratory distress in the preterm infants results in alterations in cerebral hemodynamics that can also lead to impaired brain development.57 In our study, indicators of respiratory illness such as the length of oxygen support were inversely associated with third-trimester brain volumes in preterm infants, consistent with previous findings reported at TEA.9,31,34
Prenatal, intrapartum, and/or postnatal infections result in inflammatory substances that have been linked to both brain injury and altered brain development in the preterm population.58,59 In this study, we also report an association between confirmed sepsis and stunted cerebral growth. This specific finding contrasts with 2 other studies, which found no relationship between brain volumes and sepsis in preterm infants at TEA.9,31 The severity, time, and occurrence of sepsis might play a role in the varied findings. Longitudinal data sets will allow elucidating whether there is a catch-up in brain growth by TEA in preterm infants with confirmed sepsis. A large, longitudinal population-based study has shown a negative independent effect of sepsis on neurodevelopmental impairments at 2 years of age.46 Conversely, we found no relationship between chorioamnionitis and third-trimester brain volume, which is in agreement with previous studies.35,60 Chorioamnionitis has been associated with an increased risk for developing brain injury,61,62 and it has been especially correlated with severe IVH.63,64 The direct influence of chorioamnionitis on neurodevelopmental outcome is unclear, and it is likely mediated by the increased risk of postnatal morbidities associated with prenatal infection, including brain injury and neonatal sepsis.61,65–67
The presence of extra-axial blood in our preterm cohort was linked to decreased brain volumes. Low-grade IVH has previously been related to decreased brain volumes in near-term and preterm infants at TEA.9,51 Potential mechanisms include free radical injury mediated by extra-axial blood and IVH, which adversely affects the proliferating cells and leads to subsequent downstream events in cerebral and cerebellar development.68–70 Additional studies are needed to quantify the effects of blood on the developing preterm brain.
Surprisingly, the need for surgical PDA ligation was not associated with impaired brain volumes over the third trimester in the ex utero preterm infants in our study. This finding conflicts with previous literature in which PDA ligation was associated with reduced brain volumes at term age,8,9 but it is in line with a recently published study. Lemmers et al71 postulated that the duration of reduced cerebral oxygenation associated with PDA is likely the main factor responsible for brain growth impairment associated with PDA ligation rather than the need for surgery itself. Ongoing research is warranted to address this intriguing question.
Our study provides new insights into the role of prematurity-related clinical risk factors on brain maturation during the third trimester in ex utero preterm infants with no evidence of parenchymal brain injury. Ongoing prospective studies are urgently needed to better ascertain what aspects of neonatal intensive care may be harmful versus protective for the developing preterm brain.72
Our study has several limitations. First, the design of the study was cross-sectional; thus, the brain growth trajectories examined are population based (ie, the trajectories are computed from a cohort with a single observation rather than from individuals with longitudinal MRI data). Serial MRI scans would allow for better delineation of third-trimester longitudinal brain growth in normal in utero versus ex utero environments, and study of this topic is currently underway. Second, we combined participants from 2 regionally distinct cohorts, introducing differences in the MRI scanners used and variability in clinical care. However, all participants were scanned with comparable T2-weighted anatomic sequences, and we found no significant difference after controlling for GA at MRI in brain volumes in both fetal and preterm cohorts performed at the 2 sites. Furthermore, the clinical risk factors were similar in the 2 preterm cohorts. The third limitation relates to our sample size that restricted the number of clinical risk factors we could investigate. We decided to include in our multivariate analysis only the clinical risk factors that were correlated with brain volumes on our univariate analysis. Other statistical approaches could lead to different models; consequently, our results are only hypothesis-generating and need to be evaluated and confirmed by other studies. Moreover, our findings are not brain tissue specific. A segmentation pipeline applied to this data set could provide additional information about different tissue types (ie, cortical gray versus white matter) that may be preferentially affected by preterm birth. Finally, although we specifically excluded preterm infants with evidence of structural brain injury on conventional MRI, it is possible that smaller brain lesions below the current resolution of clinical MRI field strength were missed.73 Specifically, we cannot exclude the presence of white matter microscopic necrosis, which can only be resolved by using a higher MRI field strength (eg, 12 T).74
Conclusions
Our data strongly support the notion that preterm delivery is associated with disturbances in third-trimester global and regional brain growth even in the absence of parenchymal brain injury. Clinical risk factors associated with preterm birth and impaired brain development include the use of steroids, greater respiratory illness, sepsis, and the presence of extra-axial hemorrhage. Ongoing studies are needed to clarify the effects of altered third-trimester regional brain growth on neurodevelopmental outcome.
