Supplemental digital content is available in the text.
Keywords: diagnosis, imaging, longitudinal, neuropsychology, psychosis, schizophrenia
Abstract
Despite several decades of research, our knowledge of the long-term course of schizophrenia (SZ) is hampered by a lack of homogeneity of both research methods and phenotypic definitions of SZ’s course. We provide a comprehensive review of the course of SZ by applying stringent methodological and diagnostic study-selection criteria. We report on positive and negative symptoms, cognition, and findings obtained by neuroimaging. In addition, we perform a meta-analysis of longitudinal studies of cognition in humans. We selected 35 human studies focusing on a narrow SZ phenotype, employing a follow-up duration of six months or more and consistent methodology at the different measurement points. For the meta-analysis on global cognitive change, eight and four studies were used to compare SZ to healthy and psychiatric controls, respectively. We find that the course of SZ is characterized by a constancy or even improvement of positive and negative symptoms and by fairly stable cognitive impairment, reflecting structural frontal and temporal cortical pathology. Progressive changes of the frontal cortex appear to develop in parallel with changes in symptomatology and executive impairment. Despite stable differences in cognition between patients and controls over the time intervals studied, high heterogeneity in the magnitude of effect sizes is present, and age is identified as one of its potential sources. Meta-regression shows these magnitudes to depend on the age at study inclusion. For future research, a combination of longitudinal and cross-sectional research designs is warranted to better account for potential cohort effects.
INTRODUCTION
Schizophrenia (SZ)—characterized by delusions, hallucinations, psychomotor disturbances, and cognitive impairment, or a combination thereof—is a psychiatric disorder with a sometimes unfavorable course potentially leading to life-long disability. SZ has a point prevalence of approximately 4.5 per 1000 population. Its heritability is high, with estimates hovering around 0.8.1 The long-term course of SZ has been of particular interest to scholars,2–4 resulting in an ample body of work addressing various aspects of SZ’s progression (Supplemental Table 1, available at http://links.lww.com/HRP/A30). Many studies are population based, relying on samples from specific catchment areas. These studies were instrumental in describing outcomes and variations in variables of interest for psychotic disorders over time. This line of research has underscored that, despite the generally poor prognosis for affected individuals, disease trajectories display a high level of heterogeneity.5 In a recent review, Häfner and an der Heiden4 summarized the diverse range of course types that have been proposed for SZ. From a methodological point of view,6 numerous factors help to account for the finding of such heterogeneous courses: (1) the use of retrospective research designs, (2) broad diagnostic categories, including schizoaffective disorder and affective psychosis, (3) the change of diagnostic criteria over time, (4) the lack of control groups, (5) the scarcity of repeated measurements of neurobiological and neuropsychological variables, and (6) varying time intervals studied. In the present review and meta-analysis, we adopt an approach to minimize these potential shortcomings, though this approach itself has both merits and potential disadvantages. Whenever possible, we attempt to integrate results from different areas of research.
STUDY-SELECTION CRITERIA
The selection of controlled observational studies was determined on the basis of nosological specificity for SZ, follow-up duration (>6 months), and consistent methodology at the different measurement points (see below). When such stringent criteria are applied, the number of studies on the course of SZ (see Supplemental Figure 1, http://links.lww.com/HRP/A31) is considerably reduced. To identify studies for the present review, we systematically searched the PubMed database (see Supplemental Table 1, http://links.lww.com/HRP/A30). Additional articles were identified through Google Scholar queries and the bibliographies of articles meeting the inclusion criteria. Sample characteristics of the studies included are listed in Supplemental Table 2, http://links.lww.com/HRP/A32.7–17 We focused on the following aspects of the longitudinal course of SZ in humans: symptomatology, cognition, and neuroimaging, as these are key topics in SZ research. We found that by distinguishing short-term (∼5 years or less) from long-term follow-up studies and by discussing SZ in late adulthood separately, we were best able to present the results in a structured manner.
Figure 1.
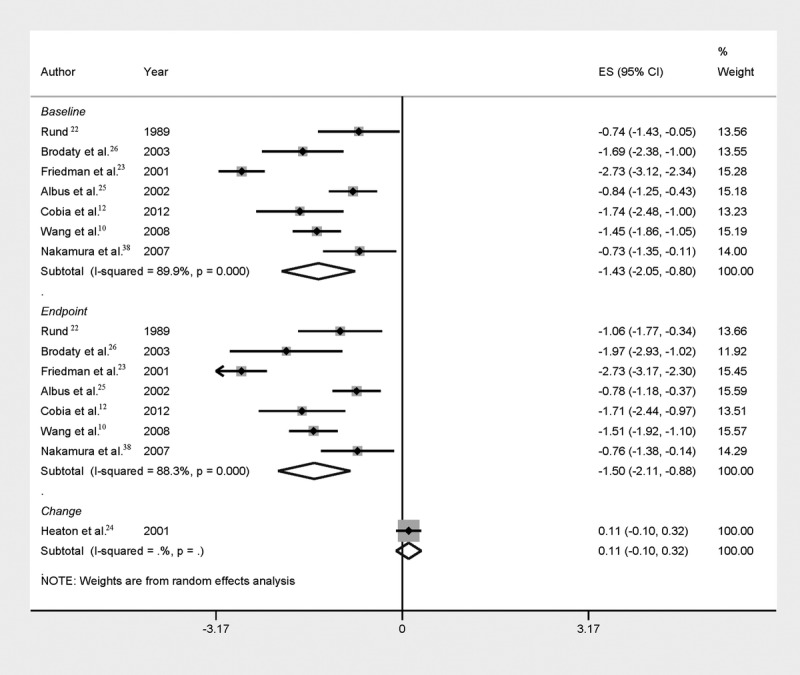
Forest plot of mean global cognitive impairment at the different time points (baseline and endpoint) of SZ patients versus healthy controls. For one study,24 change scores are reported. See Supplemental Table 4, http://links.lww.com/HRP/A36, for sample sizes.
