Summary
The effect of dried pods of Caesalpinia spinosa, known as tara, on pH, cooking loss, lipid oxidation, colour stability and texture of model meat systems stored at 4 °C for 21 days was investigated. Tara pod powder showing a potential antioxidant activity was added at 0.02, 0.04 and 0.08% (by mass) directly to the pork batter and compared with a synthetic antioxidant, butylated hydroxyanisole (BHA) and control (no added antioxidants). The addition of tara pod powder at 0.02% was as effective as BHA (0.02%) in retarding lipid oxidation in pork products during storage. Results showed that redness increased after the addition of tara pod powder. Specifically, 0.02% of tara pod powder was effective in keeping the red colour of meat batter stored under illumination at 4 °C for 48 h. Hardness of pork products was the lowest in samples manufactured with tara pod powder compared with control. Results highlight the potential of using tara pod powder as natural functional ingredient in the development of pork products with enhanced quality and shelf life.
Key words: Caesalpinia spinosa, cooked pork batter, lipid oxidation, natural antioxidants
Introduction
Lipid oxidation is the main cause of deterioration and reduced shelf life of cooked meat (1, 2). This process may cause changes in meat quality parameters such as colour, flavour, odour, texture, and even nutritional value. The heating process leads to increased oxidation of lipids in meat, which causes a warmed-over flavour in chilled cooked meat products (3, 4). The rate and extent of oxidation deterioration can be reduced through various procedures like curing, vacuum packaging, modified atmosphere packaging and, most importantly, adding synthetic or natural antioxidants (5). Although synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl gallate and tertiary butylhydroquinone (TBHQ) have been used extensively, recent studies have revealed that they have various negative health effects on animal and human cells (6). Concerns regarding the safety and toxicity of synthetic antioxidants have motivated research into natural antioxidants derived from plant sources (such as oilseeds, cereal crops, vegetables, fruits, leaves, roots, spices and herbs) (7–9). In recent times, the functional antioxidant properties of numerous plant extracts containing phenolic compounds have been investigated in cooked meat products (10–12).
Tara (Caesalpinia spinosa) is a leguminous tree indigenous to South America, having 8 to 10 cm long red or pale yellow pods. It can be found in the regions of Venezuela, Colombia, Ecuador, Peru, Bolivia, up to the north of Chile. It grows wild on the Peruvian coast and in Andean region at altitudes from 1000 to 2900 m above sea level (13). Peru is considered the most important producer worldwide with more than 80% of the world production (14). Tara infusions have been traditionally and extensively used in Peruvian folk medicine to treat inflamed tonsils, fever, cold and stomachaches (15). Tara pods (without seeds) represent approx. 65% (by mass) of the fruit. Ground tara pods are rich in hydrolysable tannins (between 40–60%, by mass), with gallic acid as the main constituent (16). Tara tannins are used in the manufacture of leather furniture, as a wine clarifier, and as a source for obtaining the antioxidant gallic acid used in the oil industry (13). Anti-inflammatory, antifungal, antibacterial and antiseptic properties have been attributed to tara tannins (15–18).
Tara pod extracts rich in gallic acid and tannins were successfully applied to increase the oxidation stability of oils (14, 19) and oil-in-water emulsions (20). Although not yet reported in the literature, tara pods could be incorporated in cooked meat products as a source of natural antioxidants to prolong quality and stability. For all these reasons, the aim of the present study is to evaluate the effect of tara pods on lipid oxidation, colour stability and textural properties of cooked meat model systems stored at 4 °C.
Materials and Methods
Plant and meat material
Fruit pods of Caesalpinia spinosa (tara) were a commercial product from Peru (Mabratan tara powder, Agrotara S.A.C., Lima, Peru). The dried powder of tara pods was stored in darkness, at room temperature. Post-rigour pork ham and fresh pork back fat were obtained from a meat plant ‘Jadwiga, Edward, Grzegorz Dworeccy’ placed in Golejewo, Poland. The meat was trimmed of visible fat and connective tissue. Lots of approx. 500 g were packed, frozen and stored at –20 °C until use.
Preparation of the extract and hydrolysis
The extraction of phenolic compounds was carried out at 4 °C for 20 h using 50% (by volume) methanol/water as solvent and a material/solvent ratio of 1:30 (by mass per volume). Then, the mixture was centrifuged at 10 000×g for 15 min and the supernatant was concentrated under vacuum at 38 °C until dryness. The resulting product was dissolved with Milli-Q water (Milipore Corporation, Bedford, MA, USA). This aqueous solution was cooled at approx. 4 °C for 16 h and then again centrifuged at 10 000×g for 10 min to obtain a clarified solution. Thus, the whole extract was obtained.
The extract was hydrolysed following the procedure of Chambi et al. (14). Briefly, the whole extract was mixed with sulphuric acid until a final concentration of 1 M H2SO4 and a phenolic compound concentration of 20 mg of gallic acid equivalent (GAE) per mL were reached. The mixture was left to stand at 100 °C for 9 h. The obtained hydrolysed tara extract was centrifuged at 10 000×g for 10 min and then subjected to liquid-liquid extraction with ethyl acetate (1:2, by volume) twice consecutively. The ethyl acetate was removed under vacuum with a rotary evaporator at 38 °C until dryness and the remaining pellet was dissolved in absolute ethanol. This was purified and hydrolysed extract of tara. The remaining aqueous phase rich in sulphuric acid was discarded.
Determination of gallic acid by high-performance liquid chromatography
High-performance liquid chromatography analyses of the tara pod extracts were carried out using an Acquity UPLC System (Waters, Milford, MA, USA) with photodiode array (PDA) detector. Tara pod extracts (10 µL) were injected into an analytical C18 column (5 µm, 3.9×150 mm; Symmetry, Waters) and the column temperature was set to 25 °C. The mobile phase was composed of 0.5% formic acid (by volume) in acetonitrile (eluent A) and 0.5% formic acid (by volume) in water (eluent B). The gradient program was as follows: 5% A (6 min), 5–95% A (2 min), 95% A (5 min), 95–5% A (1 min), 5% A (6 min). Total run time was 20 min. The detection of gallic acid was made at 273 nm. The standard was identified by its retention time and the concentration of gallic acid was calculated by comparing the peak area of samples with that of the standard. Calibration curve was made for gallic acid using different concentrations (10–100 ppm) and a high linearity (R2>0.999) was obtained.
