Abstract
Background
Atrial fibrillation (AF) is the most common sustained arrhythmia with high risk for many cardiovascular (CV) complications. Adherence to recommended management guidelines is important to avoid complications. In India, there is little knowledge on how AF is managed in real world.
Methods
This is a cross-sectional study of patients in India enrolled in RealiseAF survey between February 2010 and March 2010 with a diagnosis of AF within the last 12 months.
Results
From 15 centers, 301 patients {mean age 59.9 years (14.4); 52.5% males} were recruited. AF was controlled in 50% of patients with 77 (26.7%) in sinus rhythm and 67 (23.3%) with heart rate <80 beats/min. Hypertension (50.8%), valvular heart disease (40.7%), heart failure (25.9%), and diabetes (20.4%) were the most common underlying CV diseases. Increased risk for stroke (CHADS2 score ≥ 2) was present in 36.6%. Most of the patients (85%) were symptomatic. AF was paroxysmal, persistent, and permanent in 28.7%, 22.7%, and 34.3% respectively. In 14%, AF was diagnosed as first episode. Forty-six percent of patients had rate control, 35.2% rhythm control, 0.3% both strategies, and 18.4% received no therapy for AF before the visit. At the end of the visit, adoption to rate control strategy increased to 52.3% and patients with no therapy decreased to 7%.
Conclusion
AF in India is not adequately controlled. Concomitant CV risk factors and risk of stroke are high. The study underscores the need for improved adoption of guideline-directed management for optimal control of AF and reducing the risk of stroke.
Keywords: Atrial fibrillation, Cardiovascular risk factors, India, Guidelines, Stroke
1. Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia with a prevalence of 1–2% in the general population.1, 2 The prevalence of AF increases with age and, in the elderly population, cardiovascular (CV) risk factors and co-morbidities such as hypertension, diabetes, congestive heart failure, coronary artery disease, valvular heart disease (VHD), and stroke are common.3, 4, 5, 6 As CV risk factors are important predictors of AF, the rapidly aging population of the world would substantially increase the prevalence of AF in the coming years. By 2050, the number of people with AF is projected to be 15 million in the United States.1, 7 A similar trend is projected for the European population8 too. For India, the United Nations Populations Fund has projected a 326% increase in the number of people aged between 60 and 80 years by the year 2050 (from the year 2000).9 A 700% increase is projected in the number of people above the age of 80 years.9 Though large-scale population-based studies on the prevalence of AF are not available in India, the projected high increase in the aged population only warns us of the concomitant increase in the prevalence of AF.
AF poses a high risk for complications such as thromboembolism and heart failure (HF). Uncontrolled AF also leads to frequent hospitalizations and reduced quality of life.10 To reduce the clinical and economical burden occurring due to complications, optimal control of AF is essential. For this, knowledge on the prevalent practice in the management of AF is important. The data that are available today are from studies mainly on American or European population.11, 12, 13 The Euro Heart Survey14 which assessed the management of AF in over 5000 ambulant and hospitalized patients reported lack of adherence to guidelines. RECORD AF study assessed management of paroxysmal/persistent AF in recently diagnosed patients.15 It was observed that the young, frequently symptomatic and recently diagnosed patients received rhythm control strategy, while patients with history of HF, VHD, and persistent AF received rate control therapy.15 These data pertain to certain types of AF and do not provide representative information on characteristics and management of patients with the whole spectrum of AF. The management of AF also may vary significantly according to different medications administered and country-specific therapeutic strategies. There are very little data from India on management practices of AF and the prevalence of CV risk factors in this population. Small studies like CRAFT16 provide some insight on treatment of AF only in patients with rheumatic VHD. This underscores the need for a study on up-to-date, real-life management of patients with AF in India.
The current study presents data on Indian patients enrolled in the REal Life global Survey Evaluating17 patients with atrial fibrillation (RealiseAF).
