ABSTRACT
Recent reports of the use of transgenic mice targeting orexin neurons show that the ablation of orexin neurons in the hypothalamus causes hypothermia during cold exposure. This suggests the importance of orexin neurons for cold-induced autonomic and physiological defense responses, including brown adipose tissue (BAT) thermogenesis and vasoconstriction in thermoregulatory cutaneous vascular bed. The present study investigated whether the ablation of orexin neurons attenuated cold-elicited BAT thermogenesis and cutaneous vasoconstriction. The study took advantage of our established conscious rat experimental model of direct measurement of BAT and body temperature and tail cutaneous blood flow. The study used transgenic orexin neurons-ablated (ORX-AB) rats and wild type (WT) rats. BAT temperature and tail artery blood flow with pre-implanted probes were measured, as well as behavioral locomotor activity under conscious free-moving condition. Gradually, the ambient temperature was decreased to below 5°C. ORX-AB rats showed an attenuated cold-induced BAT thermogenesis and behavioral activity, and delayed tail vasoconstriction. An ambient temperature that initiated BAT thermogenesis and established full cutaneous vasoconstriction was 14.1 ± 1.9 °C, which was significantly lower than 20.5 ± 1.9 °C, the corresponding value in WT rats (n = 10, P < 0.01). The results from this study suggest that the integrity of orexin-synthesising neurons in thermoregulatory networks is important for full expression of the cold defense responses.
KEYWORDS: brown adipose tissue, cold defense, cutaneous vasoconstriction, orexin
Introduction
When experimental rats and mice are exposed to cold environments, they maintain core body temperature via thermoregulatory defense responses including thermogenesis in brown adipose tissue (BAT) and vasoconstriction in cutaneous vascular beds. The central thermoregulatory pathways mediating these integrated physiological responses includes nuclei in the preoptic area, the hypothalamus and the rostral medullary raphé.1-3
Hypothalamic orexin-synthesising neurons localize in a small perifornical region of the lateral hypothalamus.4,5 Projecting widely in the brain, orexin neurons contribute to a wide range of physiological functions including regulation of arousal, feeding and autonomic physiological responses.6-11 The role of orexin neurons in thermoregulatory function has been investigated in transgenic mice with ataxin-3-mediated destruction of orexin synthesising neurons. These animals are intolerant to cold exposure and they have attenuated emotional hyperthermia.12,13
The availability of orexin neurons-ablated rats (ORX-AB) with the same ataxin-3-mediated destruction approach used in the transgenic mice 8,14 has provided further opportunities to explore the role of orexin in the central thermoregulatory pathways. Using rats including ORX-AB rats, we have established direct measurements of BAT and body temperatures as well as thermoregulatory cutaneous blood flow (tail artery) in the conscious freely-moving animal.9,15,16 With the advent of ORX-AB rats, we recently showed that orexin-synthesising neurons are part of the CNS control of BAT thermogenesis and constriction of the tail vascular bed during emotional hyperthermia.9
In the present study, we investigated whether destruction of orexin neurons attenuates BAT thermogenesis and thermoregulatory cutaneous vasoconstriction in response to exposure to a cold ambient environment. We measured BAT and body temperature in addition to the tail artery blood flow in conscious freely moving ORX-AB rats and in their wild type (WT) littermates. We compared cold-elicited changes in the physiological parameters between the ORX-AB and WT rats.
Results
Autonomic physiological and behavioral responses to cold exposure in WT rats
Rats in the soundproof closed chamber were exposed to the caged-environmental cooling. After cooling began, the cage temperature gradually decreased and reached 5°C within 70 min. The cage temperature was maintained at a cold state that ranged between 3°C and 5°C for 180 min, and then returned to pre-cooling level within 40 min after the end of the cold state.
During the cold exposure BAT temperature initially decreased in 6 of 12 cases and then started increasing within 30 min (Figs 1A and 2A). BAT temperature increased by 1.0 ± 0.1°C from the pre-cooling level (n = 12, P < 0.01). Body temperature increased by 0.9 ± 0.1°C. The slope of the increase in BAT temperature during the initial 10 min from the onset of the increase was 0.06 ± 0.01°C/min, which was greater than 0.04 ± 0.01°C/min (n = 12, P < 0.05), the corresponding slope for body temperature (Fig 3). After reaching the maximum values, both BAT and body temperatures gradually returned to the pre-cooling level during the cold exposure.
Figure 1.
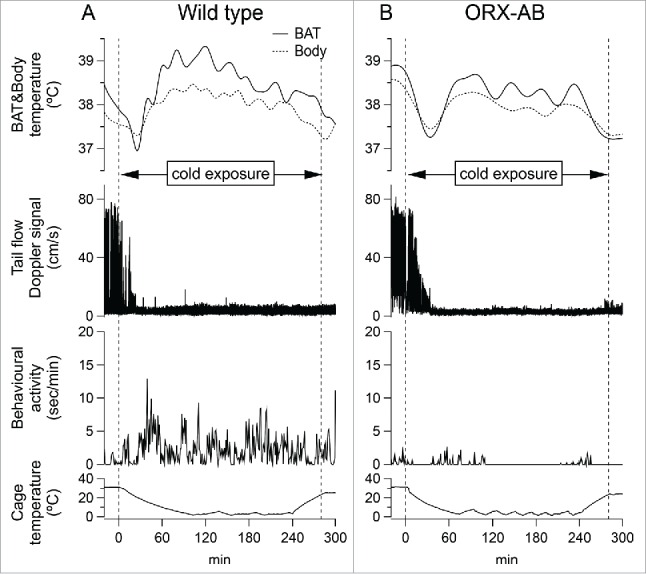
Effect of cold exposure on BAT temperature (solid line), body temperature (dashed line), tail artery blood flow Doppler signal and behavioral locomotor activity from an individual wild type rat (A) and an individual ORX-AB rat (B). Two vertical dashed lines in each graph indicated the onset and the end time of cold exposure, respectively.