DPPH free radical scavenging capacity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of tara pod extract was determined according to the method described by Skowyra et al. (21). A solution of DPPH (5.07 mM) in pure methanol was prepared. Appropriate dilutions were made for the study of the samples to allow the decrease in DPPH concentration to be in the range of 10–90%. Then the solution of DPPH and samples (in a volume fraction of 10% of sample and 90% of the radical) was added to the well of a microplate. Absorbance was measured at 517 nm, every 15 min for 60 min. The ability to scavenge the DPPH free radical was calculated with the following equation:
where Acontrol is the absorbance of the control sample and Asample is the absorbance of the DPPH solution containing the sample extract. A weaker absorbance of the reaction mixture indicated a stronger DPPH scavenging activity. The IC50 value, corresponding to the concentration of tara pod extract required to scavenge the DPPH radical by 50% in comparison with the control was also determined. The lower the IC50, the higher the antioxidant activity. Gallic acid was used as a standard.
Meat system formulation and processing
Meat and back fat packages were thawed (approx. 18 h at (3±2) °C). After this the materials were passed through a grinder with a 0.6-cm plate model Diana 887.84 (Zelmer, Rzeszów, Poland). Five different meat model systems (meat batters) were made up with the same amounts of pork ham (55%), pork back fat (24%), curing salt (Solino S.A., Inowroclaw, Poland) (1.6%) and iced water (19.4%). A control formulation (control) was prepared without the addition of antioxidant. Three other formulations were prepared with three different mass fractions of dried tara pod powder: 0.02, 0.04 and 0.08%. The last meat batter was prepared with a synthetic antioxidant, 0.02% butylated hydroxyanisole (BHA; Sigma- -Aldrich, St. Louis, MO, USA). A total of 15 batches (three independent replications per treatment) of pork batters were manufactured. All ingredients were placed in a cutter (Büchi Mixer B-400; Labortechnik GmbH, Essen, Germany) and mixed for approx. 5 s at 28 000×g. Finally, samples were manually stuffed into the polypropylene tubes (diameter of 30 mm). The batters were then held for 2 h at 4 °C to allow the ingredients to equilibrate. The batters were cooked by immersion in a water bath until the temperature of products reached 72 °C (measured with a thermometer inserted into the centre of the batter). The cooked batters were cooled down on ice, then taken out of the plastic tubes, dried, packaged in polyethylene bags and stored at 4 °C for 1, 7, 14 and 21 days.
Proximate composition and pH
Moisture and fat content of final products were determined in triplicate by AOAC methods (22, 23). Protein content was measured in duplicate using nitrogen analyzer KjeltecTM 2300 (FOSS, Hillerřd, Denmark). Acidity of model meat products was measured directly using an Orion 3-Star pH Benchtop Meter (Thermo Fisher Scientific, Waltham, MA, USA).
Cooking loss
Cooking losses were determined immediately after the production and were expressed as mass differences of samples before and after cooking. Before evaluation, the final products were dried using a paper towel.
Colour measurement
The surface colour of model meat products was evaluated using a reflectance colourimeter Minolta CR-400 (Konica Minolta, Tokyo, Japan) and it was expressed against the scale of L* (lightness), a* (redness) and b* (yellowness) in the CIELab colour space system. Before each measuring session (light source of D65 and 10° standard observer), the instrument was calibrated (white reference: Y=93.8; x=0.315; y=0.332). Colour measurement was conducted six times for each variant of meat products directly after production. The evaluation was repeated after 7, 14 and 21 days of cool storage in the darkness. Also, the slices of pork products were displayed under white fluorescent light (250 lx) at 4 °C for 48 h, simulating retail display conditions, and the colour measurement was carried out after 1, 3, 6, 24 and 48 h.
Lipid oxidation
The effect of tara pods on lipid oxidation of the chilled batters (initially and after 7, 14 and 21 days of storage) was evaluated using a spectrophotometric 2-thiobarbituric acid (TBA) extraction method described by Grau et al. (24) with slight modifications. Briefly, the TBARS reagent was prepared by mixing 15% (by mass per volume) trichloroacetic acid and 0.375% (by mass per volume) 2-thiobarbituric acid in 0.25 M hydrochloric acid. The procedure was as follows: 1 g of each sample was weighed in a centrifuge tube, and 1 mL of 0.3% aqueous EDTA (Sigma-Aldrich, St. Louis, MO, USA) was added immediately to stop the progression of fat oxidation. Then, just before homogenisation, 5 mL of TBARS reagent were added to the tube, and the content was homogenised for 1 min at 11 800×g using a Miccra D-1 homogeniser (ART Prozess & Labortechnik GmbH & Co., Müllheim, Germany), covered, placed in a boiling water bath for exactly 10 min and then cooled for 30 min at room temperature. The mixture was centrifuged (Sigma 3K30; Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) at room temperature and 3400×g for 10 min. The absorbance of supernatant was measured at 532 nm (spectrophotometer UV-1800; BRAIC, Beijing, PR China). The TBARS values were expressed as mg of malondialdehyde (MDA) per kg of sample calculated using 1,1,3,3-tetraethoxypropane (Sigma-Aldrich) as the standard. TBARS determinations for each sample were performed in triplicate.
Texture profile analysis
The texture profile analysis (TPA) of model meat products was conducted using Zwick/Roell Z010 testing machine (Zwick Testing Machines Ltd., Leominster, Herefordshire, UK) and TPA 50 test (50% deformation, head speed 60 mm/min, relaxation time 30 s). The samples (slices of 15 mm×25 mm) were compressed twice to 50% of their original height. The textural parameters of hardness, cohesiveness, springiness, gumminess and chewiness were measured (25). The evaluation was conducted at room temperature of (22±1) °C, directly after production and after two weeks of storage.