The primary objectives of the study were to assess the proportion of patients with control of AF and investigate the CV risk profile of patients with AF. Control of AF was defined as presence of sinus rhythm in patients with history of AF or a heart rate (HR) ≤80 beats/min in patients who were in AF. The secondary objectives were: to describe the characteristics of the AF; describe the management of AF in terms of treatment strategy (rate vs. rhythm control); to assess the proportion of patients being treated with evidence-based medicine and the proportion of patients with specific CV events/interventions leading to hospitalization within the past 12 months. We also aimed to assess the health-related quality of life (QoL) associated with AF.
2. Methods
RealiseAF is an international, observational, cross-sectional survey aimed at evaluating AF management and CV risk profile of patients with different types of AF. The global study was conducted in 26 countries worldwide, and the baseline data of the study have been published.17 The total duration of recruitment was 5 months with six weeks recruitment phase per country. The study was conducted in accordance with the Helsinki principles,18 guidelines for Good Epidemiological Practices,19, 20 and local regulations. The study was approved in the participating centers by the respective Institutional Ethical Committee. In India, the clinical operations were managed by Medical Affairs Clinical Operation unit of Sanofi, India.
2.1. Physician selection
Physicians were selected randomly from a pan-Indian, extended list of office or hospital-based cardiologists. The list comprised ≥10 times the total number of cardiologists required to participate. The recruitment of sites was done carefully to accurately reflect the practices according to each region.
2.2. Patient selection
Patients meeting the following eligibility criteria were considered for enrolment in the study.
Inclusion criteria: All consenting patients with documented current AF or with history of AF in the last 12 months (treated/not treated and whatever the rhythm at inclusion) were included in ReleaseAF registry. Patients with history of AF were qualified for enrollment if at least one AF episode was present within the last 12 months, documented by either standard electrocardigram (ECG) or by Holter monitoring. The patients or legal representatives (for minors) signed the written informed consent form approved by the ethics committee of the respective participating centers.
Exclusion criteria: Mentally disabled patients (including dementia and significant cognitive disorders) unable to understand or sign the written informed consent, patients within three months after surgery, and patients participating concomitantly in a clinical trial in the field of AF or in the field of antithrombotic treatment in the previous month were not included.
The selected physicians included 10 consecutive eligible patients (maximum of 30 patients) meeting the inclusion and exclusion criteria. The maximum duration of recruitment per site was six weeks. Patient information was collected using a centralized case report form which included baseline characteristics, symptoms, ECG data, medical history, risk factors, current medications, history and characteristics of AF, management of AF in terms of strategy chosen (rate vs. rhythm control), antiarrhythmic drugs, and/or rate control agents chosen, and use of antithrombotics (in relation to CHADS2 score). The QoL of patients was measured using EQ 5-D instrument,21 a standardized instrument for describing health status.
2.3. Statistical analysis
Descriptive statistics for the categorical variables were performed by computing the frequencies (percentages) in each category. For the quantitative variables, variables following normal distribution were summarized by mean and standard deviation (SD) and the remaining as median and range. The detailed statistical analyses and sample size calculation of the global RealiseAF study were described elsewhere.17
3. Results
3.1. Demographics and CV risk profile
Between 2nd February 2010 and 31st March 2010, a total of 301 consecutive patients were enrolled in the study from 15 centers across India. Of the total 301 patients, 107 (35.5%) were inpatients and 194 (64.5%) were outpatients. The mean age was 59.9 (14.4) years, range 14–89 years. Fifty-three percent were males. The body mass index systolic and diastolic blood pressure were 24.5 (4.5) kg/m2 and 124.7 (18.1) and 77.4 (10.1) mmHg respectively. Eighty-five percent of patients were symptomatic with 90% of them having at least one symptom within the last one week of assessment. Dyspnea was the most common symptom observed during the previous 12 months and also within the previous one week, followed by palpitations and fatigue. The demographic and clinical data are presented in Table 1.
Table 1.
