Abstract
Transcatheter aortic valve implantation (TAVI) has revolutionized the management of elderly patients with symptomatic severe aortic stenosis in the western world. It is a valuable alternative to surgical aortic valve replacement in patients, who are inoperable or at high surgical risk due to co-morbidities. The prevalence of aortic stenosis increases sharply with age after the sixth decade and is expected to have a significant impact on the geriatric health care system of India, given the rapid increase in life expectancy in recent years. Although a decade has passed since the first TAVI implantation, it is yet to penetrate most of the developing countries in a major way. This short review focuses on fundamentals of initiating a TAVI program based on the experience of a high volume TAVI center with a successful program in Germany.
Keywords: Trans-catheter aortic valve implantation, Aortic stenosis, Self-expanding device, Balloon-expandable device, TAVI techniques
1. Introduction
Rheumatic heart disease forms the major burden of valvular heart disease in developing countries.1, 2, 3 However, age related degeneration of the aortic valve is the most common cause of isolated acquired aortic stenosis (AS) in developed and developing countries alike.1, 3 In an Indian hospital-based echocardiographic survey, prevalence of isolated AS was as high as 7.3%, with the vast majority of subjects being 60 years and above.3 Studies on valvular heart disease in elderly from developing nations are few and hence most of the prevalence data are derived from western studies. The estimated prevalence of AS increases with age, being present in 2% of the adults above the age of 65 years4 and increases up to 12.4% for those above the age of 75 years.5 The prevalence of symptomatic severe AS in the elderly is 3.4%.5 The recent decade has witnessed a steady increase in life expectancy in India due to improved nutrition, health facilities and living conditions. According to a United Nations report, India had 10 million persons above the age of 80 years in 2013.6 Based on recent Indian demographics profile data, 5–6% of the total population of 1.25 billion (approximately 70 million people) are above the age of 65 years.7 Extrapolating western prevalence data to the Indian population, nearly 300,000 patients with AS are likely to be eligible for transcatheter aortic valve implantation (TAVI), a percutaneous procedure that has transformed care of high risk or inoperable AS in the elderly. This hypothetical number of patients, who would potentially benefit from TAVI is slightly more than the combined number of TAVI eligible patients in Europe and North America estimated using a multiple Monte Carlo simulation model.5
TAVI program was introduced at the Segeberger Kliniken, Germany in 2007, and over 600 implantations have been performed since then, with excellent procedural success and outcomes. A successful procedure relies on multiple factors such as appropriate patient selection involving a multidisciplinary ‘Heart Team’, meticulous planning, and maintaining high standards of care during and after the procedure (Table 1). By this short review, we hope to provide some insight into successful implementation of a TAVI program based on our experience.
Table 1.
Steps to set up a TAVI program.
| Regulatory body approval |
| Identification of TAVI capable centers and funding |
| TAVI training Didactic lectures, simulator training, live case observations ‘Mini fellowship’ at high volume centers Proctored training at high volume centers, home institution Integrated Team Training |
| Patient selection Heart Team Approach |
| TAVI Infrastructure Availability of CT, MRI and transesophageal echocardiography Spacious and sterile room – Hybrid operative theater or dedicated catheterization lab with high quality imaging availability Availability of experienced echocardiographer Capable of emergency heart surgery Availability of bail out materials – coronary and peripheral stents Facilities for renal support if necessary Capability of dedicated post-procedure care |
| TAVI program evaluation through registries |
2. Hurdles for starting a TAVI program
Cost concerns and regulatory body approval are the two main challenges for starting a TAVI program in the developing world.
One of the major hurdles for starting a TAVI program is the prohibitantly high device and procedure related costs that can be as high as 12–15 lakhs Indian rupees (15,000–20,000 US $). At current costs, it is certainly a mammoth task for any government to support such an expensive program for the entire country. Technological advancements and procedural simplification, availability of locally manufactured devices and increase in volume of cases performed may ultimately reduce the overall cost of a TAVI procedure. Till then, alternative means of dedicated funding for a TAVI program need to be actively pursued, which could be in the form of institutional funding, support from research organizations or public funding by charitable societies. Alternatively, the government could identify limited regional centers of excellence having necessary infrastructure and allocate funds to perform a certain number of procedures annually.
Currently, India has still to get approval for a TAVI program from its regulatory body although procedures have been performed through individual license. Ever growing data from TAVI trials on the benefits and effectiveness of the procedure supplemented by reports on cost effectiveness from independent experts could accelerate the process of regulatory body approval.
It is likely that volumes of TAVI cases performed in some centers will be low, which has implications for efficacy and safety outcomes, as there appears to be a ‘learning curve’ for operators. However, contrary to the popular belief, the learning curve for TAVI is not too steep or long, and several institutions have implemented the program successfully. Nevertheless, during initial years of implementation of a nationwide TAVI program, it may be prudent to limit the number of centers performing TAVI in order to maintain a sufficient volume of procedures in each center.
