ABSTRACT
Sago (Metroxylin sagu), a carbohydrate (CHO) based dietary staple of Southeast Asia is easily digestible and quickly absorbed, and thus has potential to be prescribed as an affordable pre-and post-exercise food in this part of the world. Compared to other CHO staples, research into the physiological response to sago ingestion is sparse, and only a few recent studies have investigated its value before, during, and after exercise. The purpose of this review is to describe the published literature pertaining to sago, particularly as a supplement in the peri-exercise period, and suggest further avenues of research, principally in an environment/climate which would be experienced in Southeast Asia i.e. hot/humid.
KEYWORDS: Carbohydrate, Cycling, Heat, Physiology, Performance, Recovery, Southeast Asia, Starch, Tropical
Introduction
The relationship between reduced carbohydrate (CHO) status and the onset of fatigue has been known for some time.1 Specifically, the progressive depletion of skeletal muscle's limited glycogen stores and reduction in circulating blood glucose as exercise progresses are linked to performance deterioration and volitional fatigue.2 It follows that supplementing CHO can delay the onset of fatigue and improve work output and capacity.3 The right combination of an appropriate CHO composition and administration regimen can potentiate this ergogenic effect;4 hence, for example, the consensus prescription of CHO before, during and after exercise to maximise performance and optimise recovery.5
Many major sporting events take place during the hottest season, in warm-to-hot environments/climates or at the hottest part of a day.6 During exercise with heat stress, there is consensus that endurance performance is decreased compared with cooler conditions, and there is an increased risk of heat illness, especially with high humidity.7 Another consequence of exercise heat stress is an alteration in CHO metabolism. Yaspelkis and Ivy8 demonstrated that exercise in the heat accelerated fatigue because of an increase in reliance upon CHO as a substrate. Further, Jentjens and Jeukendrup9 demonstrated that when ambient temperatures increase so does CHO oxidation rate during exercise largely due to an increased muscle glycogen use. This led the same authors10 to suggest that glycogen stores may be sub-optimal in athletes training or competing multiple times daily or on successive days in hot environments. Accordingly, the accepted paradigm is that increasing CHO availability before and during exercise can enhance performance when exercise is completed in the heat, even for shorter (<1 h), more intense (∼80% O2max) bouts (see refs. 11,12).
The hot/humid environment awaiting athletes at the upcoming 2016 Summer Olympics in Rio de Janeiro, the 2018 Commonwealth Games on the Gold Coast and the 2022 Soccer World Cup in Qatar, requires athletes and their support teams to take these environmental conditions into consideration for best preparation. Furthermore, at least a third of the world's population now live in the Tropical Zone, close to the equator where ambient temperatures are hotter and more physically challenging than in the Temperate or Frigid Zones.13 Therefore optimisation of physical work capacity in the tropics requires understanding the risks and ways in which these can be minimised. These include best practice nutrition and hydration in the peri-event period.
Where commercially available CHO products are unaffordable or inaccessible to those competing in sport or exercise, there is a need to investigate local food sources as suitable alternatives. Southeast Asia is a region with over 600,000,000 inhabitants and a year-round tropical (warm-humid) climate, where sago (Metroxylin sagu) palms are widely distributed and their starch is used as an important dietary CHO source;14 for example, Malaysia, Indonesia and Papua New Guinea are the world's leading countries in the production of sago.15 Previous research has identified sago as being rapidly digestible, quickly absorbed and therefore suitable for consumption before, during and in recovery from exercise,16 however only one study had initially tested this notion17 before our recent series of investigations developing a reliable protocol to test whether supplementing sago before, during and in recovery indeed proved ergogenic.18-20
Therefore, this review will provide physiological rationale for and appraisal of the performance-enhancing effect(s) of sago supplementation when exercising in a hot environment, and scope for future research.
Exercise with heat stress: The case for carbohydrate
Greater rates of CHO and a concomitant reduction in fat oxidation are consistently reported during exercise in the heat when compared to exercise at cooler ambient temperatures. For example, Fink et al.21 compared 60 min of intermittent exercise in 41°C and 9°C observing muscle glycogen utilization to be higher and triglyceride utilization lower in the heat. This was accompanied by greater hepatic glucose production and a higher respiratory exchange ratio (RER). Similarly, Febbraio and colleagues22 observed muscle glycogen utilization to be elevated by 20–80% as a result of non-exhaustive bouts of exercise in 40°C versus 20°C. From such measurement, Yaspelkis and Ivy8 suggested that heat-related fatigue is accelerated in endurance exercise due to an increase in reliance upon CHO as a substrate. However, not all studies report an increased RER and total CHO oxidation is often lower as fatigue is reached at an earlier time-point during (fixed-intensity) exercise in the heat when compared to cooler conditions.23,24 Similarly, Young et al.25 observed no differences for muscle glycogen utilization during exercise with heat stress or when heat acclimated. An explanation for this discrepancy is that in the study by Young et al., participants began their 30 min of cycling at 70% O2max in the heat with low pre-exercise muscle glycogen content, with previous research26 demonstrating that the rate of glycogenolysis during submaximal exercise is heavily influenced by pre-exercise glycogen levels.
The most detailed investigation of muscle metabolism during exercise heat stress has come from the aforementioned group of Febbraio and colleagues. They were able to identify that the increased glycogenolysis, CHO oxidation and muscle lactate production during exercise in the heat (40°C) when compared to the cool (20°C), was a function of higher muscle temperature and adrenergic response secondary to a greater rise in core temperature (see ref. 27 for review).
To our knowledge only one published work has addressed the issue of whether pre-exercise CHO supplementation improves performance in the heat. In this study, Pitsiladis and Maughan11 provided participants with an iso-caloric diet with either a low (10%) or high (80%) CHO content for 3 d following glycogen depletion and then cycled them to exhaustion at 30°C. Diets were prescribed in a randomized, but balanced fashion. Following the high CHO diet participants improved their endurance time by 8–73% (53 vs 44 min) with their RER, rate of and total CHO oxidation, and lactate concentration being higher. The high CHO diet also reduced perceived exertion.