Statistical analysis
All experiments were performed in triplicate. Mean values of different parameters were calculated and compared by analysis of variance (two-way ANOVA) using the STATISTICA software v. 10 (StatSoft Inc., Tulsa, OK, USA). Two-way ANOVA was carried out to test the significance of the effect of tara pod powder addition and storage time. The significance of differences between samples at the same storage time and the same sample at different storage times was determined using Duncan’s test at the 95% confidence level (p<0.05).
Results and Discussion
Extract characterization
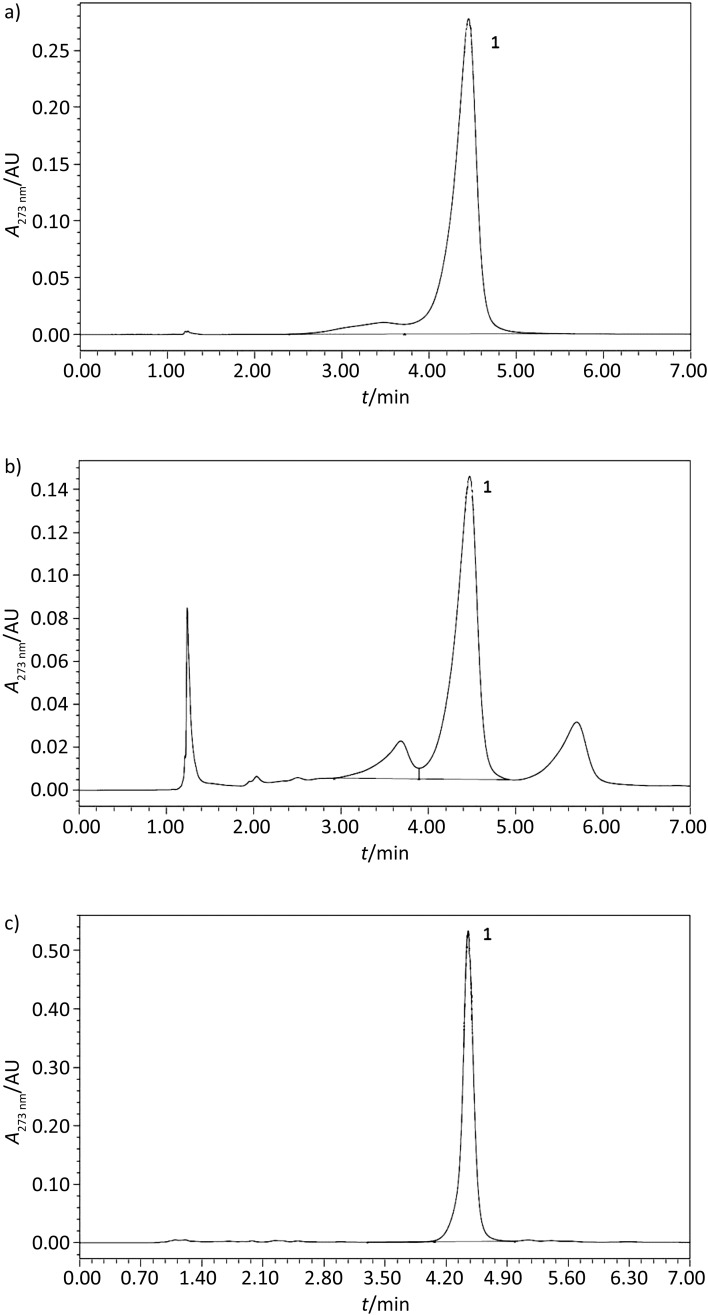
Hydrolysable tannins are common secondary metabolites in vascular plants and they are mainly found in leaves, buds, seeds, roots and tissues. Due to their antimicrobial activity, their main functions include plant defence against many pathogen attacks, and also against herbivorous animals, making assimilation of substances (gallotannins and ellagitannins) contained in the plants difficult, and giving them an unpleasant taste. Useful tannic compounds were found in the gall of walnut, such as chestnut and oak, in pomegranate (Punica granatum L.) and in tara pods (26). It has been reported that 40–65% of the fruit mass of C. spinosa corresponds to gallotannins. Hagerman (27) mentioned that gallotannins are hydrolysed, bonds between gallic acid and the polyol centre (ester bonds) are broken and depside bonds, which are more easily hydrolysed than the ester bonds, are formed between gallic acids in meta- or para-position. The results of HPLC analysis (Fig. 1) revealed that gallic acid is present in small quantities (16.9 mg of gallic acid per g of tara pods), but after hydrolysis, it is liberated to a large extent (130.7 mg of gallic acid per g of tara pods). Our results are similar to the results found by Chambi et al. (14).
Fig. 1.
HPLC chromatograms of tara pod extracts: a) external standards, b) tara pod extract, c) hydrolysed tara pod extract. 1=gallic acid
The scavenging activity of tara pod extracts, BHA and gallic acid (used as control) is shown in Table 1. Gallic acid and hydrolysed extract of tara pod were the strongest radical scavengers with IC50 of 7.3 and 8.3 µmol of GAE per L, respectively. It has been demonstrated that with the increase of the number of hydroxyl groups in the molecule, the antioxidant capacity of a phenolic compound increases. Gallic acid has three hydroxyl groups, which increases its ability to donate electrons or hydrogens. Therefore, the antioxidant capacity of hydrolysed extracts of tara pod increases when gallic acid is released (14). The IC50 value of BHA was 50.8 µmol/L. Lower IC50 values of gallic acid than of samples with BHA found in this study were also reported by Zhang et al. (28). The IC50 value of tara pod extract (468.2 µmol/L) was considerably higher than that of the hydrolysed extract. Similar results showing the increase in the antioxidant activity after tannin processing have previously been reported (16).