Figure 2.
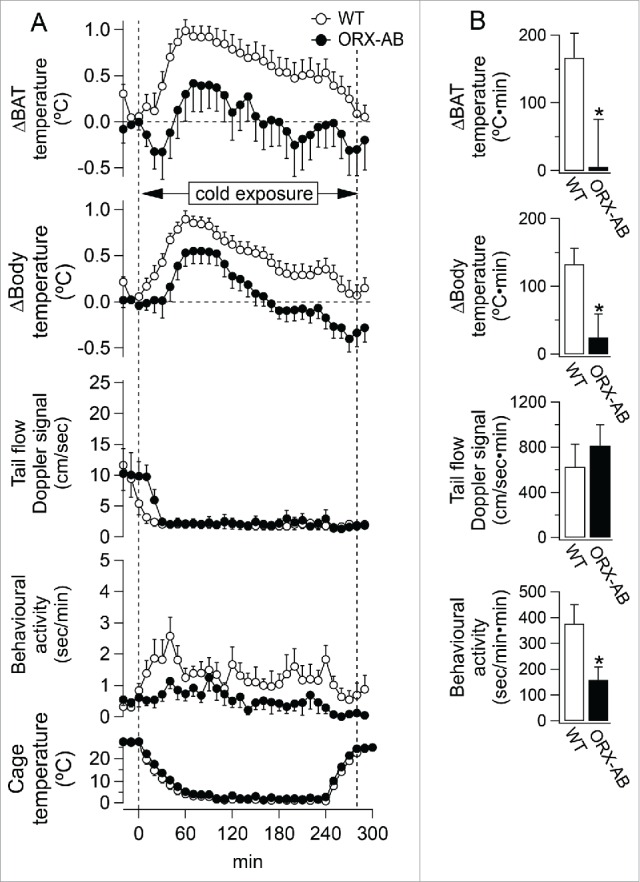
Averaged group data (mean ± SEM) showing effect of cold exposure on physiological parameters from wild type rats (open circle) and ORX-AB rats (closed circle). A: Relative changes from pre-cooling level were shown in BAT temperature (n = 12 for WT, n = 10 for ORX-AB) and body temperature (n = 12 for WT, n = 10 for ORX-AB) from pre-cooling level. Absolute value was shown in tail artery blood flow Doppler signal (n = 7 for WT, n = 8 for ORX-AB), behavioral locomotor activity (n = 7 for WT, n = 10 for ORX-AB) and cage temperature (n = 12 for WT, n = 10 for ORX-AB). Two vertical dashed lines in the graph indicated the onset and the end time of cold exposure, respectively. B: The changes during the 290 min cold exposure were expressed as the area under the curve. *P < 0.05 compared with WT.
Figure 3.
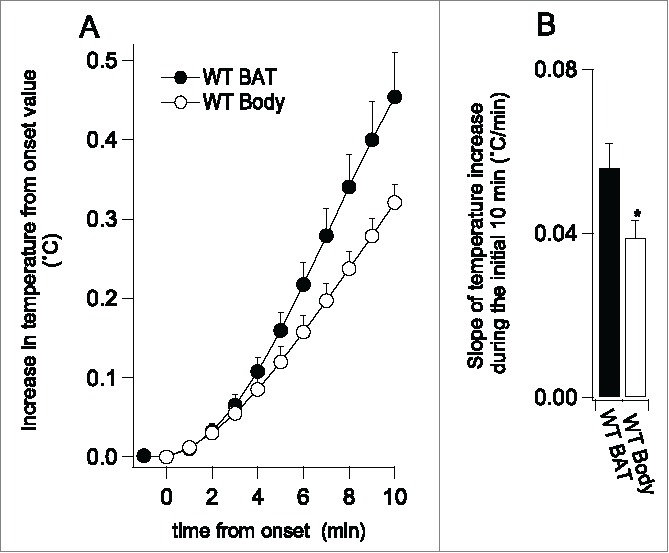
(A) showed average (means±SE) of BAT and body temperature records (normalized to commence at zero) for the initial 10 min after the onset of an increase in temperature elicited by the cold exposure. (B) showed slopes of the increase in the initial 10 min (the black bar for BAT, the white bar for body, n = 12). *P < 0.05 compared with BAT.
We also measured the amount of food eaten. The rats consumed 3.3 ± 0.3 g per 100 g of body weight during the cold exposure (n = 12).
In 6 of the 12 WT rats, recordings of tail flow were made. Tail flow decreased from 10 ± 3 to 2 ± 1 cm/sec (n = 6, P < 0.01) within 15 min after the onset of the cold exposure, and remained at the low flow level during the cold exposure. In 5 of the 6 animals, we evaluated a cage temperature when the tail flow reached the low flow level. The cage temperature was 21.6 ± 2.1°C, which was not different from 23.6 ± 1.1°C, threshold cage temperature when BAT temperature started increasing (n = 5, P > 0.05) (Fig. 4A), indicating that the threshold temperature for BAT thermogenesis was lower than that for the cutaneous vasoconstriction.
