Summary
The study focuses on developing novel cottage cheese containing spices with acceptable sensory properties, increased biological value and extended shelf life. Thirty types of cheese with added fresh or dried parsley, dill, pepper, garlic and rosemary were produced. Characterisation of phenolic compounds, antioxidant capacity and antibacterial activity of spices and cheese samples were evaluated. The cheese containing fresh pepper and fresh and dried herbs showed excellent sensory properties, with the best results obtained with fresh sweet red pepper. Dry rosemary had the highest antioxidant and antibacterial activity due to high mass fractions of caffeic and rosmarinic acids as well as high mass fractions of flavones and phenolic diterpenes. The plant extracts examined in vitro and in situ effectively reduce numbers of foodborne pathogens like Salmonella typhimurium, Escherichia coli, Staphylococcus aureus and Listeria monocytogenes, and therefore have potential as natural preservatives and antioxidants.
Key words: fresh pepper, spices, cheese, food spoilage bacteria, phenols, antioxidant activity
Introduction
Cottage cheese is a highly regarded dairy product. There has been increased interest in specialty cheese including cheese with additives like herbs, spices, or vegetables. The popularity of these cheese products is increasing due to their better biological value and improved flavour. Herbs and spices are used in different forms in food and traditional medicine because of their beneficial impact on health (1, 2). Spices contain phenolic compounds, one of the most important groups of natural antioxidants, which can reduce oxidative cell damage (3). In food production, phenols can reduce lipid oxidation and increase food stability (4). Numerous studies have reported antioxidant properties of many plants and spices (5–11), but total phenolic content and polyphenol profile of many of them are unknown due to the complex and varying chemical composition. Antimicrobial activity of herbal and spice extracts against different types of microbes, including some foodborne pathogens have been investigated (4, 12–15). Nevertheless, antibacterial activity of many spices is still unexplored, as well as their antimicrobial activity in real food system.
Although there are many artisan and hand crafted cheese, cream cheese, butter or yogurt with vegetables on the market, to the best of our knowledge there are not any investigations of the effects of polyphenolic compounds on biological value of these milk products. Hayaloglu and Farkye (16) gave examples of specific European cheese with spices like Otlu cheese, Surk cheese, Kanterkaas and methods of their manufacture, but without reference to their biological value and microbiological safety. Prgica, Turoš or Kvargli are small, cone shaped, dried sour cheese with salt and dry red pepper, which are produced under different names on the family farms and in small dairy plants in some parts of Croatia. Chemical composition and microbiological quality of Prgica and Turoš cheese have been investigated but data on the impact of red pepper on the biological value and shelf life of these cheese types were not given (17, 18).
For this purpose, the major phenolic substances in fresh pepper and in some fresh herbs and corresponding dried spices as well as in the mixtures with chesse have been analysed and identified. In addition, we report their antioxidant capacity and reducing power. Antibacterial activity of fresh pepper and fresh and dried herbs was determined in vitro and in situ.
Materials and Methods
Plant material and cheese
Dried spices, i.e. parsley, dill, pepper, garlic and rosemary were purchased at the store in the original packaging from different origins and manufacturers: dill from Hungary and rosemary from Morocco (Kotányi GmbH, Obersdorf, Austria), pepper from Hungary (AGZ d.o.o., Zagreb, Croatia), and garlic from China and parsley from Hungary (Derma d.d., Varaždin, Croatia). During the summer, the fresh plants were collected from the areas of Slavonski Brod, Croatia. All the herbs were stored at 4 °C for a few days or were frozen at –20 °C before analysis. Fresh pasteurised cheese with less than 10% fat in dry matter served as a base for the addition of fresh pepper or fresh and dried herbs. A total of 30 combinations were made for testing sensory properties, since each plant (fresh or dried) was added at three different mass fractions (0.5, 1 and 2 g per 100 g of cheese). Dill, parsley and rosemary were added in the form of chopped dried or fresh leaves. Cloves of garlic and sweet red horn pepper were cleaned and finely cut in a blender 800 ES (Snijders Scientific, Tilburg, The Netherlands).
Preparation of plant extracts
Samples of fresh pepper and fresh and dried herbs were treated with 30% ethanol or acetone. These solvents were selected with regard to the results achieved by Dragović-Uzelac et al. (19). Exactly 1 g of samples was weighed, extracted using 40 mL of 30% aqueous ethanol (Merck, Darmstadt, Germany) or acetone (Merck) and homogenized. Extract mixtures were put in a water bath WNE 10 (Memmert, GmbH+Co. KG, Schwabach, Germany) at 60 °C for 60 min to reflux. After extraction, the content was filtrated in 50-mL volumetric flasks and the volume was adjusted with 30% aqueous ethanol or acetone up to the mark. Three replicates were done for each sample. The obtained extracts were used for spectrophotometric and high-performance liquid chromatography (HPLC) determinations as well as of antibacterial activity.
Total phenolic content
For the determination of total phenolic content (TPC), method with Folin-Ciocalteu reagent (Kemika, Zagreb, Croatia) was used (20). The absorbance was measured at 760 nm with the spectrophotometer DR 4000 U (Hach, Loveland, CO, USA). The results were calculated according to the calibration curve for gallic acid (Sigma-Aldrich, St. Louis, MO, USA),
where y is absorbance at 760 nm and x is concentration of gallic acid in mg/L, R2=0.999. The TPC was expressed in mg of gallic acid equivalents (GAE) per 100 g of spices. Results are presented as mean value of three replicates with standard deviation (S.D.).
