ABSTRACT
Charles Darwin noted that natural selection applies even to the hourly organization of daily life. Indeed, in many species, the day is segmented into active periods when the animal searches for food, and inactive periods when the animal digests and rests. This episodic temporal patterning is conventionally referred to as ultradian (<24 hours) rhythmicity. The average time between ultradian events is approximately 1–2 hours, but the interval is highly variable. The ultradian pattern is stochastic, jaggy rather than smooth, so that although the next event is likely to occur within 1–2 hours, it is not possible to predict the precise timing. When models of circadian timing are applied to the ultradian temporal pattern, the underlying assumption of true periodicity (stationarity) has distorted the analyses, so that the ultradian pattern is frequently averaged away and ignored. Each active ultradian episode commences with an increase in hippocampal theta rhythm, indicating the switch of attention to the external environment. During each active episode, behavioral and physiological processes, including changes in body and brain temperature, occur in an integrated temporal order, confirming organization by programs endogenous to the central nervous system. We describe methods for analyzing episodic ultradian events, including the use of wavelet mathematics to determine their timing and amplitude, and the use of fractal-based procedures to determine their complexity.
KEYWORDS: basic rest-activity cycle, brown adipose tissue, chronobiology, circadian rhythms, eating, homeostasis, natural selection, thermogenesis, wavelets, fractals, ultradian rhythms
Introduction
Referring to natural selection as “Darwin's Demon”, Pittendrigh, one of the founders of chronobiology, wrote1 (p. 28) that “from life's outset, the major environmental cycles of day, tide, month, and year have confronted natural selection with windows of opportunity and hazard that recur with precisely predictable frequency; and the Demon has exploited that predictability by elaborating innate temporal programs that phase many undertakings in the life of cell or organism, metabolic or behavioral, to an appropriate time in the outside day. Such programs offer the clear advantage of anticipatory preparation for predictably recurrent conditions”. Darwin himself went further than Pittendrigh, writing2 that “natural selection is daily and hourly scrutinizing, throughout the world, every variation, even the slightest; rejecting that which is bad, preserving and adding up all that is good”.
Thus evolution via natural selection suggests that the temporal patterning of behavioral, psychological and physiological processes is of utmost importance for the survival and reproduction of individual members of a species. Even temporal organization over hours is a potential target of natural selection. Understanding this patterning, and its underlying mechanisms should be central tasks of integrative physiology.
However most standard physiological textbooks pay scant, if any, attention to the timing of the naturally occurring “intra-daily” behavioral and physiological processes. The “set-point, feedback-mediated error-correction” model of homeostasis is usually presented without consideration of the timing of the correction process. By default, rapid timing is assumed, with correction commencing as soon as the deviation from set-point is detected and signaled back to the central nervous system (CNS). Thus the emphasis is still on the traditional concept of homeostasis, with physiological variations blunted and smoothed out, despite reasonable attempts to modernize the concept (see below).
The episodic nature of normal daily life
Because of the difficulty of measuring physiological parameters in conscious freely moving animals, early studies of the timing of intra-day activities were limited to behavioral observations. It became apparent that parameters previously assumed to be constant or regular in fact displayed surprising variability. Szymanski3 reported that, during the 24 hour day, activity is “polyphasic”. Richter4 reported that spontaneous activity in the rat, in a quiet dark environment, is “periodic”, with about 10 active episodes per 24 hours. Observation of human infants revealed generally similar episodic patterning of rest, activity and food intake.4 Similar observations of the behavior of human infants, together with the discovery of the temporal pattern of REM (rapid eye movement) sleep, led Kleitman to his concept of the basic rest activity cycle (BRAC), the idea that the temporal pattern of REM sleep extends throughout the 24 hour day (as summarized in his review5). Halberg introduced the term “ultradian” to describe the timing of events with a periodicity less than 24 hours (see below). Polyphasic “intra-daily” activity rhythms were subsequently described for many species. The examples in Figure 1 are taken from the review by Daan and Aschoff6 who state that “these short-term rhythms…sometimes display striking precise oscillatory features and cannot be overlooked as an important ingredient in the temporal organization of behavior” (p. 491). Useful “classical” critical discussions of the ultradian concept can be found in Aschoff and Gerkema,7 in Aschoff's foreword to the book edited by Stampi,8 and in the paper by Stupfel and colleagues.9
Figure 1.
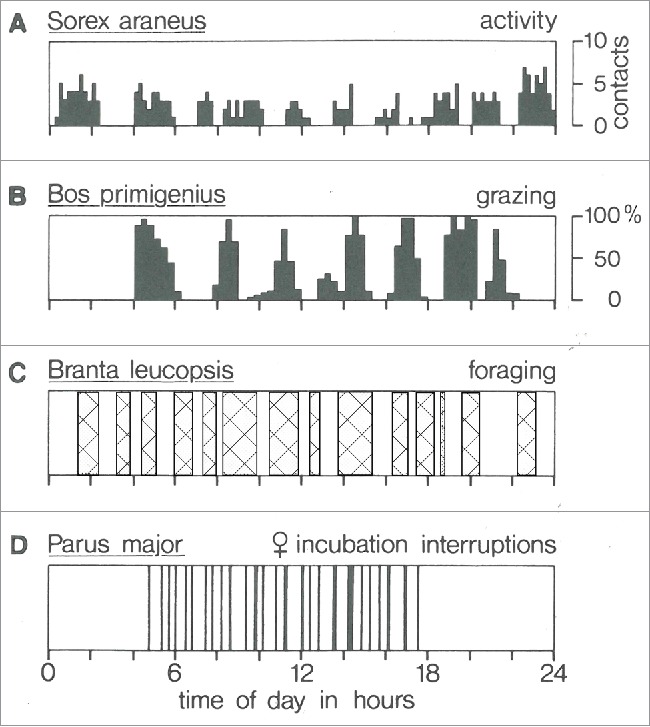
Examples of short-term rhythms in activity. (A) Locomotor activity in a common shrew. (B) Grazing activity (percent of time) in 8 bullocks. (C) Foraging bouts in a family of barnacle geese. (D) Absence from the nest in an incubating female great tit. Modified from Figure 1 of Daan and Aschoff 6 with permission.
As will be documented in our paper, quantifying the interval between individual ultradian episodes is difficult. The terms stochastic, non-stationary or even random have been applied. Random occurrences seem mysterious, especially when the underlying process is referred to as a rhythm. Perhaps partially because of this, as well as for all the reasons discussed below, the concepts of ultradian rhythmicity and the basic rest-activity cycle have been relegated to physiological limbo, and omitted from most text-books of physiology.