Acknowledgments
The authors are grateful to the families who participated in the study.
Glossary
- GA
gestational age
- ICV
intracranial cavity volume
- IVH
intraventricular hemorrhage
- PDA
patent ductus arteriosus
- TBV
total brain volume
- TEA
term-equivalent age
Footnotes
Ms Bouyssi-Kobar coordinated and supervised data collection at 1 site, conducted the MRI processing of the data, analyzed the results, and drafted the initial manuscript; Dr du Plessis co-conceptualized and designed the study, and critically reviewed the manuscript; Dr McCarter determined and performed the statistical analysis and contributed to the writing of the manuscript; Dr Brossard-Racine coordinated and supervised data collection and MRI studies at 1 site, contributed to the analysis of the data, and critically reviewed the manuscript; Drs Murnick and Robertson reviewed the MRI studies at 1 site and critically reviewed the manuscript; Ms Tinkleman contributed to the analysis of the MRI data and critically reviewed the manuscript; Dr Limperopoulos co-conceptualized and designed the study, coordinated and supervised the progress of the study at the 2 sites, analyzed the results, and contributed to the writing of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by the Canadian Institutes of Health Research (MOP-81116), the SickKids Foundation (XG 06-069), and the National Institutes of Health (UL1TR000075 and P30 HD040677). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Blencowe H, Lee AC, Cousens S, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013;74(suppl 1):17–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/133/2/e444 [DOI] [PubMed] [Google Scholar]
- 3.Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32(9):496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortinau C, Neil J. The neuroanatomy of prematurity: normal brain development and the impact of preterm birth. Clin Anat. 2015;28(2):168–183 [DOI] [PubMed] [Google Scholar]
- 5.Bennet L, Van Den Heuij L, Dean JM, Drury P, Wassink G, Gunn AJ. Neural plasticity and the Kennard principle: does it work for the preterm brain? Clin Exp Pharmacol Physiol. 2013;40(11):774–784 [DOI] [PubMed] [Google Scholar]
- 6.Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356(9236):1162–1163 [DOI] [PubMed] [Google Scholar]
- 7.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med 2006;3(8):e265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115(3):688–695 [DOI] [PubMed] [Google Scholar]
- 9.Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Ådén U. Brain growth gains and losses in extremely preterm infants at term. Cereb Cortex. 2015;25(7):1897–1905 [DOI] [PubMed] [Google Scholar]
- 10.Ball G, Srinivasan L, Aljabar P, et al. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci USA. 2013;110(23):9541–9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb Cortex. 2016;26(1):322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheinost D, Kwon SH, Shen X, et al. Preterm birth alters neonatal, functional rich club organization. Brain Struct Funct. 2016;221(6):3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131(pt 8):2028–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viola A, Confort-Gouny S, Schneider JF, et al. Is brain maturation comparable in fetuses and premature neonates at term equivalent age? AJNR Am J Neuroradiol. 2011;32(8):1451–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nossin-Manor R, Card D, Raybaud C, Taylor MJ, Sled JG. Cerebral maturation in the early preterm period—a magnetization transfer and diffusion tensor imaging study using voxel-based analysis. Neuroimage. 2015;112(C):30–42 [DOI] [PubMed] [Google Scholar]
- 16.Schneider J, Kober T, Bickle Graz M, et al. Evolution of T1 relaxation, ADC, and fractional anisotropy during early brain maturation: a serial imaging study on preterm infants. AJNR Am J Neuroradiol. 2016;37(1):155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makropoulos A, Aljabar P, Wright R, et al. Regional growth and atlasing of the developing human brain. Neuroimage. 2016;125(C):456–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouchoux C, Guizard N, Evans AC, Plessis du AJ, Limperopoulos C. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol 2012;206(2):173.e1–8 [DOI] [PubMed] [Google Scholar]
- 19.Clouchoux C, Kudelski D, Gholipour A, et al. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct. 2012;217(1):127–139 [DOI] [PubMed] [Google Scholar]