There have been a number of reports of CHO supplementation during exercise heat stress proving ergogenic (see refs. 12, 28), however the mechanism(s) responsible remain poorly understood, and not all studies have observed significant performance benefits.29 Of particular note should be that most often the form of CHO administered is that of a fluid or beverage i.e., with/-out CHO and often this is consumed ad libitum. Therefore, on first appearance it is difficult to appraise whether it is the separate level of hydration having an effect, as more fluid is often consumed during a CHO trial e.g. ref. 12. Correspondingly, Horswill et al.30 provided iso-volumetric electrolyte-beverages to participants during 1-h of fixed-intensity cycling at 30°C distinguished only by their CHO content (∼94 g vs 0 g) and observed no physiological differences. Similarly, Below et al.28 replicated this observation (of no physiological difference) during 50 min of fixed-intensity cycling at 31°C when fluid was matched but CHO content different. However, in the immediately proceeding work-dependent time-trial both fluid and CHO independently (6%) improved performance compared to a placebo, and the combined effects were additive (12%). While other self-paced cycling protocols have demonstrated that CHO supplementation during exercise heat stress improves performance,31 at least one report32 indicates that when double-blinded, CHO supplementation during exercise in the heat does not improve performance. Nevertheless, a neural or psychological effect may be present; an idea borne out from the results of carbohydrate mouth-rinse studies, some of which do suggest a change in brain activity with simply a sweet taste in the mouth.33
The observation that performance is improved despite no changes in whole-body fuel selection could reflect dissonance with specific tissue metabolism or because of an imbalance between hepatic glucose production and glucose uptake by the muscle and/or other tissues.34,35 It is also now acknowledged that, particularly in shorter (<1 h), more intense (∼80% O2max) bouts of exercise an improved CHO status may have a central ergogenic effect that is not related to or detectable by whole body (indirect) calorimetry.2 Conflicting results may also reflect the specific type of exercise test employed; self-paced tests of endurance with a known end-point prove far more reliable than open-ended tests (a coefficient of variation [CV] of ∼3–4% vs. ∼30%, respectively) where the end-point, and thus primary dependent variable, is a subjective notion of fatigue.36
Much like the evidence for pre-exercise CHO supplementation, there is scant confirmation that concerns the ingestion of CHO in recovery from and for subsequent exercise in the heat. However, indirect evidence has been reported by Davis and colleagues37 who noted that a second (work-dependent) cycle bout in conditions of heat stress following a 30-min recovery period was completed faster when 6% CHO was consumed vs. a fluid-matched placebo. This is further supported by a recent investigation38 demonstrating that following exercise heat stress the re-synthesis of muscle glycogen (within 3h) is affected by the CHO consumed and not by rehydration. Therefore, these results above point to CHO supplementation in recovery from exercise heat stress being beneficial and subsequently ergogenic. The latter, is the true test of recovery.
Supplementation for sport: Starch and sago
One of several ways of classifying CHOs is whether they are simple (mono-, di- or oligo-saccharides) or complex (poly-saccharides).39 Although extensive research has been carried out on developing and marketing (simple) CHOs for exercise supplementation, such as glucose, fructose, maltose, sucrose, maltodextrin and also galactose data concerning the effect of complex CHOs (specific starches) on exercise performance remains scarce. The limited studies investigating starch on exercise performance can be seen in Table 1, with these investigations having used waxy40-42 and corn43 starches. Irrespective of the type of starch, their basic units contain the glucose polymers amylose and amylopectin that classify the CHO as complex. A primary interest for sports nutritionists is the rate of digestion which largely dictates the glycaemic response.44 The utility of these starches, in terms of digestion, absorption (as glucose) and thence oxidation rates during exercise are thus dependent on their amylopectin:amylose ratio.2 Some starches appear suitable when consumed before40 or in recovery from43 exercise as a performance improvement has been observed in specific conditions for specific starch-containing foods.45 Moreover, a low glycaemic index (GI) CHO appears to hold some advantage over a high GI CHO when ingested prior to exercise,45 and the reverse is thought to occur post-exercise when the recovery period is short,46 but not long.47
Table 1.
Studies investigating the effect of starches on exercise performance.
| Study | Starch type | Ingestion Timing | Protocol | Performance |
|---|---|---|---|---|
| Goodpaster et al.40 | Waxy (100% amylopectin), or resistant (100% amylose) | 30 min before exercise | 60 min cycling at 75% O2max, 30 min TT | 6.3% improved performance with waxy corn |
| Jozsi et al.41 | Waxy (100% amylopectin) or resistant (100% amylose) | Post-exercise consumption over 12 h | 60 min cycling at 75% O2max, 6 × 1 min at 125% O2max, 24 h rest, 30 min TT | No effect |
| Roberts et al.42 | Waxy (95% amylopectin), hydrothermally modified | 30 min before exercise | 150 min cycling at 70% O2max, TTE at 100% O2max | No effect |
| Stephens et al.43 | Low and high molecular weight modified corn starches | Post-exercise feeding 2 h prior to second bout | TTE cycling at 73% O2max, 2 h rest, 15 min TT | 2.3% improved TT performance with high molecular modified starches |
Notes: TTE, time to exhaustion; TT, time trial; O2max, maximal O2 consumption.
