Abstract
Objective
Alcohol septal ablation (ASA) is a therapeutic alternative to surgical myectomy in patients with hypertrophic obstructive cardiomyopathy (HOCM). However, the anatomical variability of the septal branch, risk of complete heart block, and late onset ventricular arrhythmias are limitations to its therapeutic usage. There is recent interest in the use of radiofrequency catheter ablation (RFCA) as a therapeutic option in HOCM. We aimed to assess the safety and efficacy of RFCA in the treatment of symptomatic HOCM.
Methods
Seven patients with symptomatic HOCM (mean age 43.7 ± 15.6 years, five males), and significant left ventricular outflow tract (LVOT) gradient despite optimal drug therapy, underwent ablation of the hypertrophied interventricular septum. These patients had unfavorable anatomy for ASA. Ablation was performed under 3D electro-anatomical system guidance using an open irrigated tip catheter. The region of maximal LV septal bulge as seen on intracardiac echocardiography was targeted. Patients were followed up at 1, 6, and 12 months post-procedure.
Results
The mean baseline LVOT gradient by Doppler echocardiography was 81 ± 14.8 mm of Hg which reduced to 48.5 ± 22.6 (p = 0.0004), 49.8 ± 19.3 (p = 0.0004), and 42.8 ± 26.1 mm of Hg (p = 0.05) at 1, 6, and 12 months respectively. Symptoms improved at least by one NYHA class in all but one patient. One patient developed transient pulmonary edema post-RFA. There were no other complications.
Conclusion
RFCA of the hypertrophied septum causes sustained reduction in the LVOT gradient and symptomatic improvement among patients with HOCM. Electroanatomical mapping helps to perform the procedure safely.
Abbreviations: LVOT, left ventricular outflow tract obstruction; ASA, alcohol septal ablation; RFCA, radio frequency catheter ablation; HOCM, hypertrophic obstructive cardiomyopathy; LAD, left anterior descending artery; ICE, intra cardiac echocardiography; LBB, left bundle branch; CHB, complete heart block
Keywords: Alcohol septal ablation, Hypertrophic obstructive cardiomyopathy, Left ventricular outflow tract, Radiofrequency ablation
1. Introduction
Hypertrophic cardiomyopathy is a genetic condition affecting one in 500 of the general population.1 It is characterized by hypertrophy of the interventricular septum caused by mutations in the genes encoding sarcomere or sarcomere-associated proteins.2 About two-third of the patients have significant left ventricular outflow tract obstruction (LVOTO) at rest (≥30 mmHg) or at physiologic provocation (≥50 mmHg).2 Outflow obstruction causes increased LV systolic pressure which is responsible for myocardial ischemia, mitral regurgitation, and impairment in diastolic and systolic function leading to pulmonary hypertension and heart failure.
In the treatment of hypertrophic obstructive cardiomyopathy (HOCM), surgical myectomy is widely employed for symptomatic patients with elevated LV outflow gradient despite optimal medical therapy.2 A non-surgical alternative to myectomy is alcohol septal ablation (ASA).3, 4, 5 It involves catheter-based alcohol infusion into the septal coronary arteries which reduces LVOTO by causing a limited infarction of the hypertrophied septum.6 However, the outcome of ASA largely depends on the anatomy of the septal arteries and therefore, the procedure is not a suitable in many patients.5, 7
Radiofrequency catheter ablation (RFCA) is a newer and a well-established technique in the management of cardiac arrhythmias.8, 9 Recent studies have demonstrated that RFCA of the hypertrophied septum in patients with HOCM is feasible and effective in reducing LVOT gradients by the mechanism of discrete septal hypokinesia.10, 11 Patients who are not suitable candidates for surgical myectomy and ASA can get benefit of RFCA of hypertrophied septum. However, atrioventricular blocks and cardiac tamponade are known to occur with this procedure.10, 11 We hypothesized that the effective use of multimodality imaging can mitigate these complications. We present here our experience with RFCA in the treatment of septal hypertrophy in patients with symptomatic HOCM. In our study, we aimed to assess the immediate and long-term safety and efficacy of this procedure.
2. Methods
This is a retrospective study done on patients who have undergone RFCA for septal hypertrophy in HOCM at our center. Data of patients were retrieved from archived medical records.
2.1. Patient selection
Patients with HOCM having an LVOT gradient of >50 mmHg at rest and symptomatic on adequate medical therapy were first considered for surgical myectomy. Those patients who were unwilling for surgical myectomy or deemed high-risk for surgery were assessed for the suitability of ASA. RFCA was employed on patients who were not considered as optimal candidates for ASA. All patients were taken up for the procedure after obtaining a written informed consent.