- 20.Habas PA, Scott JA, Roosta A, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 2012;22(1):13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Awate SP, Licht DJ, et al. Assessment of MRI-based automated fetal cerebral cortical folding measures in prediction of gestational age in the third trimester. AJNR Am J Neuroradiol. 2015;36(7):1369–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright R, Kyriakopoulou V, Ledig C, et al. Automatic quantification of normal cortical folding patterns from fetal brain MRI. Neuroimage. 2014;91(C):21–32 [DOI] [PubMed] [Google Scholar]
- 23.Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2013;23(12):2932–2943 [DOI] [PubMed] [Google Scholar]
- 25.Brossard-Racine M, du Plessis AJ, Vezina G, et al. Prevalence and spectrum of in utero structural brain abnormalities in fetuses with complex congenital heart disease. AJNR Am J Neuroradiol. 2014;35(8):1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gholipour A, Estroff JA, Barnewolt CE, Connolly SA, Warfield SK. Fetal brain volumetry through MRI volumetric reconstruction and segmentation. Int J CARS. 2011;6(3):329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128 [DOI] [PubMed] [Google Scholar]
- 29.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Selected Papers of Hirotugu Akaike. Springer Series in Statistics. New York, NY: Springer New York; 1998:199–213 [Google Scholar]
- 30.Akaike H. Likelihood of a model and information criteria. J Econom. 1981;16(1):3–14 [Google Scholar]
- 31.Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294 [DOI] [PubMed] [Google Scholar]
- 32.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5 pt 1):939–948 [DOI] [PubMed] [Google Scholar]
- 33.Mewes AU, Hüppi PS, Als H, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118(1):23–33 [DOI] [PubMed] [Google Scholar]
- 34.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(pt 3):667–677 [DOI] [PubMed] [Google Scholar]
- 35.Parikh NA, Lasky RE, Kennedy KA, McDavid G, Tyson JE. Perinatal factors and regional brain volume abnormalities at term in a cohort of extremely low birth weight infants. PLoS ONE 2013;8(5):e62804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124(6). Available at: www.pediatrics.org/cgi/content/full/124/6/e1161 [DOI] [PubMed] [Google Scholar]
- 37.Keunen K, Išgum I, van Kooij BJ, et al. Brain volumes at term-equivalent age in preterm infants: imaging biomarkers for neurodevelopmental outcome through early school age. J Pediatr. 2016;172(0):88–95 [DOI] [PubMed] [Google Scholar]
- 38.Lind A, Parkkola R, Lehtonen L, et al. ; PIPARI Study Group . Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41(8):953–961 [DOI] [PubMed] [Google Scholar]
- 39.Nosarti C, Nam KW, Walshe M, et al. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin. 2014;6(C):180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheinost D, Kwon SH, Lacadie C, et al. Alterations in Anatomical Covariance in the Prematurely Born. Cereb Cortex. 2015;(October):bhv248–bhv10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjuland KJ, Rimol LM, Løhaugen GCC, Skranes J. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur J Paediatr Neurol. 2014;18(5):578–590 [DOI] [PubMed] [Google Scholar]
- 42.Lefèvre J, Germanaud D, Dubois J, et al. Are developmental trajectories of cortical folding comparable between cross-sectional datasets of fetuses and preterm newborns? Cereb Cortex. 2016;26(7):3023–3035 [DOI] [PubMed] [Google Scholar]
- 43.Kostović I, Jovanov-Milosević N. The development of cerebral connections during the first 20-45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11(6):415–422 [DOI] [PubMed] [Google Scholar]
- 44.Kostovic I, Vasung L. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol. 2009;33(4):220–233 [DOI] [PubMed] [Google Scholar]
- 45.Stoll BJ, Hansen NI, Adams-Chapman I, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 [DOI] [PubMed] [Google Scholar]
- 46.Schlapbach LJ, Aebischer M, Adams M, et al. ; Swiss Neonatal Network and Follow-Up Group . Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128(2). Available at: www.pediatrics.org/cgi/content/full/128/2/e348 [DOI] [PubMed] [Google Scholar]
- 47.Mitha A, Foix-L’Hélias L, Arnaud C, et al. ; EPIPAGE Study Group . Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2). Available at: www.pediatrics.org/cgi/content/full/132/2/e372 [DOI] [PubMed] [Google Scholar]
- 48.Steinhorn R, McPherson C, Anderson PJ, Neil J, Doyle LW, Inder T. Neonatal morphine exposure in very preterm infants-cerebral development and outcomes. J Pediatr. 2015;166(5):1200–1207.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rozé JC, Denizot S, Carbajal R, et al. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Arch Pediatr Adolesc Med. 2008;162(8):728–733 [DOI] [PubMed] [Google Scholar]