Sago is one of the oldest agricultural crops in Southeast Asia including in Sarawak, Malaysia.48 In fact, since the 1970s Sarawak has been one of the world's biggest producers of sago and contributes to the country's economy through a large export industry.49 Sago starch, derived from the stem or trunk of the palm, is a versatile food ingredient and widely used in numerous food and industrials applications either in its native form or as a food ingredient modified as a texturizer, gelling agent, and thickener.50 It is also commonly used for food production in ‘Western’ food products such as vermicelli, bread, crackers and biscuits.14 More recent developments in the food industry include its use as a raw material for maltodextrins and cyclodextrins in infant formulas, sport drinks, and carriers in pharmaceuticals.51
Goodpaster and colleagues40 demonstrated that certain forms of CHO, such as starch, produce a slightly different metabolic profile during exercise because of their physical or chemical structure; this should be similar for sago. Mohamed et al.52 reviewed the literature and as sago starch contains 27% amylose and 73% amylopectin, it was suggested that due to the high(er) amylopectin content sago may be digested and absorbed quicker when compared to other starches high in amylose i.e. a higher-GI.53 Ahmad et al.16 demonstrated that sago porridge provides a robust glycaemic response but with a lower insulinaemic response compared to sago gel and paste at rest, and suggested that this form would be suitable before, during and after endurance exercise. The high-GI reported by Ahmad16 is further supported by Hassan and co-workers54 who calculated blood glucose responses (area under the curve) of a number of CHO-based foods, reporting a GI of ∼80 for sago, using glucose as the standard reference food (GI of ∼97).54
Performance as a nutrition intervention outcome measure: Influence of study design
Saris and colleagues55 state that any study protocols evaluating the effects of nutritional supplementation on physical work capacity must be reliably proven in that population to detect a small difference in exercise performance. This raises 2 interesting and important questions: (1) what determines ‘reliable’, and (2) what is a ‘small’ difference? The answers are, of course, not simple or singular. For cyclists, it appears as though it is of no consequence whether they are a novice, trained or elite athlete as interventions that have improved simulated ∼1h time-trial performance, including nutrition, have done so by more than 1%.56 Interestingly, Hopkins and colleagues57 report that ∼1% is also the typical within-athlete variation at an elite level when conveyed as the typical percent error or standard error of measurement, expressed as CV. They further suggest that for an athlete to substantially increase their chance of success (winning), they have to improve their performance by just less than one-half (0.3) the typical race-to-race variation.57 Thus, it appears that even a 1% performance improvement for an athlete can be worthwhile, but more importantly possessing knowledge of the typical or “fixed” variance of a given performance test when repeated (including the participant, protocol and equipment) is essential.
Sago supplementation for performance improvement: The evidence
To our knowledge, only 3 studies have made (5) comparisons between sago formulation and a placebo/control; details of these studies can be seen in Table 2. Ghosh et al.17 were the first to investigate feeding 60g sago or 53g sago with 15g soy protein at 20-min intervals during cycling at 60% O2max on subsequent exhaustion time at 90% O2max. They reported an increased work capacity at 90% O2max from 4.1 ± 1.3 min (placebo) to 5.5 ± 1.2 min with sago and 7.5 ± 2.0 min with sago + soy, only the latter proving significant (p = 0.22 and p < 0.001, respectively). Although the double-blind design and inclusion of a flavour-matched placebo strengthens these observations, the time-to-exhaustion protocol and participants of low aerobic fitness make the results difficult to interpret and generalize to an athletic population. Further, that it was conducted in thermoneutral conditions does not easily enable translation to performance in hot conditions such as those found where sago is a staple.
Table 2.
Studies investigating the effect of sago on exercise performance.
| Study | Participants | Protocol | Comparison | Measures |
|---|---|---|---|---|
| Ghosh et al. (17) | 8 recreational male cyclists, O2max: 40 ± 1 ml·kg−1·min−1 | 60 min cycling at 60% O2max, TTE at 90% O2max in 25 ± 1 °C and 70 ± 5 % rh | 60g sago porridge vs. 53g sago porridge + 15 g soy isolate vs. flavour-matched placebo equally divided and supplemented at 0, 20 and 40 min; double-blind, cross-over design | TTE performance; plasma glucose, lactate, ammonia and insulin |
| Che Jusoh et al. (19) | 8 trained male cyclists, O2max: 65 ± 10 ml·kg−1·min−1 | 45 min cycling at 55% O2peak, 15 min TT in 30 ± 2 °C and 78 ± 3 % rh | 63g sago porridge 1h before exercise vs. 63g sago gel equally divided and supplemented at 0, 15, 30 and 45 min during exercise vs. fluid-matched control; crossover design | TT performance; plasma glucose, lactate, sodium and potassium; substrate oxidation; body temperatures; fluid consumption and sweat loss; heart rate |
| Che Jusoh et al. (20) | 8 trained male cyclists, O2max: 70 ± 10 ml·kg−1·min−1 | 15 min warm-up cycling, 15 min TT in 30 ± 1 °C and 71 ± 4 % rh, 2 h thermoneutral rest, 15 min warm-up cycling, 15 min TT in 30 ± 1 °C and 71 ± 4 % | 56g sago porridge post-exercise feeding 2h prior to second bout vs. fluid-matched control; cross-over design | TT performance; plasma glucose, lactate, sodium and potassium; substrate oxidation; body temperatures; fluid consumption and sweat loss; heart rate |
Notes: TTE, time to exhaustion; TT, time trial; O2max, maximal O2 consumption; O2 peak, peak O2 consumption; rh, relative humidity.
Therefore, we recently undertook a series of investigations19,20 into whether supplementing sago before, during and in recovery indeed proved ergogenic to an athletic/trained population exercising in thermally stressful conditions. First we developed suitable protocols (see Fig. 1) and determined their reproducibility from a test-retest following a full familiarisation trial.18,20 We observed CV's of 3.6% and 2.3% for a 15-min time-trial performed following a fixed-intensity pre-load of 45 (steady-state)18 and 15 (warm-up)20 min on a cycle ergometer, respectively, for trained, familiarized males under warm-humid laboratory conditions. Then, using these protocols, we compared the ingestion of 0.8 g·kg−1 bodyweight sago – prepared using the same traditional double-boil method as Ghosh et al.17 – one hour before, during or immediately following exercise and compared this to a fluid-matched control trial to prevent dehydration. Knowing the typical variance of these tests under these conditions we were able to determine the “real” magnitude of our treatment i.e., defining the signal above the noise for the sago interventions. When comparing mean power output for the 15-min time-trial to the control, we observed a performance change of +1 ± 9 W when sago was consumed an hour before exercise (p = 0.77), of −2 ± 6 W when sago was consumed during exercise (p = 0.33), and of +9 ± 10 W when sago was consumed in recovery from a first bout to a second bout of exercise (p = 0.04), respectively.