Antioxidant activity assays
The antioxidant activity of the fresh pepper and fresh and dried herbs was determined by two different methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay and ferric reducing antioxidant power (FRAP) assay. DPPH method was based on the reduction of stable DPPH nitrogen radicals in the presence of antioxidants in the samples (21). DPPH values are expressed in mmol of Trolox equivalents (TE) per 100 g of the sample. On the other hand, the FRAP assay was conducted according to Benzie and Strain (22). This method is based on an increase of the absorbance at 593 nm due to the formation of tripyridyl-s-triazine complexes with Fe2+[TPTZ-Fe(II)] in the presence of a reductive agent from the samples. Results are expressed as mean value of three replicates±S.D.
High-performance liquid chromatography analyses of phenols
The HPLC system used was ProStar Varian equipped with a Varian Pro Star 330 photodiode array detector (Varian, Walnut Creek, CA, USA). The HPLC column was Nucleosil® 5 µm C18 100A (Phenomenex, Torrance, CA, USA). The solvents for gradient elution were: A 0.2% o- -phosphoric acid, B methanol (J.T. Baker®, Deventer, The Netherlands), C acetonitrile (J.T. Baker) in ultrapure water. The following gradient was used: 96% A, 2% B and 2% C. The flow rate was 1.5 mL/min. Operating conditions were as follows: column temperature 30 °C and injection volume 20 µL. Chromatograms were recorded at 280 nm for phenolic acid and at 360 nm for flavonoids. The identification of the compounds was achieved by comparing the UV spectra and the retention times of the separated peaks with the retention times of the standards. Quantification was made by the external standard method using calibration of standards as a reference and was based on peak area from HPLC analyses and from mass fraction of compound. Calibration curves of the standards were made by diluting the stock solutions in 30% aqueous ethanol. The mass fractions of phenolic acids were determined according to the calibration curve of corresponding standards. Total flavones were quantified as apigenin and total flavonols as quercetin.
Antibacterial activity of plant extracts in vitro
Antibacterial activity was tested by disk diffusion method. Five pure cultures of foodborne pathogens were used: Salmonella typhimurium ATTC 14028, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Listeria monocytogenes ATCC 23074 and Staphylococcus aureus ATCC 25923 (Becton Dickinson, Le Pont de Claix, France). Strains of bacterial cultures were prepared using McFarland turbidity (Densimat, bioMérieux, Marcy I’Etoile, France) with 0.4 standard (107–108 CFU/mL). The suspension of bacteria was spread on the surface of Müller-Hinton agar (Merck), or Oxford agar (Merck) for L. monocytogenes, with sterile swabs. Previously sterilized 6-mm paper disks (Schleicher & Schuell BioScience GmbH, Dassel, Germany) were placed on the surface of the inoculated plates and then 10 µL of ethanol extracts of plants were added. A volume of 30% ethanol was used as a negative control. The inoculated plates were incubated at 37 °C for 24 h. After incubation, inhibition was measured as clear zone diameter (in mm). Results are expressed as mean value of three replicates±S.D.
Antibacterial activity of fresh and dried plants and their extracts in situ
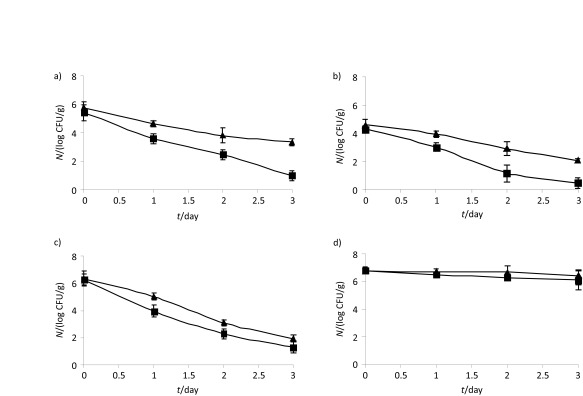
In order to determine the antibacterial activity of the fresh and dried plants or their ethanol extracts in situ, pure culture of bacteria was incubated in nutrient broth, harvested, washed, resuspended in sterile water solution and added to cheese. Also, 0.5 to 2 g of fresh or dried plants or 2 mL of their ethanol extract (originally 0.04 g of spices) were added to 100 g of cheese and homogenized. The initial number of cells (given in Fig. 1 for each strain) and total viable cells after cheese storage (three days at 4 °C) were counted by standard dilution method on nutrient agar after incubation at 37 °C for 48 h. Results were expressed as mean value of three replicates±S.D.
Fig. 1.
Antibacterial activity of rosemary extract against food pathogenic bacteria: a) Salmonella typhimurium, b) Escherichia coli, c) Staphylococcus aureus and d) Listeria monocytogenes in cheese (–▲–) and cheese supplemented with rosemary extract (–■–) during 3 days of storage at 4 °C
Sensory analysis of cheese samples
Consumer sensory tests were conducted to evaluate the acceptability of the cheese with fresh pepper or fresh and dried herbs by the evaluation committee of twenty members, according to the method of Tratnik et al. (23). Taste, smell, texture and appearance were evaluated, where the taste was the most significant attribute. The maximum possible points were 20, and depending on the scores, cheese samples were grouped as follows: excellent (17.6 to 20.0), good (15.2 to 17.5), moderate (13.2 to 15.1), acceptable (11.2 to 13.1) and unacceptable (<11.2).
Results
Phenolic content and antioxidant activity of extracts of fresh and dried plants and cheese samples
Total phenolic content and antioxidant activities measured by DPPH and FRAP assays in five fresh domestic plants and dried commercial spices are shown in Table 1. Extracts of fresh plants in acetone had lower phenolic content except garlic and rosemary, while the extracts of dried plants in acetone had a slightly higher phenolic content than the ethanol extracts. Total phenolic content in fresh plants increased in the following order: red pepper or garlic, followed by parsley, dill and rosemary. Phenolic content of dried plants was 2–4 times higher than of fresh plants, which is a consequence of the drying process and suggests that most of the antioxidants remain intact in the final dried product. Among the tested plants, rosemary had the highest antiradical capacity, with the highest FRAP value of 17.1 to 26.4 mmol per 100 g, followed by dill, parsley, sweet red peppers and garlic. Total antioxidant capacity and reducing power are proportional to the content of phenolic compounds, which was confirmed by high correlation coefficients between TPC and reducing power (data not shown).