Figure 4.
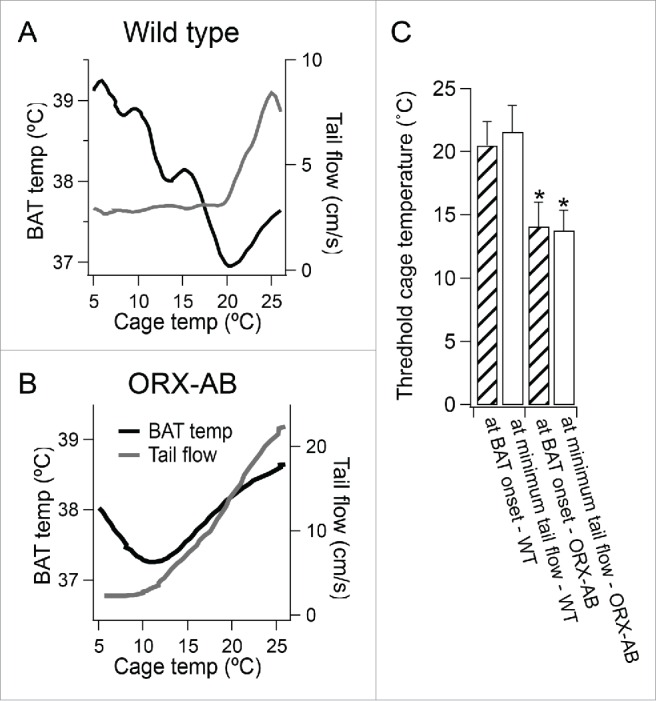
(A and B) showed changes in BAT temperature and tail flow plotted against changes in experimental cage temperature for the corresponding traces in the Figure 1. (C) showed cage temperatures at the onset of increase in BAT temperature (at BAT onset, n = 10 for WT and ORX-AB rats) (checked bars) or at time when tail flow reached the minimum level (at minimum tail flow, n = 5 for WT and n = 8 for ORX-AB) (open bars) from WT and from ORX-AB rats. *P < 0.05 compared with WT.
In 7 of the 12 WT rats, recordings of behavioral locomotor activity were made. Behavioral locomotor activity increased from 0.4 ± 0.1 to 1.3 ± 0.2 sec/min during the cold exposure (n = 7, P < 0.05).
Attenuated cold-elicited physiological and behavioral responses in ORX-AB rats
In ORX-AB rats, BAT temperatures initially decreased in 8 of 10 cases and then started increasing within 30 min after the onset of the cold exposure (Figs 1B and 2A). The increase in BAT temperature from the pre-cooling level, however, was not statistically significant. Body temperature increased by 0.5 ± 0.2 °C from the pre-cooling level (n = 10, P < 0.05). The increase in body temperature was significantly less in ORX-AB rats comparing to WT rats (Fig 2). For further comparison of the cold-elicited increases in BAT and body temperature between ORX-AB and WT rats, we evaluated the magnitude of increases in BAT and body temperature as area under the curve (AUC) during 290 min of the cold exposure (Fig. 2B). The magnitude of increases in BAT and body temperature was significantly attenuated in the ORX-AB rats (n = 10) compared to the WT rats (n = 12) (p < 0.05). We also compared threshold cage temperature when BAT temperature started increasing between groups. The threshold cage temperature in ORX-AB rats was 14.1 ± 1.9 °C, which was significantly lower than 20.5 ± 1.9 °C, the corresponding value in WT rats (n = 10, P < 0.01). There was no significant difference in body temperature at the onset of the BAT temperature increase between ORX-AB and WT rats.
In 8 of the 10 ORX-AB rats, recordings of tail flow were made. The cold exposure decreased tail flow from 10 ± 3 to 2 ± 1 cm/sec (n = 8, P < 0.05) within 60 min after the onset of the cold exposure. When tail flow reached the low level, BAT temperature started increasing (Fig. 4B). The cold-induced low tail flow level was not significantly different between ORX-AB and WT rats. We also compared a threshold cage temperature when tail flow reached the low level between groups. The threshold temperature in ORX-AB rats was 13.8 ± 1.6 °C (n = 8), which was significantly lower than 21.6 ± 2.1 °C (n = 5) (P < 0.05), the corresponding value in WT rats (Fig. 4C).
During cold exposure, behavioral locomotor activity was significantly less in ORX-AB comparing to WT rats (Fig 2). The ORX-AB rats consumed 2.6 g per 100 g of body weight (n = 10), which was not different from the corresponding value for WT rats (P < 0.05).
Histology
In ORX-AB rats, few or no orexin-synthesising neurons were demonstrated by appropriate immunohistochemistry using the orexin antibody (Fig. 5B), while many orexin neurons were clearly visible in WT rats (Fig. 5A).
Figure 5.
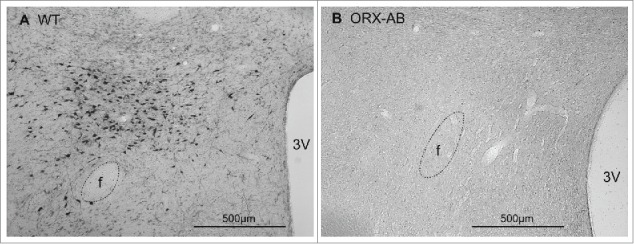
Immunohistochemical demonstration of orexin-contained neurons in coronal sections through the caudal hypothalamus from a wild-type (WT) rat (A) and from an ORX-AB rat (B). In the WT animal, but not in the ORX-AB animal, there were orexin-positive neuronal cell bodies in the perifornical region. f, Fornix; 3V, third ventricle.