It is also important to educate wider medical fraternity regarding TAVI in the form of seminars or discussions with general practitioners, physicians as well as ethical use of media to disperse knowledge in the community.
3. TAVI training
Multisocieties (Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American College of Cardiology Foundation, and Society of Thoracic Surgeons) have jointly identified operator and institutional requirements for performing TAVI.8 TAVI operators should have wide ranging knowledge of aortic valvular heart disease and its natural history, diagnostics and hemodynamics, medical and surgical therapy, and their outcomes. They should also have sufficient echocardiographic skills and expertise in interpreting computed tomography scans of the iliofemoral vessels and aortic valvular anatomy. It is also important to have experience with a variety of interventional techniques including coronary and peripheral diagnostic procedures and interventions, balloon valvuloplasties, aortic endovascular repairs and large vessel access closures. Consensus statement recommends a prior experience of 400 percutaneous coronary interventions per year with acceptable outcomes for centers and 100 structural procedures for operators, as prerequisite skills for performing TAVI.8 For surgeons, 100 aortic valve replacement during their career (at least 10 of which are high risk) or 25 per year and at least 20 in the last year prior to TAVI initiation, are recommended.8
The device companies involved in the manufacture of prostheses have a structured training and proctoring program for TAVI teams, which is usually provided at one of the high volume centers around the world. Operator aspirants are usually provided with a ‘training package’ which consists of educational material, case studies, screening advice and materials, and instructions for device handling. On site training usually involves interactive lectures, simulations and live case observations. These sessions usually focus on patient selection, procedural steps, complications, and troubleshooting. Metric-based simulations are also provided by medical device companies. A ‘mini-fellowship’ training at a high volume center prior to the proctored training phase may be useful in acquiring proper foundation skills necessary for performing TAVI. On site proctor services may also be available for certain centers with aims to ensure procedural success and safety. After a certain adequate number of proctored cases, the trainee may start performing TAVI independently or in some instances, further training may be recommended by the proctor in order to perform the procedure without supervision. Clinical support for an additional 10 or 12 cases following proctoring phase are sometimes provided. Once a trainee is fit to perform the procedure independently, it is important not to have a long gap between the training and start of the TAVI program.
4. ‘Heart Team’ approach
One of the important essentials for a successful TAVI program is the selection of the most appropriate patient for TAVI. At our German center, we follow a multidisciplinary heart team approach, which involves cardiologists, angiologists, cardiothoracic surgeons, anesthetists, intensive care specialists, and importantly patients themselves. Elderly patients with symptomatic severe AS often have significant associated co-morbidities, and predictive scores such as the EuroScore and Society of Throacic Surgeons’ score are valuable for therapy selection. All patients are assessed and thoroughly investigated by blood tests, echocardiography, right and left heart catheterization, and CT Imaging prior to discussion at the heart team conference. During the heart team meet, decision regarding the access route for TAVI (trans-femoral versus trans-apical or trans-aortic routes) is also arrived at if a patient is planned for TAVI.
5. TAVI procedure
5.1. Technique
TAVI is performed in a hybrid operating suite in our center, although it is possible to perform the procedure in a routine angiography lab, provided it has sufficient space needed to accommodate device preparation, echocardiographic device, anesthetic machine, and surgical set-up including certain specifications for a sterile environment. The majority of TAVI procedures are performed through the trans-femoral route. Almost all of our trans-femoral TAVI procedures are done under conscious sedation with general anesthesia reserved for those with compromised hemodynamics or patient preference. With conscious sedation, we have observed better hemodynamic status, lesser need for ionotropes, shorter procedural duration and length of intensive care unit stay. We perform the procedure without transesophageal echocardiography (TEE) guidance. However, some centers do perform intra-procedural TEE. Use of intraprocedural TEE is associated with disadvantages that can potentially outweigh its benefits. TEE usually requires general anesthesia, and severe AS patients are known to tolerate hypotension poorly. TEE probe may also partially obstruct the fluoroscopic view necessitating multiple retractions and advancements of the TEE probe during the procedure. However, TEE guidance is beneficial in patients with limited native valve calcifications and valve-in-valve procedures especially involving a stentless surgical bioprosthesis. A temporary pacing catheter (balloon tipped or helical screw temporary lead) is always positioned in the right ventricle prior to the procedure, usually through the right jugular route. External defibrillator pads and venous access lines are also placed. For trans-femoral TAVI, both groins are used for vascular access – a 6 or 7F access for a pigtail catheter on one side, and contralaterally, an initial 10F sheath which is later upgraded to the larger TAVI introducer sheath. Single Proglide (Abbott Vascular, Santa Clara, CA, USA) pre-sutures are laid on the side used for TAVI device. After aortography, the aortic valve is crossed by a guidewire followed by measurement of pressure gradients. Balloon dilatation if required is performed using an undersized balloon under a brief period of rapid ventricular pacing.