Table 1. Total phenolic content (TPC) and antioxidant activities of the ethanol and acetone extracts of fresh and dried plants.
| Plant extract |
w(TPC as GAE) mg/100 g |
DPPH mmol TE/100 g |
FRAP mmol Fe2+/100 g |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Acetone | Ethanol | Acetone | Ethanol | Acetone | ||||
| Parsley | Fresh | 56.9±0.4 | 51.3±0.2 | 23.6±1.9 | 21.0±1.1 | 1.3±1.3 | 1.0±0.5 | ||
| Dried | 218.4±0.4 | 235.4±5.2 | 115.1±6.0 | 112.2±0.8 | 13.0±2.3 | 11.3±6.4 | |||
| Dill | Fresh | 75.8±1.4 | 43.6±6.9 | 137.5± 0.5 | 135.2±1.0 | 4.4±4.3 | 3.3±2.0 | ||
| Dried | 317.1± 6.7 | 345.8±0.6 | 137.5±1.6 | 111.7±0.8 | 19.8±6.7 | 17.8±5.9 | |||
| Pepper | Fresh | 13.6±1.7 | 10.0±2.2 | 15.5±1.6 | 15.0±3.3 | 0.4±0.2 | 0.3±0.4 | ||
| Dried | 176.8±1.9 | 182.9±0.1 | 119.9±4.4 | 116.0±4.4 | 8.1±6.2 | 7.6±6.7 | |||
| Garlic | Fresh | 36.3±9.6 | 39.9±9.1 | 59.8±3.8 | 35.7±4.9 | 0.5±1.2 | 0.6±0.4 | ||
| Dried | 85.0±0.8 | 97.4±7.7 | 92.7±6.6 | 88.0±4.2 | 2.8±3.4 | 2.7±3.4 | |||
| Rosemary | Fresh | 142.2±3.4 | 197.5±3.5 | 139.7±0.5 | 137.5±0.5 | 17.1±6.9 | 20.6±0.6 | ||
| Dried | 515.3±7.9 | 709.1±7.7 | 130.0±3.0 | 130.0±1.1 | 26.4±0.9 | 26.3±3.4 |
The results are expressed as mean value±S.D. GAE=gallic acid equivalents, TE=Trolox equivalents
High-performance liquid chromatography (HPLC) is widely used for both separation and quantification of phenolic aglycones, whereas intact glycosides can be determined by HPLC with mass spectrometry. In this study, HPLC was used to quantify hydrolyzed aglycones in the samples of plants (Table 2). Gallic, p-coumaric, chlorogenic, caffeic and rosmarinic acids were monitored. Flavonoids were expressed as total flavones or total flavonols since some peaks could not be identified as aglycones. In parsley, gallic, p-coumaric and chlorogenic acids as well as apigenin and luteolin conjugates were detected. Flavonols and caffeic acid derivatives were found in dill samples, while after hydrolysis rosmarinic acid and caffeic acid conjugates were detected. The most abundant flavonols were rutin and an unidentified kaempherol derivative. After hydrolysis, these peaks disappeared and those of quercetin and kaempherol aglycone increased. In fresh dill, phenolic acids were not detected probably because of the detection limit. The HPLC analysis of the ethanol extract obtained from the sweet red pepper pericarp showed that it contained low amount of polyphenols. After acid hydrolysis, only luteolin, quercetin and a small amount of an unidentified hydroxycinamic acid were detected. Low amount of polyphenols was detected in garlic, too. Rosemary extracts contained very high mass fractions of different phenolic compounds, among which rosmarinic acid was dominant. It was also found in dry dill (Table 2). Phenolic diterpenes in rosemary: carnosic acid, carnosol and rosmanol were not detected with the applied method of extraction and HPLC analysis.
Table 2. Mass fractions of individual phenolic compounds in the extracts of fresh and dried plants.
| Plant extract |
w(phenolic acids) mg/100 g |
w(total flavones) mg/100 g |
w(total flavonols) mg/100 g |
|||||
|---|---|---|---|---|---|---|---|---|
| Gallic | p-Coumaric | Chlorogenic | Caffeic | Rosmarinic | ||||
| Parsley | Fresh | n.d. | 13.5± 2.6 | n.d. | n.d. | n.d. | 408.9±3.4 | n.d. |
| Dried | 33.0±1.5 | 7.2±1.1 | 354.3±2.5 | n.d. | n.d. | 831.8±13.5 | n.d. | |
| Dill | Fresh | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 50.2±1.6 |
| Dried | n.d. | n.d. | 221.2±1.4 | 274.9±3.2 | 417.6±3.9 | n.d. | 522.2±2.2 | |
| Pepper | Fresh | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dried | n.d. | n.d. | n.d. | n.d. | n.d. | 18.1±0.6 | 10.0±0.6 | |
| Garlic | Fresh | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dried | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| Rosemary | Fresh | n.d. | n.d. | n.d. | 1.9±0.4 | 30.4±0.7 | 25.4±2.2 | n.d. |
| Dried | n.d. | n.d. | n.d. | 460.7±8.7 | 1044.4±12.2 | 598.4±4.4 | n.d. |
The results are expressed as mean value±S.D. n.d.=not determined
The results in Table 3 show that the biological value of cheese can be improved by adding the plants rich in bioactive phenolic compounds. Cheese samples with added fresh or dried plants had higher TPC and free radical scavenging activity than the control sample. Selection of samples for further processing was based on the sensory analysis. Among different cheese samples, the cheese with dill had the highest level of TPC (37.82 mg of GAE per 100 g), on a par with rosemary (34.54 mg of GAE per 100 g) due to the highest level of phenolic compounds in these spices. Cheese with rosemary had strong antioxidant activity. The total phenolic contents of cheese samples were measured with Folin–Ciocalteu reagents, which do not measure only phenols, and can react with many substances from the cheese. Also, a number of factors affect the interactions between phenolic molecules and protein interactions (pH, temperature, phenolic structure, molecular mass and amino acid composition). This can explain the differences in phenolic content of plants and their contribution to antioxidant activity of cheese.