Discussion
Previous studies using genetically-modified mice in which orexin neurons were destroyed with ataxin-3 toxin gene show that the ablation of orexin neurons results in intolerance to cold exposure, suggesting the importance of orexin neurons in driving the cold defense responses. The present study used transgenic Sprague-Dawley rats (ORX-AB) with the same ataxin-3 toxin gene approach and demonstrated that the increase in their body temperature during cold exposure was less than that for WT rats, reflecting attenuated cold-elicited BAT thermogenesis, cutaneous vasoconstriction and behavioral locomotion. These findings provide direct evidence that supports the current concept that orexin neurons are involved in maintaining body temperature during cold. Before further discussion, we firstly address technical issues.
In this study, BAT temperature initially decreased during cold exposure. The temperature probe was positioned near Sulzer's vein under interscapular BAT.9,15-18 Since interscapular BAT is close to the skin, temperature readings from the probe may also be influenced by skin temperature. Shaving the fur of the skin above the interscapular BAT, necessary for implant surgery, may augment the cold effect. Our previous study showed that during BAT thermogenesis under thermoneutral environments, the initial slope of the BAT temperature was greater than the corresponding slope of body temperature,9 indicating that measurement from an implanted BAT temperature probe is a valid index of BAT thermogenesis. In a cold environment, readings from the BAT temperature probe may be affected by skin cooling, especially during an initial phase of cold exposure, before the cold response is triggered. Nevertheless, in the present study, we again demonstrated that once BAT thermogenesis was initiated, the initial slope of the BAT temperature trace was greater than the corresponding slope for body temperature, suggesting that the BAT temperature probe still provides a valid index of BAT thermogenesis.
Body temperature increased rather than stayed the same during the cold exposure, unlike in our previous studies.19,20 The studies measured temperature of abdominal cavity as body core temperature with a data logger (1 cm diameter coin shape), while the present study measured temperature of the mediastinum (close to heart) with a thermistor (less than 2 mm square shape). Heated blood from interscapular BAT flows into the heart with a short distance via the Sulzer's and the Azygos vein.21 Therefore, it is likely that the mediastinum temperature reflects BAT temperature. Once heated blood is distributed to peripheral tissues, an increase in peripheral temperature may be dampen due to heat capacity of surrounding peripheral tissues. When we measured both mediastinum and abdominal temperatures with the same type of the thermistor simultaneously, the amplitude of spontaneous changes in the abdominal temperature was 30%–50% less than that in the mediastinum temperature (unpublished data). Large size of the data logger also contributes to increasing heat capacity, and thus to dampening changes in temperature.
In the present study, the cold exposure decreased ambient temperature gradually over 60 min without any physical interventions in a sound-insulated and temperature-controlled chamber. In our previous study, we used a two-chambers system and performed cold exposure by transferring an animal to the cold chamber. Our present exposure method would be a better model than the two-chambers system that require transferring, which may cause physical and/or psychological stress. Therefore, it is unlikely that the increase in the body temperature was triggered by factors other than the cold exposure in the present study. Another group reported a similar increase in body temperature during cold exposure, using a temperature-controlled chamber without a transferring procedure.22
In both studies, rats had free access to food and water during experiments. Our rats consumed approximately 3 g per 100 g body weight during 5-hour cold exposure. When rats are kept under 26°C ambient temperature without any intervention, they usually consume 5–6 g per 100 g body weight in 24 hours, i.e. 1 g per 100 g body weight in five hours.9 That means that rats consumed almost three times more food than usual during cold exposure. In our pervious non-food rat studies, body temperature was not increased during cold exposure.19,23 Food deprivation augments hypothermic response to cold.24 It is reasonable to conclude that food consumption during cold exposure may contribute to the increase in the body temperature.
Comparison of BAT thermogenesis and cutaneous vasoconstriction sensitivities to cold exposure in WT rats
The experimental procedure involved a gradual decrease in ambient temperature. In this situation, cutaneous vasoconstriction was triggered at the onset of cold exposure, while BAT thermogenesis was initiated after cutaneous vasoconstriction was fully established. This result is consistent with our previous findings using anesthetized rats with sympathetic nerve recording, in which BAT and tail cutaneous sympathetic outflows have unequal sensitivities to skin surface temperature.25 These results support the current concept that central neural pathways regulating BAT thermogenesis and cutaneous vasoconstriction are independent of each other, although sympathetic premotor neurons for BAT and those for tail cutaneous bed are located in the same medullary raphé (for a review see refs. 1,26). The cutaneous vasoconstriction requires less bodily fuel than BAT thermogenesis. Therefore, it is reasonable to recruit cutaneous vasoconstriction as the first-choice among cold defense responses.
Thermoregulatory responses to the cold exposure are attenuated in ORX-AB rats
The present study demonstrated that the cold exposure was essentially able to induce BAT thermogenesis and cutaneous vasoconstriction responses in ORX-AB rats, but the responses were attenuated. The results suggest that ORX-AB rats have less ability to maintain the cold defense responses, while fundamental thermoregulatory central mechanisms triggering the responses are intact.
