Abstract
In this Opinion article, we discuss the function of tissues as a crucial checkpoint for the regulation of effector T cell responses, and the notion that interleukin-15 (IL-15) functions as a danger molecule that communicates to the immune system that the tissue is under attack and poises it to mediate tissue destruction. More specifically, we propose that expression of IL-15 in tissues promotes T helper 1 cell-mediated immunity and provides co-stimulatory signals to effector cytotoxic T cells to exert their effector functions and drive tissue destruction. Therefore, we think that IL-15 contributes to tissue protection by promoting the elimination of infected cells but that when its expression is chronically dysregulated, it can promote the development of complex T cell-mediated disorders associated with tissue destruction, such as coeliac disease and type 1 diabetes.
The integrity of our tissues is regularly challenged by intracellular infection, in particular by viruses. In response, T helper 1 (TH1) cell-mediated immunity, which is characterized by the production of interferon-γ (IFNγ) by T cells and a concomitant increase in the number of tissue-resident cytotoxic T cells, is thought to have a key role in tissue protection by promoting the elimination of infected cells1–3. However, concurrent TH1 cell-mediated immunity and cytotoxic T cell responses are also associated with autoimmunity and tissue destruction4–6. Thus, how tissues control the initiation of TH1 cell responses and regulate cytotoxic T cells is key to maintaining their integrity.
Interleukin-15 (IL-15) is a member of the four α-helix bundle family of cytokines that includes IL-2, IL-4, IL-7, IL-9 and IL-21. IL-15 shares the common cytokine receptor γ-chain (γc; also known as CD132) of its heterodimeric receptor with the receptors for IL-2, IL-7, IL-4, IL-9 and IL-21, and it shares the β-chain (IL-2/IL-15Rβ; also known as CD122) with the receptor for IL-2 (REFS 7,8). IL-15 functions mainly in a cell contact-dependent manner through the trans-presentation of membrane-bound IL-15–IL-15Rα (IL-15 receptor α-subunit) complexes to responding cells that express IL-2/IL-15Rβ–γc8. Signalling by cis-presentation or through soluble complexes of IL-15–IL-15Rα9–11 also contributes, but to a lesser extent, to IL-15-induced responses. By contrast, IL-2, which also signals through IL-2/IL-15Rβ–γc, is mainly produced as a soluble factor by activated T cells12 (FIG. 1a) and, unlike IL-15, trans-presentation by the α-chain of the IL-2 receptor may be only a minor mechanism for IL-2-induced signalling13. IL-15 receptor signalling induces JAK1 (Janus kinase 1)–STAT3 (signal transducer and activator of transcription 3) and JAK3–STAT5 activation via the β-chain and γc, respectively14. IL-15 is notable among cytokines for being produced by a wide range of cells14, including non-myeloid cells such as epithelial and stromal cells, antigen-presenting cells and other myeloid cells such as mastocytes, and B cells and T cells of the adaptive immune system. Furthermore, IL-15 can be induced in response to innate microbial triggers15,16 and under conditions of sterile inflammation, reflecting the presence of ongoing cellular distress17,18. Notably, most — if not all — organ-specific autoimmune disorders are associated with IL-15 overexpression in the affected tissue19–26, whereas the opposite holds true for the related cytokine IL-2, deficiency of which leads to autoimmunity27,28. The absence of IL-15 in solid tumours is associated with defective lymphocyte activation in the tumour environment and decreased patient survival, which further supports a role for IL-15 in tissue immunity and destruction29.
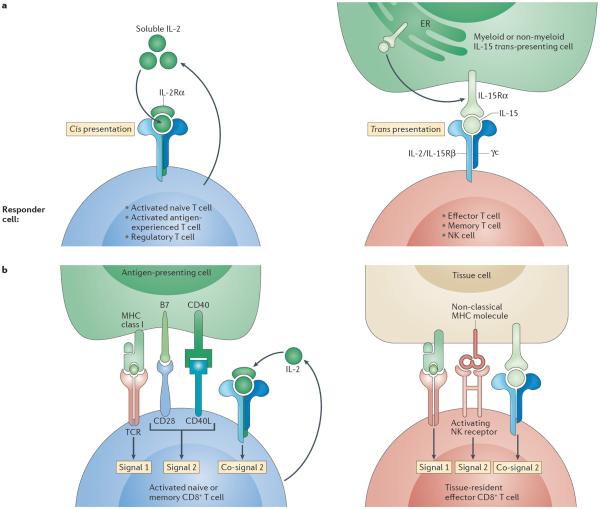
Figure 1. Models contrasting IL-15 and IL-2 signalling and the regulation of naive versus tissue-resident effector memory T cells.
a | Interleukin-15 (IL-15) signalling compared with IL-2 signalling. The main mechanism by which IL-15 interacts with its receptor in vivo is trans-presentation. IL-15 is assembled as an IL-15–IL-15 receptor α-subunit (IL-15Rα) complex intracellularly in the endoplasmic reticulum (ER), then shuttled to the cell surface and presented by distressed cells in trans to responder cells expressing a heterodimer of the IL-2/IL-15 receptor β-chain (IL-2/IL-15Rβ) and the common cytokine receptor γ-chain (γc). This receptor is constitutively expressed by effector and memory T cells, as well as by natural killer (NK) cells. Unlike IL-15, IL-2 is mainly secreted as a soluble factor by T cells in response to co-stimulation. IL-2 can bind the IL-2/IL-15Rβ–γc receptor with low affinity and interacts with high affinity in an autocrine manner with the trimeric receptor IL-2Rα–IL-2/IL-15Rβ–γc. This trimeric receptor is only transiently expressed on all activated T cells and NK cells. b | Regulation of naive versus effector cytotoxic T lymphocytes (CTLs). Naive or memory CD8+ T cells require, in addition to T cell receptor (TCR) signals (signal 1), co-stimulation (signal 2) provided by CD28 and CD40 ligand (CD40L) — which recognize B7 and CD40, respectively, expressed by dendritic cells — to become activated and undergo differentiation. In the absence of co-stimulation, very little IL-2 is produced by T cells, and cells that receive a TCR signal die or become anergic. IL-2, which is induced in response to signal 2, promotes T cell proliferation and prevents anergy159, and it therefore functions as a co-signal. By contrast, tissue-resident effector memory CD8+ T cells classically do not express CD28 and do not require signal 2 for survival. Furthermore, tissue cells do not express B7. However, we propose that a different form of co-stimulation is required for tissue effector CTLs to exert their effector function: signal 2 and co-signal 2 are provided by activating NK receptors recognizing non-classical MHC class I molecules and by IL-15, respectively, that are induced on tissue cells under conditions of stress and infection.
Considerable effort has focused on deciphering the role of IL-15 expressed by myeloid cells in the survival and proliferative expansion of natural killer (NK) cells, memory CD8+ T cells and innate-like intestinal intraepithelial lymphocytes (IELs)7,8,30 (BOX 1). However, the role of IL-15 expressed by non-haematopoietic and haematopoietic tissue-resident cells in the regulation of tissue effector T cell responses and tissue immunity in general is less well recognized. In this Opinion article, we suggest that tissues constitute a crucial checkpoint for the initiation and execution of destructive T cell responses and that IL-15 should thus be recognized as a master regulatory cytokine with regard to tissue immunity. More specifically, we propose that IL-15 is a cytokine that communicates the health status of the tissue to the immune system and has a key role in promoting immune responses that drive tissue destruction through its effects on dendritic cells (DCs) and tissue-resident effector cytotoxic T lymphocytes (CTLs). Finally, we discuss our belief that IL-2 cannot fulfil the same role as IL-15 in tissue immunity and the possible mechanisms that underlie the postulated opposing roles of IL-15 and IL-2 in tissue immunopathology.
Tissue-specific regulation of CTL responses
It has long been thought that the nature of the infectious agent and the innate pathways activated in DCs in response to infection determine which type of immune response (for example, a TH1, TH2, TH17 or regulatory T (TReg) cell response) is generated, based on the level of co-stimulatory molecules expressed and the types of cytokines produced by DCs31. However, different tissues have different challenges and requirements for ensuring their survival. For example, the eye lacks self-renewal properties and, by impeding TH1 cell responses while promoting TH2 cell and TReg cell responses, it promotes immune responses that effectively eliminate ocular pathogens while minimizing tissue damage that can cause blindness32–34. In the gut, TH17 cell responses and peripherally derived TReg cell responses are promoted to enable the maintenance of intestinal immune homeostasis through the production of secretory IgA and antimicrobial peptides35, while still allowing for protective immune responses against pathogens. Although the cellular and molecular mechanisms underlying the role of tissues in directing T cell differentiation are still being uncovered, known examples have been linked to the functional properties of tissue DCs imparted by the tissue environment. For example, intestinal DCs, which are in an environment rich in retinoic acid, transforming growth factor-β (TGFβ) and IL-6, tend to induce gut-homing receptors on T cells36–38 and to promote TReg cell39–41 and TH17 cell35,39,42 responses. Furthermore, oral but not systemic infection with the pathogen Yersinia enterocolitica triggers the selective induction of TH17 cell responses instead of TH1 cell responses through engagement of Toll-like receptor 1 (TLR1) in the presence of TGFβ and retinoic acid43.