Figure 1.
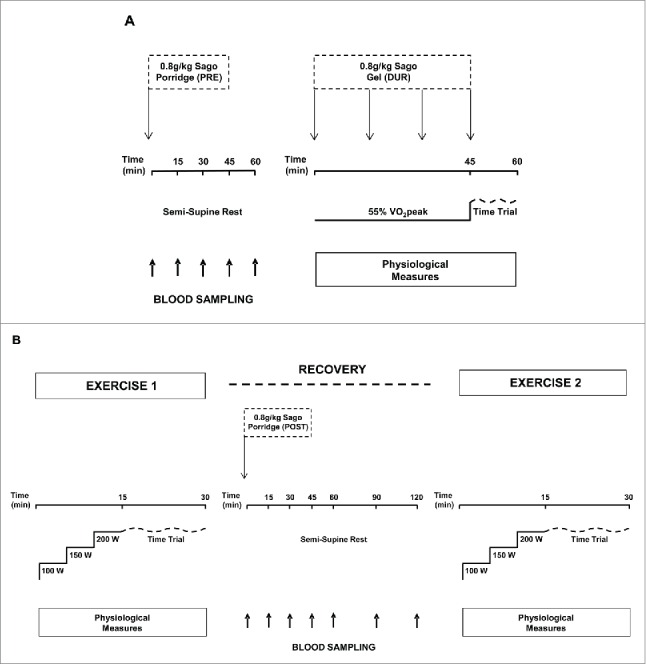
A schematic overview of the experimental protocol for sago supplementation before and during (A) and in recovery (B) from exercise. See refs. 19, 20.
The dosage of 0.8 g·kg−1 bodyweight was chosen for 3 reasons: 1) because the absolute amount for a 70 kg male lies within the American College of Sports Medicine (ACSM) Guidelines of 30–60 g per hour; 2) it is very close to the dosage applied by Ghosh et al.17, the latter enabling some comparison; and 3) it is well within what is able to be absorbed and metabolised during exercise.58
The question is whether using sago to improve performance is worthwhile. The performance results reviewed above can be interpreted by more than just null-hypothesis testing. Table 3 displays this research as evidence-based conclusions (inference) that can be made about the likely “true” effect (population effect), not just these samples. This conclusion should be useful to practitioners and athletes, not just other researchers – for further discussion see refs. 59, 60. Thus in Table 3, the smallest worthwhile change (0.3 x within-athlete variation57) for comparisons 1. and 2. are 8% (0.3 × 27%), for comparisons 3. and 4. are 1.1% (0.3 × 3.6%) and for comparison 5. is 0.7% (0.3 × 2.3%) when using the known CV for these performance tests (see previous section). Therefore, the inference is that sago (and sago + soy) consumed during exercise improves high-intensity fixed-intensity endurance time to a large extent and that sago improves recovery of moderate-intensity self-paced work performed to a small extent, whereas sago consumed before and during exercise does not appear to improve performance of moderate-intensity self-paced work.
Table 3.
Statistical inference and magnitude of sago's effect on exercise performance.
| Study | Comparison | % Change in Performance (90% CI) | Cohen's d | Pearson's r | Statistical Inference |
|---|---|---|---|---|---|
| Ghosh et al. (17) | 1. 60g sago porridge vs. flavour-matched placebo | 34 (18 to 68) | 1.1 | 0.5 | Large, Major |
| 2. 53g sago porridge + 15 g soy isolate vs. flavour-matched placebo | 84 (44 to 40) | 2.0 | 0.7 | Large, Major | |
| Che Jusoh et al. (19) | 3. 63g sago porridge 1h before exercise vs. fluid-matched control | 1 (−2 to 3) | 0.0 | 0.0 | Very Small, Trivial |
| 4. 63g sago gel during exercise vs. fluid-matched control | 1 (−2 to 1) | 0.1 | 0.0 | Very Small, Trivial | |
| Che Jusoh et al. (20) | 5. 56g sago porridge post-exercise feeding 2h prior to second bout vs. fluid-matched control | 4 (0 to 7) | 0.2 | 0.1 | Small, Minor |
Notes: CI, confidence interval; statistical inference of % change in performance due to sago from d and r based on Cohen.61
The physiological responses to these sago interventions, and therefore mechanisms responsible for these performance effects, almost certainly concern an enhanced or at least maintained, supply of CHO. Plasma concentrations of glucose were higher at rest and during recovery when fed sago19,20 with concentrations of glucose17,19 and insulin17 higher during exercise when sago was fed during exercise. Other notable responses to sago include an attenuated rise in rectal temperature when consumed before exercise and an increased fluid retention when consumed during exercise.19
Guidelines (Position Stands) are available from well-regarded bodies such as the ACSM5,62,63 to help with athlete safety and ensure optimal performance. However, they do not necessarily reflect nor refer to regional/local environments and foods. Information which can assist those in these regions to tailor recommendations from ACSM (or similar) to local conditions and resources is necessary; hopefully the findings of these studies goes some way to enabling that to occur.