Intellectual traditions that have led to the lack of appropriate emphasis on ultradian timing
Bernard's milieu intérieur and cannon's homeostasis
Cannon explicitly based his concept of homeostasis on Bernard's conclusion that “it is the fixity of the milieu interieur which is the condition of free and independent life…. all the vital mechanisms, however varied they may be, have only one object, that of preserving constant the conditions of life in the internal environment”10 (p. 400). Although the idea initially had little influence,11 after approximately 50 years British and American physiologists embraced the concept so that J. S. Haldane12 (p. 383) wrote that “no more pregnant sentence was ever framed by a physiologist.”
The time relations implicit in Cannon's concept are apparent in his discussion of the naming of homeostasis. “As in the branch of mechanics called “statics”, the central concept is that of a steady state produced by the action of forces; homeostatics might therefore be regarded as preferable to homeostasis.” Cannon noted that, given the complexity of physiological processes, simple mechanical explanations are insufficient, so the term homeostasis was chosen. However Cannon seemed pleased that homeostatic, with its mechanical associations, would be the appropriate adjectival form.
Darwinian natural selection implies that the “object” of the “vital mechanisms” is survival for reproduction, not homeostasis per se. Homeostatic principles may be core aspects of physiological functioning, but they should be seen as mechanisms for survival rather than as the object of survival. The traditional homeostatic model is hardly helpful in understanding natural physiological events occurring, for example, when we climb a hill or, more dramatically, during the chase between predator and prey. The reality is that correction of deviations from physiological norms (lactic acidosis, increased temperature etc) is “postponed” until we rest, or until the chase is resolved. With respect to temperature regulation, Darwin13 noted that sudden attention to the external environment interrupts the routine maintenance of homeostasis. “A dog when panting after exercise, or on a hot day, breathes loudly; but if his attention be suddenly aroused, he instantly pricks his ears to listen, shuts his mouth, and breathes quietly”. Thus active engagement with the external environment “suspends” the homeostatic process.
Wiener's cybernetics
Arturo Rosenblueth, Cannon's principal collaborator, provided a physiological background for Wiener's application of the mechanical principles of cybernetics (the art of steering, including feedback) to the understanding of biological processes. In Wiener's14 book, notably subtitled “control and communication in the animal and the machine”, there is a constant interchange of mechanical and physiological examples. Clearly, the rapid timing paradigm implicit in mechanical feedback greatly influenced expectations of the timing of homeostatic feedback.
Cannon had already noted that body temperature is controlled by a “delicate thermostat which…… appears to be located in the subthalamus” (p. 422). Wiener's discussion of negative feedback in the design of temperature thermostats moves easily from the mechanical to the physiological. “A badly designed (house) thermostat may send the temperature of the house into violent oscillations, not unlike the motions of a man suffering from cerebellar tremor” (p. 97).
Over-emphasis on the importance of signals from peripheral sense organs
In their foundation cybernetics paper Rosenblueth, Wiener and Bigelow15 acknowledged that some purposive behavior proceeds without feedback, as, in their example, when a snake strikes at a frog. Once initiated, the movement is so fast that no biological feedback is possible. The author's inclination, however, was to emphasize the importance of negative feedback in the regulation of all purposeful behavior, analogous to the feedback that controls the path of a guided missile. J. S. Haldane had a similar emphasis on peripheral control. “Another tendency has been to regard the nervous system as the primary autonomous regulator of breathing and circulation….however the regulative influence of the nervous system is not autonomous, but dependent on conditions of environment determined mainly by varying tissue activity”12 (p. 383). Curt Richter also considered that signals from the periphery dominate the timing process, proposing that gradually increasing contractions of the stomach, endogenously generated in this organ, functioned as the pacemaker for the active behavioral periods.
Other discoveries have emphasized the need to modify the traditional concept of homeostasis. When food is presented at fixed times each day, animals learn the feeding schedule and display meal-anticipatory physiological responses, including secretion of insulin that lessens meal-induced increases in plasma glucose.16,17 These “cephalic phase” anticipatory behaviors fulfill metabolic requirements before peripheral signaling systems are activated, entailing a much more complex view of homeostasis.18 Although Cannon himself appreciated the role of “central command” in regulating, for example, the amount of blood flowing to exercising muscle (see discussion in19), the idea that peripheral signals dominate regulatory control in the conscious animal is a still an established part of physiological thinking. In many textbook diagrams of physiological regulation, central command is relegated to an arrow entering the control process from a small isolated box.
The traditional division of physiology into organ-based regulatory systems
The complexity of behavior and the underlying bodily functions makes it difficult to achieve a deep understanding of the physiology of all the bodily organs. The consequent specialization assigned the different organs to separate regulatory systems; cardiovascular, respiratory, gastrointestinal, renal, hemopoietic, musculo-skeletal, etc. The concept of the “thermoregulatory system” also became firmly established, although no specialized organ was assignable to the regulation of body temperature. The additional division of body processes into somatic and visceral categories exacerbated the isolation of each regulatory system.
When the integrative control of any one of these systems was under investigation, the relevant afferent and efferent loops of the control process tended to be confined to that particular system. Thus it was common to study how arterial baroreceptors regulate heart rate, or how gastric vagal afferents regulate digestive function. But the idea that stimulation of vagal afferents could modify, for example, the knee jerk reflex, was regarded as exotic (see for example Schweitzer and Wright20). The isolation of bodily functions into different “systems” reduced any emphasis on their integrated function in the execution of temporally patterned bodily processes. Indeed the concept of “system” is itself problematical, sometimes suggesting the operation of opposing physiological forces. Thus a prominent chronobiologist concludes a major review21 (p. 575) by referring to “violation of homeothermy” by the circadian rhythm of body temperature, and to “the opposition between the thermoregulatory system and the circadian system”.
Halberg, pittendrigh, and aschoff; founders of chronobiology
Halberg,22 tells us that in his investigation of “the rules of variability in time” he endeavored to “replace truisms such as a relative constancy or homeostasis…… They were never intended, however, to lower a curtain of ignorance over everyday physiology”. Halberg gave us the terms “circadian”, “infradian” and “ultradian” (see Appendix A in ref. 22). Ultradian rhythms are those with a periodicity of less than 24 hours. He was familiar with Richter's accounts of short-term periodicities in the daily life of rats, but Halberg himself was not particularly interested in these short-term events. The cosine function dominated Halberg's conception of biological timing, so that he insisted that time-series observations be fitted to a sinusoidal model, the cosine wave that Aschoff dubbed “the cosinor beast” (cited in23). The statistical averaging required for the appropriate cosine fit, and the emphasis on the “MESOR” (Midline Estimating Statistic of Rhythm) obliterated all traces of ultradian timing. Indeed, in Halberg's22 major review of chronobiology there is no mention of ultradian events.