Demographics, cardiovascular risk profile, and symptom history of variables.
| Variables | Observation |
|---|---|
| Mean age in years (SD) | 59.9 (14.4) |
| No. of patients above 60 years | 176/301 (58.5%) |
| No. of males | 158/301 (52.5%) |
| Mean body mass index (kg/m2) (SD) | 24.5 (4.5) |
| No. of obese patients | 28/289 (9.7%) |
| Mean systolic blood pressure in mmHg (SD) | 124.7 ± 18.1 |
| Mean diastolic blood pressure in mmHg (SD) | 77.4 ± 10.1 |
| No. of patients with uncontrolled blood pressurea | 109/299 (36.5%) |
| No. of patients (%) with at least one symptom | 256 (85.0%) |
| No of patients (%) with at least one symptom within the past one week | 203/256 (89.8%) |
| Palpitations | 112/203 (55%) |
| Dyspnea | 134/203 (66%) |
| Fatigue | 99/203 (48.7%) |
| Light headedness/dizziness | 38/203 (18.7%) |
| Chest pain | 43/203 (21%) |
| Syncope | 9/203 (4.4%) |
| No. of patients (%) with at least one cardiovascular event within the preceding 12 months | 92/295 (31.2%) |
| No. of patients (%) with at least one cardiovascular intervention within the preceding 12 months | 70/300 (23.3%) |
| Valve surgery | 44/300 (14.6%) |
| Percutaneous coronary intervention | 13/300 (4.3%) |
| Coronary artery bypass graft | 12/300 (4%) |
| Others | 5/300 (1.6%) |
SD = standard deviation.
Controlled blood pressure: for diabetes patients – systolic blood pressure (SBP) < 130 mmHg or diastolic blood pressure (DBP) < 80 mmHg, and for non-diabetic patients – SBP < 140 mmHg or DBP < 90 mmHg.
3.2. Cardiovascular events leading to hospitalization
A total of 92 (31.9%) patients with at least one CV event were admitted to hospitals during the previous 12 months. Acute decompensated HF was the most common CV event leading to hospitalization, followed by acute coronary syndrome and stroke (Fig. 1). Thirty-eight percent of ACS events: 52% of stroke, 62% of decompensated HF, and 67% of non-neurological embolic events occurred after AF was diagnosed.
Fig. 1.
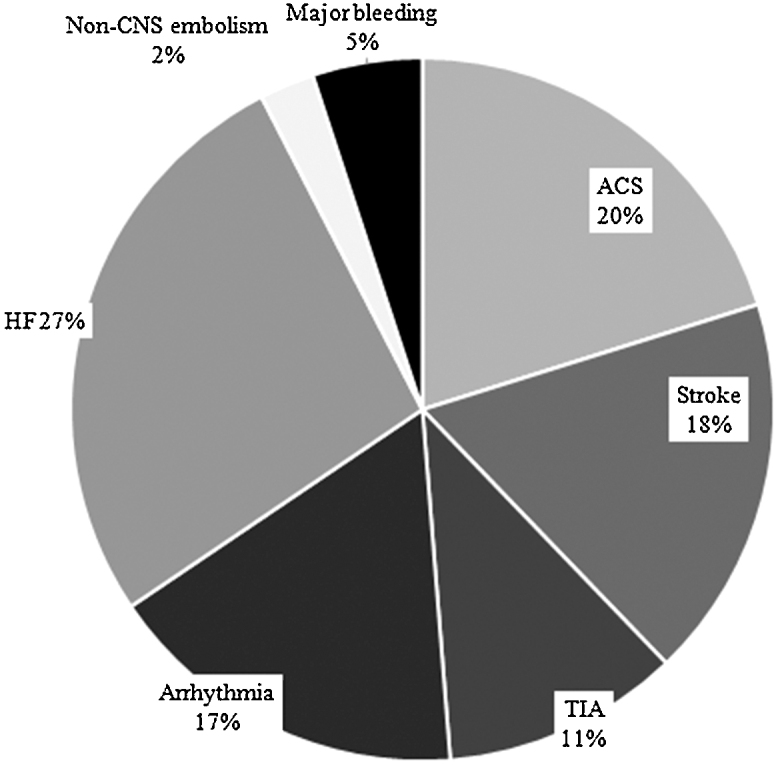
Major cardiovascular events leading to hospitalization. Abbreviations: ACS, acute coronary syndrome; HF, heart failure (decompensated); CNS, central nervous system; TIA, transient ischemic attack.