However, although the role of the tissue environment in guiding the early differentiation of T cells is now well recognized, the role of the tissue in controlling the activation status of existing effector T cells — which encompass both tissue-resident effector memory T cells (TRM cells) and recently differentiated effector T cells that have emigrated from lymph nodes44,45 — is less well understood. Effector CTLs contain granules that are armed with cytolytic molecules (such as granzyme and perforin) and pro-inflammatory molecules (such as IFNγ). A prototype of TRM cells are IELs, which express CD69 and CD103 (also known as integrin αE), are located between epithelial cells in the intestine and have an important role in immune protection against pathogens17. Such tissue-resident effector memory CTLs must provide rapid protection against infection while preventing indiscriminate tissue destruction. Classical immunology textbooks teach us that whereas naive T cells and central memory T cells require co-stimulation (also known as signal 2) in addition to T cell receptor (TCR) stimulation (signal 1) for their activation (FIG. 1b), effector T cells require only signal 1 to mediate their effector function. We propose that this notion is only partially correct because although effector CTLs do have the potential to induce cytolysis and produce cytokines in response to TCR stimulation in the absence of co-stimulation, their activity in these circumstances is largely suboptimal as shown by the very high levels of TCR stimulation that are required in vitro and the low levels of cytolysis and cytokines produced46–48.
We therefore suggest that the tissue environment functions as a second checkpoint for effector CTL activation but that this role for the tissue has frequently been overlooked because most studies are not designed to address it. In our view, the best controlled in vivo experiment to address this issue showed that forced migration of effector CTLs in a healthy tissue that expressed the cognate antigen was not sufficient to induce tissue destruction — and, more specifically, diabetes — using a TCR- and β-islet-transgenic mouse model49. Moreover, there are several examples of mouse models in which the induction of an inflammatory adaptive immune response specific for dietary antigens is insufficient to cause tissue damage when it takes place in an intestine where epithelial cells are originally healthy50–53. In humans, two disease examples support this concept. One is latent autoimmune diabetes in adults (LADA), in which the presence of adaptive immunity against antigens expressed by β-islet cells is predictive of but not sufficient for the development of type 1 diabetes26. The other is potential coeliac disease54, in which the presence of an adaptive immune response specific for gluten does not result in the activation of intraepithelial CTLs or villous atrophy in the absence of epithelial stress (as measured by the expression of heat shock proteins and IL-15)55. The proposed tissue-specific second checkpoint for effector CTL activation has a teleological foundation in that it ensures that tissues are not unnecessarily destroyed in response to microorganisms that can activate pattern recognition receptors on DCs and hence induce T cell responses but that are not harmful to the tissue. Furthermore, this checkpoint would provide an evolutionary advantage by allowing tissues to overcome pathogen evasion strategies that depend on MHC class I downregulation leading to defective TCR activation. Based on these observations and the functional properties of IL-15 described below, we propose that IL-15 and stress inducible non-classical MHC class I molecules expressed by distressed tissue cells provide co-stimulatory signals to cytotoxic TRM cells that license them to become killer cells and destroy tissue cells (FIG. 1b).
Comparing roles of IL-2 and IL-15 in vivo
The contrasting associations of IL-15 overexpression and IL-2 deficiency with autoimmunity27,28, which have led to the development of therapies for autoimmune disorders based on selectively blocking IL-15 signalling18 but on providing low doses of IL-2 (REF. 56), suggest that even though IL-2 and IL-15 signal through a common receptor and share some important biological functions8,12, they have distinct roles in vivo. We first present evidence suggesting that there are key similarities between IL-2 and IL-15 biology, before discussing in greater depth the underlying mechanisms that may explain their apparent opposing in vivo roles in autoimmunity.
Similar functions of IL-2 and IL-15 include stimulating the generation, proliferation and activation of NK cells, promoting the proliferation of activated T cells and facilitating the induction of CTLs, as well as inducing the proliferation of, and immunoglobulin synthesis by, pre-activated B cells8. In addition, IL-2 and IL-15 induce a similar gene expression profile in peripheral blood CD8+ T cells, in particular when saturating concentrations of each cytokine are used57. However, strikingly, IL-2-deficient mice develop autoimmune and inflammatory disorders, whereas IL-15-deficient mice do not. Conversely, IL-15 overexpression has been reported in numerous organ-specific autoimmune disorders19–26, whereas to our knowledge there are no reports suggesting a link between IL-2 overexpression and autoimmunity in humans. Furthermore, IL-15-deficient mice have a reduction in the number of NK cells, central memory CD8+ T cells and resident IELs58, whereas IL-2-deficient mice develop major hyper-lymphoproliferative disorders27,28. These in vivo differences suggest that, although IL-15 and IL-2 exert some common in vivo functions, they also have key distinct functions, as illustrated by the following observations. Although IL-15 was reported to enhance the proliferative expansion of forkhead box P3 (FOXP3)+ TReg cells59–63 and to have a role in the development of CD25−FOXP3+ T cells62, the development of CD25+FOXP3+ TReg cells is acknowledged to be strictly IL-2 dependent, and IL-2 deficiency is overall associated with a major defect in the homeostatic maintenance of TReg cells62,64–66. Furthermore, IL-15 was shown to block the differentiation of peripherally derived TReg cells, especially in the presence of retinoic acid51. Finally, IL-2 promotes the elimination of potentially harmful self-reactive T cells through activation-induced cell death (AICD)67, whereas IL-15 is an anti-apoptotic factor in several systems and inhibits AICD68,69. The opposing roles of IL-15 and IL-2 in vivo in terms of AICD are in particular illustrated by a report showing that blocking IL-2 enhances the rate of appearance of dividing CD8+ memory T cells, whereas the absence of IL-2/IL-15Rβ decreases it70.
To explain the apparent paradox that IL-2 and IL-15 have distinct roles in vivo yet they signal through a common receptor, investigators in the field have relied on the explanation that IL-2 mainly functions as a soluble factor, whereas IL-15 mainly functions in a cell contact-dependent manner and hence induces signalling in the presence of other co-stimulatory signals provided by the cell trans-presenting IL-15 (REF. 8). The main mechanisms by which IL-2 and IL-15 interact with their receptors in vivo are cis-presentation and trans-presentation, respectively (FIG. 1a), although IL-15 signalling by cis-presentation or through soluble complexes of IL-15Rα–IL-15 (REFS 9–11) and IL-2 signalling by trans-presentation13 have also been described.
Ultimately, we propose that, in addition to the fact that IL-15, but not IL-2, mainly functions in a cell contact-dependent manner, two crucial differences drive the apparent paradoxical opposing roles of IL-15 and IL-2 in tissue immunopathology. First, IL-2 is typically not produced by antigen-presenting cells or non-haematopoietic cells such as epithelial or stromal cells, and thus it cannot function in a similar manner to IL-15 as a first messenger relaying tissue distress. Second, because IL-2 is secreted transiently and at low levels by tissue-resident effector CTLs45, and the produced IL-2 is rapidly consumed by the activated effector T cells and TReg cells residing in the tissue12, we suggest that IL-2 fails to reach sufficient levels in tissues to allow for signalling through the constitutively expressed dimeric IL-2/IL-15Rβ–γc receptor that is shared with IL-15. Consequently, IL-2-mediated signalling by effector T cells in these environments would mainly, if not selectively, take place through the high-affinity trimeric IL-2Rα–IL-2/IL-15Rβ–γc receptor, which is upregulated only in response to TCR signalling. This is in contrast to IL-15, which achieves much higher concentration levels locally because it is trans-presented by the distressed cell to the responder cells71,72 and which has the ability to signal selectively through the dimeric receptor because of its tenfold higher affinity than IL-2 for this receptor57. If correct, IL-2 and IL-15 would thus signal in T cells that have different activation statuses. Furthermore, the presence or absence of IL-2Rα (also known as CD25) in combination with the different affinity of the two cytokines for their receptors would lead to differences in the physical engagement and signalling of the IL-2/IL-15Rβ chain and γc. This may explain why, although IL-2 and IL-15 regulate a very large set of common genes (that is, 4,284 genes) in peripheral blood CD8+ T cells in vitro, there are 406 and 492 genes that are uniquely regulated by IL-2 and IL-15, respectively57, suggesting that the two cytokines also have unique transcriptional properties. This difference might be even more pronounced if studies were carried out on peripheral blood effector CTLs and cytotoxic TRM cells, rather than on global peripheral blood CD8+ T cells, which mainly include central memory and naive T cells.