Table 3. Total phenolic content (TPC) and antioxidant activities of ethanol extracts of cheese supplemented with fresh and dried plants.
| Cheese extract with | w(g/100 g) |
w(TPC as GAE) mg/100 g |
DPPH mmol TE/100 g |
FRAP mmol Fe2+/100 g |
|---|---|---|---|---|
| Parsley | Fresh 1 | 6.3±2.0 | 10.3±3.7 | 10.2±2.9 |
| Dried 0.5 | 28.4±0.1 | 23.7±2.9 | 9.7±0.6 | |
| Dill | Fresh 1 | 10.3±0.2 | 10.0±.1 | 10.9±1.7 |
| Dried 0.5 | 37.8±0.2 | 41.8±3.5 | 13.3±3.0 | |
| Pepper | Fresh 2 | 3.5±0.7 | 8.5±0.6 | 12.5±2.1 |
| Dried 0.5 | 20.3±0.8 | 14.8±5.3 | 15.5±0.6 | |
| Garlic | Fresh 0.5 | 7.1±0.2 | 16.5±2.7 | 7.1±1.4 |
| Dried 1 | 28.1±0.3 | 14.2±3.4 | 7.9±0.3 | |
| Rosemary | Fresh 0.5 | 8.3±0.3 | 16.2±0.4 | 9.4±1.0 |
| Dried 1 | 34.5±0.2 | 80.8±1.3 | 15.2±2.4 | |
| Control | (without spices) | 2.1±0.6 | 2.8±0.6 | 4.0±0.5 |
The results are expressed as mean value±S.D.
In vitro and in situ antibacterial properties of plant extracts
Antibacterial activity of the ethanolic extracts of plants against some foodborne pathogens, Gram-positive and Gram-negative bacteria, is shown in Table 4. These bacterial species are the most common contaminants of traditional homemade cheese and cream (24). Plant extracts inhibited the growth of most bacterial isolates with the wide inhibition zone in the range from 9.3 to 15.3 mm (McFarland standard 0.4) even up to 19.3–20.7 mm of rosemary extract against Gram-positive bacteria. Salmonella typhimurium and Enterococcus faecalis showed resistance to the extracts of fresh parsley, dill, garlic and sweet red pepper probably due to the lower mass fraction of phenolic compounds in these plants. However, these extracts showed significant antibacterial activity against Escherichia coli. Extracts of dried plants inhibited the growth of more or less all tested microorganisms. Among the extracts, rosemary showed very promising and significant antibacterial activity, especially against Gram-positive bacteria (Table 4). Only dill and rosemary reduced Listeria monocytogenes growth. Significantly different results were achieved in the real system when the antimicrobial activity of plant extracts and fresh plants in cheese samples was tested. Although the number of pathogens was lower in the cheese with added supplements, statistically significant difference was recorded only in the cheese supplemented with spice extracts. Rosemary extract notably inhibited bacterial growth, except L. monocytogenes (Fig. 1).
Table 4. Antimicrobial activity of the ethanol extracts of fresh and dried plants examined in vitro against foodborne pathogens (McFarland standard 0.4).
| Plant ethanol extract |
d(inhibition zone)/mm | |||||
|---|---|---|---|---|---|---|
|
Salmonella typhimurium ATCC 14028 |
Escherichia coli ATCC 25922 |
Enterococcus faecalis ATCC 29212 | Staphylococcus aureus ATCC 25923 |
Listeria monocytogenes ATCC 23074 |
||
| Parsley | Fresh | n.d. | 10.7±1.2 | n.d. | n.d. | n.d. |
| Dried | 12.7±1.2 | 10.0±2.0 | 12.0±0.0 | 14.0±2.0 | n.d. | |
| Dill | Fresh | n.d. | 13.3±1.2 | n.d. | 9.3±1.2 | 12.0±0.8 |
| Dried | 10.0±2.0 | 14.0±2.0 | 15.3±1.2 | 11.3±1.2 | 16.0±0.6 | |
| Pepper | Fresh | 9.3±1.2 | 10.0±2.0 | n.d. | 9.3±2.0 | n.d. |
| Dried | 13.3±1.2 | 9.3±2.3 | 15.3±1.2 | 11.3±1.2 | n.d. | |
| Garlic | Fresh | n.d. | 9.3±1.2 | 10.0±1.0 | 10.7±1.2 | n.d. |
| Dried | 10.7±1.2 | 10.7±1.2 | 12.7±1.2 | 12.7±1.2 | n.d. | |
| Rosemary | Fresh | 12.7±1.2 | 14.0±0.0 | 13.3±1.2 | 13.3±1.2 | 16.0±1.0 |
| Dried | 12.7±2.3 | 13.3±1.2 | 19.3±1.2 | 20.7±1.2 | 14.0±1.0 |
The results are expressed as mean value±S.D. n.d.=not detected
Sensory scores of cheese samples
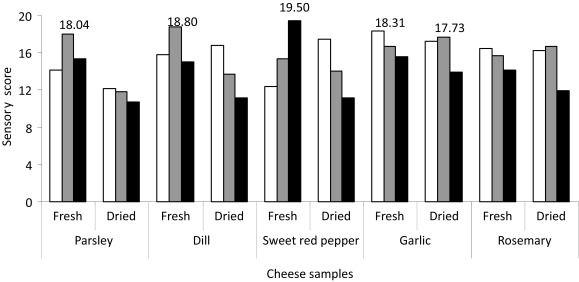
Cheese samples with the addition of 0.5, 1 and 2 g of fresh or dried plants per 100 g were selected for sensory analysis (Fig. 2). Acceptance of 30 cheese samples on the basis of overall liking by a consumer panel was conducted. The results showed better acceptability of cheese with fresh pepper or herbs in relation to the dry spices. Therefore, 80% of cheese samples with the addition of fresh plants received excellent quality scores: cheese with fresh parsley 18.04, fresh garlic 18.31, fresh dill 18.80, fresh pepper 19.50, and dry garlic 17.73. Cheese with fresh rosemary (16.46), dry pepper (17.49), dry dill (16.81) and dry rosemary (16.7) received slightly lower scores. Cheese with dried parsley got only 12.11 points and thus was classified in the category of acceptable quality. The cheese with the highest score (19.50) contained 2 g of fresh red pepper per 100 g.