In reality, biological processes are rarely if ever sinusoidal. Circadian rhythms are related to the onset and offset of the light period, not to some parameter varying sinusoidally with the 24 hour cycle. Thus visual inspection shows that the circadian pattern underlying the temperature and activity shown in Refinetti24 is much more rectangular than sinusoidal. Indeed Refinetti himself tells us (p. 566) that the wave form “often differs considerably from a sine or cosine wave and sometimes approximates a square wave”. Yet in his 1999 paper and in a major review21 Refinetti uses Halberg's cosine procedure as the basis of his analysis of circadian changes in body temperature.
A further reason for the chronobiologists' lack of attention to ultradian variability was the belief that a “proper rhythm” should have a stable phase relation with an environmental periodicity (Aschoff's zeitgeber), like the day-night cycle for circadian rhythms. For ultradian events, no such relation has been described. This may have been a factor in Pittendrigh's selective concentration on circadian rhythmicity; in his major reviews1,25 there is no mention of ultradian rhythms.
Pittendrigh and Aschoff independently introduced the oscillator, based on mechanical engineering models, as an explanatory concept into the field of chronobiology1 (pp. 32-33). Although neither investigator espoused the sinusoidal conception of biological events, the oscillator idea reinforced the assumption that biological rhythmicity is intrinsically sinusoidally-based as well as truly periodic. Lehmann26 emphasized the deficiencies of oscillator models of ultradian rhythmicity. “The conventional approach to all these patterns with doubtful rhythmicity is to consider that the underlying (endogenous or geophysical) periodicity is disturbed and superimposed by random events; or that activity and the oscillator are partially uncoupled; or that the oscillating systems are internally desynchronized; or that the system has degenerated. Thus patterns with ill-defined rhythmicity - if they are discussed at all – are usually submitted to one of the various methods of periodogram-analysis in order to detect the obscured “basic” rhythm. The biological relevance in describing these patterns a priori in the fixed terms of oscillating systems, however, seems to be rather poor….The nature and the mode of action of the assumed random disturbances as well as the mechanisms that are responsible for coupling or uncoupling or for internal desynchronization can hardly be investigated.”
Our rediscovery of ultradian episodic patterning
We developed instrumentation to simultaneously measure a number of physiological parameters, including temperatures of body, brain and brown adipose tissue (BAT), in conscious freely-moving Sprague-Dawley rats. We decided to observe what normally happens to these temperatures in singly caged animals maintained at 24–26°C in a quiet environment with ad libitum access to food and water in a 12 hour light/12 hour dark cycle.
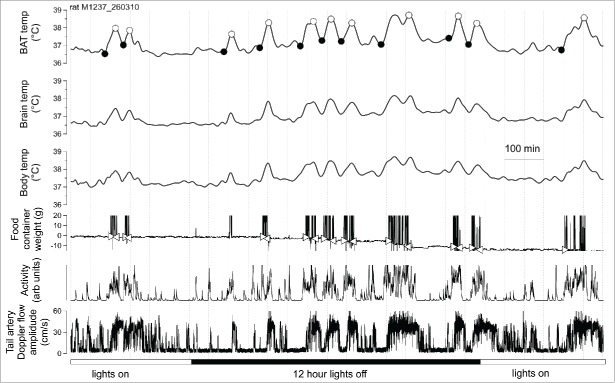
Being unfamiliar with the concept of ultradian patterning, we were surprised when BAT, body and brain temperatures suddenly increased, for no apparent reason, every 1–2 hours in both the dark and light phases. Traces from an individual rat are shown in Figure 2. The temperature increases were substantial (BAT >1°C, brain approximately 0.8°C and body approximately 0.6°C for dark period ultradian episodes).
Figure 2.
Experimental record from an individual rat, maintained in a quiet environment at 24–26°C with ad libitum access to food and water. Disturbances of the food container are shown as sudden large variations in the weight of the container. Consumption of food is indicated by the progressive fall in the weight of the container. Filled and open circles in the top trace indicate onset and peaks of episodic ultradian increases in BAT temperature. Open triangles in the food trace indicate onset and offset of eating. From ref. 28 with permission.
Our recording techniques measured whenever the rat disturbed the food container and how much food was taken from the container during the disturbance. Time relations between the temperatures and the other behavioral and physiological parameters with respect to the onset of eating during active ultradian episodes are shown in Figure 3. The increases in BAT thermogenesis commences at approximately the same time as the increases in activity, approximately 15 min before the onset of eating. It can be seen in Figure 2 that the rat rarely disturbs the container in the inter-meal period. When food was removed from the container for approximately 18 hours, ultradian episodic activity still occurred, accompanied by BAT thermogenesis that was similarly integrated with other physiological parameters. Remarkably, in the absence of food, it was again apparent that the rat disturbs the (empty) food container only approximately 15 min after the onset of an active phase.27
Figure 3.
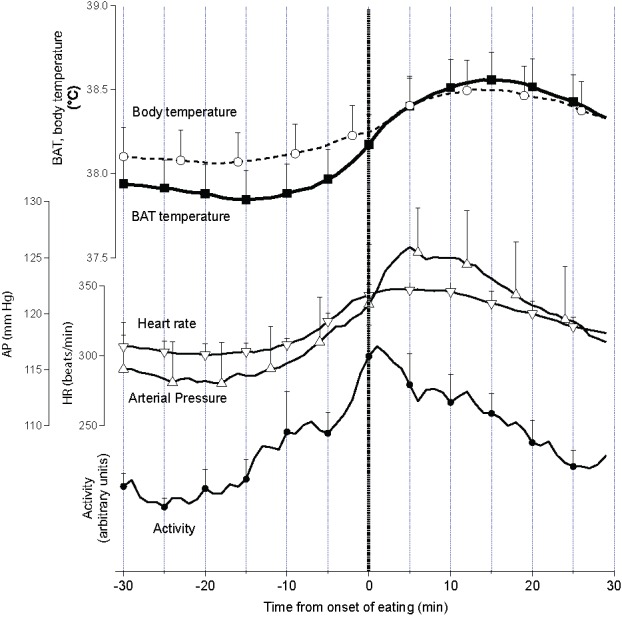
Group data (mean ± SEM) from dark period showing episodic increases in BAT and body temperature, arterial pressure (AP), heart rate (HR), tail artery blood flow and behavioral activity recorded from 30 min before the onset of eating (time zero) until 30 min after the onset of eating in rats maintained with ad libitum access to food. From ref. 28 with permission.
The ambient temperature in our studies was constant (24–26°C) and the BAT thermogenesis was not triggered by a fall in body temperature.28
Clearly it makes no sense to wonder whether an ultradian episode is primarily thermoregulatory, primarily behavioral or primarily cardiovascular. The various behavioral and physiological processes constituting each ultradian BRAC episode occur in a highly regulated temporal order. The similarity of the time relations between the different variables when food is present or absent strongly suggests that ultradian episodes are endogenously programmed. CNS involvement is implied by the observation that active ultradian episodes commence with the sudden appearance of theta activity in the hippocampal EEG,29 indicating that the animal has directed its attention to the external environment and commenced an exploratory phase30 (p. 308).