Table 1. Scavenging DPPH free radical activity of tara pod extracts in comparison with the control samples (gallic acid and BHA).
| Sample | IC50 µmol/L |
|---|---|
| Gallic acid BHA Tara pod extract as GAE Tara pod hydrolyzed extract as GAE |
(7.30±0.08)a (50.8±3.8)b (468.2±12.2)c (8.3±0.5)a |
All values are expressed as mean±standard deviation. Values with different letters are statistically different at p<0.05. BHA=butylated hydroxyanisole, GAE=gallic acid equivalents
Proximate composition, pH and cooking loss of pork meat batters
Table 2 shows chemical composition, pH and cooking loss of pork meat batters. The differences in moisture, fat and protein content, and pH among samples of cooked pork batter were not significant. Cooking loss measures the ability of the system to bind water and fat after protein denaturation and aggregation (4). Cooking mass losses are one of the main parameters that affect meat quality and can be mainly ascribed to water exudates (29). Cooking loss (Table 2) ranged from (5.2±0.4) to (6.3±0.2) %, and was the same in all samples containing tara pod powder and the control.
Table 2. Proximate composition, pH and cooking loss of cooked pork batters.
| Treatment |
w(moisture) % |
w(fat) % |
w(protein) % |
pH |
w(cooking loss) % |
|---|---|---|---|---|---|
| Control | 56.3±2.1 | 21.2±0.9 | 13.4±0.3 | 6.45±0.02 | (6.1±0.6)ab |
| BHA | 56.4±1.2 | 21.8±0.4 | 13.3±0.2 | 6.46±0.02 | (5.2±0.4)a |
| T1 | 58.0±0.7 | 20.4±0.1 | 13.5±0.4 | 6.45±0.01 | (5.7±0.3)ab |
| T2 | 58.2±0.9 | 20.8±0.3 | 13.8±0.8 | 6.45±0.01 | (5.95±0.03)ab |
| T3 | 57.6±2.3 | 21.5±0.1 | 13.6±0.02 | 6.44±0.06 | (6.3±0.2)b |
All values are expressed as mean±standard deviation of three replicates. Mean values in the same column (variants) with different lowercase letters are significantly different (p<0.05). Control=meat batter without antioxidants, BHA=meat batter with 0.02% of butylated hydroxyanisole, T1=meat batter with 0.02% of tara pod powder, T2=meat batter with 0.04% of tara pod powder, T3=meat batter with 0.08% of tara pod powder
Colour values of cooked pork batter
Instrumental colour parameters measured on the surface of the cooked pork batters during 21 days of refrigerated storage are shown in Table 3. Lightness (L*) values of pork batter samples with BHA, 0.02 and 0.04% of tara pod powder decreased (p<0.05) during 21 days of storage. The decrease of L* value of pork meat products was also observed by Wójciak et al. (30) in organic pork sausages. On the other hand, L* values of the sample with 0.08% of tara pod powder and the control sample were stable during this period of storage. The most important colour parameter of meat products is the redness (a*) value. Measured a* values ranged from 6.1 to 7.6 immediately after packaging to 7.1 to 9.0 after 21 days of storage. Samples with 0.02 and 0.04% of tara pod powder had significantly (p<0.05) higher redness values compared to the sample with BHA and the control sample on day 21 of refrigerated storage. Yellowness (b*) values of samples with 0.02 and 0.04% of tara pod powder were significantly (p<0.05) lower than of the sample with BHA and control sample during entire storage period. On the other hand, redness values of the cooked batters stored under illumination at 4 °C for 48 h (conditions similar to those in supermarkets and evaluated after 1, 3, 6, 24 and 48 h) ranged from 6.1 to 7.6 immediately after packaging to 3.1 to 5.2 after 48 h of storage (Table 4). The a* value (5.2) of the sample with 0.02% of tara pod powder was significantly (p<0.05) higher than of other samples after 48 h of storage under illumination. In addition, this sample had significantly (p<0.05) lower lightness and yellowness values than control samples (without antioxidant and with BHA) after 48 h of storage under illumination.
Table 3. The colour values of cooked pork batters during refrigerated storage for 21 days.
| Treatment | t(storage)/day | ||||
|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | ||
| L* | Control | (72.6±1.8)aB | (71.2±1.0)bA | (71.6±1.2)aAB | (71.9±0.2)aAB |
| BHA | (72.7±1.0)aB | (72.5±0.5)cB | (71.5±0.2)aA | (71.5±1.2)aA | |
| T1 | (69.9±0.9)bB | (70.4±0.5)aB | (68.9±0.2)bA | (68.5±1.3)bA | |
| T2 | (71.4±1.4)cB | (70.11±0.05)aA | (70.2±0.8)cA | (70.1±0.5)cA | |
| T3 | (72.8±0.5)aA | (70.7±0.4)abB | (72.1±2.3)aA | (73.1±0.7)dA | |
| a* | Control | (6.1±0.2)aB | (7.7±0.9)bA | (7.3±0.9)cA | (7.3±1.2)aA |
| BHA | (6.4±0.9)aB | (7.2±0.7)cA | (7.9±1.0)acA | (8.0±1.4)aA | |
| T1 | (7.0±0.2)bB | (8.0±0.3)abC | (8.5±0.8)bA | (9.0±0.8)bA | |
| T2 | (7.1±0.3)bC | (8.2±0.5)aA | (8.5±0.7)abAB | (8.8±0.6)bB | |
| T3 | (7.6±0.5)cA | (8.4±0.1)aB | (8.2±0.3)abB | (7.1±1.4)aA | |
| b* | Control | (8.7±0.5)bB | (7.9±0.5)bA | (8.0±0.5)aA | (8.2±0.4)bA |
| BHA | (8.5±1.4)bB | (7.7±0.4)bA | (8.0±0.07)aA | (8.18±0.06)abAB | |
| T1 | (7.8±0.6)aB | (7.0±0.7)aA | (7.1±0.1)bA | (7.4±0.2)cA | |
| T2 | (7.6±0.7)aB | (6.7±0.4)aA | (7.0±0.3)bA | (7.8±0.2)aB | |
| T3 | (7.3±0.5)aAB | (6.8±0.4)aA | (7.7±0.2)aB | (9.0±0.7)dC |
All values are expressed as mean±standard deviation of three replicates. Mean values in the same column (variants) with different lowercase letters are significantly different (p<0.05). Mean values in the same row (storage time) with different capital letters are significantly different (p<0.05). L*=lightness, a*=redness, b*=yellowness, control=meat batter without antioxidants, BHA=meat batter with 0.02% butylated hydroxyanisole, T1=meat batter with 0.02% of tara pod powder, T2=meat batter with 0.04% of tara pod powder, T3=meat batter with 0.08% of tara pod powder
Table 4. The colour values of cooked pork batter under illumination at 4 °C for 48 h.