Future functional and transcriptional profiling studies that compare the effects of different concentrations of IL-2 and IL-15 in resting and TCR-activated memory T cells and TRM cells in the absence or presence of a blocking IL-2Rα-specific antibody will help to further dissect the mechanisms underlying the differential roles of IL-2 and IL-15 in vivo. Similar studies carried out with different DC and innate lymphocyte subsets would also help to delineate the functional impact of these cytokines on tissue DCs and innate lymphocytes. Finally, when analysing the roles of IL-15 and IL-2 in vivo, it is important to take into account where these cytokines are actually expressed and can signal to responder cells under physiopathological conditions. This is illustrated, for example, by the observation that whereas IL-2 and IL-15 can both prevent FOXP3+ TReg cells from exerting their suppressive functions on effector T cells in vitro73,74, IL-15 but not IL-2 was shown to be present in the joint fluid of patients with juvenile arthritis in vivo, and therefore it can be suggested that IL-15 and not IL-2 is responsible for the TReg cell-inhibitory effect in this case74. Furthermore, there is some evidence that IL-15 overexpression in the intestinal epithelium68, but not in the lamina propria51,55, promotes the acquisition of activating NK receptors by IELs, and IL-15 (but not IL-2) was reported to be expressed by intestinal epithelial cells55,75,76.
Role of IL-15 in tissue immunity
Where IL-15 is upregulated and in which cell type it signals have a defining impact on its role in tissue immunity. Here, we discuss in detail the regulation of IL-15 and its immuno pathological effects on DCs and T cells. IL-15 also affects NK cells and group 1 innate lymphoid cells (ILC1s). ILC1s were shown to produce large amounts of IFNγ in response to IL-15 stimulation and are thought to have a role in the early protection of tissues against pathogens and potentially in intestinal inflammatory disorders77 (FIG. 2).
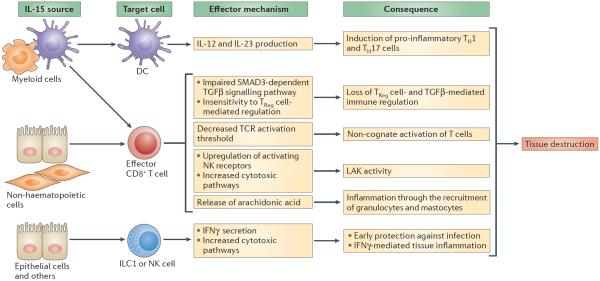
Figure 2. IL-15 has pleiotropic effects on tissue-resident cells that promote TH1 cell-mediated responses and tissue destruction.
Interleukin-15 (IL-15), produced by cells of haematopoietic or non-haematopoietic origin, can act on dendritic cells (DCs) — possibly in an autocrine manner if the source of IL-15 is a DC — and endow them with the ability to secrete IL-12 and IL-23 and to promote the differentiation of T helper 1 (TH1) and TH17 cells. In addition, IL-15 blocks the ability of transforming growth factor-β (TGFβ) to suppress the activation of T cells by impairing SMAD3-dependent TGFβ-induced signalling. By activating the phosphoinositide 3-kinase (PI3K) pathway, IL-15 renders effector CD8+ T cells unresponsive to the suppressive effect of forkhead box P3 (FOXP3)+ regulatory T (TReg) cells. In addition to its ability to upregulate the expression of activating natural killer (NK) receptors such as natural killer group 2, member D (NKG2D), which endows cytotoxic T cells with lymphokine-activated killer (LAK) activity, IL-15 lowers the T cell receptor (TCR) activation threshold. IL-15 synergizes with the NKG2D cytolytic signalling pathway, leading to the activation of cytosolic phospholipase A2 (cPLA2), which in turn crucially regulates NKG2D-mediated degranulation and cytolysis and induces the release of arachidonic acid. Arachidonic acid can promote inflammation and the recruitment and activation of granulocytes. Finally, IL-15 promotes interferon-γ (IFNγ) production by group 1 innate lymphoid cells (ILC1s) and NK cells and cytotoxic pathways in NK cells. All of these IL-15-mediated immunological effects are directed towards promoting protection against intracellular pathogens but can also lead to tissue destruction.
IL-15 regulation
IL-15 has a uniquely wide cellular distribution compared with other cytokines and can be expressed by both haematopoietic and non-haematopoietic cells. More specifically, IL-15 can be expressed by monocytes and macrophages15,78,79, DCs15,80,81, mast cells82, B cells and T cells83,84, endothelial cells78, bone marrow and lymph node stromal cells78,85, and tissue cells such as fibroblasts86, intestinal and respiratory epithelial cells75,76,87–89, hair follicles90 and keratinocytes91. Bacterial and viral infections associated with innate signals such as type I IFN, double-stranded RNA and TLR signalling through MYD88 have been reported to induce IL-15 upregulation15,16. Intriguingly, however, IL-15 has also been reported to be induced in tissues under conditions of sterile inflammation, such as in autoimmune disorders19–26,92, coeliac disease75,76,93, inflammatory bowel disease94–96, alopecia areata90 and sarcoidosis97, although the mechanisms underlying these observations remain poorly understood. Key transcription factors that are induced under inflammatory conditions and that regulate IL15 transcription are nuclear factor-κB (NF-κB; in particular p50) and IFN regulatory factor 1 (IRF1), the binding motifs for which are found in the promoter region of IL15 (REF. 98). Whether other factors associated with tissue stress, such as oxidative stress, DNA damage, endoplasmic reticulum stress or metabolic alterations, are associated with IL-15 upregulation remains to be determined. Interestingly, many of the susceptibility genes for coeliac disease and, more generally, for autoimmune and inflammatory disorders have a functional connection with IL-15, which suggests that IL-15 dysregulation in these diseases may result from alterations of this network. Whatever the mechanisms underlying IL-15 overexpression, the fact that it functions in a cell contact-dependent manner has major implications because the location of IL-15 upregulation will determine its biological effect.
Effect on DCs and the development of TH1 cells
Because of the importance of TH1 cell-mediated immunity in the protection against intracellular microorganisms and as a pathological mediator of autoimmunity, the factors that are required to promote DC production of IL-12p70, a major cytokine involved in TH1 cell differentiation, are of major interest. Importantly, the lack of IL-2/IL-15Rβ expression by DCs has been shown to affect IL-12 production and signalling in DCs ex vivo and in vitro99. In addition, IL-15-deficient mice but not IL-2-deficient mice mimic this defect in IL-12 production, which indicates a crucial role for IL-15-induced signalling in DCs for the induction of TH1 cell-mediated immunity through IL-12 production99 (FIG. 2). Furthermore, it has been demonstrated that IL-15 promotes TH1 cell-mediated immune responses in the intestinal environment by synergizing with retinoic acid to induce the differentiation of inflammatory DCs in a JUN N-terminal kinase 2 (JNK2; also known as MAPK9)-dependent manner51. Finally, the acquisition of an inflammatory phenotype by DCs upon stimulation with IL-15 is also made evident by the increased ability of IL-15-stimulated DCs to secrete IFNγ, to stimulate the proliferation of antigen-specific CD8+ T cells and to activate NK cells81,100. This ability of IL-15 to promote DC-mediated TH1 cell responses led to efforts to use IL-15-treated DCs as a potential vaccine for cancer therapy101,102. In addition, IL-15 was shown to promote IL-23 secretion by intestinal DCs and TH17 cell differentiation in the presence of IL-6 (REFS 51,103). Thus, a role for IL-15 has also been uncovered in diseases such as psoriasis22,23 and autoimmune encephalomyelitis103, in which IL-17 is thought to have a more important role in immunopathology than IFNγ.
Licensing of effector CTLs to mediate tissue destruction
The focus of this Opinion is not to discuss the largely reviewed effects of IL-15 on the homeostasis of NK cells, invariant NKT cells and CD8+ T cells8,14,104 but to address its role in the regulation of effector CTL responses in tissues. Here, we discuss the ability of IL-15 to reduce the TCR activation threshold of CTLs by providing co-stimulation, to promote lymphokine-activated killer activity (LAK activity) in CTLs (defined as the ability of a cytokine to enable a CTL to mediate cytolysis independently of the TCR and in the absence of MHC restriction) and to render CTLs resistant to TReg cells.
The ability of IL-15 to promote the cytolytic functions of effector CTLs and their production of IFNγ was first suggested by studies looking at the effect of IL-15 on IELs, which are a prototypical example of cytotoxic TRM cells105,106. Follow-up studies showed that IL-15 increased TCR-mediated cytolysis in IELs48. This is made possible because, similarly to CD28, IL-15 can activate phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signalling pathways107–109 and thereby functions as a co-stimulatory molecule for the TCR (FIG. 3). The ability of IL-15 to reduce the TCR activation threshold for effector CTLs may provide a mechanistic basis to explain the ability of CD8+ T cells to recognize self-antigens in vitro in a TCR-dependent process46 and to mediate in vivo the destruction of solid tumours lacking expression of cognate antigen in a TCR-dependent manner47 when IL-15 is present.