Methods used to describe ultradian temporal patterns in our laboratory
Perhaps it is reasonable to note that it was very difficult to have our BAT-body-brain temperature study accepted for publication; one referee memorably dismissed the ultradian events as “blips”. Other referees insisted on analytical procedures that, as we already understood, are strictly applicable only to rhythms with true periodicity. Here we present what we hope will be a helpful summary of our published methodology for episodic ultradian events (see details in refs.27-29) as well as a description of the use of fractal analyses to quantify their complexity.
It is very important to examine the record from each individual animal, presented graphically with appropriate time intervals, preferably 1 min bins. The initial inspection should be done before the records are averaged, either within or between animals, and before the records are subjected to further complex analytical procedures. Figure 4 (modified from the methodology paper from Refinetti and colleagues31) presents the final 5 days of locomotor activity of an individual horse measured 1 per min for 10 days. Inspection of Figure 4 shows a total of approximately 40 dark phase activity peaks. The inter-peak interval, estimated from inspection of the record, is variable, but the mean is approximately 90 minutes. The Fourier analysis presented in the lower panel of Figure 4 apparently fails to detect this 90 min periodicity. Surely it must be of physiological interest. Yet extensive analysis of results from 5 horses32 makes no mention of the ultradian rhythmicity apparent in the original record.
Figure 4.
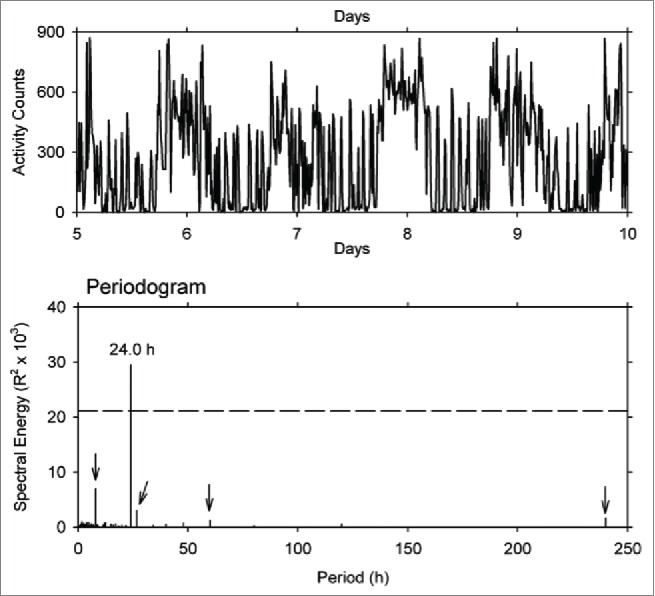
Record (days 5–10) of locomotor activity of a horse monitored by an activity data logger attached to the animal's neck, recorded at 1-min intervals for 10 consecutive days (top panel), and the periodogram generated by Fourier analysis of the complete 10 day time series (bottom panel). The dashed line in the periodogram indicates 0.05 level of significance. Modified from ref. 31 with permission.
In our chronic recordings in conscious rats we discarded the first 12 hours of each recording after experience taught us that it takes many hours for the rat to become accustomed to the recording cage. In their new surroundings, animals often avoid eating for many hours. Analog input signals were digitized in PowerLab (ADInstruments) and saved as IgorPro (Wavemetrics, Lake Oswego, Oregon) files (called waves). We are grateful to Wavemetrics for the flexibility, user-friendliness, ability to handle waves with large numbers of data points, powerful inbuilt routines and graphical excellence of IgorPro. We found the discrete wave transform (DWT) function in IgorPro to be very useful for “smoothing” the wave in a flexible manner, without “averaging away” what we judged to be physiologically important variations in the signal. Figure 5A is a record of BAT temperature (1 per min bins) and Figure 5B shows the DWT function of this wave.
Figure 5.
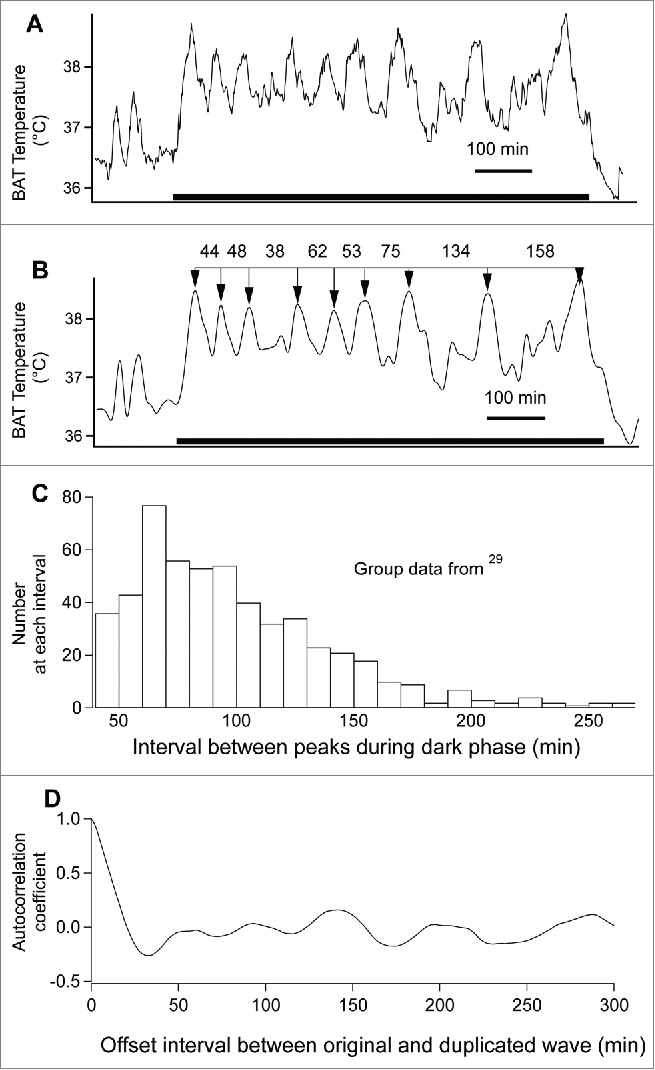
(A) Record (1 min bins) of brown adipose tissue (BAT) temperature, from just before lights-off to just after lights-on. The 12 hour dark period is indicated by the filled black bar. (B) IgorPro DWT function applied to the record in (A). The numbers are time (min) between sequential dark period ultradian peaks (arrows). (C) Group data showing frequency histogram distribution of the time between ultradian peaks during the dark phase. (D) Autocorrelation of the dark phase BAT temperature record shown in (A). From ref. 28 with permission.