Considerations and future research avenues
Although the primary aim with our series of investigations19,20 was to identify whether there was any difference between supplementing sago at different time-points i.e. before vs. during vs. in recovery from exercise, we used a control condition, where nothing was consumed other than water to maintain similar levels of hydration. The next logical step would be to assess sago against a suitable and known CHO source (e.g., vs. pasta/porridge or glucose) to determine efficacy, as, for example, it has previously been shown that waxy starch for 12h following glycogen-depleting exercise restores muscle glycogen and influences work completed in a subsequent 30-min time-trial similarly as equicaloric solutions with glucose or maltodextrin.41 Including a whole-food or fluid placebo would also be worthwhile, as a placebo effect has been demonstrated previously with CHO,64 something that cannot be determined from our results. However, Ghosh et al.17 still observed a performance improvement with sago when including a flavour-matched placebo and using a double-blind design. It must also be emphasized that all the performance tests investigating sago17-20 were completed in a post-prandial state to mimic pre-competition behavior, whereas several previous studies (see refs. 12, 28, 29) that have investigated CHO supplementation during exercise heat stress had participants complete their exercise following an overnight fast. This may go some way in explaining any performance discrepancy as the maintenance of glycaemia becomes more challenging in overnight fasted exercise.45
Although the mechanisms through which performance improved with sago (Table 3) very likely were related to enhancing or maintaining CHO status, it was beyond the resources of these studies to confirm this or to be able to partition the source of this CHO i.e., exogenous vs. endogenous (hepatic vs. muscular vs. circulating). Any future study should attempt to investigate this. Study participants (Table 2) were not heat-adapted as would likely be the case with those completing exercise regularly outdoors or indoors without air-conditioning in Southeast Asia. As acclimation to heat influences the metabolic (and thermoregulatory) response to exercise65 it is unknown whether supplementing sago to those regularly completing exercise in a tropical climate (thus heat-adapted) would yield similar results. Equally, the mode of exercise chosen within these studies (cycle-ergometry) cannot necessarily be extrapolated to that of (treadmill) running as gastro-intestinal distress due to supplementation of carbohydrate can occur more frequently and last for longer with running;66 both of these avenues warrant further research.
It has been suggested that when exercise heat stress is compensable, and the thermoregulatory system is not necessarily the primary limiting factor, endogenous carbohydrate stores become further depleted and that exogenous supplementation may be more effective.67 The combination of exercise intensity and ambient heat stress for our investigations18-20 was uncompensable, most likely a result of the high(er) ambient humidity. Therefore, future studies should investigate sago supplementation during more compensable heat stress i.e. lower-intensity, longer-duration exercise and/or lower-humidity environments, as ingestion of other CHO sources have been shown to be ergogenic in these conditions e.g. ref. 12.
Numerous studies, reviewed carefully by Cermak and van Loon,2 and the consensus position statement by the ACSM5 recommend that for endurance athletes protein (or amino acid) ingestion should be consumed ‘near’ to exercise in order for maintenance of or enhancing gains in skeletal muscle. While there is some evidence that adding protein to carbohydrate during exercise enhances performance,17 there is stronger evidence that adding protein to carbohydrate in recovery from exercise enhances muscle repair and elicits a more anabolic hormonal profile.5 In fact, Ghosh et al.17 observed a significant improvement in time to exhaustion following sago-soy supplementation than sago alone although these results may have been due to a greater overall caloric content. Nevertheless, it would be worthwhile to determine whether iso-caloric supplementation of sago-soy (or other suitable protein) enhances performance and/or the hormonal profile during and in recovery from exercise when compared to sago alone. Equally, as the form of carbohydrate provided does not alter the ergogenic effect2 and taking into consideration the need for athletes exercising in hot (especially humid) environments to prevent dehydration, there is merit in developing sago into a soluble form (perhaps with added electrolytes) that can be consumed in place of existing commercially-available carbohydrate-electrolyte drinks/powders.
Conclusion
Sago (Metroxylin sagu) proves suitable in a majority of the published research as a high CHO food or supplement source in the peri-exercise period for maintaining performance and improving recovery. Most importantly sago seems to possess the physical and chemical properties to be suitable as a supplement during exercise in a warm, humid environment where it is commonly grown and consumed, although further research is needed to compare it to other known CHO sources. This information could provide sports people in Southeast Asia an affordable alternative to costly commercially available CHO-containing sport nutrition products. Future research, however, could be conducted to further investigate sago in different environmental conditions, with other ingredients added (e.g., electrolytes) and for different modes of exercise.