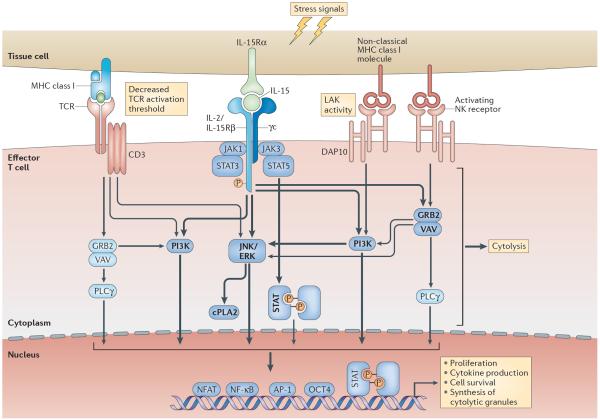
Figure 3. IL-15, NKG2D and the TCR function in synergy to enable CTLs to kill distressed target cells.
Interleukin-15 (IL-15) and non-classical MHC class I molecules are induced on tissue cells by cellular stress. IL-15-induced signalling in T cells activates phosphoinositide 3-kinase (PI3K), extracellular signal-regulated kinase (ERK), JUN N-terminal kinase (JNK) and cytosolic phospholipase A2 (cPLA2). Natural killer group 2, member D (NKG2D), which associates with the adaptor molecule DNAX-activation protein 10 (DAP10; containing a PI3K activation motif), additionally activates VAV–growth factor receptor-bound protein 2 (GRB2) and phospholipase Cγ (PLCγ)119. Both IL-15 and NKG2D can hence co-stimulate T cell receptor (TCR) signalling and enhance TCR-mediated effector functions and cell survival. As a result, IL-15-induced signalling in cytotoxic T lymphocytes (CTLs) substantially reduces the TCR activation threshold, enabling CTLs to recognize low-avidity antigens and acquire the potential for autoreactivity. Furthermore, by functioning as a co-stimulatory molecule for the NKG2D-mediated signalling pathway, IL-15 enables NKG2D to mediate direct cytolysis (that is, lymphokine-activated killer (LAK) activity), independently from signalling through the TCR. In addition, IL-15 activates the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway, leading to the phosphorylation of STAT3 and STAT5 and the formation of STAT dimers that traffic to the nucleus for transcriptional activation. How the JAK–STAT pathway intersects with TCR and NKG2D signalling remains to be determined. Arrows and words in bold highlight pathways and molecules activated by IL-15. γc, common cytokine receptor γ-chain; IL-2/IL-15Rβ, IL-2/IL-15 receptor β-subunit; IL-15Rα, IL-15 receptor α-subunit; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; NK, natural killer.
In addition to directly functioning as a co-stimulatory signalling molecule for the TCR, IL-15 promotes the expression of the NK receptors NKG2D (natural killer group 2, member D)48,107,110 and CD94 (REFS 75,111), which themselves can function as co-stimulatory receptors for the TCR in effector CTLs5. Importantly, human tissue cells can upregulate expression of the non-classical MHC class I or class I-like molecules HLA-E and MICA (MHC class I polypeptide-related sequence A) under conditions of stress or activation, which are ligands for CD94–NKG2 receptor family and NKG2D receptors, respectively107,112,113. NKG2D, like CD28, associates with the adaptor molecule DNAX-activation protein 10 (DAP10), which contains a PI3K motif114,115. Shortly after its discovery, NKG2D was shown to co-stimulate the TCR in virus-specific T cells116 and in cytolytic IELs48. Furthermore, NKG2D was proposed to have a key role in the regulation of tissue-resident effector T cells48. This is particularly relevant for human physiopathology because cytotoxic TRM cells generally lack expression of the co-stimulatory receptor CD28 (REFS 48,117), and human tissue cells lack expression of its B7 ligands (also known as CD80 and CD86).
Finally, IL-15 has been shown to increase the expression of cytotoxic effector molecules such as TNF-related apoptosis-inducing ligand (TRAIL; also known as TNFSF10) and perforin118, and to endow effector CTLs with LAK activity — in other words, to enable NKG2D–DAP10 signals to mediate TCR-independent cytolysis48,107. This property of IL-15 is linked to its ability to function as a co-stimulatory molecule for the NKG2D-mediated cytolysis signalling pathway by synergizing with NKG2D to promote the activation of PI3K, JNK, extracellular signal-regulated kinase (ERK) and cytosolic phospholipase A2 (cPLA2)107,109,119 (FIG. 3). By promoting the activation of cPLA2, IL-15 also induces the release of arachidonic acid to promote tissue inflammation together with cell lysis48,107,109. Whether and how STAT3 and STAT5 (which are downstream of the IL-15 receptor) are involved in the regulation of NK receptor expression and function in CTLs remain to be determined.
In summary, we propose that IL-15 has the ability to enable effector CTLs to kill damaged tissue cells based on the recognition of stress and inflammatory signals rather than a cognate antigen through two distinct, but not mutually exclusive, mechanisms: by decreasing the TCR activation threshold of effector CTLs and by inducing TCR-independent LAK activity in CTLs (FIG. 2). In the absence of specific antigen recognition, the targeting of tissue cells by effector CTLs remains highly specific as it involves receptor–ligand interactions that direct CTLs exclusively against cells that upregulate expression of IL-15 and non-classical MHC class I or class I-like molecules. Furthermore, IL-15 confers such properties to effector but not central memory CTLs — in other words, only to T cells that have undergone recent activation through their TCR, which further helps to contain and restrict the potent effects of IL-15.
Rendering CTLs resistant to TReg cells
Following the discovery that TReg cells have an important role in preventing autoimmunity120, it was surprising to find that the number of TReg cells is increased in tissues of patients with autoimmune diseases121. In particular, studies have shown that there is an increase in the number of CD4+CD25hi T cells that have suppressive functions and express cytotoxic T lymphocyte-associated antigen 4 (CTLA4), glucocorticoid-induced TNFR-related protein (GITR; also known as TNFRSF18) and OX40 (also known as TNFRSF4) — the classical TReg cell phenotype — in patients with rheumatoid arthritis122,123 and inflammatory bowel disease124,125. This suggests that, at least in these cases, the increase in the number of FOXP3+ T cells that is observed in humans is probably not due to a failure to discriminate between effector and regulatory T cells. This led to further analysis of the ability of TReg cells to exert their regulatory functions and to the discovery that several cytokines of the γc family, including IL-15, IL-7 and IL-2, could prevent TReg cells from exerting their suppressive effect on effector CD4+ T cells in vitro. However, only IL-15 and IL-7 were present in the synovial fluid, suggesting that these cytokines, but not IL-2, limit the suppressive effect of TReg cells in patients with juvenile arthritis74. Later, it was shown that IL-15 renders human peripheral blood-derived CD4+ and CD8+ T cells resistant to the suppressive effects of thymus-derived CD4+CD25+FOXP3+ TReg cells by activating the PI3K pathway in the effector T cells73 (FIG. 2). These direct effects of IL-15 on TReg cells and their suppressive functions, together with the ability of IL-15 to inhibit IL-2-induced AICD of effector lymphocytes126, underline the ability of IL-15 to interfere with immune tolerance.
Hence IL-15, a cytokine that is induced in distressed tissues, profoundly changes the functional phenotype of differentiated immune cells, favouring the development of TH1 cell responses, decreasing the TCR activation threshold of effector CTLs and endowing them with LAK activity, and rendering effector T cells insensitive to the suppressive effects of TReg cells. All of these properties are tailored towards favouring the destruction of tissue cells (FIG. 2), which may be beneficial in the context of intracellular infection but is deleterious in the context of autoimmunity.
Role of IL-15 in health and disease
Taking into account the proposed role of IL-15 in the tissue-specific regulation of effector T cell responses, here we discuss its role in protection against infection and in autoimmunity. Conversely, we suggest that the lack of IL-15 and stress signals in solid tumours may have a crucial role in the inability of CTLs to eliminate such tumours (FIG. 4).
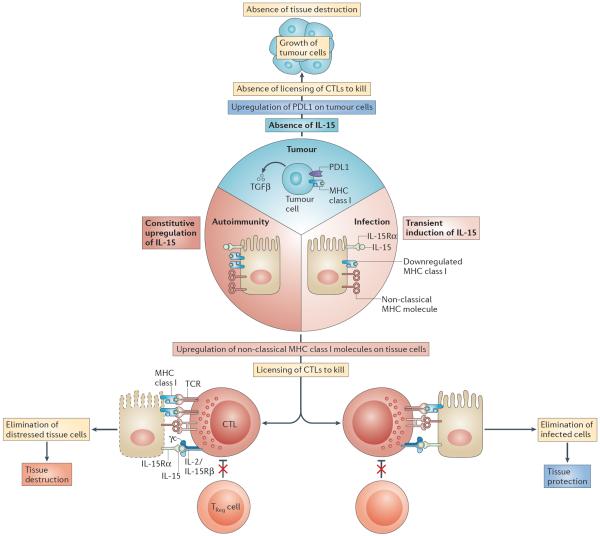
Figure 4. Proposed roles of IL-15 in tissue protection and tissue destruction.