Fig. 2.
The mean scores for the sensory characteristics of cheese supplemented with different fresh and dried plants in mass fractions of (in g/100 g): □ 0.5, ■ 1.0 and ■ 2
Discussion
In this paper we investigated the possibility of the use of fresh or dried pepper and herbs as cost-effective natural antioxidants and food preservatives in the production of cottage cheese with extended shelf life, acceptable sensory properties and beneficial antioxidant capacity. Herbs and spices possess essential, aromatic oils, which show antimicrobial activity and contribute to the aroma and taste of food, as well as phenolic compounds, one of the most important groups of natural antioxidants. Some researchers have already described the potential of milk or cheese supplementation with polyphenols with the aim to develop novel milk products with incorporated nutraceuticals and bioactive compounds. Cinnamon, oregano, clove, pomegranate peel, grape, green tea extract, dehydrated cranberry powder, cardamom and nutmeg were added as functional ingredients during the cheese or yogurt making process (4, 25–28). Except these, common herbs and spices added to cheese include green peppers, black peppercorns, horseradish, thyme, cumin, caraway, tarragon, basil, onion, as well as sun-dried tomatoes, depending on the country and tradition (16). Some spice extracts like oregano and clove can effectively inhibit lipid oxidation in cheese during storage and thus help to preserve the cheese sensory characteristics (4). Adding herbs or spices must not affect the metabolism and activities of the applied starter culture during fermentation and ripening. The addition of plants to the cottage cheese after cheese making process annulled this problem and it did not have an impact on the physical and chemical characteristics of the cheese in our research (protein and fat content, dry matter, ash and pH; data not shown). Parsley, dill, pepper, garlic and rosemary were investigated because these plants are commonly consumed.
Dill and parsley belong to the same Apiaceae family, and their TPC values were in close proximity. Rosemary belongs to botanical family Labiatae, also called Lamiaceae, comprising many widely used culinary herbs, such as oregano, basil, mint, sage or marjoram, all rich in phenolic compounds (25, 29–31). The literature data about total phenolic content of spices is very difficult to compare since it can be influenced by chemical structure, the extraction method employed, sample particle size, storage time and conditions, as well as presence of interfering substances. However, the results of Alezandro et al. (1) and Santos et al. (31) are similar to those obtained here, confirming the high TPC and antioxidant activity of the alcoholic extract of rosemary. The literature data about TPC in parsley and dill range from 960 to 1584 and 129 to 1250 mg of GAE per 100 g of fresh mass, respectively (5, 32, 33). Bayili et al. (34) determined total phenolic content of 59.4–74.1 mg of GAE per 100 g of fresh garlic, a value similar to our results. Tangkanakul et al. (35) reported TPC of 112.33 mg of GAE per 100 g in dried pepper while we determined 176.75 mg of GAE per 100 g of fresh mass.
Chlorogenic acid was the most abundant phenolic acid detected in parsley, different from Kaiser et al. (36), who detected p-coumaric acid derivatives as the most abundant class of phenolics. Flavones are present in significant concentrations and apigenin-7-O-apiosylglucoside (apiin) is the major compound in parsley (32, 36). Phenolic compounds are found in dill flower, leaf and seed but the flower extract had the highest total amount of polyphenols (37). Chlorogenic, p-coumaric, gallic, benzoic and p-anisic acids are the major phenolic acids detected in dill flower extract, while myricetin, quercetin, luteolin and kaempferol are the most abundant flavonoids (37). Chlorogenic, caffeic and rosmarinic acids, quercetin and kaempferol, as well as high antioxidant activity were determined, probably due to the presence of quercetin and rosmarinic acid. Quercetin has a hydroxyl group at C-3 position in the ring which results in greater free radical scavenging efficiency (38). Rosmarinic acid has two ortho-dihydroxy groups, which is the most important structural feature for strong antioxidant activity (38). Rosemary, containing rosmarinic acid, had the highest antioxidant activity. Consequently, cheese with rosemary had strong antioxidant activity. Phenolic diterpenes, rosmarinic acid and hydroxycinnamic acid ester are the major antioxidant compounds existing in rosemary (39). In peppers and garlic, low amounts of polyphenols were detected. Nevertheless, total antioxidant capacity and reducing power of peppers were high due to the presence of other health-related compounds such as ascorbic acid, carotenoids, tocopherols and capsaicinoids (40). Bae et al. (41) extracted quercetin, luteolin, kaempferol and myricetinin in descending order from ripe peppers with different extraction solvents. There are only a few research papers about the phenolic composition of garlic (42, 43). Beato et al. (43) determined total phenolic content in garlic to be in the range from 3.4 to 10.8 mg of GAE per g of dry mass and caffeic and ferulic acids were the major phenolic acids.