3.3. Cardiovascular risk factors and risk of stroke
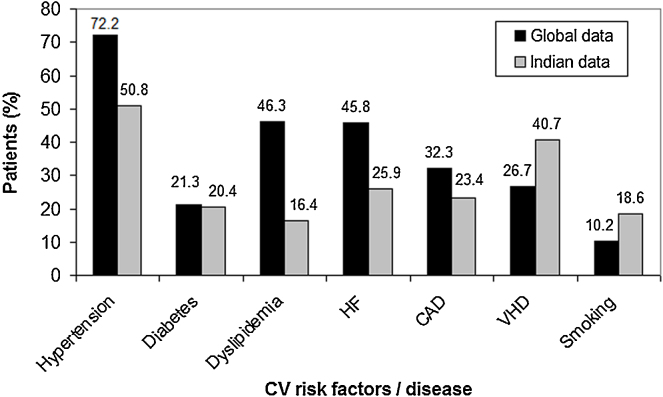
Hypertension was the most common underlying CV condition (50.8%), followed by VHD (40.7%), HF (25.9%), coronary artery disease (32.3%), diabetes (20.4%), and dyslipidemia (16.4%) (Fig. 2). Mitral valve was the most frequently involved valve (87.7%). The risk of stroke as estimated using CHADS2 score was ≥2 in 36% of patients. The CV risk factors and history of CV and non-CV diseases are presented in Table 2.
Fig. 2.
Major cardiovascular risk factors/diseases in global and Indian patients in RealiseAF study. Reference: Steg et al.17 Abbreviations: CAD, coronary artery disease; CV, cardiovascular; HF, heart failure; VHD, valvular heart disease.
Table 2.
Cardiovascular risk factors and history of cardiovascular and non-cardiac co-morbidities.
| Variables | n (%) |
|---|---|
| Arterial hypertension, (n = 297) | 151 (50.8) |
| Diabetes mellitus, (n = 299) | 61 (20.4) |
| Valvular heart disease, (n = 300) | 122 (40.7) |
| Mitral | 107 (36.4) |
| Aortic | 26 (8.8) |
| Other | 9 (3.1) |
| Coronary artery disease, (n = 286) | 67 (23.4) |
| Dyslipidemia, (n = 250) | 41 (16.4) |
| Physical inactivity, (n = 301) | 153 (50.8) |
| Family history of AF, (n = 300) | 7 (2.3) |
| History of heart failure, (n = 297) | 77 (25.9) |
| Smoking, (n = 301) | 56 (18.6) |
| Peripheral arterial disease, (n = 292) | 6 (2.1) |
| Past stroke/TIA, (n = 295) | 28 (9.5) |
| CHADS2 scorea, (n = 287) | |
| Missing, n | 14 |
| 0 | 78 (27.2) |
| 1 | 104 (36.2) |
| 2 | 61 (21.3) |
| 3 | 30 (10.5) |
| 4 | 12 (4.2) |
| 5 | 2 (0.7) |
| Patients with at least one co-morbidity, (n = 296) | 229 (77.4) |
| Bradycardia, (n = 297) | 16 (5.4) |
| Atrial flutter (other than 1 to 1), (n = 297) | 14 (4.7) |
| Sick sinus syndrome, (n = 297) | 7 (2.4) |
| Non-cardiovascular diseases | |
| Chronic pulmonary disease, (n = 291) | 26 (8.9) |
| At least 1 thyroid disease, (n = 271) | 29 (10.7) |
| Hypothyroidism | 27/271 (9.9) |
| Hyperthyroidism | 2/271 (0.7) |
| Liver diseases, (n = 292) | 12 (4.1) |
| Chronic advanced liver failure, (n = 296) | 9 (3.0) |
| Malignancies, (n = 289) | 4 (1.4) |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; NYHA, New York Heart Association, TIA, transient ischemic attack.
CHADS2 score: 1 point each for history of heart failure, arterial hypertension, age > 75 years, history of diabetes, 2 points for history of stroke or history of TIA.