Abbreviations
- CHO
Carbohydrate
- CI
Confidence interval
- CV
Coefficient of variation
- GI
Glycemic index
- RER
Respiratory exchange ratio
- rh
Relative humidity
- O2
Volume of O2 consumed
- O2max
Maximal O2 uptake
- O2peak
Peak O2 uptake
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Christensen EH, Hansen O. Arbeitsfahigkeit und Ernahrung. Scand Arch Physiol 1939; 81:160-71; http://dx.doi.org/ 10.1111/j.1748-1716.1939.tb01320.x [DOI] [Google Scholar]
- [2].Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med 2013; 43:1139-55; PMID:23846824; http://dx.doi.org/ 10.1007/s40279-013-0079-0 [DOI] [PubMed] [Google Scholar]
- [3].Burke LM, Hawley JA. Carbohydrate and exercise. Curr Opin Clin Nutr Metab Care 1999; 6:515-20; http://dx.doi.org/ 10.1097/00075197-199911000-00015 [DOI] [PubMed] [Google Scholar]
- [4].Vandenbogaerde TJ, Hopkins WG. Effects of acute carbohydrate supplementation on endurance performance A Meta-Analysis. Sports Med 2011; 41:773-92; PMID:21846165; http://dx.doi.org/ 10.2165/11590520-000000000-00000 [DOI] [PubMed] [Google Scholar]
- [5].Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc 2009; 41:709-31; PMID:19225360; http://dx.doi.org/ 10.1249/MSS.0b013e31890eb86 [DOI] [PubMed] [Google Scholar]
- [6].Burke LM. Nutritional needs for exercise in the heat. Comp Biochem Physiol A Mol Integr Physiol 2001; 128:735-48; PMID:11282317; http://dx.doi.org/ 10.1016/S1095-6433(01)00279-3 [DOI] [PubMed] [Google Scholar]
- [7].Wendt D, van Loon LJ, Lichtenbelt WD. Thermoregulation during exercise in the heat: strategies for maintaining health and performance. Sports Med 2007; 37:669-82; PMID:17645370; http://dx.doi.org/ 10.2165/00007256-200737080-00002 [DOI] [PubMed] [Google Scholar]
- [8].Yaspelkis BB, Ivy JL. Effect of carbohydrate supplements and water on exercise metabolism in the heat. J Appl Physiol 1991; 71:680-7; PMID:1938742 [DOI] [PubMed] [Google Scholar]
- [9].Jentjens RL, Wagenmakers AJ, Jeukendrup AE. Heat stress increases muscle glycogen use but reduces the oxidation of ingested carbohydrates during exercise. J Appl Physiol 2002; 92:1562-72; PMID:11896023; http://dx.doi.org/ 10.1152/japplphysiol.00482.2001 [DOI] [PubMed] [Google Scholar]
- [10].Jentjens RL, Underwood K, Achten J, Currell K, Mann CH, Jeukendrup AE. Exogenous carbohydrate oxidation rates are elevated after combined ingestion of glucose and fructose during exercise in the heat. J Appl Physiol 2006; 100:807-16; PMID:16282436; http://dx.doi.org/ 10.1152/japplphysiol.00322.2005 [DOI] [PubMed] [Google Scholar]
- [11].Pitsiladis YP, Maughan RJ. The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans. J Physiol 1999; 517:919-30; PMID:10358130; http://dx.doi.org/ 10.1111/j.1469-7793.1999.0919s.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carter J, Jeukendrup AE, Mundel T, Jones DA. Carbohydrate supplementation improves moderate and high-intensity exercise in the heat. Pflug Arch 2003; 446:211-9; http://dx.doi.org/ 10.1007/s00424-003-1020-4 [DOI] [PubMed] [Google Scholar]
- [13].Harding S. The tropical agenda. J Tropical Psychol 2011; 1:2-5; http://dx.doi.org/ 10.1375/jtp.1.1.2 [DOI] [Google Scholar]
- [14].Abd-Aziz S. Sago starch and its utilisation. J Biosci Bioeng 2002; 94:526-9; PMID:16233345; http://dx.doi.org/ 10.1016/S1389-1723(02)80190-6 [DOI] [PubMed] [Google Scholar]
- [15].Singhal RS, Kennedy JF, Gopalakrishnan SM, Kaczmarek A. Industrial production, processing, and utilization of sago palm-derived products. Carbohyd Polym 2008; 72:1-20; http://dx.doi.org/ 10.1016/j.carbpol.2007.07.043 [DOI] [Google Scholar]
- [16].Ahmad H, Singh R, Ghosh AK. Glycaemic & insulinaemic responses in men at rest following sago meal. Indian J Med Res 2009; 130:160-5; PMID:19797813 [PubMed] [Google Scholar]
- [17].Ghosh AK, Rahaman AA, Singh R. Combination of sago and soy-protein supplementation during endurance cycling exercise and subsequent high-intensity endurance capacity. Int J Sport Nutr Exerc Metab 2010; 20:216-23; PMID:20601739 [DOI] [PubMed] [Google Scholar]
- [18].Che Jusoh MR, Morton RH, Stannard SR, Mündel T. A reliable pre-loaded cycling time-trial for use in conditions of significant thermal stress. Scand J Med Sci Sports 2015; 25:S296-301; http://dx.doi.org/ 10.1111/sms.12332 [DOI] [PubMed] [Google Scholar]
- [19].Che Jusoh MR, Stannard SR, Mündel T. Physiologic and performance effects of sago supplementation before and during cycling in a warm-humid environment. Temperature 2016; 3:318-27; http://dx.doi.org/ 10.1080/23328940.2016.1159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Che Jusoh MR, Stannard SR, Mündel T. Sago supplementation for recovery from cycling in a warm-humid environment and its influence on subsequent cycling physiology and performance. Temperature 2016; 3:444-54; http://dx.doi.org/ 10.1080/23328940.2016.1179382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fink WJ, Costill DL, Van Handel PJ. Leg muscle metabolism during exercise in the heat and cold. Eur J Appl Physiol Occup Physiol 1975; 34:183-90; PMID:1181181; http://dx.doi.org/ 10.1007/BF00999931 [DOI] [PubMed] [Google Scholar]