Food safety is a fundamental concern for both consumers and food producers. In addition to the antioxidant activity, spices have a strong antibacterial activity and can be used as food preservatives (12, 14, 15, 44). Shan et al. (12) have suggested that the antibacterial activity of spices and herbs extracts is associated with their phenolic constituents and essential oils. Our results do not show close correlation between the mass fraction of phenolic compounds and the extract inhibition zones probably due to the presence of other compounds with antibacterial activity. Fresh and dry plants showed a variable antibacterial activity against the tested strains despite the higher mass fraction of phenolics in dry spices. Garlic and peppers have a low content of polyphenols, but significant antibacterial activity was observed. Allicin, an organosulphur compound present in garlic, acts as a growth inhibitor of both Gram-positive and Gram-negative bacteria (45, 46). Lanzotti et al. (47) have reported that a bactericidal property of garlic-derived organosulphur compounds was much greater compared to phenolic compounds. Capsaicinoid, a naturally occurring alkaloid group in peppers, contributes to the pungency, taste and aroma of peppers and acts as the main antibacterial component in pepper (48). Rosemary showed the strongest antimicrobial activity, especially towards the Gram-positive bacteria. Gram-positive bacteria were more sensitive to rosemary extract and other plant extracts than Gram-negative ones due to the difference in the structure of cell membrane (49). Only dill and rosemary reduced L. monocytogenes growth probably due to the presence of rosmarinic acid in these spices. Moreno et al. (50) concluded that carnosic and rosmarinic acids are the main antimicrobial compounds present in rosemary extracts. Phenolic compounds containing hydroxyl group are more effective against microorganisms compared to those that contain the carbonyl group. The –OH group can easily bind the active site of enzymes, altering cell metabolism of microorganisms. Additionally, the position of the –OH group in the phenolic ring structure influences antibacterial and antioxidant activities (38).
There is little information about the antimicrobial activity of spices used in real food systems. The effect of spices on food pathogens depends on their phenolic content and interaction with various food components. Zaika (51) summarised the factors that can affect the antimicrobial activity of herbs and spices. The medium conditions like pH, NaCl content and temperature influenced the antibacterial activity of the rosemary extract and can have a synergistic effect with the extract of spices (29). Lipids, surface-active agents, and some proteins can influence the antibacterial activity of spices. Because of that, a large number of results that confirmed the antimicrobial activity of plants achieved in vitro must be verified in real media. Our study did not confirm all antimicrobial activity results achieved in vitro. Rosemary extract in the real system did not inhibit the L. monocytogenes growth. L. monocytogenes is more tolerant than most foodborne pathogens over a wide range of environmental conditions (52), among others, due to resistance to low pH, which was in cheese samples pH=4.28–4.31. On the other hand, some authors explained that the amounts of spices and herbs added to food for flavour are generally too low to prevent spoilage by microorganisms (53). Our results refute this view and confirm that phenolic compounds, among other compounds, are responsible for the antimicrobial activity of plants. Namely, ethanolic extract of phenols from only 0.04 g of plants efficiently reduced bacterial growth (Fig. 1), contrary to the ten times higher mass fraction of fresh plants (data not shown). We assume that the extraction of phenolic compounds was not sufficient to reduce the bacterial growth during cheese production. The extraction efficiency of phenolic compounds from plant material greatly depends on the solvent (Table 1) and water is less effective than ethanol, methanol and acetone or their aqueous mixtures (19). Also, it is generally supposed that the high levels of fat and/or protein in foodstuffs (cheese contained 10.86–11.81% of proteins and 8.33–9.75% of lipids on dry mass basis) can protect the bacteria from the activity of the phenols and essential oils. In the lipid phase, the solubility of hydrophobic compounds increases, and they are less available to act on bacteria present in the aqueous phase (54).
Conclusion
Recent trends in food industry and thus in cheese manufacture focus on the production of novel functional products with health benefits. The results show that cheese samples with fresh pepper and fresh or dried herbs are naturally flavoured products with acceptable sensory properties, highly nutritive value and good microbiological quality, which can be produced in short time. Fresh plants are more acceptable by consumers, but dry ones contribute more to the biological value. The antibacterial activity and antioxidant activity of plants originates from their phenolic compounds, which are considered as powerful bioactive compounds expressing strong antibacterial and antioxidant activities. The fresh and dried plants mixed with cheese to add flavour could aid in prolonging storage at refrigeration temperatures, at which the multiplication of microorganisms is slow. Phenolic extracts from plants have a potential as natural preservatives and antioxidants.
Acknowledgement
The authors are grateful for the support of the Institute of Public Health of Brod-Posavina County, Slavonski Brod, Croatia, and Faculty of Food Technology and Biotechnology, Zagreb, Croatia.