We tested assumptions concerning minimum amplitude and minimum inter-peak intervals for an episode to qualify as an ultradian event, as discussed in ref. 29. For BAT temperature we adopted a minimum amplitude of 0.5C° and a minimum peak interval of 30 min. IgorPro routines were then used to define the onset and the peak time of each ultradian peak, for both the 12 hour light and 12 hour dark periods, over the 4 day recording period. Interpeak time intervals were calculated for each dark period ultradian event (see Fig. 5B) and the results plotted as frequency distributions (Fig. 5C). In our initial study29 we found the interpeak interval for the dark period to be 94±43 min (mean±standard deviation), with the large standard deviation (almost 50% of the mean value) emphasizing the substantial variability of the ultradian timing. Now that we are familiar with Lehmann's 1977 paper, we realize that the (Poisson) negative exponential shape of the distribution in Figure 5C suggests that each ultradian event occurs independently of the previous one. Bash33 reported a similar negative exponential distribution of feeding intervals, both in intact rats and in animals who recovered from vagotomy performed just above the diaphragm. Baker34 presents strong statistical evidence of aperiodicity in feeding behavior.
We found the paper by Refinetti and colleagues31 to be a very useful summary of the methods used to assess circadian rhythmicity. Autocorrelation, Enright chi square periodograms35 and Lomb-Scargle (Fourier transform-based) periodograms36,37 all search for regular events, spaced evenly apart in time. As noted earlier in our paper, ultradian events do not have this property; they are non-stationary or stochastic. Nevertheless we applied each of these procedures to test for underlying periodicity in our records. When we examined 12 hour dark and 12 hour light periods separately, no substantial autocorrelations were observed in individual records (Fig. 6D). Periodogram results of the dark period record shown in Figure 5A are shown in Figures 6A and 6B. Although statistically significant ultradian events were detected, it can be seen that no dominant ultradian periodicity is demonstrated by either procedure, consistent with the frequency distribution and the autocorrelation results.
Figure 6.
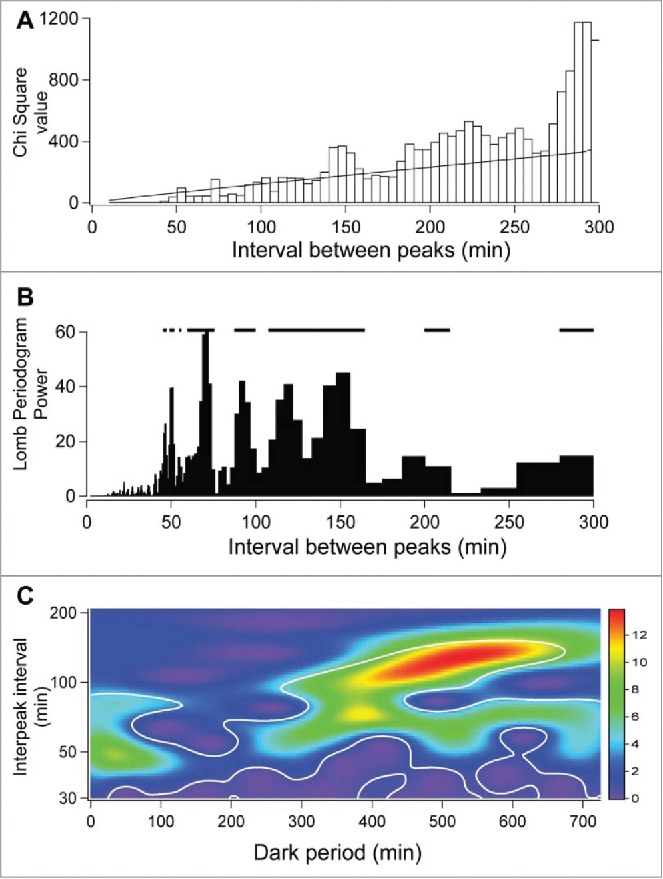
Chi square periodogram (A) and Lomb-Scargle periodogram (B) and continuous wavelet transform (CWT) (C) of the dark phase BAT temperature record shown in Figure 5A. Significance at the 0.05 level of confidence is shown by the sloping line in A, by the interrupted horizontal line in (B) and by the white lines in (C). The statistical significance of wavelet-power was assessed using software for wavelet analysis incorporating algorisms for Brownian noise in MATLAB (http://noc.ac.uk/using-science/crosswavelet-wavelet-coherence/).38 Modified from ref. 28 with permission.
Continuous wavelet transform of episodic ultradian events
Fourier transformation is a frequency analysis that decomposes a signal into a series of sine/cosine waves, with no indication of which particular frequencies occur at any particular time in that particular signal. To obtain an indication of which frequencies occur at a particular time it is necessary to subdivide the signal into smaller time segments, a process that compromises the frequency resolution. In contrast, the continuous wave transform (CWT) is a time-frequency analysis that describes signals by decomposing them into short waves, called ‘wavelets”.38,39 This form of analysis can assess frequency changes over time, analogous to a musical score that indicates that a certain frequency of sound is played at a certain time, with the superimposed sounds becoming music. The wavelet size can be changed depending on the frequency at that time, so that the wavelet transform can provide better time resolution without compromising the frequency resolution. Thus the continuous wavelet transform is suitable for non-stationary signals. The Morlet wavelet used in our ultradian analysis detects both amplitude and frequency at given time points of the physiological signal.40,41
As an example, we applied the IgorPro CWT procedure to the dark period of the BAT record shown in Figure 5A, with the resulting image shown in Figure 6C. The X axis represents sequential dark period time. The Y axis is the ultradian period in minutes and the Z (colored) axis is the power of the CWT function for each dark period time, an indication of the robustness of the interpeak interval and the amplitude of the BAT temperature signal at that portion of the dark period. The power of the portion of the image outlined by the white line is significant (see reference in legend).
We have used the CWT procedure to demonstrate that transgenic rats with ataxin-induced lesions of hypothalamic orexin have reduced ultradian BAT thermogenesis in comparison with wild type animals.42
Fractal dimension as a measure of complexity of ultradian events
The fractal dimension of any signal provides a measure of its complexity. Higuchi43 described how fractal theory can be used to measure the complexity of a signal varying in time. The method calculates the theoretical “length” of the signal when it is analyzed using different bin widths. For a straight line (amplitude always zero) the “length” of the signal is the same for all bin widths. For more complex signals, as the bin width decreases smaller jaggier elements of the signal will be included so that the overall “length” of the signal will increase. As the signal becomes even more complex this effect will be amplified. Higuchi's FD, defined as the absolute slope of the log relation between bin width and the calculated length of the signal, provides a measure of signal complexity, ranging from 1 for a straight line to 2 for a random signal because a random signal requires a line long enough to fill the whole “area” of the time-amplitude graph.