3.4. Control of atrial fibrillation
One hundred and forty-four of 288 patients (50%) had controlled AF of whom 77 (53.4%) were in sinus rhythm and the remaining (46.5%) had HR ≤80 beats/min.
3.5. Atrial fibrillation characteristics
The duration since the first diagnosis of AF was 6.5 months median ranging from 0 to 278 months. Eighty-six (28.6%) patients had paroxysmal AF, 68 (22.6%) patients had persistent AF, and 103 (34.3%) patients had permanent AF. In 43 (14.3%) patients, AF was diagnosed for the first time. Table 3 presents AF characteristics.
Table 3.
Atrial fibrillation characteristics.
| Variable | Observation (n = 301) |
|---|---|
| Lone AFa | 24 (8.1) |
| Time since first AF diagnosis in months, median (range) | 6.5 (0–278) |
| Missing, n | 28 |
| <3 months | 113 (41.4) |
| 3–6 months | 22 (8.1) |
| 6–12 months | 23 (8.4) |
| >12 months | 115 (42.1) |
| Type of AF | |
| Missing, n | 1 |
| First episode | 43 (14.3%) |
| Paroxysmal | 86 (28.7%) |
| Persistent | 68 (22.7%) |
| Permanent | 103 (34.3%) |
Patients younger than 60 years of age without clinical or echocardiographic evidence of any sort of cardiopulmonary disease, including hypertension.
3.6. Treatment strategy for atrial fibrillation
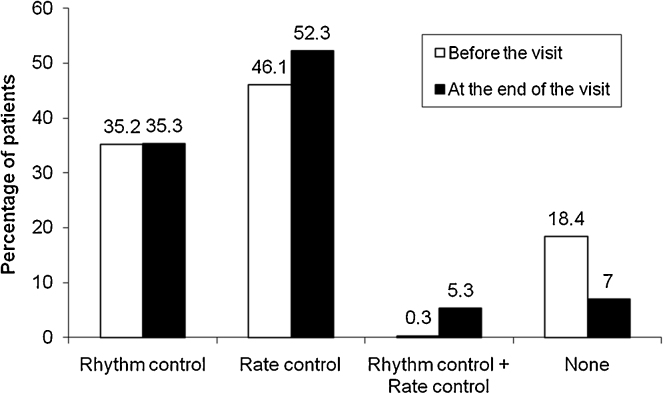
Data were available for 293 patients and of these, 135 (46.1%) received rate control treatment, 103 (35.2%) received rhythm control treatment, 1 (0.3%) patient received both while 54 (18.4%) patients did not receive any drugs for AF before the study visit (Fig. 3). At the end of the visit, more patients received both rate control (157, 52.3%) and rhythm control (106, 35.3%) strategies. After the study visit, there were 16 (5.3%) patients receiving both rate and rhythm control strategies, and 21 (7.0%) patients who did not receive any treatment.
Fig. 3.
Type of therapeutic strategy used for management of atrial fibrillation.
3.7. Pharmacologic treatment for AF management
On the day of study visit, Class III drugs (39.2%) was the most common pharmacologic treatment prescribed, followed by beta-blockers (38.5%) and cardiac glycosides (31.9%). Class IC antiarrhythmic drugs (AADs) were prescribed to four patients, while class IAAADs were not prescribed at all (Table 4).
Table 4.
Antiarrhythmic use on the day of the visit.
| Atrial fibrillation treatment | n (%) (n = 301) |
|---|---|
| Patients with at least one AAD | 249 (82.7) |
| Class IC (Flecainide, other) | 4 (1.3) |
| Class II, beta-blockers | 116 (38.5) |
| Class III | 118 (39.2) |
| Amiodarone | 112 (37.2) |
| Sotalol | 6 (2.0) |
| Others | 4 (1.3) |
| Class IV, calcium-channel blockers | 75 (24.9) |
| Cardiac glycosides | 96 (31.9) |
AAD, antiarrhythmic drug.
3.8. Stroke risk and prevention
As the risk of stroke increased, increasing number of patients received antiplatelet agent. Among 105 patients who were at high risk for stroke, 15.2% patients received both antiplatelet and anticoagulant, 38% received anticoagulation, and 58% received antiplatelet but no anticoagulation therapy (Fig. 4).