The present study is for the first time to compare threshold ambient temperatures for tail vasoconstriction and BAT thermogenesis in conscious rats with simultaneous direct measurements of tail blood flow and BAT temperature. We found that an ambient temperature required to trigger BAT thermogenesis and to accomplish full cutaneous vasoconstriction was lower in ORX-AB rats comparing to WT rats. The lower ambient threshold temperature may augment an initial decrease in BAT temperature during cold exposure in ORX-AB rats (Fig. 1B).
During the cold exposure behavioral locomotor activity was increased in WT rats, but not in ORX-AB rats. Behavioral locomotor activity during active phase of ultradian pattern is reduced in ORX-AB rats,9 while circadian pattern of basal behavioral locomotor activity is normal in this mutant.27 Therefore, the diminished ability to maintain body temperature in ORX-AB rats during the cold exposure is partly due to a defect in behavioral response to cold. This view is not constituent with ORX-AB mice studies in which cold exposure increased behavioral locomotor activity in similar manner both in ORX-AB and WT mice.12 Differences in cold exposure procedures and/or in species may explain the inconsistency.
ORX-AB mice were unable to maintain body temperature during the cold exposure,12 while ORX-AB rats were able to defend body temperature. Rats need less heat production per body weight to maintain their body temperature than mice, as explained by Bergmann's rule.28 In addition, rats have a better core-shell insulation with thicker skin and longer fur than mice. Consequently, heat production in ORX-AB rats could be sufficient to maintain their body temperature during cold exposure.
In the ORX-AB mice study,12 they performed the cold exposure in the light phase of their circadian cycle, while we used the dark phase. Behavioral and physiological parameters change in the circadian cycle manner.18,27,29 The difference of the timing of the cold exposure in the circadian cycle may affect general thermoregulatory responses to the cold exposure.
Possible role of orexin neurons in the cold defense responses
Considering the lower ambient threshold temperature to trigger thermoregulatory responses, ORX-AB rats may have lower thermal sensitivity to cooling skin stimuli than WT rats. Cutaneous thermosensation involves thermal transduction via transient receptor potential (TRP) channels.30-32 Evidence indicates that expression of orexin peptides and their receptors is not restricted to the CNS (For a review see refs.33-36), but it is also found in peripheral tissue 37-41 including skin.42 Expression of prepro-orexin and orexin receptor mRNAs is found in peripheral tissue in rats and humans.37,40,43-46 Thus, it is worth investigating whether peripheral orexin-synthesising cells are involved in the TRP-mediated thermal transduction.
Central injection of orexin peptide shifts rats' thermal preference to warmer ambient temperature.47 The median subnucleus of the preoptic area (MnPO), which is the most upstream nucleus in the central thermoregulatory networks, receives peripheral thermal sensory information via the lateral parabrachial nucleus.26,48,49 Numerous orexin projections are present in the MnPO.7 Hence, it is possible that orexin neurons may involve in central processing signals from peripheral thermosensors.
We have shown that BAT thermogenesis and cutaneous vasoconstriction elicited by emotional events are reduced in ORX-AB rats.9,50 ORX-AB rats show attenuated spontaneous BAT thermogenesis that occurs during active phases of the basic rest-activity cycles (BRAC) as part of normal daily life.9 Recent studies using ORX-AB mice show that BAT thermogenesis elicited by febrile and handling stress is also attenuated.12,13 Furthermore, hypothermic response to general anesthesia is larger in ORX-AB mice comparing to WT mice.51 These findings indicate that the observed abnormal response in ORX-AB rats and mice is not restricted to cold exposure stressor, but it is generalized to any types of stressors. Orexin neurons would therefore have an important role in driving BAT thermogenesis and cutaneous vasoconstriction in general manner.
ORX-AB rats and mice have abnormal sleep-wake behavior characterized by fragmented wake and sleep bouts. It has been suggested that orexin signals are important for integration in neurophysiological mechanisms for stabilizing alternation between wake and sleep states.52 When animals respond to external unexpected cue or to spontaneous internal demand, their arousal level or alertness is usually increased.15,53 The ablation of orexin neurons may cause instability in alternation between the states and result in impairing stable arousal level. It is possible that ORX-AB rats cannot stably promote alertness that is triggered by the initial decrease in ambient temperature during the cold exposure, resulting in delayed and attenuated physiological responses.
Materials and methods
All experiments were performed in male transgenic ORX-AB Sprague-Dawley rats (n = 10, 427 ± 17 g) and their WT litter mates (n = 12 including five normal Sprague-Dawley rats, 419 ± 25 g, not significantly different from ORX-AB, P > 0.05) with procedures approved by the Animal Welfare Ethical Committee of Flinders University. Care was taken to minimise the number of animals used. Since there was no difference in data between the WT littermates and the normal Sprague-Dawley rats, all data from those animals were grouped together.
ORX-AB rats were initially received from Dr Yanagisawa's laboratory at Howard Hughes Medical Institute, Dallas, Texas. The generation of transgenic rat-lines was reported earlier.8 The breeding colonies were maintained by crossing normal Sprague-Dawley rats at Flinders University's Animal Facility. Animals were identified as transgenic or wild type rats by genotyping with PCR before experiments, as described previously.9
Surgical procedures
For implantation of measuring devices, rats were anaesthetised with 2% isoflurane (Veterinary Companies of Australia Pty Ltd., Kings Park, NSW, Australia) in 100% oxygen. An ultrasonic Doppler flow probe (Iowa Doppler Products, IA, USA) was implanted around the tail artery about 2 cm distal to the bases.9,54 Flow probes were connected via subcutaneous wires to a headpiece. Small thermistor-based sensors were chronically fixed into the interscapular brown fat (BAT) and in the mediastinum just ventral to the trachea (close to the heart) to record BAT and body temperatures continuously.9,15 Thermistor cables were passed subcutaneously and attached to the same headpiece screwed to the skull. Rats were left undisturbed for one week to recover from surgical stress.