2.2. Evaluation for alcohol septal ablation
A selective left coronary angiogram was performed in multiple views to visualize the first, large septal branch of the left anterior descending coronary artery. After visualizing the first septal branch, a 0.0014 in. guidewire was inserted into the artery over which a 2.0 mm × 10 mm balloon was advanced and positioned at the proximal part of the artery. Then, one milliliter of myocardial echo contrast (MEC) was injected through the lumen of the catheter to assess the supply area of the septal branch. For a targeted flow, the balloon was first inflated and it was ensured that there was no leak of the contrast agent occurring along the sides of the balloon. Patients who either had a small first septal branch (<1.5 mm) or in whom contrast spill over was observed in the cavity of right ventricle were considered unsuitable for ASA and proceeded with RFCA.
2.3. Radiofrequency catheter ablation technique
Both right femoral arterial and venous access were obtained. The procedure was done under sedation and local anesthesia under the guidance of intracardiac echocardiography (ICE) and 3-D mapping system (CARTO-Biosense Webster, Diamond Bar, California, USA or EnSite-NavX-Endocardial Solutions, St. Jude Medical, Inc., St. Paul, MN, USA). Anatomical map of the LV was created with the help of 3-D mapping system. Bundle of His, left bundle branch (LBB), left anterior and posterior fascicular Purkinje potentials were tagged to prevent injury to these structures during the procedure.
A retrograde, trans-aortic approach was used for mapping and ablation of the LV septum in all patients. Intracavitary LV pressure was recorded through the lumen (irrigation port) of the mapping catheter. The LV cavity was divided into three zones (high pressure zone, transitional, and low pressure zone) as shown in Fig. 1. Ablation was performed with a 3.5 mm open, irrigated-tip ablation catheter on the septal surface of the transitional pressure zone at the area of maximal septal bulge avoiding tagged potentials (Fig. 1). Catheter location and stable contact with LV septum were also monitored under real-time ICE images (Fig. 2). Lesions were delivered for 60–120 s each with a power setting of 30–40 W and saline irrigation of 30 ml/min (Cool Flow Irrigation Pump, Biosense Webster, Diamond Bar, California). Patients also received adjusted doses of heparin to maintain activated clotting time of 250–300 s throughout the procedure.
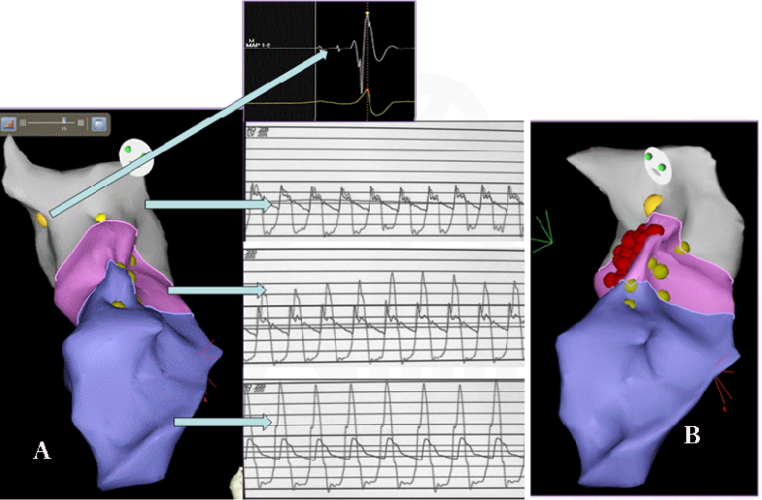
Fig. 1.
CARTO-guided 3D anatomical map of LV with three pressure zones and radiofrequency ablation lesions. (A) The three pressure zones in 3-D CARTO LAO view with their corresponding pressure tracings. Yellow dots indicate the site of conduction system signals. The insert shows A-H-V signals (Site of left side His bundle). The gray color indicates the area of normal pressure zone with no difference in gradient observed in the pressure tracing. Pink zone reflects transition zone and purple, high pressure zone with corresponding pressure tracings. Note that in (B), the RF lesions (red dots) are delivered in the transition zone, away from the conduction system (yellow dots).