- [22].Febbraio MA, Snow RJ, Stathis CG, Hargreaves M, Carey MF. Effect of heat stress on muscle energy metabolism during exercise. J Appl Physiol 1994; 77:2827-31; PMID:7896628 [DOI] [PubMed] [Google Scholar]
- [23].Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 1997; 29:1240-9; PMID:9309637; http://dx.doi.org/ 10.1097/00005768-199709000-00018 [DOI] [PubMed] [Google Scholar]
- [24].Parkin JM, Carey MF, Zhao S, Febbraio MA. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol 1999; 86:902-8; PMID:10066703; http://dx.doi.org/ 10.1063/1.370821 [DOI] [PubMed] [Google Scholar]
- [25].Young AJ, Sawka MN, Levine L, Cadarette BS, Pandolf KB. Skeletal muscle metabolism during exercise is influenced by heat acclimation. J Appl Physiol 1985; 59:1929-35; PMID:4077800 [DOI] [PubMed] [Google Scholar]
- [26].Chesley A, Hultman E, Spriet LL. Effects of epinephrine infusion on muscle glycogenolysis during intense aerobic exercise. Am J Physiol 1995; 268:127-34. [DOI] [PubMed] [Google Scholar]
- [27].Mündel T. Exercise heat stress and metabolism. In: Thermoregulation and Human Performance: Physiological and Biological Aspects Medicine and Sports Science, ed. Marino FE. 2008; 53:121-9. Karger, Basel. [DOI] [PubMed] [Google Scholar]
- [28].Below PR, Mora-Rodríguez R, González-Alonso J, Coyle EF. Fluid and carbohydrate ingestion independently improve performance during 1 h of intense exercise. Med Sci Sports Exerc 1995; 27:200-10; PMID:7723643; http://dx.doi.org/ 10.1249/00005768-199502000-00009 [DOI] [PubMed] [Google Scholar]
- [29].Febbraio MA, Murton P, Selig SE, Clark SA, Lambert DL, Angus DJ, Carey MF. Effect of CHO ingestion on exercise metabolism and performance in different ambient temperatures. Med Sci Sports Exerc 1996; 28:1380-7; PMID:8933488; http://dx.doi.org/ 10.1097/00005768-199611000-00006 [DOI] [PubMed] [Google Scholar]
- [30].Horswill CA, Stofan JR, Lovett SC, Hannasch C. Core temperature and metabolic responses after carbohydrate intake during exercise at 30 °C. J Athl Train 2008; 43:585-91; PMID:19030136; http://dx.doi.org/ 10.4085/1062-6050-43.6.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abbiss CR, Peiffer JJ, Peake JM, Nosaka K, Suzuki K, Martin DT, Laursen PB. Effect of carbohydrate ingestion and ambient temperature on muscle fatigue development in endurance-trained male cyclists. J Appl Physiol 2008; 104:1021-8; PMID:18218905; http://dx.doi.org/ 10.1152/japplphysiol.00683.2007 [DOI] [PubMed] [Google Scholar]
- [32].Nassif C, Gomes AR, Peixoto GH, Chagas MH, Soares DD, Silami-Garcia E, Drinkwater EJ, Cannon J, Marino FE. The effect of double-blind carbohydrate ingestion during 60 km of self-paced exercise in warm ambient conditions. PLoS One 2014; 9:e104710; PMID:25110952; http://dx.doi.org/ 10.1371/journal.pone.0104710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Che Muhamed AM, Mohamed NG, Ismail N, Aziz AR, Singh R. Mouth rinsing improves cycling endurance performance during Ramadan fasting in a hot humid environment. Appl Physiol Nutr Metab 2014; 39:458-64; PMID:24669987; http://dx.doi.org/ 10.1139/apnm-2013-0276 [DOI] [PubMed] [Google Scholar]
- [34].Hargreaves M, Angus D, Howlett K, Conus NM, Febbraio M. Effect of heat stress on glucose kinetics during exercise. J Appl Physiol 1996; 81:1594-7; PMID:8904574 [DOI] [PubMed] [Google Scholar]
- [35].Angus DJ, Febbraio MA, Lasini D, Hargreaves M. Effect of carbohydrate ingestion on glucose kinetics during exercise in the heat. J Appl Physiol 2001; 90:601-5; PMID:11160059 [DOI] [PubMed] [Google Scholar]
- [36].Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc 1996; 28:266-70; PMID:8775164; http://dx.doi.org/ 10.1097/00005768-199602000-00017 [DOI] [PubMed] [Google Scholar]
- [37].Davis JM, Burgess WA, Slentz CA, Bartoli WP, Pate RR. Effects of ingesting 6% and 12% glucose/electrolyte beverages during prolonged intermittent cycling in the heat. Eur J Appl Physiol Occup Physiol 1988; 57:563-9; PMID:3396573; http://dx.doi.org/ 10.1007/BF00418463 [DOI] [PubMed] [Google Scholar]
- [38].Fernández-Elías VE, Ortega JF, Nelson RK, Mora-Rodriguez R. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur J Appl Physiol 2015; 115:1919-26; http://dx.doi.org/ 10.1007/s00421-015-3175-z [DOI] [PubMed] [Google Scholar]
- [39].Burke LM. Dietary Carbohydrates. London: Blackwell Science Ltd., 2000. [Google Scholar]
- [40].Goodpaster BH, Costill DL, Fink WJ, Trappe TA, Jozsi AC, Starling RD, Trappe SW. The effects of pre-exercise starch ingestion on endurance performance. Int J Sports Med 1996; 17:366-372; PMID:8858409; http://dx.doi.org/ 10.1055/s-2007-972862 [DOI] [PubMed] [Google Scholar]
- [41].Jozsi AC, Trappe TA, Starling RD, Goodpaster B, Trappe SW, Fink WJ, Costill DL. The influence of starch structure on glycogen resynthesis and subsequent cycling performance. Int J Sports Med 1996; 17:373-8; PMID:8858410; http://dx.doi.org/ 10.1055/s-2007-972863 [DOI] [PubMed] [Google Scholar]
- [42].Roberts MD, Lockwood C, Dalbo VJ, Volek J, Kerksick CM. Ingestion of a high-molecular-weight hydrothermally modified waxy maize starch alters metabolic responses to prolonged exercise in trained cyclists. Nutrition 2011; 27:659-65; PMID:20951003; http://dx.doi.org/ 10.1016/j.nut.2010.07.008 [DOI] [PubMed] [Google Scholar]
- [43].Stephens FB, Roig M, Armstrong G, Greenhaff PL. Post-exercise ingestion of a unique, high molecular weight glucose polymer solution improves performance during a subsequent bout of cycling exercise. J Sports Sci 2011; 26:149-54; http://dx.doi.org/ 10.1080/02640410701361548 [DOI] [PubMed] [Google Scholar]