Cold exposure protocol
One day before the experiment, the rats were transferred to an experimental cage that was placed in a temperature-controlled (26°C) chamber, with light on at 7:00 pm and off at 7:00 am. The temperature-controlled chamber is a modified freezer fitted with an electrical heater. Temperature was controlled by a computer-controlled switching between the refrigeration system and the electrical heater. The chamber was ventilated continuously by a fan. Rats were housed singularly in the experimental cage and provided with food and water ad-libitum. On the day of experiment, between 10:00 am and 11:00 am (dark/active period), cold exposure trial commenced. A cage temperature gradually decreased from 26°C to below 5°C. The cage temperature started returning to pre-cooling temperature 26°C approximately after three hours of the cold state below 5°C.
Data recording
Animals were connected to a swivel device (SL12C, PlasticOne, Roanoke, VA, USA) with a flexible cable and a counter-balanced swivel device at the top of the cage. Temperature signals from the BAT were measured by a bridge amplifier (BME, Flinders University) and then digitised (1Hz) with an analog/digital converter (PowerLab, ADInstruments Inc., Castle Hill, Australia). The tail artery Doppler signal was converted to voltage signals (flow speed, cm/s) by a Doppler flow amplifier (System 6 Model 200, Triton Technology, San Diego, CA, USA). The voltage signal was then digitised with the Powerlab for signal sampling (40 Hz). Locomotor activity was measured with a pyro-electric passive infrared sensor (NaPiOn, AMN1111, Panasonic, Osaka, Japan). All recorded data were stored on a computer with a data acquisition software (Chart, ADInstruments Inc., Castle Hill, NSW, Australia). The data were analyzed with IgorPro software (Wavemetrics, Lake Oswego, OR, USA). We measured total amount of time when the sensor detected animal movements per minute. The amount of food eaten was measured using the method reported previously.18
Immunohistochemistry
Rats were deeply anaesthetised with pentobarbital (100 mg/kg i.p.) and perfused transcardially with 0.5 M phosphate buffer, which was followed by fixative solution containing 4% formaldehyde and 15% picric acid in 0.5 M phosphate buffer. This was followed by fixative solution containing 4% formaldehyde, 15% picric acid and 20% sucrose in in 0.5 M phosphate buffer. The brain was removed and post-fixed in the same formaldehyde-picric acid-sucrose fixative. Serial sections (50 μm) were cut from the forebrain using a cryostat (Cryocut 1800, Leica Microsystem Pty Ltd, North Ryde, NSW, Australia). The sections were incubated with 0.1 M TBS contained 50% ethanol for 30 min, 20% normal horse serum for one hour, rabbit anti-orexin antiserum (1:5000 in 0.1 M TBS containing 0.1% normal horse serum, Peptide Institute, Minohshi, Japan) for overnight at 4°C, and biotinylated goat anti-rabbit IgG antibodies (1:200 in 0.1 M TBS containing 0.1% normal horse serum, Vector Laboratories Inc., CA) for overnight at 4°C. Between incubations, section were washed thoroughly with 0.1 M TBS containing 0.1% normal horse serum. Finally, tissues were treated with avidin-biotin complex (Vectastain ABC kit, Vector Laboratories Inc., Burlington, CA, USA) for 1 hour and reacted with 0.05% di-amino benzidine (DAB) for 10 min and intensified with 0.01% H2O2. Tissues sections were then mounted on gelatin-coated microscopic slides, dehydrated with graded alcohol and cover-slipped for histological examinations.
Statistical analysis
Statistical analysis was performed using SPSS (IBM Corporation, Armonk, NY, USA). Response magnitude in each parameter over time was calculated as the area under the curve above the baseline. Group data are shown as mean ± SEM. The statistical significance of mean difference was assessed with paired t-test within a group or unpaired t-test between groups.
Abbreviations
- BAT
brown adipose tissue
- CNS
central nervous system
- DAB
di-amino benzidine
- ORX-AB
orexin neurons ablated
- PCR
polymerase chain reaction
- TBS
Tris-buffered saline
- WT
wild type
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Jessi Moore, Pam Simpson and Robyn Flook for technical assistance, and Professor Tomoyuki Kuwaki for constructive comments on our manuscript.
Funding
This work was supported by National Health and Medical Research Council Project Grant (38414 and 1101677) and the Flinders Medical Center Research Foundation.