- 50.Short EJ, Klein NK, Lewis BA, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112(5). Available at: www.pediatrics.org/cgi/content/full/112/5/e359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasileiadis GT, Gelman N, Han VK, et al. Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics. 2004;114(3). Available at: www.pediatrics.org/cgi/content/full/114/3/e367 [DOI] [PubMed] [Google Scholar]
- 52.Murphy BP, Inder TE, Huppi PS, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107(2):217–221 [DOI] [PubMed] [Google Scholar]
- 53.Parikh NA, Lasky RE, Kennedy KA, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119(2):265–272 [DOI] [PubMed] [Google Scholar]
- 54.Cheong JL, Burnett AC, Lee KJ, et al. ; Victorian Infant Collaborative Study Group . Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J Pediatr. 2014;164(4):737–743.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howard E. Reductions in size and total DNA of cerebrum and cerebellum in adult mice after corticosterone treatment in infancy. Exp Neurol. 1968;22(2):191–208 [DOI] [PubMed] [Google Scholar]
- 56.Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology. 2006;31(1):12–35 [DOI] [PubMed] [Google Scholar]
- 57.Neubauer V, Junker D, Griesmaier E, Schocke M, Kiechl-Kohlendorfer U. Bronchopulmonary dysplasia is associated with delayed structural brain maturation in preterm infants. Neonatology. 2015;107(3):179–184 [DOI] [PubMed] [Google Scholar]
- 58.du Plessis AJ, Volpe JJ. Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol. 2002;15(2):151–157 [DOI] [PubMed] [Google Scholar]
- 59.Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11(4):192–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reiman M, Kujari H, Maunu J, et al. ; PIPARI Study Group . Does placental inflammation relate to brain lesions and volume in preterm infants? J Pediatr. 2008;152(5):642–647, 647.e1–e2 [DOI] [PubMed] [Google Scholar]
- 61.Ericson JE, Laughon MM. Chorioamnionitis: implications for the neonate. Clin Perinatol. 2015;42(1):155–165, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu HY, Zhang Q, Wang QX, Lu JY. Contribution of histologic chorioamnionitis and fetal inflammatory response syndrome to increased risk of brain injury in infants with preterm premature rupture of membranes. Pediatr Neurol. 2016;61:94–98.e1 [DOI] [PubMed] [Google Scholar]
- 63.Stark MJ, Hodyl NA, Belegar V KK, Andersen CC. Intrauterine inflammation, cerebral oxygen consumption and susceptibility to early brain injury in very preterm newborns. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F137–F142 [DOI] [PubMed] [Google Scholar]
- 64.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK, Canadian Neonatal Network A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol 2009;200(4):372.e1–6 [DOI] [PubMed] [Google Scholar]
- 65.Alexander JM, Gilstrap LC, Cox SM, McIntire DM, Leveno KJ. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol. 1998;91(5 pt 1):725–729 [DOI] [PubMed] [Google Scholar]
- 66.Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198(4):466.e1–466.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bersani I, Thomas W, Speer CP. Chorioamnionitis—the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med. 2012;25(suppl 1):12–16 [DOI] [PubMed] [Google Scholar]
- 68.Gram M, Sveinsdottir S, Ruscher K, et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J Neuroinflammation. 2013;10(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tam EW, Miller SP, Studholme C, et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J Pediatr. 2011;158(3):366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain. 2011;134(pt 5):1344–1361 [DOI] [PubMed] [Google Scholar]
- 71.Lemmers PM, Benders MJ, D'Ascenzo R, et al. Patent ductus arteriosus and brain volume. Pediatrics. 2016;137(4):e20153090. [DOI] [PubMed] [Google Scholar]
- 72.Pickler RH, McGrath JM, Reyna BA, et al. A model of neurodevelopmental risk and protection for preterm infants. J Perinat Neonatal Nurs. 2010;24(4):356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Back SA. brain injury in the preterm infant: new horizons for pathogenesis and prevention. Pediatr Neurol. 2015;53(3):185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riddle A, Dean J, Buser JR, et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann Neurol. 2011;70(3):493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]