Relevant chapters in the book by Bassingthwaighte and colleagues44 provide informative accounts of fractals in physiology. Accardo45 provides a useful account of Higuchi's method and its application to biological phenomena. Klonowski46 is also helpful, particularly because he compares results of Higuchi's procedure with Fourier-based methods. Combining sine/cosine waves with different frequencies and amplitudes does not substantially increase the FD of the resultant wave,46 indicating that Fourier-based analysis of the BAT temperature wave will not provide a valid indication of its ultradian complexity.
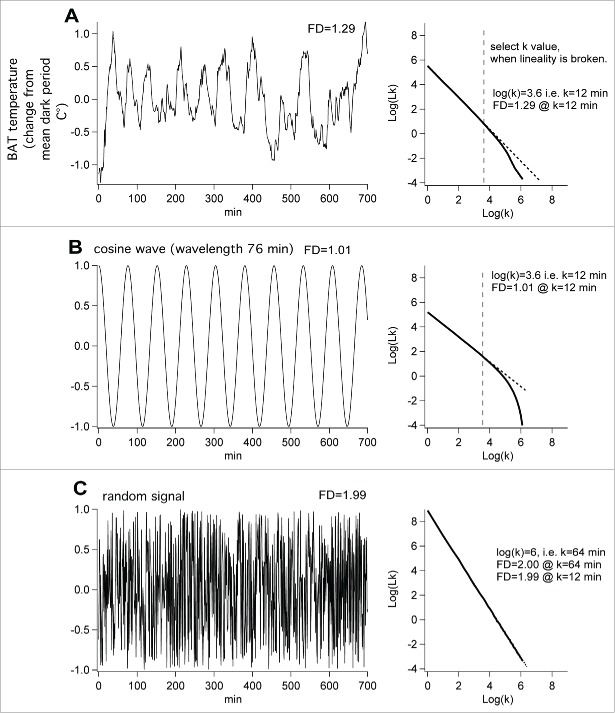
We wrote an IgorPro program for Higuchi's47 procedure, and applied it to the 700 min dark period of the same BAT temperature signal shown in Figure 5A. In this case we expressed the temperature as change from the mean dark period value (Fig. 7A). We also constructed a cosine signal (Fig. 7B) with periodicity set at the mean periodicity (76 per min) of the segment of the dark period BAT temperature signal. In addition we used the IgorPro enoise function to generate the random signal shown in Figure 7C. The FD of the cosine wave is 1.01. The FD of the enoise function is 1.99. The value of the FD for dark period BAT temperature signal is 1.29, between the 2 extremes. Thus the complexity of the ultradian signal is greater than that of the cosign function, but much less than the random signal. It may be that variations in FD will prove helpful in quantifying the factors, in both external and internal environments, that alter the frequency and amplitude of ultradian temporal patterns.
Figure 7.
Higuchi fractal dimension (FD) analysis of actual BAT temperature data (dark period BAT temperature from rat 854) and simulated data. The k values on the x axis in each right hand graph indicate bin widths used to determine the total signal length (lk on the y axis) in the Higuchi analysis. The selected bin width is the maximum k value yielding a linear relation (dotted straight line) between log(k) and log (lk). (A) The 700 min dark period portion of the BAT temperature record shown in Figure 6A. (B) Equivalent cosine wave with the average periodicity (76 min) of the BAT temperature wave. (C) Equivalent IgorPro random noise function.
Conclusions and speculations
When linked with the ultradian organization of daily life, the term rhythm so strongly implies a regularly recurring pattern that we agree with the reviewer of our original manuscript who recommended that it no longer be used. Polyphasic, periodic, stochastic, non-stationary; each of these terms has been used to describe the ultradian temporal organization of the intra-daily life of the individual. Episodic might be preferable to polyphasic, because it suggests the stochastic timing of the ultradian pattern. Jaggy might also be useful in that the term suggests variations in both the timing and the amplitude of active ultradian events.
In the analysis of ultradian organization we emphasize the importance of examining each individual record before results are averaged. If autocorrelation of individual ultradian records demonstrates no regularity then analytic “periodogram” procedures that assume stationarity are inappropriate. Fourier transformation procedures should be used cautiously, with adequate recognition that the processes underlying ultradian organization are not themselves sinusoidal. Wavelet-based mathematics may provide more useful descriptions of the jaggy timing and amplitude of ultradian events, and calculation of the fractal dimension may provide a useful measure of their complexity.
Taken together with the fact that the active ultradian episodes commence with an increase in hippocampal theta rhythm, the highly coordinated nature of the increases in so many behavioral and physiological parameters implies their generation within the CNS. This is not to deny the importance of signals arising from peripheral organs. In the case of circadian rhythmicity a reflex response, initiated by day/night transition has, in the course of the evolutionary process, become a more complex response, now generated by CNS circuitry. Similarly, in the case of the ultradian temporal pattern it may be that early in the course of evolution the timing of the next food-seeking episode was inititiated as a “hard-wired” reflex response to gastrointestinal afferent signaling. However venturing into the external environment is dangerous, much too risky to be initiated as a reflex response to input from gastrointestinal interoceptors. Thus, as for circadian rhythmicity, control has been transferred from the periphery to the CNS, so that active ultradian episodes are now initated by endogenous CNS circuitry.
Transfer of control from the periphery to the CNS ensures a more flexible, safer interaction with the external world. There is the added advantage that the individual can satisfy nutritional needs in advance of their occurrence. The ultradian pattern is a CNS-organized anticipatory pattern. Thus when food is available ad libitum, there is no relation between the amount eaten at a given meal and the time interval before the next meal (see discussion in ref. 27). When food becomes available after a period of food deprivation, the intensity of peripheral signaling to the CNS becomes more important in the timing of the next foraging episode. In situations requiring urgent attention to, and interaction with, the external world, active ultradian episodes are, in Aschoff's phase,7 “on call”.
The stochastic timing of spontaneous ultradian events may reflect the complexity of the underlying process. For successful survival and reproduction the individual must satisfy many potentially conflicting psychological, behavioral, physiological and metabolic needs. An urgent need to groom might delay the search for food. The iguana must warm up on a sunny rock in order to reach a body temperature sufficient for the next food-seeking sortie into the cold ocean. The external environment is highly contingent and potentially dangerous. It is thus not surprising that the CNS-integrated solution to all these requirements exhibits a stochastic organization of active ultradian episodes. Such an organization may also have survival value because it reduces the likelihood of a predator predicting appearance of its prey.
Major changes in body-brain temperature have occurred during the course of vertebrate evolution. The transition from ectothermy to endothermy, permitting higher basal temperatures, has been a major factor in the success of mammals. Birds, the other vertebrate species with complex cognitive abilities and complex social behaviors, have body and brain temperatures higher than mammals. Each active ultradian episode involves a further substantial increase in temperature, partially reflecting CNS-initiated BAT thermogenesis in mammals. The additional increase in brain temperature may further improve the synaptic processing necessary for making the complex cognitive and emotional decisions that ensure safe and productive execution of exploratory episodes.