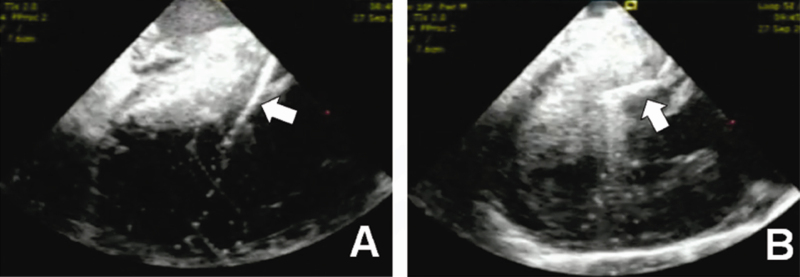
Fig. 2.
Intracardiac echocardiographic images during radiofrequency catheter ablation. (A) Arrow shows that catheter tip is not in proper contact with septum. In this situation radiofrequency lesion delivery may not be effective. (B) Arrow shows firm contact of the catheter tip with the hypertrophied septum.
While ablating near the areas of tagged potentials, extra care was taken to keep catheter tip stable and power setting was titrated down to 25 W. Bipolar electrograms from the distal tip of the ablation catheter were continuously monitored during procedure to avoid injury to the conduction system. The lesion was assumed to be adequate when local bipolar voltage reduced to 50% from the baseline value at the ablation site. Baseline and post-procedure LVOT gradients were measured invasively.
2.4. Follow-up
A comprehensive transthoracic echocardiography (iE33, Philips Medical System) performed at baseline and at each follow-up by a cardiologist specialized in echocardiography. All parameters were evaluated by two blinded experts. Echocardiographic LVOT gradients were measured at resting conditions and with Valsalva maneuver at baseline and at each follow-up: one, six, and twelve months post-procedure. Maximum gradients were noted for analysis.
Patients’ ECGs were monitored for the development of any abnormality; specifically AV conduction abnormality, immediately after the procedure until discharge and during each follow-up.
2.5. Statistical analysis
All data were analyzed using the Statistical Package for Social Sciences (SPSS; Chicago, IL, USA) program, version 15. Parametric and nonparametric paired data were analyzed with Student's paired t test and Wilcoxon signed-rank test respectively. p-Values <0.05 were considered significant.
3. Results
Between April 2011 and August 2013, seven patients underwent RFCA for HOCM: five through CARTO guidance and the remaining two under the guidance of EnSiteNavX. The mean age of patients was 43.7 ± 15.6 years (range 21–62 years). Five were males. All patients were symptomatic (Table 1) despite optimal medical management. Three patients were on betablocker, two on diltiazem and two on betablocker with disopyramide. Systemic blood pressure was normal on tolerable doses of medical therapy. One patient had automatic implantable cardioverter defibrillator (AICD) implantation before RFCA for syncopal VT. The mean number of lesions delivered during RFA was 22.2 ± 7.5.
Table 1.
Patients’ clinical and echocardiographic characteristics.
| No | Age (years) | Sex | Septal anatomy | Number of RF lesions | LVOT gradienta at baseline (mmHg) | LVOT gradient after RFCA (mmHg) |
NYHA class at baseline | NYHA class post RFCA | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 month | 6 months | 12 months | ||||||||
| 1 | 42 | M | Small | 25 | 100 | 30 | 30 | 17 | III | I |
| 2 | 36 | M | Abnormal MEC | 29 | 95 | 80 | 72 | 40 | III | II |
| 3 | 62 | M | Single coronary | 17 | 90 | 45 | 48 | 38 | III | I |
| 4 | 62 | F | Abnormal MEC | 16 | 65 | 30 | 35 | 30 | III | I |
| 5 | 31 | F | Small | 34 | 84 | 77 | 70 | 65 | III | II |
| 6 | 21 | M | Abnormal MEC | 22 | 67 | 53 | 66 | 90 | III | III |
| 7 | 52 | M | Abnormal MEC | 13 | 66 | 25 | 28 | 20 | III | I |
MEC: myocardial echocardiographic contrast; RF: radiofrequency; LVOT: left ventricular outflow tract obstruction; RFCA: radiofrequency catheter ablation.
Maximum gradient obtained either at rest or with Valsalva maneuver.
3.1. LVOT gradients
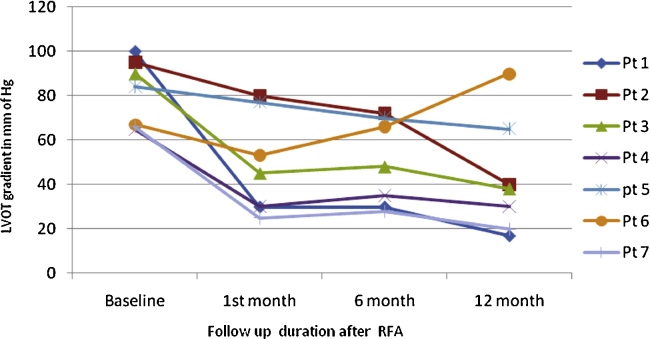
The baseline mean LVOT gradient was 81 ± 14.8 mmHg. A sustained and significant reduction in LVOT gradient from baseline was seen in all but one patient at every follow-up (Fig. 3). The mean LVEF at baseline was 70% ± 4.3%. There was no deterioration in LVEF post-procedure.