References
- 1.Alezandro MR, Lui MCY, Lajolo FM, Genovese MI. Commercial spices and industrial ingredients: evaluation of antioxidant capacity and flavonoids content for functional foods development. Cięncia Tecnol Aliment. 2011;31:527–33. 10.1590/S0101-20612011000200038 [DOI] [Google Scholar]
- 2.Dragland S, Senoo H, Wake K, Holte K, Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr. 2003;133:1286–90. [DOI] [PubMed] [Google Scholar]
- 3.Veeru P, Kishor MP, Meenakshi M. Screening of medicinal plant extract for antioxidant activity. J Med Plants Res. 2009;3:608–12. [Google Scholar]
- 4.Shan B, Cai YZ, Brooks JD, Corke H. Potential application of spice and herb extracts as natural preservatives in cheese. J Med Food. 2011;14:284–90. 10.1089/jmf.2010.0009 [DOI] [PubMed] [Google Scholar]
- 5.Proestos C, Chorianopoulos N, Nychas GJE, Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J Agric Food Chem. 2005;53:1190–5. 10.1021/jf040083t [DOI] [PubMed] [Google Scholar]
- 6.Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–59. 10.1021/jf051513y [DOI] [PubMed] [Google Scholar]
- 7.Suhaj M. Spice antioxidants isolation and their antiradical activity: a review. J Food Compos Anal. 2006;19:531–7. 10.1016/j.jfca.2004.11.005 [DOI] [Google Scholar]
- 8.Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9. 10.1016/j.foodchem.2007.04.038 [DOI] [Google Scholar]
- 9.Dudonné S, Vitrac X, Coutičre P, Woillez M, Mérillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57:1768–74. 10.1021/jf803011r [DOI] [PubMed] [Google Scholar]
- 10.Kratchanova M, Denev P, Ciz M, Lojek A, Mihailov A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim Pol. 2010;57:229–34. [PubMed] [Google Scholar]
- 11.Kim IS, Yang MR, Lee OH, Kang SN. Antioxidant activities of hot water extract from various spices. Int J Mol Sci. 2011;12:4120–31. 10.3390/ijms12064120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan B, Cai YT, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol. 2007;117:112–9. 10.1016/j.ijfoodmicro.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 13.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Contr. 2010;21:1199–218. 10.1016/j.foodcont.2010.02.003 [DOI] [Google Scholar]
- 14.Gyawali R, Ibrahim SA. Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Appl Microbiol Biotechnol. 2012;95:29–45. 10.1007/s00253-012-4117-x [DOI] [PubMed] [Google Scholar]
- 15.Witkowska AM, Hickey DK, Alonso-Gomez M, Wilkinson M. Evaluation of antimicrobial activities of commercial herb and spice extracts against selected food-borne bacteria. J Food Res. 2013;2:37–54. 10.5539/jfr.v2n4p37 [DOI] [Google Scholar]
- 16.Hayaloglu AA, Farkye NY. Cheese with added herbs, spices and condiments. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopedia of dairy sciences. San Diego, CA, USA: Academic Press; 2011. pp. 783–9. http://dx.doi.org/ 10.1016/B978-0-12-374407-4.00507-0 [DOI] [Google Scholar]
- 17.Valkaj K, Kalit S, Salajpal K, Zubović M, Marković T. Chemical and microbiological characterization of Turoš cheese. Agric Conspec Sci. 2014;79:201–7. [Google Scholar]
- 18.Valkaj K, Kalit S, Kalit MT, Wendorff WL. Hygienic indicators and chemical composition of Prgica cheese produced from raw and pasteurised milks. Czech J Food Sci. 2013;31:217–21. [Google Scholar]
- 19.Dragović-Uzelac V, Elez Garofulić I, Jukić M, Penić M, Dent M. The influence of microwave-assisted extraction on the isolation of sage (Salvia officinalis L.) polyphenols. Food Technol Biotechnol. 2012;50:377–83. [Google Scholar]
- 20.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolibdic-phosphotungistic reagents. Am J Enol Vitic. 1965;16:144–58. [Google Scholar]
- 21.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT –. Food Sci Technol (Campinas). 1995;28:25–30. 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- 22.Benzie IFF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. 10.1016/S0076-6879(99)99005-5 [DOI] [PubMed] [Google Scholar]
- 23.Tratnik Lj, Mioković G, Banović M. Sensory properties and acceptability of cottage cheese. Mljekarstvo. 1995;45:223–32. [Google Scholar]
- 24.Markov K, Frece J, Čvek D, Delaš F. Listeria monocytogenes and other contaminants in fresh cheese and cream from Zagreb city area domestic production. Mljekarstvo. 2009;59:225–31. [Google Scholar]
- 25.Olmedo RH, Nepote V, Grosso NR. Preservation of sensory and chemical properties in flavoured cheese prepared with cream cheese base using oregano and rosemary essential oils. LWT –. Food Sci Technol (Campinas). 2013;53:409–17. 10.1016/j.lwt.2013.04.007 [DOI] [Google Scholar]
- 26.Han J, Britten M, St-Gelais D, Champagne CP, Fustier P, Salmieri S, et al. Effect of polyphenolic ingredients on physical characteristics of cheese. Food Res Int. 2011;44:494–7. 10.1016/j.foodres.2010.10.026 [DOI] [Google Scholar]
- 27.Han J, Britten M, St-Gelais D, Champagne CP, Fustier P, Salmieri S, et al. Polyphenolic compounds as functional ingredients in cheese. Food Chem. 2011;124:1589–94. 10.1016/j.foodchem.2010.08.021 [DOI] [Google Scholar]
- 28.Illupapalayam VV, Smith SC, Gamlath S. Consumer acceptability and antioxidant potential of probiotic-yogurt with spices. LWT –. Food Sci Technol (Campinas). 2014;55:255–62. 10.1016/j.lwt.2013.09.025 [DOI] [Google Scholar]