References
- [1].Ootsuka Y, Tanaka M. Control of cutaneous blood flow by central nervous system. Temperature 2015; 2:392-405; http://dx.doi.org/ 10.1080/23328940.2015.1069437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 2011; 589:3641-58; PMID:21610139; 10.1113/jphysiol.2011.210047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014; 19:741-56; PMID:24630813; http://dx.doi.org/ 10.1016/j.cmet.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al.. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92:573-85; PMID:9491897 [DOI] [PubMed] [Google Scholar]
- [5].de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, et al.. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 1998; 95:322-7; PMID:9419374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al.. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999; 98:437-51; PMID:10481909 [DOI] [PubMed] [Google Scholar]
- [7].Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 1998; 18:9996-10015; PMID:9822755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci 2004; 24:4469-77; PMID:15128861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mohammed M, Ootsuka Y, Yanagisawa M, Blessing W. Reduced brown adipose tissue thermogenesis during environmental interactions in transgenic rats with ataxin-3-mediated ablation of hypothalamic orexin neurons. Am J Physiol Regul Integr Comp Physiol 2014; 307:R978-89; PMID:25324552; http://dx.doi.org/ 10.1152/ajpregu.00260.2014 [DOI] [PubMed] [Google Scholar]
- [10].Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A 2004; 101:4649-54; PMID:15070772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mullett MA, Billington CJ, Levine AS, Kotz CM. Hypocretin I in the lateral hypothalamus activates key feeding-regulatory brain sites. Neuroreport 2000; 11:103-8; PMID:10683839 [DOI] [PubMed] [Google Scholar]
- [12].Takahashi Y, Zhang W, Sameshima K, Kuroki C, Matsumoto A, Sunanaga J, Kono Y, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J Physiol 2013; 591:5623-43; http://dx.doi.org/ 10.1113/jphysiol.2013.261271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J physiol 2010; 588:4117-29; PMID:20807795; http://dx.doi.org/ 10.1113/jphysiol.2010.195099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang S, Lin L, Kaur S, Thankachan S, Blanco-Centurion C, Yanagisawa M, Mignot E, Shiromani PJ. The development of hypocretin (orexin) deficiency in hypocretin/ataxin-3 transgenic rats. Neuroscience 2007; 148:34-43; PMID:17618058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience 2009; 164:849-61; PMID:19679172; http://dx.doi.org/ 10.1016/j.neuroscience.2009.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mohammed M, Ootsuka Y, Blessing W. Brown adipose tissue thermogenesis contributes to emotional hyperthermia in a resident rat suddenly confronted with an intruder rat. Am J Physiol Regul Integr Comp Physiol 2014; 306:R394-400; PMID:24452545; http://dx.doi.org/ 10.1152/ajpregu.00475.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ootsuka Y, Kulasekara K, de Menezes RC, Blessing WW. SR59230A, a β-3 adrenoceptor antagonist, inhibits ultradian brown adipose tissue thermogenesis and interrupts associated episodic brain and body heating. Am J Physiol Regul Integr Comp Physiol 2011; 301:R987-94; PMID:21813867; http://dx.doi.org/ 10.1152/ajpregu.00085.2011 [DOI] [PubMed] [Google Scholar]
- [18].Blessing W, Mohammed M, Ootsuka Y. Heating and eating: brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol Behav 2012; 105:966-74; PMID:22115948; http://dx.doi.org/ 10.1016/j.physbeh.2011.11.009 [DOI] [PubMed] [Google Scholar]
- [19].Rusyniak DE, Ootsuka Y, Blessing WW. When administered to rats in a cold environment, 3,4-methylenedioxymethamphetamine reduces brown adipose tissue thermogenesis and increases tail blood flow: Effects of pretreatment with 5-HT(1A) and dopamine D(2) antagonists. Neuroscience 2008; 154:1619-26; PMID:18534763; http://dx.doi.org/ 10.1016/j.neuroscience.2008.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ootsuka Y, Heidbreder CA, Hagan JJ, Blessing WW. Dopamine D(2) receptor stimulation inhibits cold-initiated thermogenesis in brown adipose tissue in conscious rats. Neuroscience 2007; 147:127-35; PMID:17512675 [DOI] [PubMed] [Google Scholar]
- [21].Smith RE, Roberts JC. Thermogenesis of brown adipose tissue in cold-acclimated rats. Am J Physiol 1964; 206:143-8; PMID:14117643 [DOI] [PubMed] [Google Scholar]
- [22].Ishiwata T, Saito T, Hasegawa H, Yazawa T, Kotani Y, Otokawa M, Aihara Y. Changes of body temperature and thermoregulatory responses of freely moving rats during GABAergic pharmacological stimulation to the preoptic area and anterior hypothalamus in several ambient temperatures. Brain Res 2005; 1048:32-40; PMID:15913569 [DOI] [PubMed] [Google Scholar]
- [23].Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience 2006; 141:2067-73; PMID:16814930 [DOI] [PubMed] [Google Scholar]
- [24].Sakurada S, Shido O, Sugimoto N, Hiratsuka Y, Yoda T, Kanosue K. Autonomic and behavioural thermoregulation in starved rats. J Physiol (Lond) 2000; 526:417-24; PMID:10896730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold defense. Am J Physiol Regul Integr Comp Physiol 2006; 291:R589-95; PMID:16601257 [DOI] [PubMed] [Google Scholar]