Intracellular microorganisms, in particular viruses, cause the downregulation of expression of MHC class I molecules as a mechanism of immune evasion to prevent the destruction of infected cells by cytotoxic T lymphocytes (CTLs). The host, in turn, upregulates expression by infected cells of interleukin-15 (IL-15) and the non-classical MHC class I molecules such as MHC class I polypeptide-related sequence A (MICA), which is recognized by the activating natural killer (NK) receptor NKG2D (natural killer group 2, member D). Together, this leads to a reduced T cell receptor (TCR) activation threshold and to lymphokine-activated killer (LAK) activity in CTLs. CTLs can hence destroy infected cells despite low levels of or absent MHC class I expression. Furthermore, effector CTLs are rendered resistant to the effects of forkhead box P3 (FOXP3)+ regulatory T (TReg) cells and transforming growth factor-β (TGFβ) in the presence of IL-15. Once the infected cells are eliminated and replaced by healthy cells, CTLs return to a `resting state' and again become sensitive to immune regulation. In the context of autoimmunity, MHC class I molecules are not downregulated, and the expression of IL-15 and MICA is constitutive for unknown reasons. This leads to the chronic activation of CTLs and ongoing tissue destruction. By contrast, tumours — in addition to expressing TGFβ and PDL1, which is the ligand for the inhibitory receptor PD1 (programmed cell death protein 1) — also lack surface expression of IL-15 and MICA. Hence, CTLs lack the necessary activating signals as well as being sensitive to the inhibitory signals present in the tumour environment, resulting in a lack of CTL-mediated killing of tumour cells. γc, common cytokine receptor γ-chain; IL-2/IL-15Rβ, IL-2/IL-15 receptor β-subunit; IL-15Rα, IL-15 receptor α-subunit.
Protection of tissues against pathogens
Protection against intracellular microorganisms is primarily mediated by CTL-mediated destruction of infected cells. A key immune evasion strategy used by intracellular pathogens is to downregulate MHC class I expression by infected cells to prevent TCR-mediated recognition by CTLs127. However, the host has evolved mechanisms to counteract pathogen immune evasion and ensure protective immunity that involve upregulation of the expression of IL-15 and non-classical MHC molecules. Intracellular pathogens such as mycobacteria128, listeria129, Cryptosporidium parvum130 and a wide range of viruses89,131–133 have been reported to induce IL-15 expression, probably downstream of the activation of various innate immune receptors such as TLR4 (REFS 81,133). Furthermore, intracellular pathogens have been reported to upregulate the expression of non-classical MHC class I ligands for activating NK receptors on host cells. For example, the NKG2D ligands MICA and MICB and their mouse counterpart RAE1 (retinoic acid early transcript 1) have been shown to be upregulated on stressed infected cells89,130,134. Of note, induction of these non-classical MHC class I molecules is not directly mediated by IL-15 (REF. 135) and occurs in response to heat shock factor 1, hyper proliferation and DNA damage136,137. The ability of IL-15 to function as a co-stimulatory signalling molecule for the TCR (see earlier) and to induce the expression of NK receptors enables CTLs to bypass the effects of downregulation of MHC class I molecules and to kill infected cells based on the recognition of danger signals. In agreement, IL-15 was shown to promote the upregulation of activating NK receptors on CTLs and the induction of NK receptor-dependent cytotoxic mechanisms in several infectious contexts130,134,138–140. Interestingly, viruses have developed several strategies to prevent the expression of NK receptor ligands on the surface of infected cells141, which indicates that this is an effective host strategy and must be countered by viruses for them to survive.
In summary, in response to an infection with intracellular pathogens, IL-15 activates key immune cell types and effector functions that are crucial for the destruction of infected cells. Furthermore, because IL-15 functions in a cell contact-dependent manner and the upregulation of its expression ends with the clearance of the pathogen, CTLs are only activated in a transient manner and specifically target infected cells. This mechanism provides protection for tissues against pathogens while avoiding indiscriminate tissue destruction.
Autoimmune responses
The same properties that we believe enable IL-15 to mediate protection against pathogens are probably responsible for its role in the pathogenesis of organ-specific autoimmune disorders in which its expression is dysregulated. IL-15 expression is stringently regulated at the levels of transcription, translation and intracellular trafficking to avoid excessive protein production and secretion98 (BOX 2). However, for unknown reasons, IL-15 has been found to be constitutively upregulated in tissue cells that are targeted by a wide variety of autoimmune processes, such as rheumatoid arthritis19,20, multiple sclerosis21,142, psoriatic arthritis or psoriasis22,23, systemic lupus erythematosus24,25, type 1 diabetes26, inflammatory bowel disease94–96 and coeliac disease75,76,93.
The chronic overexpression of IL-15 in tissues is also often associated with the upregulated expression of ligands for activating NK receptors. For example, coeliac disease is characterized by the induction of stress-inducible MIC molecules107,112 and of the non-classical MHC class I molecule HLA-E113 on epithelial cells, which are ligands for NKG2D and CD94–NKG2C, respectively. IL-15 promotes the expression of these activating NK receptors by IELs107,113, which acquire cytotoxic properties and the ability to kill epithelial cells based on the recognition of stress signals48,107,109. IL-15 also upregulates NKG2D and DAP10 expression on peripheral blood CD4+NKG2D+ T cell clones from patients with Crohn disease143. Furthermore, IL-15 upregulation in patients with Crohn disease94–96 is associated with increased expression of NKG2D on CD4+ T cells in the lamina propria and of MICA on epithelial cells, which suggests that tissue damage in these patients could be mediated by the interaction between CD4+NKG2D+ T cells and MICA+ epithelial cells143. The severity of rheumatoid arthritis pathology is also associated with the degree of expansion of a population of CD4+CD28− T cells that express NKG2D upon stimulation with IL-15 and tumour necrosis factor (TNF) and with the expression level of stress-inducible MIC ligands by rheumatoid synoviocytes144. Finally, IL-15 endows these CD4+CD28− T cells with the ability to lyse microvascular endothelial cells in patients with Wegener granulomatosis145, increases the cytotoxic properties of CD8+ T cells from patients with multiple sclerosis84 and is associated with NKG2D upregulation in patients with alopecia areata90.
Inhibition of JAK1 and JAK2 in patients with alopecia areata who overexpress IL-15 in their hair follicles leads to a near-complete restoration of hair growth90, which suggests that IL-15 has a pathogenic role, although it cannot be excluded that JAK inhibition could affect signalling induced by other cytokines such as IL-2. Other strong evidence in support of a role for IL-15 in tissue destruction in the context of autoimmune disorders is provided by observations in patients with LADA or potential coeliac disease who maintain normal tissue integrity despite having developed an adaptive T cell response specific for β-islet cells or gluten, respectively (FIG. 5). Indeed, IL-15 is upregulated in the serum and β-islet cells of patients with type 1 diabetes but not with LADA26,146. Similarly, patients with active coeliac disease, but not potential coeliac disease, overexpress IL-15 in their epithelium55. Moreover, and in support of a primary role for IL-15 in mediating tissue destruction, we found that a subset of family members of patients with active coeliac disease had normal intestinal architecture and no signs of adaptive anti-gluten immunity but had epithelial cells expressing high levels of IL-15. Strikingly, IL-15 overexpression was absent in patients with potential coeliac disease. In accordance with IL-15 having a role in the licensing of cytotoxic TRM cells to kill, only IELs from family members with high levels of IL-15 expression had upregulated expression of NKG2D and activating CD94 NK receptors55. The absence of villous atrophy in these individuals correlated with the presence of IELs with high levels of inhibitory CD94–NKG2A receptors55 and of epithelial cells that failed to upregulate expression of MICA (B.J., unpublished observation). These observations in humans are consistent with reports in transgenic mouse models showing that antigen-specific CTLs fail to mediate tissue destruction despite the presence of cognate antigens when the tissue is healthy and hence fails to provide stress signals to the immune system49.
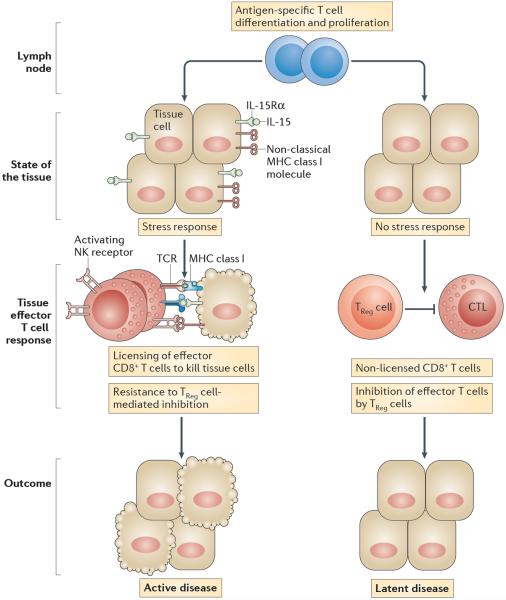
Figure 5. Lack of IL-15 expression by tissue cells is associated with latent autoimmunity.