Fig. 3.
LVOT gradient of individual patients at 1-month, 6-month, and 12-month followup.
3.2. Symptomatic status
Symptomatic status improved by at least one NYHA class in all but one patient. The pre- and post-procedure NYHA class of the individual patients is given in Table 1. One patient who had severe mitral regurgitation before the procedure continued to have severe symptoms and high LVOT gradients. This patient underwent surgical myectomy and mitral valve replacement six months following RFCA.
3.3. Complications
One patient developed acute pulmonary edema immediately after the procedure, which resolved with ventilatory support and intravenous diuretic therapy. None of the patients developed AV conduction abnormality during or after the procedure. There were no arrhythmic episodes except for the one patient with ICD who had two appropriate shocks delivered during the follow-up period. The same patient underwent surgical myectomy in the follow-up period.
4. Discussion
Our study demonstrated that RFCA of the hypertrophied interventricular septum in HOCM results in significant and sustained reduction of the LVOT gradient without compromising safety. RFCA may be considered as a therapeutic alternative to the non-surgical ASA approach in the treatment of HOCM.
Until recently, surgical myectomy, ASA, and dual-chamber pacing were the most commonly discussed non-pharmacological modalities for the treatment of patients with symptomatic HOCM.12, 13, 14, 15, 16, 17 While dual-chamber pacing reduces LVOT gradient through dyssynchronous contraction of the septum, surgical myectomy and ASA achieve the effect by physical reduction in septal mass thickness. Surgical myectomy and ASA are the most widely employed non-pharmacological treatment modalities in HOCM currently. The catheter-based approach of ASA has gained momentum in the recent years,15, 16 but has two major drawbacks: obligatory requirement of a suitable septal artery anatomy and high risk of complete AV block. In view of these, RFCA seems to be a good alternative that may overcome these issues.
4.1. Mechanism of LVOT gradient reduction by RFCA
The concept of endocardial RFCA was first demonstrated by Lawrenz and Kuhn in 2004 on a 45-year-old man with severe HOCM.18 The authors reported subaortic septal hypokinesia, enlargement of the LVOT, reduction of the septal thickness, and an increase in the exercise capacity of the patient as observed seven days after the procedure. They proposed that RF ablation produces septal hypokinesis at the treated segment and this ineffective contraction resulted in decrease in pressure gradient across the LVOT. Later, the same authors demonstrated the effectiveness of the procedure on 19 patients with HOCM.10 Their study showed significant improvement in 6-min walk test, NYHA class, and 62% reduction from the resting LVOT gradient at 6 months follow-up. Sreeram et al.11 also showed promising results in their study on 32 children with HOCM. In our patients too, the decrease in gradient and also the symptomatic relief sustained until one year post-procedure.
4.2. Multimodality imaging for prevention of complications
The development of complete AV block warranting permanent pacemaker implantation is one drawback with RFCA in HOCM. Lawrenz and Kuhn18 reported CHB in 21% of patients who underwent RFCA and Sreeram et al.,11 in 6% patients. There was no occurrence of AV block in our patient population. Tagging the left bundle and the fascicles on the 3-D map helped to avoid lesions at these sites. The power delivered and the irrigation rates were lowered if RF energy had to be delivered close to these structures. We believe that these measures helped us in avoiding injury to the conduction system.
ICE proved to be a very useful modality during the procedure. Catheter manipulation in hypertrophied LV with small cavity may be difficult and is likely to influence the result. Real time ICE images helped us to manipulate ablation catheter and have stable contact at the desired location on the septum.
We believe that the single most advantage of this method is controlled delivery of RF and the avoidance of collateral damage to conduction system. RFCA is safe and effective in reducing LVOT gradient and results in symptomatic improvement. The only complication that occurred in our study was the development of acute pulmonary edema immediately following the procedure in the first patient. This was related to fluid overload due to repeated irrigation during the ablation. To our knowledge, this study is the only one to demonstrate sustained beneficial effects (12 months follow-up) after radiofrequency ablation in an adult population.