- [44].Singh J, Dartois A, Kaur L. Starch digestibility in food matrix: a review. Trends Food Sci Technol 2010; 21:168-80; http://dx.doi.org/ 10.1016/j.tifs.2009.12.001 [DOI] [Google Scholar]
- [45].Thomas DE, Brotherhood JR, Brand JC. Carbohydrate feeding before exercise: effect of glycemic index. Int J Sport Med 1991; 12:180-6; http://dx.doi.org/ 10.1055/s-2007-1024664 [DOI] [PubMed] [Google Scholar]
- [46].Wong SH, Chen YJ, Fung WM, Morris JG. Effect of glycemic index meals on recovery and subsequent endurance capacity. Int J Sports Med 2009; 30:898-905; PMID:20013559; http://dx.doi.org/ 10.1055/s-0029-1237710 [DOI] [PubMed] [Google Scholar]
- [47].Brown LJ, Midgley AW, Vince RV, Madden LA, McNaughton LR. High vs. low glycemic index 3-h recovery diets following glycogen-depleting exercise has no effect on subsequent 5-km cycling time trial performance. J Sci Med Sport 2013; 16:450-4; PMID:23154155; http://dx.doi.org/ 10.1016/j.jsams.2012.10.006 [DOI] [PubMed] [Google Scholar]
- [48].Pei-Lang AT, Mohamed AMD, Karim AA. Sago starch and composition of associated components in palm of different growth stages. Carbohydr Polym 2005; 63:283-6; http://dx.doi.org/ 10.1016/j.carbpol.2005.08.061 [DOI] [Google Scholar]
- [49].Tek-Ann C, Md. Isa AH, Mohayidin MG. Sago (Metroxylon sagu Rottboll), the forgotten palm. J Sustain Agri 1999; 14:5-17. [Google Scholar]
- [50].Karim AA, Tie PL, Manan DMA, Zaidul ISM. Starch from the sago palm - Properties, prospects and challenges as a new industrial source for food and other uses. Compr Rev Food Sci Food Saf 2008; 7:215-28; http://dx.doi.org/ 10.1111/j.1541-4337.2008.00042.x [DOI] [PubMed] [Google Scholar]
- [51].Flores DM. The Economic Potential of Sago Starch Bioindustrial Products. Sago: Green Prospects; Proc 9th Int Sago Symp, 19-21 July 2007, Ormoc City, Philippines. [Google Scholar]
- [52].Mohamed A, Jamilah, Abbas KA, Abdul Rahman R, Roselina K. A review on physicochemical and thermorheological properties of sago starch. Am J Agric Biol Sci 2008; 3:639-46; http://dx.doi.org/ 10.3844/ajabssp.2008.639.646 [DOI] [Google Scholar]
- [53].Thorne MJ, Thompson LU, Jenkins DJ. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am J Clin Nutr 1983; 38:481-8; PMID:6310984 [DOI] [PubMed] [Google Scholar]
- [54].Hassan A, Elobeid T, Kerkadi A, Medhat M, Suheil G. Glycemic index of selected carbohydrate-based foods consumed in Qatar. Int J Food Sci Nutr 2010; 61:512-8; PMID:20141487; http://dx.doi.org/ 10.3109/09637480903517796 [DOI] [PubMed] [Google Scholar]
- [55].Saris WH, Antoine JM, Brouns F, Fogelholm M, Gleeson M, Hespel P, Jeukendrup AE, Maughan RJ, Pannemans D, Stich V. PASSCLAIM - Physical performance and fitness. Eur J Nutr 2003; 42 Suppl 1:I50-95; PMID:12664323 [DOI] [PubMed] [Google Scholar]
- [56].Jeukendrup AE, Martin J. Improving cycling performance: how should we spend our time and money? Sports Med 2001; 31:559-69; PMID:11428691; http://dx.doi.org/ 10.2165/00007256-200131070-00009 [DOI] [PubMed] [Google Scholar]
- [57].Hopkins WG, Hawley JA, Burke LM. Design and analysis of research on sport performance enhancement. Med Sci Sports Exerc 1999; 31:472-85; PMID:10188754; http://dx.doi.org/ 10.1097/00005768-199903000-00018 [DOI] [PubMed] [Google Scholar]
- [58].O'Brien WJ, Stannard SR, Clarke JA, Rowlands DS. Fructose-maltodextrin ratio governs exogenous and other CHO oxidation and performance. Med Sci Sports Exerc 2013; 45:1814-24; PMID:23949097; http://dx.doi.org/ 10.1249/MSS.0b013e31828e12d4 [DOI] [PubMed] [Google Scholar]
- [59].Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc 2009; 41:3-13; PMID:19092709; http://dx.doi.org/ 10.1249/MSS.0b013e31818cb278 [DOI] [PubMed] [Google Scholar]
- [60].Hopkins WG, Batterham AM. Error rates, decisive outcomes and publication bias with several inferential methods. Sports Med 2016; DOI: 10.1007/s40279-016-0517-x [DOI] [PubMed] [Google Scholar]
- [61].Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- [62].Armstrong L, Casa D, Millard-Stafford M, Moran D, Pyne S, Roberts W. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc 2007; 39:556-72; PMID:17473783; http://dx.doi.org/ 10.1249/MSS.0b013e31802fa199 [DOI] [PubMed] [Google Scholar]
- [63].Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 2007; 39:377-90; PMID:17277604; http://dx.doi.org/ 10.1249/01.mss.0000272779.34140.3b [DOI] [PubMed] [Google Scholar]
- [64].Clark VR, Hopkins WG, Hawley JA, Burke LM. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med Sci Sports Exerc 2000; 32:1642-7; PMID:10994918; http://dx.doi.org/ 10.1097/00005768-200009000-00019 [DOI] [PubMed] [Google Scholar]
- [65].Febbraio MA, Snow RJ, Hargreaves M, Stathis CG, Martin IK, Carey MF. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol 1994; 76:589-97; PMID:8175568 [DOI] [PubMed] [Google Scholar]
- [66].Peters HP, van Schelven FW, Verstappen PA, de Boer RW, Bol E, Erich WB, van der Togt CR, de Vries WR. Gastrointestinal problems as a function of carbohydrate supplements and mode of exercise. Med Sci Sports Exerc 1993; 25:1211-24; PMID:8289607; http://dx.doi.org/ 10.1249/00005768-199311000-00003 [DOI] [PubMed] [Google Scholar]
- [67].Febbraio MA. Temperature, muscle metabolism and performance In: Perspectives in exercise science and sports medicine: the metabolic basis of performance in exercise and sport. Ed. Lamb DR, Murray R, 1999; 315-53. Cooper, Carmel. [Google Scholar]