Effector cytotoxic T lymphocytes (CTLs) residing in healthy tissue fail to receive the necessary signals to exert their effector functions and mediate tissue destruction. Only when these effector CTLs are in contact with non-haematopoietic tissue cells that upregulate expression of interleukin-15 (IL-15) and non-classical MHC class I molecules do they become licensed to kill the distressed tissue cells. Latent autoimmune diseases such as potential coeliac disease and latent autoimmune diabetes in adults (LADA) are characterized by the presence of a dysregulated immune response to gluten and β-islet self-antigens, respectively, with the preservation of functional tissue. Conspicuously, IL-15 upregulation is absent in the intestinal epithelial cells and β-islet cells of these patients, which supports the hypothesis that, to mediate tissue destruction, CTLs require signals that license them to kill their target cells. IL-15 upregulation in intestinal epithelial cells and β-islet cells is associated with the licensing of CTLs to promote tissue destruction and the development of active coeliac disease and overt type 1 diabetes, respectively. Licensing of CTLs comprises a reduction in the T cell receptor (TCR) activation threshold, the acquisition of lymphokine-activated killer activity and resistance to immune regulation. IL-15Rα, IL-15 receptor α-subunit; NK, natural killer; TReg cell, regulatory T cell.
These observations in the context of autoimmunity suggest that the inability of tumour-specific CTLs to eliminate solid tumours may not only be due to the expression of TGFβ147 and ligands for the inhibitory receptor PD1 (programmed cell death protein 1)148,149 but also due to the lack of expression of stress signals and IL-15 overexpression by tumour cells. In particular, it was shown that loss of IL-15 expression in solid tumours such as colorectal cancers was associated with decreased inflammation in the tumour environment and poor clinical prognosis29. Conversely, in head and neck cancer, in which inflammation promotes tumour growth, it was shown that a high level of expression of IL-15 was associated with increased inflammation and poor clinical outcome150. More systematic analysis of IL-15 in tumours is of high interest and warrants further investigation. Furthermore, studies in mice have suggested that IL-15 overexpression in solid tumours can lead to their effective elimination by CTLs even in the absence of a cognate antigen47. IL-15 should therefore be considered for cancer therapy101,102 in patients with tumours for which inflammation does not constitute a selective advantage. Delivery to the tumour of soluble IL-15 complexes that have been shown to stimulate potent NK cell and CD8+ T cell responses in vivo151,152 may be especially effective in patients with cancer and may not have the high toxicity reported for high-dose IL-2 therapy153.
Concluding remarks
Individual tissues have unique challenges and needs; therefore, endowing tissues with the ability to control the type of immune response that is generated and whether TRM cells should exert their effector functions has a clear advantage for maximal tissue protection. It enables the immune response to be tailored to the individual requirements of the tissue and ensures that effector T cells exert their functions locally only if there is ongoing active tissue distress that requires control of an infectious agent. Type I IFN154, IL-33 and thymic stromal lymphopoietin (TSLP)155 are cytokines that, similarly to IL-15, are inducibly expressed by non-haematopoietic epithelial and stromal cells and have the capacity to relay the health status of the tissue to the immune system and modulate the immune response accordingly. Whereas type I IFN and IL-15 drive mostly TH1 cell-mediated immunity, IL-33 and TSLP promote TH2 cell-mediated immunity. These cytokines could thus also be considered to be master regulatory cytokines of tissue immunity. Interestingly, IL-15 has been found in all mammals sequenced so far, as well as in many reptiles and birds, which shows that this cytokine has been conserved for more than 250 million years of evolution and indicates that it is likely to have an important role in survival. The role of IL-15 and all of its pleiotropic immunological properties can be summarized in terms of its ability to promote the destruction of infected tissue cells. More generally, we propose that tissue destruction by cytotoxic TRM cells only occurs when tissue cells provide them with a `kill me' signal. Furthermore, additional cytokines associated with complex immune disorders156,157, such as IL-21, could synergize or, to some extent, have redundant functions with IL-15 to promote tissue destruction by licensing effector CTLs to kill158. Stratifying patients on the basis of tissue expression levels of IL-15, type I IFN and IL-21, determining the level of redundancy of IL-15 with type I IFN and IL-21, and understanding the mechanisms underlying IL-15 dysregulation and how IL-15 reprogrammes differentiated cells to acquire new functional properties will aid in the design of new therapeutic strategies aimed at modulating tissue immunity.
Box 1 | Role of IL-15 in the homeostasis of NK, NKT and CD8+ T cells.
The generation of mice deficient in interleukin-15 (IL-15) or in IL-15 receptor α-subunit (IL-15Rα) revealed important roles for IL-15 in the development, maintenance and proliferation of memory CD8+ T cells, natural killer (NK) cells and invariant NKT (iNKT) cells8,14,104. IL-15 receptor signalling contributes to cell proliferation and survival through the phosphorylation and activation of Janus kinase 1 (JAK1) and JAK3, which subsequently recruit and phosphorylate signal transducer and activator of transcription 5 (STAT5) and STAT3 (REF. 8). The STAT proteins dissociate from the IL-15 receptor and translocate to the nucleus, where they promote transcription of the gene encoding the anti-apoptotic factor BCL-2 and of the proto-oncogenes MYC, FOS and JUN14. IL-15 can also trigger the RAS–RAF–MEK–mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K)–AKT signalling pathways, which induce mitogenic signals and BCL-2- and BCL-Xl-mediated anti-apoptotic signals, and can limit the production of the pro-apoptotic proteins BIM (also known as BCL-2L11) and PUMA (also known as BBC3)14.
In addition, IL-15 induces iNKT cell and NK cell activation and increases the cytotoxicity of NK cells by enhancing the production of perforin and granzymes A and B14. Macrophage- and dendritic cell-derived IL-15 supports the development and maturation of memory CD8+ T cells, hepatic iNKT cells and NK cells104. The trans-presentation of IL-15 by intestinal epithelial cells is also crucial for the homeostasis of innate-like T cells such as CD8αα+ TCRαβ+ T cells and TCRγδ+ T cells30,58,104. IL-15 supports the survival of unconventional intraepithelial lymphocytes through the activation of JAK–STAT, PI3K–AKT and ERK signalling pathways that lead to the upregulation of BCL-2 and MCL1 expression14.
Box 2 | Post-transcriptional regulation of IL-15.
To tightly control the function of this potent pro-inflammatory cytokine, interleukin-15 (IL-15) expression is tightly regulated at several levels to limit its translation and secretion. First, IL-15-mediated signalling requires that the IL-15 receptor α-subunit (IL-15Rα) is expressed on the cell surface through a mechanism known as trans-presentation (FIG. 1b). More specifically, IL-15 is transported through the Golgi apparatus to the cell surface as a complex bound to IL-15Rα. The complex is then presented by membrane-bound IL-15Rα in trans to responder cells expressing the IL-2/IL-15 receptor β-chain (IL-2/IL-15Rβ) and the common cytokine receptor γ-chain (γc) of the IL-15 receptor8. In addition, IL-15 associated with IL-15Rα can be cleaved into soluble complexes to mediate IL-15 responses9,10. Second, IL-15 expression is regulated post-transcriptionally by several distinct mechanisms. Unlike the 5′ untranslated regions (UTRs) of effectively translated mRNAs, which are short and devoid of AUGs, the 5′ UTR of IL15 is long and contains many AUG sites upstream of the initiation AUG, thus restricting the translation of IL15 mRNA98. The presence of a carboxy-terminal negative regulatory element in the IL-15 mature protein also contributes to control translation98. Finally, IL-15 exists as two isoforms generated by alternative splicing. Both produce mature proteins that are associated with alternative signal peptides98. The long signal peptide is associated with the secreted soluble form of IL-15, whereas the short signal peptide is associated with a cytoplasmic or nuclear form of IL-15, the intracellular localization of which could be consistent with a role as a transcriptional regulator98. Therefore, not only do these signal peptides contribute to the regulation of IL-15 translation, but they also influence the intracellular trafficking of the protein. However, the exact roles of those isoforms are unclear, as is whether their expression and regulation are tissue specific.
Acknowledgements
The authors thank patients with coeliac disease and their family members, as well as the University of Chicago Coeliac Disease Center, for supporting their research. The authors also thank L.B. Barreiro and B. Sally for critical reading of the manuscript. The work was supported by grants from the Digestive Diseases Research Core Center (DK42086) at the University of Chicago, from the US National Institutes of Health (R01DK67180 and R01DK098435) to B.J. and from the SickKids Foundation (NI15-040) to V.A.