| Treatment | t(storage)/h | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 6 | 24 | 48 | ||
| L* | Control | (73.4±1.0)abAB | (73.3±1.4)aAB | (73.0±1.7)aAB | (73.6±1.3)aB | (73.1±2.1)aAB | (72.2±0.8)aA |
| BHA | (73.6±0.3)bBC | (73.7±0.03)aC | (72.7±0.5)aA | (73.4±1.1)aBC | (73.0±1.2)aAB | (72.6±0.1)aA | |
| T1 | (71.1±0.7)cA | (70.8±0.1)bAB | (70.4±0.7)bAB | (71.4±0.4)bA | (70.6±1.9)bAB | (69.9±0.5)bB | |
| T2 | (72.1±0.5)dA | (71.8±1.7)bA | (71.7±1.7) cA | (72.3±0.7)bcA | (71.7±1.4)cAb | (70.6±1.0) bA | |
| T3 | (72.8±0.5)aA | (73.2±0.5)aA | (73.2±0.6) aA | (73.0±0.7)acA | (72.6±1.7)acA | (71.3±1.0)cB | |
| a* | Control | (6.1±0.1)aE | (5.3±0.4)aD | (4.7±0.2)cA | (4.7±0.01)cA | (4.0±0.2)aC | (3.1±0.5)aB |
| BHA | (6.1±0.6)aE | (5.2±0.4)aD | (4.5±0.1)bA | (4.5±0.2)bA | (4.0±0.2)aC | (3.4±0.1)aB | |
| T1 | (7.0±0.2)bE | (6.3±0.5)bcD | (5.7±0.3)aB | (5.61±0.06)aAB | (5.5±0.1)cA | (5.2±0.3)cC | |
| T2 | (7.2±0.3)bE | (6.2±0.3)bD | (5.6±0.3)aA | (5.5±0.3)aA | (5.0±0.4)bC | (4.0±0.8)bB | |
| T3 | (7.6±0.5)cE | (6.4±0.3)cD | (5.76±0.03)aA | (5.5±0.2)aA | (5.0±0.4)bC | (4.3±0.7)bB | |
| b* | Control | (8.7±0.5)cB | (9.4±0.5)bA | (9.5±0.8)cA | (9.4±0.9)bA | (9.6±0.6)bA | (9.8±0.9)aA |
| BHA | (8.6±1.4)cB | (9.4±1.2)bAB | (9.4±1.4)cAB | (9.2±1.4)bAB | (9.7±1.1)bA | (9.7±1.1)aA | |
| T1 | (7.9±0.6)bB | (8.7±0.5)aA | (8.7±0.9)bA | (8.6±1.0)aA | (9.0±0.8)aA | (8.9±0.6)bcA | |
| T2 | (7.5±0.5)abB | (8.5±0.5)aA | (8.7±0.7)abA | (8.4±0.8)aA | (8.6±0.6)aA | (8.6±0.2)bA | |
| T3 | (7.3±0.5)aB | (8.4±0.4)aA | (8.4±0.5)aA | (8.2±0.6)aA | (8.5±0.2)aA | (9.2±0.8)acC |
All values are expressed as mean±standard deviation of three replicates. Mean values in the same column (variants) with different lowercase letters are significantly different (p<0.05). Mean values in the same row (storage time) with different capital letters are significantly different (p<0.05). L*=lightness, a* =redness, b*=yellowness, control=meat batter without antioxidants, BHA=meat batter with 0.02% butylated hydroxyanisole, T1=meat batter with 0.02% of tara pod powder, T2=meat batter with 0.04% of tara pod powder, T3=meat batter with 0.08% of tara pod powder
The colour of food in retail exerts a strong influence on the consumer’s decision to purchase. Maintaining colour attractiveness is of primary importance since colour is the first attribute consumers use to evaluate meat quality and, therefore, it plays a major role in influencing purchase decisions (2). Meat colour is determined by its haem pigment concentration, oxidation-reduction state and light- -scattering properties (31). In the present study, the addition of tara powder (0.02 and 0.04%) resulted in redder colouration of meat product in comparison with control samples (without antioxidants and with added BHA) during the entire storage period (21 days and 48 h under illumination) (p<0.05). In addition, L* and b* values of samples containing tara powder were reduced during storage in comparison with control (p<0.05). Thus, the addition of tara pod powder changed the colour attributes of cooked pork batters by increasing redness and decreasing lightness and yellowness.
The stabilising effect of natural antioxidants on colour has been observed and verified in several studies with other natural antioxidants such as rosemary extracts (1) or ellagic acid in cooked pork (32).