The magnitude of the increase in metabolic rate that characterizes each ultradian episode in the Djungarian hamster (Phodopus sungorus), even when the animal is inactive during torpor, is shown in Figure 8 from Heldmaier et al.48 Thus the ultradian temperature increases are metabolically expensive, substantially increasing daily food requirements.48 It is economically inefficient to spend energy continuously at high levels.7 A more efficient organization alternates between active phases involving energy expenditure and rest phases during which energy levels can be restored, metabolic homeostatic requirements can be met, and memories of survival-relevant events occurring during the active phase can be consolidated.49
Figure 8.
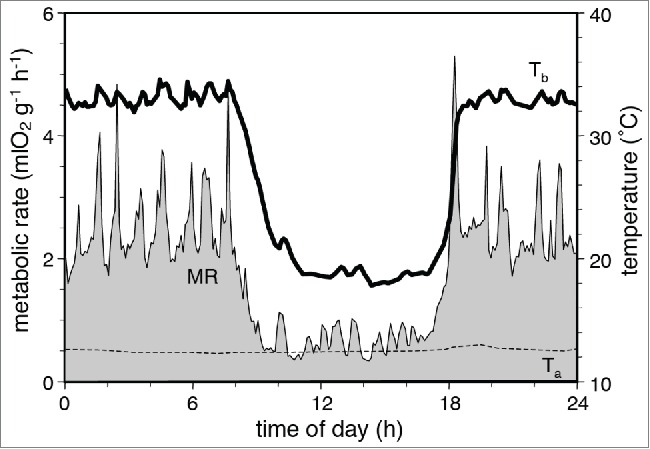
Daily torpor in the Djungarian hamster (Phodopus sungorus). During the nocturnal activity period the hamster displayed an ultradian pattern of metabolic rate and body temperature which is largely associated with the ultradian pattern of locomotor activity. MR; metabolic rate. Ta; ambient temperature. Tb; body temperature. From ref. 48 with permission.
In many species active ultradian episodes occur throughout the 24 hour day/night period. Inspection of relevant records (e.g., Figs. 4, 8) suggests that the circadian pattern is a modulation by the light/dark transition of the more fundamental ultradian pattern. The ultradian episodic pattern remains intact when circadian rhythmicity is eliminated by lesioning the suprachiasmatic nucleus.50 The CNS neural circuitry responsible for ultradian temporal organization is yet to be discovered.
Abbreviations
- BAT
brown adipose tissue
- BRAC
basic rest-activity cycle
- CNS
Central nervous system
- CWT
continuous wavelet transform
- DWT
discrete wavelet transform
- EEG
electroencephalogram
- FD
fractal dimension
- REM
rapid eye movement
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank John Furness, Marcello Costa and Susan O'Brien for their constructive criticisms of our ideas and for reviewing drafts of our paper.
Funding
Our research was supported by NHMRC Grants APP535025 and APP105826.
References
- [1].Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Ann Rev Physiol 1993; 55:17-54; PMID:8466172; http://dx.doi.org/ 10.1146/annurev.ph.55.030193.000313 [DOI] [PubMed] [Google Scholar]
- [2].Darwin C. On the Origin of Species by Means of Natural Selection. The Floating Press, ISBN: 9781775415374 (ebook) 2009 p151. Originally published in 1859. [Google Scholar]
- [3].Szymanski JS. Aktivität und ruhe bei tieren und menschen. Zeitschrift Fjur Allgerneine Physiol 1920; 18:105-62. [Google Scholar]
- [4].Richter CP. A behavioristic study of the activity of the rat. Comp Psychol Monogra 1922; 1:1-55. [Google Scholar]
- [5].Kleitman N. Basic rest-activity cycle–22 years later. Sleep 1982; 5:311-7; PMID:6819628 [DOI] [PubMed] [Google Scholar]
- [6].Daan S, Aschoff J. Short-term rhythms in activity In: Aschoff J, ed. Biological rhythms. Boston: Springer, 1981:491-8. [Google Scholar]
- [7].Aschoff J, Gerkema M. On diversity and uniformity of ultradian rhythms. Exp Brain Res 1985; 12(Supplement):321-34; http://dx.doi.org/ 10.1007/978-3-642-70483-3_21 [DOI] [Google Scholar]
- [8].Stampi C, ed. Why We Nap. Evolution, Chronobiology and Functions of Polyphasic and Ultrashort Sleep. New York: Springer Science, 1992. [Google Scholar]
- [9].Stupfel M. Metabolic and behavioural long period ultradian rhythms in endotherms In: Lloyd D, Rossi EL, eds. Ultradian Rhythms in Life Processes: An Inquiry into Fundamental Principles of Chronobiology and Psychobiology. London; New York: Springer-Verlag, 1992:208-39. [Google Scholar]
- [10].Cannon W. Organization for physiological homeostasis. Physiol Rev 1929; 9:399-431 [Google Scholar]
- [11].Gross CG. Claude Bernard and the constancy of the internal environment. Neuroscientist 1998; 4:380-5; http://dx.doi.org/ 10.1177/107385849800400520 [DOI] [Google Scholar]
- [12].Haldane JS. Respiration. Yale University Press: New Haven, 1922. [Google Scholar]
- [13].Darwin C. The Expression of the Emotions in Man and Animals. Oxford: Oxford University Press, 1872. (third edition, 1998). [Google Scholar]
- [14].Wiener N. Cybernetics: Or Control and Communication in the Animal and the Machine. Massachusetts: MIT Press, 1948. [Google Scholar]
- [15].Rosenblueth A, Wiener N, Bigelow J. Behavior, purpose and teleology. Philosophy of Science 1943; 10:18-24; http://dx.doi.org/ 10.1086/286788 [DOI] [Google Scholar]
- [16].Strubbe JH, Woods SC. The timing of meals. Psychol Rev 2004; 111:128-41; PMID:14756590; http://dx.doi.org/ 10.1037/0033-295X.111.1.128 [DOI] [PubMed] [Google Scholar]
- [17].Woods S, Ramsay D. Homeostasis: beyond curt richter. Appetite 2007; 49:388-98; PMID:17524521; http://dx.doi.org/ 10.1016/j.appet.2006.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev 2014; 121:225-47; PMID:24730599; http://dx.doi.org/ 10.1037/a0035942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29:1195-200; PMID:16213079; http://dx.doi.org/ 10.1016/j.pnpbp.2005.08.005 [DOI] [PubMed] [Google Scholar]
- [20].Schweitzer A, Wright S. Effects on the knee jerk of stimulation of the central end of the vagus, and of various changes in the circulation and respiration. J Physiol 1937; 88:454-75; PMID:16994837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Refinetti R. The circadian rhythm of body temperature. Front Biosci 2010; 15:564-94; http://dx.doi.org/ 10.2741/3634 [DOI] [PubMed] [Google Scholar]
- [22].Halberg F. Chronobiology. Annu Rev Physiol 1969; 31:675-725; PMID:4885778; http://dx.doi.org/ 10.1146/annurev.ph.31.030169.003331 [DOI] [PubMed] [Google Scholar]