Glossary
- Activation-induced cell death (AICD)
A phenomenon in T cells, in which activation through the T cell receptor results in apoptosis. CD95 (also known as Fas) and its ligand (CD95L) are the main regulators of AICD, and the engagement of CD95 ultimately leads to DNA cleavage by caspase-activated DNase (CAD).
- Latent autoimmune diabetes in adults (LADA)
A disorder characterized by the presence of diabetes-associated autoantibodies and islet-reactive T cells in the absence of β-cell destruction and overt diabetes.
- Lymphokine-activated killer activity (LAK activity)
The ability of T cells to lyse target cells in the absence of specific antigenic stimuli and MHC restriction. LAK cells can be generated in vitro in the presence of interleukin-15 (IL-15) or high concentrations of IL-2.
- Potential coeliac disease
A form of coeliac disease defined by the presence of transglutaminase- and gluten-specific antibodies and compatible HLA molecules in the absence of villous atrophy.
- Tissue-resident effector memory T cells (TRM cells)
A population of non-circulating memory T cells with an effector-like phenotype that have entered tissues during the effector phase of immune responses and can permanently reside in tissues.
Footnotes
Competing interests statement The authors declare no competing interests.
References
- 1.Cousens LP, et al. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor GA, Feng CG, Sher A. Control of IFN-γ-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Valentine L, Potts R, Premenko-Lanier M. CD8+ T cell-derived IFN-γ prevents infection by a second heterologous virus. J. Immunol. 2012;189:5841–5848. doi: 10.4049/jimmunol.1201679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015;14:174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat. Rev. Immunol. 2009;9:858–870. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 6.Walker LS, von Herrath M. CD4 T cell differentiation in type 1 diabetes. Clin. Exp. Immunol. 2015 doi: 10.1111/cei.12672. http://dx.doi.org/10.1111/cei.12672. [DOI] [PMC free article] [PubMed]
- 7.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 9.Bergamaschi C, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood. 2012;120:e1–e8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ota N, Takase M, Uchiyama H, Olsen SK, Kanagawa O. No requirement of trans presentations of IL-15 for human CD8 T cell proliferation. J. Immunol. 2010;185:6041–6048. doi: 10.4049/jimmunol.0901834. [DOI] [PubMed] [Google Scholar]
- 12.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuest SC, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 2011;17:604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin. Cancer Res. 2014;20:2044–2050. doi: 10.1158/1078-0432.CCR-12-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colpitts SL, et al. Cutting edge: the role of IFN-α receptor and MyD88 signaling in induction of IL-15 expression in vivo. J. Immunol. 2012;188:2483–2487. doi: 10.4049/jimmunol.1103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou R, Wei H, Sun R, Zhang J, Tian Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl Acad. Sci. USA. 2007;104:7512–7515. doi: 10.1073/pnas.0700822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abadie V, Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunol. Rev. 2014;260:221–234. doi: 10.1111/imr.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J. Investig. Dermatol. Symp. Proc. 2013;16:S28–S30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
- 19.Harada S, et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1508–1516. doi: 10.1002/1529-0131(199907)42:7<1508::AID-ANR26>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.McInnes IB, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat. Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 21.Vaknin-Dembinsky A, Brass SD, Gandhi R, Weiner HL. Membrane bound IL-15 is increased on CD14 monocytes in early stages of MS. J. Neuroimmunol. 2008;195:135–139. doi: 10.1016/j.jneuroim.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchaud G, et al. Epidermal IL-15Rα acts as an endogenous antagonist of psoriasiform inflammation in mouse and man. J. Exp. Med. 2013;210:2105–2117. doi: 10.1084/jem.20130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villadsen LS, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Invest. 2003;112:1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aringer M, et al. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford) 2001;40:876–881. doi: 10.1093/rheumatology/40.8.876. [DOI] [PubMed] [Google Scholar]
- 25.Robak E, et al. Proinflammatory interferon-gamma-inducing monokines (interleukin-12, interleukin-18, interleukin-15)—serum profile in patients with systemic lupus erythematosus. Eur. Cytokine Netw. 2002;13:364–368. [PubMed] [Google Scholar]
- 26.Chen J, et al. Insulin-dependent diabetes induced by pancreatic β cell expression of IL-15 and IL-15Rα. Proc. Natl Acad. Sci. USA. 2013;110:13534–13539. doi: 10.1073/pnas.1312911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadlack B, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 28.Willerford DM, et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 29.Mlecnik B, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl Med. 2014;6:228ra37. doi: 10.1126/scitranslmed.3007240. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan BS, Lefrancois L. Intraepithelial lymphocytes: to serve and protect. Curr. Gastroenterol. Rep. 2010;12:513–521. doi: 10.1007/s11894-010-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 32.Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372–381. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- 33.Simpson E. A historical perspective on immunological privilege. Immunol. Rev. 2006;213:12–22. doi: 10.1111/j.1600-065X.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 34.McKenna KC, Kapp JA. Ocular immune privilege and CTL tolerance. Immunol. Res. 2004;29:103–112. doi: 10.1385/IR:29:1-3:103. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 2009;9:883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 36.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Johansson-Lindbom B, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 39.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 41.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.DePaolo RW, et al. A specific role for TLR1 in protective TH17 immunity during mucosal infection. J. Exp. Med. 2012;209:1437–1444. doi: 10.1084/jem.20112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–435. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deshpande P, et al. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. J. Immunol. 2013;190:1416–1423. doi: 10.4049/jimmunol.1201620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu RB, et al. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc. Natl Acad. Sci. USA. 2013;110:8158–8163. doi: 10.1073/pnas.1301022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts AI, et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 2001;167:5527–5530. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 49.Lang KS, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 50.de Kauwe AL, et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J. Immunol. 2009;182:7440–7450. doi: 10.4049/jimmunol.0900233. [DOI] [PubMed] [Google Scholar]
- 51.DePaolo RW, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 53.Marietta E, et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J. Clin. Invest. 2004;114:1090–1097. doi: 10.1172/JCI21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husby S, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 55.Setty M, et al. Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology. 2015;149:681–691. doi: 10.1053/j.gastro.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long SA, Buckner JH, Greenbaum CJ. IL-2 therapy in type 1 diabetes: “Trials” and tribulations. Clin. Immunol. 2013;149:324–331. doi: 10.1016/j.clim.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Ring AM, et al. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat. Immunol. 2012;13:1187–1195. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4+CD25hi cells expressing FOXP3. Eur. J. Immunol. 2008;38:1621–1630. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin SJ, et al. Expansion of regulatory T cells from umbilical cord blood and adult peripheral blood CD4+CD25+ T cells. Immunol. Res. 2014;60:105–111. doi: 10.1007/s12026-014-8488-1. [DOI] [PubMed] [Google Scholar]
- 61.Litjens NH, et al. Allogeneic mature human dendritic cells generate superior alloreactive regulatory T cells in the presence of IL-15. J. Immunol. 2015;194:5282–5293. doi: 10.4049/jimmunol.1402827. [DOI] [PubMed] [Google Scholar]
- 62.Marshall D, Sinclair C, Tung S. Seddon, B.Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J. Immunol. 2014;193:5525–5533. doi: 10.4049/jimmunol.1402144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raynor J, et al. IL-15 fosters age-driven regulatory T cell accrual in the face of declining IL-2 levels. Front. Immunol. 2013;4:161. doi: 10.3389/fimmu.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 65.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 66.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 67.Lenardo M, et al. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 68.Ohta N, et al. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 αβ+NK1.1+ T cells for the development of small intestinal inflammation. J. Immunol. 2002;169:460–468. doi: 10.4049/jimmunol.169.1.460. [DOI] [PubMed] [Google Scholar]
- 69.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 70.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 71.Burkett PR, et al. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl Acad. Sci. USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 73.Ben Ahmed M, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J. Immunol. 2009;182:6763–6770. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 74.Ruprecht CR, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J. Exp. Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jabri B, et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology. 2000;118:867–879. doi: 10.1016/S0016-5085(00)70173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mention JJ, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 77.Fuchs A, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui G, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl Acad. Sci. USA. 2014;111:1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 80.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc. Natl Acad. Sci. USA. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J. Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 82.Orinska Z, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 83.Miranda-Carus ME, et al. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54:1151–1164. doi: 10.1002/art.21731. [DOI] [PubMed] [Google Scholar]