Oxidation stability of cooked pork batter
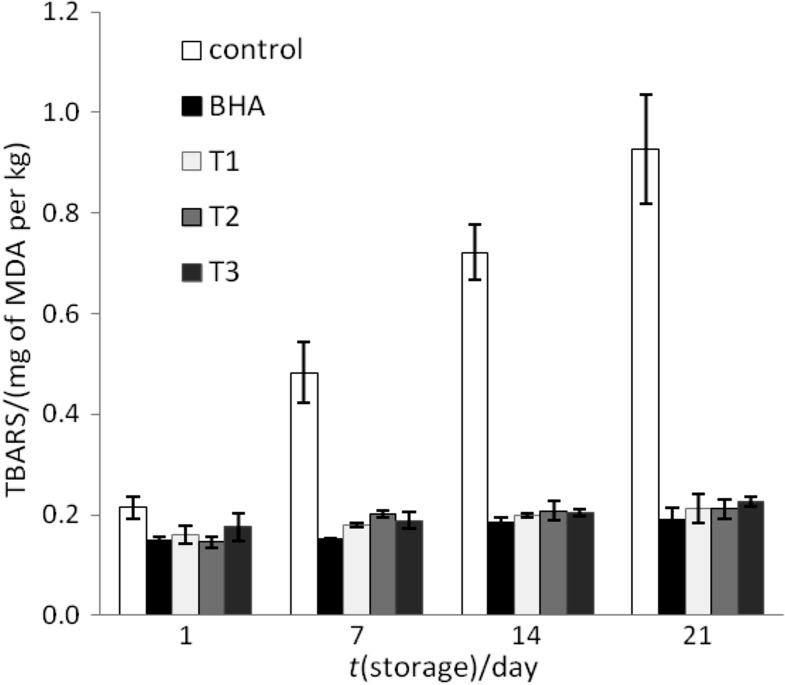
The effect of tara powder on TBARS values in cooked pork batters during storage at 4 °C for 21 days is shown in Fig. 2. In general, storage time has a significant influence on lipid oxidation in the cooked pork batters. TBARS values in all samples containing tara pod powder and in the sample with BHA were considerably lower (p<0.05) than in the control (without antioxidants). Tara pod powder showed high protection against lipid oxidation in cooked pork batters and no differences were observed between the samples containing 0.02, 0.04 and 0.08% tara pod powder. The lowest mass fraction of tara pod powder (0.02%) in cooked pork batters showed strong lipid stabilisation during storage, similar to BHA at the same mass fraction (0.02%). Initial (day 1) TBARS values in all treated cooked pork batters were significantly lower than those in the control (p<0.05), suggesting that the antioxidants retarded lipid oxidation during and immediately after cooking. In all samples with tara pod powder and in the sample containing BHA, TBARS values were at the same level on day 21 ((0.19–0.23) mg of MDA per kg of product) as in the control sample on day 1 (0.21 mg of MDA per kg of product).
Fig. 2.
The TBARS values of cooked pork batters during refrigerated storage for 21 days. Control=meat batter without antioxidants, BHA=meat batter with 0.02% butylated hydroxyanisole, T1=meat batter with 0.02% of tara pod powder, T2=meat batter with 0.04% of tara pod powder, T3=meat batter with 0.08% of tara pod powder, MDA=malondialdehyde
These oxidation/reduction effects of tara pod powder may be due to the presence of phenolic compounds such as phenolic acids. Tara pod extracts were reported to contain free gallic acid and gallotannins (1.7 and 50.4 g of GAE per 100 g, respectively). These two compounds represented approx. 95% of the total phenolics present in tara pods. The remaining 5% of tara phenolics possibly comprise ellagitannins and other phenolic compounds (14). Romero et al. (19) found that tara pod extract obtained by supercritical fluid extraction with CO2 had high antioxidant activity and was efficient in inhibiting rancidity deterioration of sunflower oil by improving its stability. Skowyra et al. (20) also reported a high level of antioxidant protection in oil-in-water emulsion with the addition of tara extracts obtained with 75% ethanol. The addition of 48 µg/mL of this extract to the emulsion with 10% oil delayed oxidation to the same extent as 17.8 µg/mL of Trolox. Bastida et al. (7) reported extracts from carob fruit rich in condensed tannins which were successfully applied to reduce fat deterioration in cooked meat during chilled and frozen storage. Retardation of lipid oxidation by different plants was demonstrated also using lotus leaf powder (0.1 and 0.5%) in cooked ground pork (10). Similar results were shown with other natural antioxidants such as rosemary extracts (0.03%) (1) or radix puerariae extracts (1%) in precooked pork sausage (33).
Texture parameters of cooked pork batter
The addition of tara pod powder to cooked pork batters reduced (p<0.05) texture parameters such as: hardness, chewiness, gumminess and cohesion (Table 5) in comparison with control (without antioxidants and with added BHA) samples on days 1 and 14. Lipid and protein oxidation are closely associated deteriorative processes occurring in meat products during storage. It has been reported that protein oxidation can negatively affect the sensory quality of meat products in terms of texture, tenderness and colour (34). Some authors have related an increase in hardness of fresh pork during storage to higher intensity of protein oxidation reactions, leading to the formation of crosslinking bonds and polymerisation in proteins (35). Results suggest that tara pod powder may have reduced the hardness of cooked pork product through its protective role against oxidation. Furthermore, these results indicate that the addition of tara powder was efficient in preparing precooked pork products with softer textural properties.
Table 5. Texture profile analysis of cooked pork batter on day 1 and 14 of refrigerated storage.