- [23].Halberg F, Cornelissen G, Katinas G, Syutkina EV, Sothern RB, Zaslavskaya R, Halberg F, Watanabe Y, Schwartzkopff O, Otsuka K, et al.. Transdisciplinary unifying implications of circadian findings in the 1950s. J Circadian Rhythms 2003; 1:2; PMID:14728726; http://dx.doi.org/ 10.1186/1740-3391-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Refinetti R. Relationship between the daily rhythms of locomotor activity and body temperature in eight mammalian species. Am J Physiol 1999; 277:R1493-500; PMID:10564224 [DOI] [PubMed] [Google Scholar]
- [25].Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 1960; 25:159-84; PMID:13736116; http://dx.doi.org/ 10.1101/SQB.1960.025.01.015 [DOI] [PubMed] [Google Scholar]
- [26].Lehmann U. Stochastic principles in the temporal control of activity behaviour. Int J Chronobiol 1977; 4:223-66; PMID:591134 [PubMed] [Google Scholar]
- [27].Blessing W, Mohammed M, Ootsuka Y. Heating and eating: brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol Behav 2012; 105:966-74; PMID:22115948; http://dx.doi.org/ 10.1016/j.physbeh.2011.11.009 [DOI] [PubMed] [Google Scholar]
- [28].Blessing W, Mohammed M, Ootsuka Y. Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats. Physiol Behav 2013; 121:61-9; PMID:23562865; http://dx.doi.org/ 10.1016/j.physbeh.2013.03.028 [DOI] [PubMed] [Google Scholar]
- [29].Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience 2009; 164:849-61; PMID:19679172; http://dx.doi.org/ 10.1016/j.neuroscience.2009.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buzsáki G. Rhythms of the Brain. Oxford; New York: Oxford University Press, 2006. [Google Scholar]
- [31].Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 2007; 38:275-325; PMID:23710111; http://dx.doi.org/ 10.1080/09291010600903692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Piccione G, Caola G, Refinetti R. Temporal relationships of 21 physiological variables in horse and sheep. Comp Biochem Physiol A Mol Integr Physiol 2005; 142:389-96; PMID:16290083; http://dx.doi.org/ 10.1016/j.cbpa.2005.07.019 [DOI] [PubMed] [Google Scholar]
- [33].Bash KW. An investigation into a possible organic basis for the hunger drive. J Comp Physiol Psychol 1939; 28:109-33; http://dx.doi.org/ 10.1037/h0061206 [DOI] [Google Scholar]
- [34].Baker RA. Aperiodic feeding behavior in the albino rat. J Comp Physiol Psychol 1953; 46:422-6; PMID:13109064; http://dx.doi.org/ 10.1037/h0062279 [DOI] [PubMed] [Google Scholar]
- [35].Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol 1978; 72:131-60; PMID:566361; http://dx.doi.org/ 10.1016/0022-5193(78)90022-X [DOI] [PubMed] [Google Scholar]
- [36].Lomb NR. Least-squares frequency analysis of unequally spaced data. Astrophysics and Space Science 1976; 39:447-62; http://dx.doi.org/ 10.1007/BF00648343 [DOI] [Google Scholar]
- [37].Press WH, Rybicki GB. Fast algorithm for spectral-analysis of unevenly sampled data. Astrophys J 1989; 338:277-80; http://dx.doi.org/ 10.1086/167197 [DOI] [Google Scholar]
- [38].Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Proc Geoph 2004; 11:561-6; http://dx.doi.org/ 10.5194/npg-11-561-2004 [DOI] [Google Scholar]
- [39].Leise TL. Wavelet analysis of circadian and ultradian behavioral rhythms. J Circadian Rhythms 2013; 11:5; PMID:23816159; http://dx.doi.org/ 10.1186/1740-3391-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Price TS, Baggs JE, Curtis AM, Fitzgerald GA, Hogenesch JB. WAVECLOCK: wavelet analysis of circadian oscillation. Bioinformatics 2008; 24:2794-5; PMID:18931366; http://dx.doi.org/ 10.1093/bioinformatics/btn521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Leise TL, Indic P, Paul MJ, Schwartz WJ. Wavelet meets actogram. J Biol Rhythms 2013; 28:62-8; PMID:23382592; http://dx.doi.org/ 10.1177/0748730412468693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mohammed M, Ootsuka Y, Yanagisawa M, Blessing W. Reduced brown adipose tissue thermogenesis during environmental interactions in transgenic rats with ataxin-3-mediated ablation of hypothalamic orexin neurons. Am J Physiol Regul Integr Comp Physiol 2014; 307:R978-89; PMID:25324552; http://dx.doi.org/ 10.1152/ajpregu.00260.2014 [DOI] [PubMed] [Google Scholar]
- [43].Higuchi T. Approach to an irregular time series on the basis of the fractal therory. Physica D 1988; 31:277-83; http://dx.doi.org/ 10.1016/0167-2789(88)90081-4 [DOI] [Google Scholar]
- [44].Bassingthwaighte JB, Liebovitch LS, West BJ. Fractal physiology. New York: Oxford University Press, 1994. [Google Scholar]
- [45].Accardo A, Affinito M, Carrozzi M, Bouquet F. Use of the fractal dimension for the analysis of electroencephalographic time series. Biol Cybern 1997; 77:339-50; PMID:9418215; http://dx.doi.org/ 10.1007/s004220050394 [DOI] [PubMed] [Google Scholar]
- [46].Klonowski W. From conformons to human brains: an informal overview of nonlinear dynamics and its applications in biomedicine. Nonlinear Biomed Phys 2007; 1:5; PMID:17908344; http://dx.doi.org/ 10.1186/1753-4631-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Higuchi T. Fractal analysis of time series (in Japanese). Proc Inst Stat Math 1989; 37:209-33 [Google Scholar]
- [48].Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 2004; 141:317-29; PMID:15288602; http://dx.doi.org/ 10.1016/j.resp.2004.03.014 [DOI] [PubMed] [Google Scholar]
- [49].Buzsaki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015; 25:1073-188; PMID:26135716; http://dx.doi.org/ 10.1002/hipo.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Baker FC, Angara C, Szymusiak R, McGinty D. Persistence of sleep-temperature coupling after suprachiasmatic nuclei lesions in rats. Am J Physiol Regul Integr Comp Physiol 2005; 289:R827-38; PMID:15860650; http://dx.doi.org/ 10.1152/ajpregu.00093.2005 [DOI] [PubMed] [Google Scholar]