Prosthesis preparation is done simultaneously by another operator or an assistant. Annulus size and optimal angiographic projection for device implantation is predetermined from the information obtained from computed tomography (CT). Some operators recommend a minimum distance of 10 mm between the valve cusp hinge point and the coronary ostium on CT to perform TAVI. However, this should not be stressed as an absolute requirement, and factors such as sinus width, leaflet size, and calcification are also taken into consideration.
Balloon expandable valves are always deployed under rapid ventricular pacing. Aortic valve gradients are then again measured along with angiographic and transthoracic echocardiographic assessments for leaks. Post-dilatation is performed in case of significant leaks. The catheters and guidewires are withdrawn, and hemostasis achieved using the Proglide suture (Abbott Vascular). It is essential to have bailout materials such as snares, coronary guidewires, coronary and peripheral stents in the room where TAVI is performed.
An optimal TAVI program should incorporate other routes for the TAVI procedure such as trans-apical, trans-aortic or trans-subclavian, which may be necessary in the event of small femoral access vessels or severe peripheral artery diseases. For the trans-apical procedure, anterolateral mini thoracotomy is performed and the valve delivered through a sheath that is inserted via the apical puncture site.
6. Devices
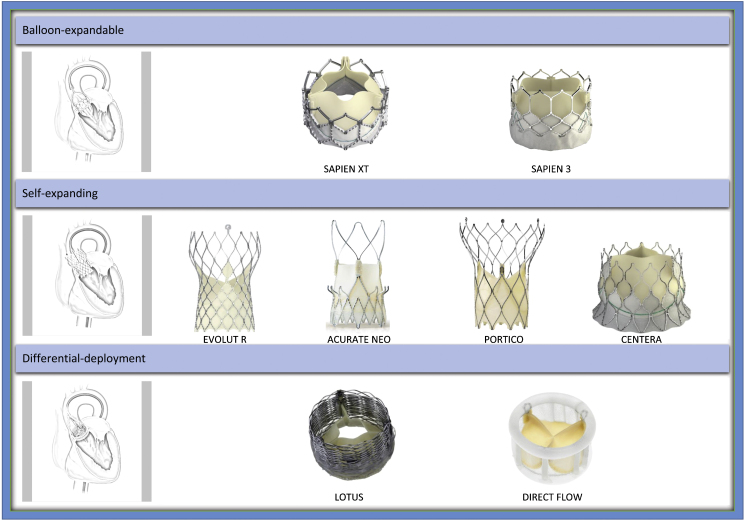
Currently several devices have Conformité Européene mark approval for use in Europe. All these devices can be classified into one of balloon-expandable, self-expanding or differential deployment device categories (Fig. 1).9 Of these devices, SAPIEN 3 and CoreValve Evolut are in widespread clinical use, and there are several others in early clinical trials phase. The newer generation devices have low profile delivery systems (as low as 14F) to reduce access site complications, design modifications to reduce paravalvular leaks, and some are even capable of repositionability or retrievability. Direct comparison studies between different classes of TAVI devices have been a few and till date, the CHOICE randomized trial is the only trial directly comparing balloon-expandable and self-expanding devices.10 One year outcomes of this trial showed no significant difference in clinical outcomes despite higher post-procedural device success with balloon-expandable valves (SAPIEN XT; Edwards Lifesciences, Irvine, CA, USA). However, the self-expanding valve (CoreValve; Medtronic, Minneapolis, MN, USA) was associated with higher rates of paravalvular regurgitation.11 There are no long-term randomized trials comparing newer generation devices.
Fig. 1.
Transfemoral TAVI devices.
Pictures provided courtesy of Edwards Lifesciences, Irvine, CA, USA; Medtronic, Minneapolis, MN, USA; Symetis SA, Ecublens, Switzerland; St. Jude Medical, St. Paul, MN, USA; Direct Flow Medical Inc., Santa Rosa, CA, USA and Boston Scientific, Marlborough, MA, USA. Modified and reprinted from “Abdel-Wahab M, Jose J, Richardt G. Transfemoral TAVI devices: design overview and clinical outcomes. EuroIntervention. 11(suppl. W):W118–W122. Copyright (2015) with permission from Europa Digital & Publishing”.