| Treatment |
t(storage) day |
Hardness N |
Springiness mm |
Cohesiveness | Chewiness Nm |
Gumminess N |
|---|---|---|---|---|---|---|
| Control | 1 | (49.1±4.0)ab | (2.8±0.2)d | (0.59±0.03)d | (41.3±3.4)e | (27.4±0.1)cd |
| BHA | (48.8±2.9)ab | (1.6±0.1)c | (0.54±0.06)cd | (33.7±3.0)bcd | (26.4±3.5)cd | |
| T1 | (38.8±4.1)a | (2.2±0.2)a | (0.42±0.06)ab | (26.8±0.6)ab | (15.2±2.8)a | |
| T2 | (41.7±2.4)a | (1.9±0.1)abc | (0.41±0.07)a | (29.2±3.0)abc | (17.3±1.9)ab | |
| T3 | (37.7±4.4)a | (2.0±0.5)ab | (0.42±0.07)ab | (27.1±3.1)ab | (17.6±1.3)ab | |
| Control | 14 | (56.2±5.1)e | (1.9±1.9)abc | (0.53±0.05)cd | (51.7±2.8)f | (30.6±1.8)d |
| BHA | (52.8±5.4)ce | (1.8±1.8)bc | (0.49±0.06)bc | (38.2±4.7)de | (24.2±2.8)c | |
| T1 | (45.3±5.3)bd | (2.0±0.1)ab | (0.43±0.04)ab | (35.9±4.0)cde | (19.6±2.9)b | |
| T2 | (39.4±1.5)a | (2.1±0.2)ab | (0.43±0.05)ab | (27.56±3.1)ab | (17.1±2.3)ab | |
| T3 | (39.8±1.5)a | (2.23±0.02)a | (0.37±0.02)a | (24.7±3.3)a | (14.8±1.2)a |
All values are expressed as mean±standard deviation of three replicates. Mean values in the same column (variants and storage time) with different lowercase letters are significantly different (p<0.05). Control=meat batter without antioxidants, BHA=meat batter with 0.02% butylated hydroxyanisole, T1=meat batter with 0.02% of tara pod powder, T2=meat batter with 0.04% of tara pod powder, T3=meat batter with 0.08% of tara pod powder
Cohesiveness measures the degree of difficulty in breaking down the internal structure of the sausage, while springiness represents the extent of recovery of sausage height and sometimes is referred to as elasticity (33). The springiness values of treated samples were considerably lower (p<0.05) than that of the negative control on day 1, but after 14 days of chilled storage, the values in batters containing antioxidants (BHA or tara pod powder) showed an increasing trend. The findings observed by TPA were confirmed by the sensory evaluation, where significant differences in textural properties among cooked pork batters were identified. Estevez et al. (36) also reported that the addition of natural antioxidants may enhance texture characteristics of emulsion-type meat products by reducing hardness, adhesiveness, gumminess and chewiness. Hayes et al. (4) found that the application of lipid-soluble ingredients such as ellagic acid protected the muscle membrane from lipid oxidation and therefore reduced moisture loss, which in turn had an effect on the sausages textural properties.
Conclusions
The results presented here suggest that tara (Caesalpinia spinosa) pod powder is a potential source of natural antioxidants and can be successfully used to decrease lipid oxidation and improve the shelf life and colour stability of cooked pork products in meat industry. In addition, from a nutritional point of view, the addition of a natural functional ingredient to cooked pork products can provide bioactive compounds (polyphenols) and it also addresses consumer demands for healthier functional food products.
Acknowledgement
The authors would like to thank Laura Muińos for technical support.
References
- 1.Lara MS, Gutierrez JI, Timón M, Andrés AI. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L.) as antioxidants in cooked pork patties packed in MAP. Meat Sci. 2011;88:481–8. 10.1016/j.meatsci.2011.01.030 [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Escalante A, Torrescano G, Djenane D, Beltrán JA, Giménez B, Roncalés P. Effect of antioxidants and lighting conditions on color and lipid stability of beef patties packaged in high-oxygen modified atmosphere. CyTA – J Food. 2011;9:49–57. 10.1080/19476330903572945 [DOI] [Google Scholar]
- 3.Brewer MS. Irradiation effects on meat flavor: a review. Meat Sci. 2009;81:1–14. 10.1016/j.meatsci.2008.07.011 [DOI] [PubMed] [Google Scholar]
- 4.Hayes JE, Stepanyan V, Allen P, O’Grady MN, Kerry JP. Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. LWT-Food Sci Technol. 2011;44:164–72. 10.1016/j.lwt.2010.05.020 [DOI] [Google Scholar]
- 5.Banerjee R, Verma AK, Das AK, Rajkumar V, Shewalkar AA, Narkhede HP. Antioxidant effects of broccoli powder extract in goat meat nuggets. Meat Sci. 2012;91:179–84. 10.1016/j.meatsci.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 6.Pop A, Berce C, Bolfa P, Nagy A, Catoi C, Dumitrescu IB, et al. Evaluation of the possible endocrine disruptive effect of butylated hydroxyanisole, butylated hydroxytoluene and propyl gallate in immature female rats. Farmacia. 2013;61:202–11. [Google Scholar]
- 7.Bastida S, Sanchez-Muniz FJ, Olivero R, Perez-Olleros L, Ruiz-Roso B, Jimenez-Colmenero F. Antioxidant activity of carob fruit extracts in cooked pork meat systems during chilled and frozen storage. Food Chem. 2009;116:748–54. 10.1016/j.foodchem.2009.03.034 [DOI] [Google Scholar]
- 8.Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–47. 10.1111/j.1541-4337.2011.00156.x [DOI] [Google Scholar]
- 9.Cofrades S, Salcedo Sandoval L, Delgado-Pando G. Lopez- -Lopez I, Ruiz-Capillas C, Jimenez-Colmenero F. Antioxidant activity of hydroxytyrosol in frankfurters enriched with n-3 polyunsaturated fatty acids. Food Chem. 2011;129:429–36. 10.1016/j.foodchem.2011.04.095 [DOI] [PubMed] [Google Scholar]
- 10.Choe JH, Jang A, Lee ES, Choi JH, Choi YS, Han DJ, et al. Oxidative and color stability of cooked ground pork containing lotus leaf (Nelumbo nucifera) and barley leaf (Hordeum vulgare) powder during refrigerated storage. Meat Sci. 2011;87:12–8. 10.1016/j.meatsci.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 11.Qin YY, Zhang ZH, Li L, Xiong W, Shi JY, Zhao TR, et al. Antioxidant effect of pomegranate rind powder extract, pomegranate juice, and pomegranate seed powder extract as antioxidants in raw ground pork meat. Food Sci Biotechnol. 2013;22:1063–9. 10.1007/s10068-013-0184-8 [DOI] [Google Scholar]