- [26].Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011; 16:74-104; PMID:21196160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schwimmer H, Stauss HM, Abboud F, Nishino S, Mignot E, Zeitzer JM. Effects of sleep on the cardiovascular and thermoregulatory systems: a possible role for hypocretins. J Appl Physiol 2010; 109:1053-63; PMID:20705949; http://dx.doi.org/ 10.1152/japplphysiol.00516.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zamora-Camacho FJ, Reguera S, Moreno-Rueda G. Bergmann's Rule rules body size in an ectotherm: heat conservation in a lizard along a 2200-metre elevational gradient. J Evol Biol 2014; 27:2820-8; PMID:25387908 [DOI] [PubMed] [Google Scholar]
- [29].Tankersley CG, Irizarry R, Flanders S, Rabold R. Circadian rhythm variation in activity, body temperature, and heart rate between C3H/HeJ and C57BL/6J inbred strains. J Appl Physiol 2002; 92(1985):870-7; PMID:11796704 [DOI] [PubMed] [Google Scholar]
- [30].Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 2014; 210:498-507; PMID:24716231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 2003; 4:529-39; PMID:12838328 [DOI] [PubMed] [Google Scholar]
- [32].Wang H, Siemens J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015; 2:178-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tsunematsu T, Yamanaka A. The role of orexin/hypocretin in the central nervous system and peripheral tissues. Vitam Horm 2012; 89:19-33; PMID:22640606; http://dx.doi.org/ 10.1016/B978-0-12-394623-2.00002-0 [DOI] [PubMed] [Google Scholar]
- [34].Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M. Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci CMLS 2002; 60:72-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol 2014; 171:332-50; PMID:24102345; http://dx.doi.org/ 10.1111/bph.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am J Physiol Cell Physiol 2013; 304:C2-32; PMID:23034387; http://dx.doi.org/ 10.1152/ajpcell.00227.2012 [DOI] [PubMed] [Google Scholar]
- [37].Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron 1999; 24:941-51; PMID:10624957 [DOI] [PubMed] [Google Scholar]
- [38].de Miguel MJ, Burrell MA. Immunocytochemical detection of orexin A in endocrine cells of the developing mouse gut. J Histochem Cytochem 2002; 50:63-9; PMID:11748295 [DOI] [PubMed] [Google Scholar]
- [39].Ouedraogo R, Naslund E, Kirchgessner AL. Glucose regulates the release of orexin-a from the endocrine pancreas. Diabetes 2003; 52:111-7; PMID:12502500 [DOI] [PubMed] [Google Scholar]
- [40].Lopez M, Senaris R, Gallego R, Garcia-Caballero T, Lago F, Seoane L, Casanueva F, Dieguez C. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinol 1999; 140:5991-4 [DOI] [PubMed] [Google Scholar]
- [41].Blanco M, Garcia-Caballero T, Fraga M, Gallego R, Cuevas J, Forteza J, Beiras A, Dieguez C. Cellular localization of orexin receptors in human adrenal gland, adrenocortical adenomas and pheochromocytomas. Regul Pept 2002; 104:161-5; PMID:11830291 [DOI] [PubMed] [Google Scholar]
- [42].Beiras-Fernandez A, Gallego R, Blanco M, Garcia-Caballero T, Dieguez C, Beiras A. Merkel cells, a new localization of prepro-orexin and orexin receptors. J Anat 2004; 204:117-22; PMID:15032918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinol 2001; 142:3324-31. [DOI] [PubMed] [Google Scholar]
- [44].Nakabayashi M, Suzuki T, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Date F, Takeyama J, Darnel AD, Moriya T, et al.. Orexin-A expression in human peripheral tissues. Mol Cell Endocrinol 2003; 205:43-50; PMID:12890566 [DOI] [PubMed] [Google Scholar]
- [45].Mazzocchi G, Malendowicz LK, Gottardo L, Aragona F, Nussdorfer GG. Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade. J Clin Endocrinol Metab 2001; 86:778-82; PMID:11158046 [DOI] [PubMed] [Google Scholar]
- [46].Liguori G, Pavone LM, Assisi L, Langella E, Tafuri S, Mirabella N, Costagliola A, Vittoria A. Expression of orexin B and its receptor 2 in rat testis. Gen Comp Endocrinol 2015; S0016–6480(15):30029-0; PMID:26631456; http://dx.doi.org/ 10.1016/j.ygcen.2015.11.015 [DOI] [PubMed] [Google Scholar]
- [47].Viggiano E, Messina G, Viggiano A, Viggiano A, Luca VD, Messina A, Monda M. Effects of Orexin A on thermal behaviour: substantial evidences for thermoregulatory role of Orexin A. J Bioanalysis Biomed 2014; 6:40-4. [Google Scholar]
- [48].Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 2008; 11:62-71; PMID:18084288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tupone D, Madden CJ, Morrison SF. Autonomic regulation of brown adipose tissue thermogenesis in health and disease: potential clinical applications for altering BAT thermogenesis. Frontiers Neurosci 2014; 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kleitman N. Basic rest-activity cycle-22 years later. Sleep 1982; 5:311-7; PMID:6819628; http://dx.doi.org/ 10.3389/fnins.2014.00014 [DOI] [PubMed] [Google Scholar]
- [51].Kuroki C, Takahashi Y, Ootsuka Y, Kanmura Y, Kuwaki T. The impact of hypothermia on emergence from isoflurane anesthesia in orexin neuron-ablated mice. Anesth Analg 2013; 116:1001-5; PMID:23477964; http://dx.doi.org/ 10.1213/ANE.0b013e31828842f0 [DOI] [PubMed] [Google Scholar]
- [52].Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci 2004; 24:6291-300; PMID:15254084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].de Menezes RC, Ootsuka Y, Blessing WW. Sympathetic cutaneous vasomotor alerting responses (SCVARs) are associated with hippocampal theta rhythm in non-moving conscious rats. Brain Res 2009; 1298:123-30; PMID:19699727; http://dx.doi.org/ 10.1016/j.brainres.2009.08.042 [DOI] [PubMed] [Google Scholar]
- [54].Garcia JN, Pedersen NP, Nalivaiko E, Blessing WW. Tail artery blood flow measured by chronically implanted Doppler ultrasonic probes in unrestrained conscious rats. J Neurosci Methods 2001; 104:209-13; PMID:11164247 [DOI] [PubMed] [Google Scholar]