- 29.Del Campo J, Nguyen-The C, Sergent M, Amiot MJ. Determination of the most bioactive phenolic compounds from rosemary against L. monocytogenes: influence of concentration, pH and NaCl. J Food Sci. 2003;68:2066–71. 10.1111/j.1365-2621.2003.tb07019.x [DOI] [Google Scholar]
- 30.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40:223–31. 10.1080/10715760500473834 [DOI] [PubMed] [Google Scholar]
- 31.Santos RD, Shetty K, Cecchini AL, da Silva Miglioranza LH. Phenolic compounds and total antioxidant activity determination in rosemary and oregano extracts and its use in cheese spread. Semin-Cięnc Agrár. 2012;33:655–66. 10.5433/1679-0359.2012v33n2p655 [DOI] [Google Scholar]
- 32.Luthria DL, Mukhopadhyay S, Kwansa AL. A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate. J Sci Food Agric. 2006;86:1350–8. 10.1002/jsfa.2521 [DOI] [Google Scholar]
- 33.Lisiewska Z, Kmiecik W, Korus A. Content of vitamin C, carotenoids, chlorophylls and polyphenols in green parts of dill (Anethum graveolens L.) depending on plant height. J Food Compos Anal. 2006;19:134–40. 10.1016/j.jfca.2005.04.009 [DOI] [Google Scholar]
- 34.Bayili RG, Abdoul-Latif F, Kone OH, Diao M, Bassole IHN, Dicko MH. Phenolic compounds and antioxidant activities in some fruits and vegetables from Burkina Faso. Afr J Biotechnol. 2011;10:13543–7. 10.5897/AJB10.2010 [DOI] [Google Scholar]
- 35.Tangkanakul P, Auttaviboonkul P, Niyomwit B, Lowvitoon N, Charoenthamawat P, Trakoontivakorn G. Antioxidant capacity, total phenolic content and nutritional composition of Asian foods after thermal processing. Int Food Res J. 2009;16:571–80. [Google Scholar]
- 36.Kaiser A, Carle R, Kammerer DR. Effects of blanching on polyphenol stability of innovative paste-like parsley (Petroselinum crispum (Mill.) Nym ex A. W. Hill) and marjoram (Origanum majorana L.) products. Food Chem. 2013;138:1648–56. 10.1016/j.foodchem.2012.11.063 [DOI] [PubMed] [Google Scholar]
- 37.Shyu YS, Lin JT, Chang YT, Chiang CJ, Yang DJ. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chem. 2009;115:515–21. 10.1016/j.foodchem.2008.12.039 [DOI] [Google Scholar]
- 38.Kazazić SP. Antioxidative and antiradical activity of flavonoids. Arh Hig Rada Toksikol. 2004;55:279–90. [in Croatian] [PubMed] [Google Scholar]
- 39.Almela L, Sánchez-Muńoz B, Fernández-López JA, Roca MJ, Rabe V. Liquid chromatograpic–mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A. 2006;1120:221–9. 10.1016/j.chroma.2006.02.056 [DOI] [PubMed] [Google Scholar]
- 40.Wahyuni Y, Ballester AR, Sudarmonowati E, Bino RJ, Bovy AG. Metabolite biodiversity in pepper (Capsicum) fruits of thirty-two diverse accessions: variation in health-related compounds and implications for breeding. Phytochemistry. 2011;72:1358–70. 10.1016/j.phytochem.2011.03.016 [DOI] [PubMed] [Google Scholar]
- 41.Bae H, Jayaprakasha GK, Jifon J, Patil BS. Extraction efficiency and validation of an HPLC method for flavonoid analysis in peppers. Food Chem. 2012;130:751–8. 10.1016/j.foodchem.2011.07.041 [DOI] [Google Scholar]
- 42.Bozin B, Mimica-Dukic N, Samojlik I, Anackov G, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem. 2008;111:925–9. 10.1016/j.foodchem.2008.04.071 [DOI] [Google Scholar]
- 43.Beato VM, Orgaz F, Mansilla F, Montańo A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum Nutr. 2011;66:218–23. 10.1007/s11130-011-0236-2 [DOI] [PubMed] [Google Scholar]
- 44.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11:1451–60. 10.2174/0929867043365107 [DOI] [PubMed] [Google Scholar]
- 45.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–9. 10.1016/S1286-4579(99)80003-3 [DOI] [PubMed] [Google Scholar]
- 46.Fujisawa H, Watanabe K, Suma K, Origuchi K, Matsufuji H, Seki T, et al. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci Biotechnol Biochem. 2009;73:1948–55. 10.1271/bbb.90096 [DOI] [PubMed] [Google Scholar]
- 47.Lanzotti V, Barile E, Antignani V, Bonanomi G, Scala F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera. Phytochemistry. 2012;78:126–34. 10.1016/j.phytochem.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 48.Leng TW, Muhamad II, Zaidel DNA, Khairuddin N. Evaluation of capsaicinoids extracts as bioactive substance for antimicrobial films. Jurnal Teknologi. 2013;64:269–74. [Google Scholar]
- 49.Dziri S, Hassen I, Fatnassi S, Mrabet Y, Casabianca H, Hanchi B, et al. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J Funct Foods. 2012;4:423–32. 10.1016/j.jff.2012.01.010 [DOI] [Google Scholar]
- 50.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40:223–31. 10.1080/10715760500473834 [DOI] [PubMed] [Google Scholar]
- 51.Zaika LL. Spices and herbs – their antimicrobial activity and its determination. J Food Saf. 1988;9:97–118. 10.1111/j.1745-4565.1988.tb00511.x [DOI] [Google Scholar]
- 52.Gandhi M, Chikindas ML. Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol. 2007;113:1–15. 10.1016/j.ijfoodmicro.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 53.Panpatil VV, Tattari S, Kota N, Nimgulkar C, Polasa K. In vitro evaluation on antioxidant and antimicrobial activity of spice extracts of ginger, turmeric and garlic. J Pharmacogn Phytochem. 2013;2:143–8. [Google Scholar]
- 54.Mejlholm O, Dalgaard P. Antimicrobial effect of essential oils on the seafood spoilage micro-organism Photobacterium phosphoreum in liquid media and fish products. Lett Appl Microbiol. 2002;34:27–31. 10.1046/j.1472-765x.2002.01033.x [DOI] [PubMed] [Google Scholar]