7. Post-procedural care
In our center, all patients are transferred to the Intensive Care Unit (ICU) after the procedure, and monitored for hemodynamic and electrocardiographic parameters. When the level of care required is deemed to be lower, those with uneventful ICU stay are discharged to the ward. A transthoracic echocardiography is performed prior to discharge and follow-up visits are scheduled at 1 month, 6 months, 1 year and yearly thereafter. Patients with uncomplicated procedures and hospital stay are usually discharged within a few days after TAVI. All patients are offered a further rehabilitative program after discharge.
TAVI patients are routinely discharged on dual antiplatelets. Those who require oral anticoagulation are discharged on a single antiplatelet drug. For those with increased bleeding risk and requiring oral anticoagulation, we tend to perform left atrial appendage closure followed by dual antiplatelet drugs.
8. TAVI program evaluation
Once a TAVI program is in place, it is necessary to have performance monitoring measures in order to recognize shortcomings of the program as well as to evaluate procedure outcomes. Follow-ups are typically scheduled at 30 days, 6 month, 1 year and yearly thereafter. Parameters assessed during follow-up are clinical and echocardiographic, and outcomes are reported in accordance with updated Valve Academic Research Consortium-2 (VARC-2) standardized endpoint definitions.12 Currently, there is limited long-term data on survival outcomes, quality of life and cost effectiveness in comparison to surgical management. It is therefore necessary to maintain a database of all TAVI procedures from the onset of the program in order to track outcomes after the procedure. Prospective cohort studies and randomized controlled trials can add to the wealth of knowledge subsequently.
9. Summary
The use of TAVI has increased exponentially in recent years in the western world and is poised to navigate the uncharted waters of the developing world in the near future. There seems to be a sizeable number of at demand elderly AS patients in India, who are deprived of this beneficial procedure owing to its current high costs and non-availability. Epidemiological studies are however needed to estimate the actual number of patients who might benefit from TAVI. A successful TAVI program provides hope to inoperable and high-risk AS patients and is an incredibly rewarding experience for operators as well. Centers with prior experience in structural interventions, endovascular repairs, and surgical valve replacements could pool resources and personnel to form a ‘Heart Team’, who would then undergo structured training for a TAVI program. India has the necessary infrastructure, but cheaper devices and innovative programs tailored to the needs of the country are keys to the successful implementation of a TAVI program.
Conflicts of interest
First author receives an educational grant in Interventional Cardiology and TAVI research from the ‘European association of Percutaneous Cardiovascular Interventions’ (EAPCI), which is partially sponsored by Medtronic. Last author is a proctor for Boston Scientifc's Lotus Valve program and reports receiving institutional research grants from St. Jude Medical and Biotronik.
References
- 1.Iung B., Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30:962–970. doi: 10.1016/j.cjca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K., Carrington M., Mayosi B.M., Zigiriadis E., Mvungi R., Stewart S. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the heart of Soweto study. Eur Heart J. 2010;6:719–727. doi: 10.1093/eurheartj/ehp530. [DOI] [PubMed] [Google Scholar]
- 3.Manjunath C.N., Srinivas P., Ravindranath K.S., Dhanalakshmi C. Incidence and patterns of valvular heart disease in a tertiary care high-volume cardiac center: a single center experience. Indian Heart J. 2014;66:320–326. doi: 10.1016/j.ihj.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart B.F., Siscovick D., Lind B.K. Clinical factors associated with calcific aortic valve disease Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 5.Osnabrugge R.L., Mylotte D., Head S.J. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 6.United Nations . 2013. World Population Ageing. Retrieved from http://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf Accessed August 2015. [Google Scholar]
- 7.Government of India. Ministry of Home Affairs. SRS Statistical Report 2013. Chapter 2 – Population composition. Retrieved from http://www.censusindia.gov.in/vital_statistics/SRS_Reports_2013.html Accessed August 2015.
- 8.Tommaso C.L., Bolman R.M., 3rd, Feldman T. Multisociety (AATS ACCF, SCAI, and STS) expert consensus statement: operator and institutional requirements for transcatheter valve repair and replacement, part 1: transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2012;143:1254–1263. doi: 10.1016/j.jtcvs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Wahab M., Jose J., Richardt G. Transfemoral TAVI devices: design overview and clinical outcomes. EuroIntervention. 2015;11(suppl.):W118–W122. doi: 10.4244/EIJV11SWA33. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Wahab M., Mehilli J., Frerker C. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311:1503–1514. doi: 10.1001/jama.2014.3316. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Wahab M., Neumann F.J., Mehilli J. 1-Year outcomes after transcatheter aortic valve replacement with balloon-expandable versus self-expandable valves: results from the CHOICE Randomized Clinical Trial. J Am Coll Cardiol. 2015;66:791–800. doi: 10.1016/j.jacc.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Kappetein A.P., Head S.J., Généreux P. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013;145:6–23. doi: 10.1016/j.jtcvs.2012.09.002. [DOI] [PubMed] [Google Scholar]