- 84.Schneider R, et al. B cell-derived IL-15 enhances CD8 T cell cytotoxicity and is increased in multiple sclerosis patients. J. Immunol. 2011;187:4119–4128. doi: 10.4049/jimmunol.1100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ogasawara K, et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature. 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 86.Rappl G, et al. Dermal fibroblasts sustain proliferation of activated T cells via membrane-bound interleukin-15 upon long-term stimulation with tumor necrosis factor-α. J. Invest. Dermatol. 2001;116:102–109. doi: 10.1046/j.1523-1747.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- 87.Ma LJ, Acero LF, Zal T, Schluns KS. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8αα IELs. J. Immunol. 2009;183:1044–1054. doi: 10.4049/jimmunol.0900420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–1713. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 89.Zdrenghea MT, et al. RSV infection modulates IL-15 production and MICA levels in respiratory epithelial cells. Eur. Respir. J. 2012;39:712–720. doi: 10.1183/09031936.00099811. [DOI] [PubMed] [Google Scholar]
- 90.Xing L, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruckert R, et al. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J. Immunol. 2000;165:2240–2250. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]
- 92.Bo H, et al. Elevated expression of transmembrane IL-15 in immune cells correlates with the development of murine lupus: a potential target for immunotherapy against SLE. Scand. J. Immunol. 2009;69:119–129. doi: 10.1111/j.1365-3083.2008.02197.x. [DOI] [PubMed] [Google Scholar]
- 93.Maiuri L, et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 94.Kirman I, Nielsen OH. Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am. J. Gastroenterol. 1996;91:1789–1794. [PubMed] [Google Scholar]
- 95.Liu Z, et al. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J. Immunol. 2000;164:3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 96.Vainer B, Nielsen OH, Hendel J, Horn T, Kirman I. Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine. 2000;12:1531–1536. doi: 10.1006/cyto.2000.0744. [DOI] [PubMed] [Google Scholar]
- 97.Agostini C, et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J. Immunol. 1996;157:910–918. [PubMed] [Google Scholar]
- 98.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 99.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15–IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 100.Yu X, et al. Artificial antigen-presenting cells plus IL-15 and IL-21 efficiently induce melanoma-specific cytotoxic CD8+ CD28+ T lymphocyte responses. Asian Pac. J. Trop. Med. 2013;6:467–472. doi: 10.1016/S1995-7645(13)60076-0. [DOI] [PubMed] [Google Scholar]
- 101.Anguille S, et al. Interleukin-15 dendritic cells as vaccine candidates for cancer immunotherapy. Hum. Vaccin. Immunother. 2013;9:1956–1961. doi: 10.4161/hv.25373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pandiyan P, et al. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J. Immunol. 2012;189:4237–4246. doi: 10.4049/jimmunol.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Castillo EF, Schluns KS. Regulating the immune system via IL-15 transpresentation. Cytokine. 2012;59:479–490. doi: 10.1016/j.cyto.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ebert EC. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439–1445. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 106.Jabri B, Ebert E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 2007;215:202–214. doi: 10.1111/j.1600-065X.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 107.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 108.Riha P, Rudd CE. CD28 co-signaling in the adaptive immune response. Self Nonself. 2010;1:231–240. doi: 10.4161/self.1.3.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang F, et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J. Exp. Med. 2009;206:707–719. doi: 10.1084/jem.20071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sutherland CL, et al. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J. Immunol. 2002;168:671–679. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 111.Jabri B, et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–499. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 112.Hue S, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–377. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 113.Meresse B, et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 2006;203:1343–1355. doi: 10.1084/jem.20060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. [PubMed] [Google Scholar]
- 115.Wu J, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 116.Groh V, et al. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 117.Thome JJ, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang C, Zhang J, Niu J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42:128–136. doi: 10.1016/j.cyto.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Upshaw JL, Leibson PJ. NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin. Immunol. 2006;18:167–175. doi: 10.1016/j.smim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 120.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 121.Buckner JH. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao D, et al. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 2003;33:215–223. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 123.Mottonen M, et al. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin. Exp. Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maul J, et al. Peripheral and intestinal regulatory CD4+ CD25high T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 125.Sitohy B, Hammarstrom S, Danielsson A, Hammarstrom ML. Basal lymphoid aggregates in ulcerative colitis colon: a site for regulatory T cell action. Clin. Exp. Immunol. 2008;151:326–333. doi: 10.1111/j.1365-2249.2007.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl Acad. Sci. USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lilley BN, Ploegh HL. Viral modulation of antigen presentation: manipulation of cellular targets in the ER and beyond. Immunol. Rev. 2005;207:126–144. doi: 10.1111/j.0105-2896.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 128.Maeurer M, Seliger B, Trinder P, Gerdes J, Seitzer U. Interleukin-15 in mycobacterial infection of antigen-presenting cells. Scand. J. Immunol. 1999;50:280–288. doi: 10.1046/j.1365-3083.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- 129.Liu T, Nishimura H, Matsuguchi T, Yoshikai Y. Differences in interleukin-12 and -15 production by dendritic cells at the early stage of Listeria monocytogenes infection between BALB/c and C57 BL/6 mice. Cell. Immunol. 2000;202:31–40. doi: 10.1006/cimm.2000.1644. [DOI] [PubMed] [Google Scholar]
- 130.Dann SM, et al. Interleukin-15 activates human natural killer cells to clear the intestinal protozoan Cryptosporidium. J. Infect. Dis. 2005;192:1294–1302. doi: 10.1086/444393. [DOI] [PubMed] [Google Scholar]
- 131.Braun M, et al. NK cell activation in human hantavirus infection explained by virus-induced IL-15/IL15Rα expression. PLoS Pathog. 2014;10:e1004521. doi: 10.1371/journal.ppat.1004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fawaz LM, Sharif-Askari E, Menezes J. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J. Immunol. 1999;163:4473–4480. [PubMed] [Google Scholar]
- 133.Zhou R, Wei H, Sun R, Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J. Immunol. 2007;178:4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]
- 134.Rausch A, et al. Interleukin-15 mediates protection against experimental tuberculosis: a role for NKG2D-dependent effector mechanisms of CD8+ T cells. Eur. J. Immunol. 2006;36:1156–1167. doi: 10.1002/eji.200535290. [DOI] [PubMed] [Google Scholar]
- 135.Perera L, et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm. Bowel Dis. 2007;13:298–307. doi: 10.1002/ibd.20026. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr. Top. Microbiol. Immunol. 2006;298:121–138. doi: 10.1007/3-540-27743-9_6. [DOI] [PubMed] [Google Scholar]
- 137.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gosselin J, TomoIu A, Gallo RC, Flamand L. Interleukin-15 as an activator of natural killer cell-mediated antiviral response. Blood. 1999;94:4210–4219. [PubMed] [Google Scholar]
- 139.Nguyen KB, et al. Coordinated and distinct roles for IFN-αβ, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 140.Sharif-Askari E, Fawaz LM, Tran P, Ahmad A, Menezes J. Interleukin 15-mediated induction of cytotoxic effector cells capable of eliminating Epstein–Barr virus-transformed/immortalized lymphocytes in culture. J. Natl Cancer Inst. 2001;93:1724–1732. doi: 10.1093/jnci/93.22.1724. [DOI] [PubMed] [Google Scholar]
- 141.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Losy J, Niezgoda A, Zaremba J. IL-15 is elevated in sera of patients with relapsing-remitting multiple sclerosis. Folia Neuropathol. 2002;40:151–153. [PubMed] [Google Scholar]
- 143.Allez M, et al. CD4+NKG2D+ T cells in Crohn's disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132:2346–2358. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 144.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl Acad. Sci. USA. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Menthon M, et al. Excessive interleukin-15 transpresentation endows NKG2D+CD4+ T cells with innate-like capacity to lyse vascular endothelium in granulomatosis with polyangiitis (Wegener's) Arthritis Rheum. 2011;63:2116–2126. doi: 10.1002/art.30355. [DOI] [PubMed] [Google Scholar]
- 146.Kuczynski S, et al. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2005;69:231–236. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 147.Malchow S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 149.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fridman WH, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr. Top. Microbiol. Immunol. 2011;344:1–24. doi: 10.1007/82_2010_46. [DOI] [PubMed] [Google Scholar]
- 151.Rubinstein MP, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl Acad. Sci. USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8 T cells into innate-like effector cells with antitumor activity. Oncoimmunology. 2013;2:e26442. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maas RA, Dullens HF, Den Otter W. Interleukin-2 in cancer treatment: disappointing or (still) promising? A review. Cancer Immunol. Immunother. 1993;36:141–148. doi: 10.1007/BF01741084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons α/β in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 155.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 156.De Nitto D, Monteleone I, Franze E, Pallone F, Monteleone G. Involvement of interleukin-15 and interleukin-21, two γ-chain-related cytokines, in celiac disease. World J. Gastroenterol. 2009;15:4609–4614. doi: 10.3748/wjg.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 158.Sutherland AP, et al. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J. Immunol. 2013;190:3977–3984. doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- 159.Umlauf SW, et al. Molecular regulation of the IL-2 gene: rheostatic control of the immune system. Immunol. Rev. 1993;133:177–197. doi: 10.1111/j.1600-065x.1993.tb01516.x. [DOI] [PubMed] [Google Scholar]