Fig. 4.
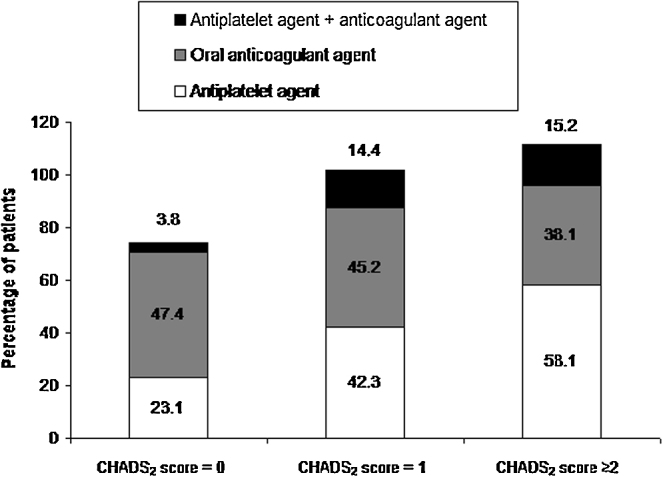
Prescription pattern of antithrombotic agents on the day of the visit according to CHADS2 score. n = 287. CHADS2 score is calculated as 1 point for ‘history of heart failure’, 1 point for ‘arterial hypertension’, 1 point for ‘age > 75 years’, 1 point for ‘history of diabetes’, 2 points for ‘history of stroke’ or ‘history of TIA’.
3.9. Quality of life
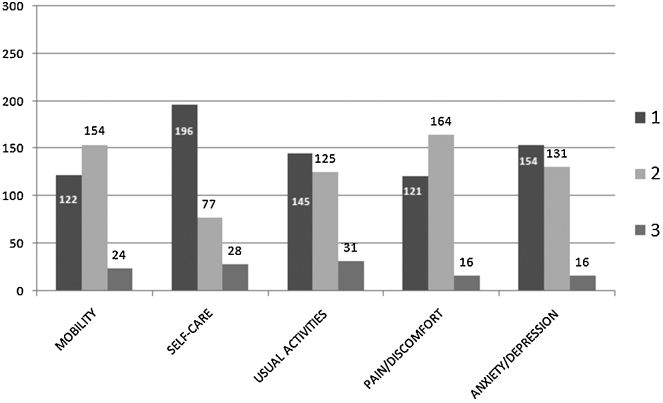
Majority of the patients had either no problem at all or some to moderate problem with all the five aspects of the questionnaire. Severe problems were reported by 8% of patients with mobility, 9% with self-care, 10% with usual activities, and 5% each with pain/discomfort and anxiety/depression (Fig. 5).
Fig. 5.
Response to EQ5D questionnaire. X axis shows the five dimensions on the health-related quality of life state and Y axis the percentage of responses given by the patients. Levels of scoring: 1 = no problem, 2 = some to moderate problems, 3 = severe problem.
4. Discussion
The data provided here on the Indian subset data of the global RealiseAF study reflect the real-life scenario of the epidemiology of AF in India in terms of patient characteristics, therapies used to control of AF, and CV risk profile of AF patients.
Our results showed that in 50% of patients, AF was not adequately controlled and majority of the patients, i.e. 85% were symptomatic within the previous one year and 90% within one week. This study illustrates the inadequate control of AF and the high prevalence of symptoms, among patients who were being treated for AF.
Earlier studies showed that hypertension, HF, diabetes, previous stroke/systemic embolism/transient ischemic attack, etc. were the most common CV risk factors.22 Current studies in AF (RELY,23 ARISTOTLE24 and ROCKET AF25) suggest hypertension as the most prevalent (80–90%) co-morbid disease. In line with the earlier evidence, in our study also, the interplay between AF and other CV conditions was evident. These patients were at high risk of CV events leading to hospitalization. In the global RealiseAF cohort,17 hypertension (72.2%), dyslipidemia (46.3%), HF (45.8%), and coronary artery disease (32.3%) were the most common risk factors. Amongst the CV disease profile in the Indian sub-study, VHD is strikingly more prevalent at 41% and the other risk factors were comparatively less (Fig. 2). However, the present study did not capture the prevalence of rheumatic VHD in this group.