- 12.Tapp WN, Yancey JWS, Apple JK, Dikeman ME, Godbee RG. Noni puree (Morinda citrifolia) mixed in beef patties enhanced color stability. Meat Sci. 2012;91:131–6. 10.1016/j.meatsci.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 13.De la Cruz-Lapa P. An integral and rational utilility of tara (Caesalpinia spinosa). Rev del Inst Investig FIGMMG. 2004;7:64–73. [in Spanish] [Google Scholar]
- 14.Chambi F, Chirinos R, Pedreschi R, Betalleluz-Pallardel I, Debaste F, Campos D. Antioxidant potential of hydrolyzed polyphenolic extracts from tara (Caesalpinia spinosa) pods. Ind Crops Prod. 2013;47:168–75. 10.1016/j.indcrop.2013.03.009 [DOI] [Google Scholar]
- 15.Bussmann RW, Sharon D. Traditional medicinal plant use in Northern Peru: tracking two thousand years of healing culture. J Ethnobiol Ethnomed. 2006;2:47. 10.1186/1746-4269-2-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilar-Galvez A, Noratto G, Chambi F, Debaste F, Campos D. Potential of tara (Caesalpinia spinosa) gallotannins and hydrolysates as natural antibacterial compounds. Food Chem. 2014;156:301–4. 10.1016/j.foodchem.2014.01.110 [DOI] [PubMed] [Google Scholar]
- 17.Kloucek P, Polesny Z, Svobodova B, Vlkova E, Kokoska L. Antibacterial screening of some Peruvian medicinal plants used in Calleria District. J Ethnopharmacol. 2005;99:309–12. 10.1016/j.jep.2005.01.062 [DOI] [PubMed] [Google Scholar]
- 18.Kondo K, Takaishi Y, Shibata H, Higuti T. ILSMRs (intensifier of beta-lactam-susceptibility in methicillin-resistant Staphylococcus aureus) from tara [Caesalpinia spinosa (Molina) Kuntze]. Phytomedicine. 2006;13:209–12. 10.1016/j.phymed.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 19.Romero N, Fernandez A, Robert P. A polyphenol extract of tara pods (Caesalpinia spinosa) as a potential antioxidant in oils. Eur J Lipid Sci Technol. 2012;114:951–7. 10.1002/ejlt.201100304 [DOI] [Google Scholar]
- 20.Skowyra M, Falguera V, Gallego G, Peiró S, Almajano MP. Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J Sci Food Agric. 2014;94:911–8. 10.1002/jsfa.6335 [DOI] [PubMed] [Google Scholar]
- 21.Skowyra M, Calvo MI, Gallego MG, Azman NAM, Almajano MP. Characterization of phytochemicals in petals of different colours from Viola × wittrockiana Gams. and their correlation with antioxidant activity. J Agric Sci. 2014;6:93–105. 10.5539/jas.v6n9p93 [DOI] [Google Scholar]
- 22.Official Method AOAC. 950.46. Moisture in meat. Gaithersburg, MD, USA: AOAC International; 2000. [Google Scholar]
- 23.Official Method AOAC. 960.39. Fat (crude) or ether extract in meat. Gaithersburg, MD, USA: AOAC International; 2000. [Google Scholar]
- 24.Grau A, Guardiola F, Boatella J, Barroeta A, Codony R. Measurement of 2-thiobarbituric acid values in dark chicken meat through derivative spectrophotometry: influence of various parameters. J Agric Food Chem. 2000;48:1155–9. 10.1021/jf990518q [DOI] [PubMed] [Google Scholar]
- 25.Bourne MC. Texture profile analysis. Food Technol. 1978;32:62–72. [Google Scholar]
- 26.Romani A, Campo M, Pinelli P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012;130:214–21. 10.1016/j.foodchem.2011.07.009 [DOI] [Google Scholar]
- 27.Hagerman AE. The tannin handbook. 2002. USA: Miami University; Oxford, OH, Ohio. [Google Scholar]
- 28.Zhang Y, Shen Y, Zhu Y, Xu Z. Assessment of the correlations between reducing power, scavenging DPPH activity and anti-lipid-oxidation capability of phenolic antioxidants, LWT –. Food Sci Technol (Campinas). 2015;63(1):569–74. 10.1016/j.lwt.2015.03.047 [DOI] [Google Scholar]
- 29.Chiavaro E, Rinaldi M, Vittadini E, Barbanti D. Cooking of pork longissimus dorsi at different temperature and relative humidity values: effects on selected physico-chemical properties. J Food Eng. 2009;93:158–65. 10.1016/j.jfoodeng.2009.01.010 [DOI] [Google Scholar]
- 30.Wójciak KM, Karwowska M, Dolatowski ZJ. Use of acid whey and mustard seed to replace nitrites during cooked sausage production. Meat Sci. 2014;96:750–6. 10.1016/j.meatsci.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 31.Brewer S. Irradiation effects on meat color – a review. Meat Sci. 2004;68:1–17. 10.1016/j.meatsci.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 32.Hayes JE, Stepanyan V, O’Grady MN, Allen P, Kerry JP. Evaluation of the effects of selected phytochemicals on quality indices and sensorial properties of raw and cooked pork stored in different packaging systems. Meat Sci. 2010;85:289–96. 10.1016/j.meatsci.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 33.Jung E, Yun I, Go G, Kim G, Seo H. Effects of radix puerariae extracts on physicochemical and sensory quality of precooked pork sausage during cold storage. LWT –. Food Sci Technol (Campinas). 2012;46:556–62. 10.1016/j.lwt.2011.11.007 [DOI] [Google Scholar]
- 34.Rowe LJ, Maddock KR, Lonergan SM, Huff-Lonergan E. Oxidative environments decrease tenderization of beef steaks through inactivation of mu-calpain. J Anim Sci. 2004;82:3254–66. [DOI] [PubMed] [Google Scholar]
- 35.Lund MN, Lametsch R, Hviid MS, Jensen ON, Skibsted LH. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007;77:295–303. 10.1016/j.meatsci.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 36.Estévez M, Ventanas S, Cava R. Effect of natural and synthetic antioxidants on protein oxidation and colour and texture changes in refrigerated stored porcine liver pate. Meat Sci. 2006;74:396–403. 10.1016/j.meatsci.2006.04.010 [DOI] [PubMed] [Google Scholar]