5. Conclusion
RFCA is a promising treatment option in HOCM. It allows significant reduction in LVOT gradient and symptomatic relief in patients who may not be suitable for surgical myectomy and ASA. Electroanatomical mapping helps to perform the procedure safely.
6. Study limitation
The main limitation of the study is the small population size. A larger prospective, multicenter study is needed to establish safety and efficacy.
Key message.
What is already known?
RFCA is effective in the reduction of septal hypertrophy in symptomatic HOCM, but the major risk of this procedure is the occurrence of conduction blocks.
What this study adds?
Multimodality imaging and electroanatomic mapping of the conduction system used in this study help prevent conduction blocks in RFCA.
Conflicts of interest
The authors have none to declare.
Acknowledgement
The authors would like to acknowledge Ms. Gomathi Sundar, Medical Writer and Manager, Clinical Research for her assistance in manuscript editing.
References
- 1.Maron B.J. Hypertrophic cardiomyopathy. Lancet. 1997;350:127–133. doi: 10.1016/S0140-6736(97)01282-8. [DOI] [PubMed] [Google Scholar]
- 2.Gersh B.J., Maron B.J., Bonow R.O. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg. 2011;142:e153–e203. doi: 10.1016/j.jtcvs.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Alam M., Dohainish H., Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006;19:319–327. doi: 10.1111/j.1540-8183.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes V.L., Nielsen C., Nagueh S.F. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC: Cardiovasc Interv. 2008;1:561–570. doi: 10.1016/j.jcin.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn H., Lawrenz T., Lieder F. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin Res Cardiol. 2008;97:234–243. doi: 10.1007/s00392-007-0616-7. [DOI] [PubMed] [Google Scholar]
- 6.Talreja D.R., Nishimura R.A., Edwards W.D. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J Am Coll Cardiol. 2004;44:2329–2332. doi: 10.1016/j.jacc.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Faber L., Welge D., Fassbender D., Schmidt H.K., Horstkotte D., Seggewiss H. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response. Clin Res Cardiol. 2007;96:864–873. doi: 10.1007/s00392-007-0578-9. [DOI] [PubMed] [Google Scholar]
- 8.Müssigbrodt A., Kosiuk J., Koutalas E. Results of catheter ablation of atrial fibrillation in hypertrophied hearts – comparison between primary and secondary hypertrophy. J Cardiol. 2015;65:474–478. doi: 10.1016/j.jjcc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson W.G., Delacretaz E. Radiofrequency catheter ablation of ventricular tachycardia. Heart. 2000;84:553–559. doi: 10.1136/heart.84.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrenz T., Borchert B., Leuner C. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months’ follow-up in 19 patients. J Am Coll Cardiol. 2011;57:572–576. doi: 10.1016/j.jacc.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 11.Sreeram N., Emmel M., de Giovanni J.V. Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children. J Am Coll Cardiol. 2011;58:2501–2510. doi: 10.1016/j.jacc.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Gersh B.J., Maron B.J., Bonow R.O. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the American Association for Thoracic Surgery, American Society of echocardiography, American Society of nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Ommen S.R., Maron B.J., Olivotto I. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura R.A., Trusty J.M., Hayes D.L. Dual-chamber pacing for hypertrophic cardiomyopathy: a randomized, double-blind, crossover trial. J Am Coll Cardiol. 1997;29:435–441. doi: 10.1016/s0735-1097(96)00473-1. [DOI] [PubMed] [Google Scholar]
- 15.Lakkis N.M., Nagueh S.F., Dunn J.K., Killip D., Spencer W.H. Nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy: one-year follow-up. J Am Coll Cardiol. 2000;36:852–855. doi: 10.1016/s0735-1097(00)00767-1. [DOI] [PubMed] [Google Scholar]
- 16.Veselka J., Zemánek D., Tomašov P., Duchoňová R., Linhartová K. Alcohol septal ablation for obstructive hypertrophic cardiomyopathy: ultra-low dose of alcohol (1 ml) is still effective. Heart Vessels. 2009;24:27–31. doi: 10.1007/s00380-008-1083-4. [DOI] [PubMed] [Google Scholar]
- 17.Gietzen F., Leuner C., Gerenkamp T., Kuhn H. Relief of obstruction in hypertrophic cardiomyopathy by transient occlusion of the first septal branch of the left coronary artery. Eur Heart J. 1994;15:125. [Google Scholar]
- 18.Lawrenz M.T., Kuhn M.H. Endocardial radiofrequency ablation of septal hypertrophy: a new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy. Z Kardiol. 2004;93:493–499. doi: 10.1007/s00392-004-0097-x. [DOI] [PubMed] [Google Scholar]