Many randomized clinical trials such as AFFIRM,26 RACE,27 PIAF,28 STAF,29 AF-CHF,30 and CAFÉ-II31 demonstrate that there is no significant difference between rhythm control and rate control strategies in terms of overall mortality or stroke rate. In fact, both these strategies are important and treatment should be individualized according to the clinical profile of patients.32 In our study, rate control (52.3%) was the preferred treatment as compared to rhythm control treatment (35.3%), while 40.5% of patients used at least one AAD per rhythm control in management of AF. The preferred treatment strategies in Indian patients were similar to those in the global patients.16
International guidelines recommend management of AF with rate control and/or rhythm control using various combinations of AADs, and use of anticoagulants, depending upon the presence of paroxysmal, persistent, or permanent AF.33, 34 Based on the guidelines, AADs are the first line treatment for patients with paroxysmal or persistent AF. Many AADs including amiodarone, propafenone, flecainide, and sotalol have been shown to be effective in the prevention of AF recurrences; however, AADs could have cardiac and extra-cardiac toxicity (i.e. amiodarone).35 The American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) 2006 guidelines also reflect the fact that there are no truly safe and effective AADs for prevention of AF.34 Non-availability of Class IC agents in India at the time of the study resulted in excess use of amiodarone for rhythm control. As a result, 61.5% of evaluated paroxysmal or persistent AF patients without congestive HF or hypertension received amiodarone as first-line treatment, despite guidelines recommending that it be used as a second-line agent. Also, as recommended by the guidelines, high-risk patients should be given vitamin K antagonist (VKA),33, 36 while in low-risk patients, an antiplatelet is recommended.33 In our study, only 38% of patients with high risk received VKA, while 58% of patients did not receive any anticoagulants. Our results are in line with earlier reports in other Asian patient populations which reported underuse of oral anticoagulant therapy.37, 38 This underscores the need for simpler and safer anticoagulants in AF patients.39
4.1. Limitations of the study
As the ethnic population of India is diverse, a breakdown in terms of ethnicity and socioeconomic status may provide insights into factors that may modulate the compliance/response to pharmacotherapy for AF. However, the study did not capture information on these variables. The cross-sectional design of the study makes interpretation of data such as control of AF and the rationale behind the choice of management strategies difficult to comprehend. The sites involved in the study were all in urban areas. All physicians involved in the study are attached to private healthcare institutions. The inclusion of study sites from rural geographical areas and also public healthcare institutions could have possibly depicted a higher prevalence of AF and the proportion of patients at risk of stroke.
5. Conclusion
This cross-sectional, observational survey of real-life clinical practice, demonstrates that AF is not adequately controlled in India with appropriate choice of antiarrhythmic drugs. A good proportion of patients who are at high risk for stroke do not receive anticoagulation. The results highlight the need for improved antiarrhythmic drugs and antithrombotic treatments for optimal management of AF.
Conflicts of interest
The authors have none to declare.
Funding source
The RealiseAF study was sponsored by Sanofi.
Acknowledgements
The authors acknowledge the assistance of Dr. (Ms.) Jai Tilak-Jain and Anahita Gouri with editing of the manuscript, references, and figures, supported by Sanofi (India).
References
- 1.Go A.S., Hylek E.M., Phillips K.A. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm A.J. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Kannel W.B., Wolf P.A., Benjamin E.J. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S., Hart C.L., Hole D.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 5.Stroke Risk in Atrial Fibrillation Working Group Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 6.Jahangir A., Lee V., Friedman P.A. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka Y., Barnes M.E., Gersh B.J. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 8.Ryder K.M., Benjamin E.J. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84:131R–138R. doi: 10.1016/s0002-9149(99)00713-4. [DOI] [PubMed] [Google Scholar]
- 9.UNFPA and HelpAge International Ageing in the twenty-first century: a celebration and a challenge. www.unfpa.org/sites/default/files/pub-pdf/Ageing%20report.pdf [accessed on 15.03.15].
- 10.Wattigney W.A., Mensah G.A., Croft J.B. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 11.Vora A., Karnad D., Goyal V. Control of rate versus rhythm in rheumatic atrial fibrillation: a randomized study. Indian Heart J. 2004;56:110–116. [PubMed] [Google Scholar]
- 12.Kerr C., Boone J., Connolly S. Follow-up of atrial fibrillation: the initial experience of the Canadian Registry of Atrial Fibrillation. Eur Heart J. 1996;17:48–51. doi: 10.1093/eurheartj/17.suppl_c.48. [DOI] [PubMed] [Google Scholar]
- 13.Nabauer M., Gerth A., Limbourg T. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garty M., Shotan A., Gottlieb S. The management, early and one year outcome in hospitalized patients with heart failure: a national Heart Failure Survey in Israel – HFSIS 2003. Isr Med Assoc J. 2007;9:227–233. [PubMed] [Google Scholar]
- 15.Nieuwlaat R., Capucci A., Camm A.J. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 16.Le Heuzey J.Y., Breithardt G., Camm J. The RecordAF study: design, baseline data, and profile of patients according to chosen treatment strategy for atrial fibrillation. Am J Cardiol. 2010;105:687–693. doi: 10.1016/j.amjcard.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Steg P.G., Alam S., Chiang C.E. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98:195–201. doi: 10.1136/heartjnl-2011-300550. [DOI] [PubMed] [Google Scholar]
- 18.Declaration of Helsinki. Article I.9. Helsinki, Finland: Adopted by the 18th World Medical Assembly, Helsinki, 1964 as amended by the 41st World Medical Assembly, Hong Kong, 1989.
- 19.International Society for Pharmocoepidemiology . 1996. Guidelines for Good Epidemiology Practices for Drug, Devise, and Vaccine Research in the United States.https://www.pharmacoepi.org/resources/guidelines_08027.cfm [accessed on 15.03.15] [DOI] [PubMed] [Google Scholar]
- 20.International Epidemiological Association: IEA guidelines for proper conduct in epidemiological research. Ieaweb.org/category/guidelines-2/ [accessed on 15.03.15].
- 21.EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin E.J., Levy D., Vaziri S.M. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 23.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 24.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 25.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 26.Wyse D.G., Waldo A.L., DiMarco J.P. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 27.Van Gelder I.C., Hagens V.E., Bosker H.A. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 28.Hohnloser S.H., Kuck K.H., Lilienthal J. Rhythm or rate control in atrial fibrillation – Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson J., Miketic S., Windeler J. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 30.Roy D., Talajic M., Nattel S. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 31.Shelton R.J., Clark A.L., Goode K. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study) Heart. 2009;95:924–930. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- 32.Bajpai A., Savelieva I., Camm A.J. Treatment of atrial fibrillation. Br Med Bull. 2008;88:75–94. doi: 10.1093/bmb/ldn046. [DOI] [PubMed] [Google Scholar]
- 33.Camm A.J., Kirchhof P., Lip G.Y. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 34.European Heart Rhythm A, Heart Rhythm S, Fuster V. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]
- 36.Lip G.Y., Boos C.J. Antithrombotic treatment in atrial fibrillation. Heart. 2006;92:155–161. doi: 10.1136/hrt.2005.066944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lok N.S., Lau C.P. Presentation and management of patients admitted with atrial fibrillation: a review of 291 cases in a regional hospital. Int J Cardiol. 1995;48:271–278. doi: 10.1016/0167-5273(94)02259-l. [DOI] [PubMed] [Google Scholar]
- 38.Lin L.J., Cheng M.H., Lee C.H. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation – a nationwide descriptive study in Taiwan. Clin Ther. 2008;30:1726–1736. doi: 10.1016/j.clinthera.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Forleo G.B., Santini L., Romeo F. Present concepts in management of atrial fibrillation: from drug therapy to ablation. World J Cardiol. 2009;1:11–22. doi: 10.4330/wjc.v1.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]