Diagnosis
We included only studies in which a diagnosis of SZ was based on the third or later edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM), the ninth or tenth editions of the International Classification of Diseases (ICD), or the Research Diagnostic Criteria.18 Due to different diagnostic criteria of DSM and ICD concerning schizo-affective disorder, only studies investigating SZ are included in the present review. One study on symptomatology (included in the present review) also investigated patients with schizophreniform disorder.7
Research Design
We considered only prospective studies that also investigated one or more control groups. Studies on the symptomatology of SZ over time constitute an exception; we know of no longitudinal study that investigated SZ symptoms in patients and, in parallel, in an unrelated, healthy control group. Thus, we also considered uncontrolled studies in that area. For other areas considered in this article, the control group could be of any kind to avoid “supernormal” control groups.19 To be included in our review, studies had to include a follow-up interval between measurement points of at least six months. Also, only research that used identical instruments for repeated measurements was considered. Although we required studies to be purely observational in order to minimize the influence of treatment effects, in most studies patients received some kind of treatment. Therefore, possible influences of medication on the results are reported and discussed alongside the primary findings. Finally, we did not take into account studies in which the majority of participants were younger than 18 years. Research had to be published in English-language, peer-reviewed journals. Single-case studies and conference abstracts were not considered.
In summary, we employed stringent inclusion criteria and focused on longitudinal studies that investigated narrowly defined SZ using, within each study, the same instrument at all measurement points. We focused on the following areas: positive and negative symptoms, cognition, and neuroimaging.
META-ANALYSIS ON LONGITUDINAL TRAJECTORIES OF COGNITION: DATA EXTRACTION AND META-ANALYTIC CALCULATIONS
Two of the authors (UH and MS) independently extracted all data. For each study, the overall means at baseline and endpoint (or, if unavailable, change scores) in cognitive tests were extracted. If the overall mean was not available, all measures of cognitive functions were extracted and averaged to obtain a single effect size per time point and study. The effect-size measure was the standardized mean difference expressed as Hedges’ adjusted g. Standard inverse of the variance weighting was used for pooling the studies. As we expected considerable heterogeneity between studies, we applied the DerSimonian and Laird random-effects model throughout.20 The degree of heterogeneity was estimated by the I2 statistics21 (I2 values >50% reflecting considerable heterogeneity) and a chi-square test of homogeneity (α set at p < .1). Age was identified as a potential reason for heterogeneity and was explored with meta-regression (Supplemental Figure 2, http://links.lww.com/HRP/A34). Intention-to-treat data sets were used whenever available. Meta-analytic calculations were performed using Comprehensive Meta-analysis version 2 (http://www.meta-analysis.com) and STATA version 12 (http://www.stata.com). Two-sided α was set at p < .05.
POSITIVE AND NEGATIVE SYMPTOMS
Short-Term Course
Arndt and colleagues7 followed a group of young patients diagnosed with SZ or schizophreniform disorder for two years. Psychotic symptoms (delusions and hallucinations) were strongly reduced at discharge compared to index hospitalization and remained stable at that level—a pattern also observed with disorganized symptoms. Negative symptoms remained stable. The course of positive and negative symptoms was also assessed in several neuroimaging studies (see below and Supplemental Table 2, http://links.lww.com/HRP/A32). Some studies found constant positive symptomatology,10–12 whereas others8,9 found improvement over the study interval. Regarding negative symptoms, a similar pattern can be observed, with some studies detecting constancy9,11,12 and others finding improvement.8,10 These mixed results may reflect differences in statistical power, as the two studies11,12 that did not detect any improvement (in either positive or negative symptoms) investigated relatively few SZ patients (n = 10 and n = 20, respectively). Conversely, three7,8,10 of the four studies that did find improvement in at least one area7–10 were based on larger samples that included at least 40 SZ subjects (see Supplemental Table 2, http://links.lww.com/HRP/A32). Concerning the effects of medication, the subjects of Arndt and colleagues' study7 probably received typical antipsychotic medication, whereas most subjects in neuroimaging studies were treated with atypical antipsychotic medication (see Supplemental Table 3, http://links.lww.com/HRP/A35). 7–17,22–45 However, no differential effects of these two classes of medication are evident.
Long-Term Course
The MUFUSSAD study reassessed a sample of SZ patients after a period of 15 years and observed rather stable negative symptomatology together with a considerable reduction in paranoid-hallucinatory symptomatology.13 The majority of patients (57%) had a chronic illness course, and 39% an episodic-remitting course. High positive-symptom scores at admission and high negative-symptom scores at discharge significantly predicted a chronic course. Two studies14,15 analyzed data from the Chicago Follow-up Study on the long-term course of positive symptoms, with measurement points at 2, 4.5, 7.5, 10, 15, and 20 years after index hospitalization. Rosen and colleagues14 longitudinally compared SZ and bipolar patients for the presence of Schneiderian first-rank symptoms (including delusional perceptions and commenting voices). Two and 4.5 years after index admission, almost half of the patients with SZ (44%) experienced first-rank symptoms, but these values declined to 30% at the 10-year follow-up and rose again to 44% at the 20-year follow-up. At three measurement points (2, 4.5, and 7.5 years), the SZ patients experienced significantly more positive symptoms than the bipolar patients. When hallucinations were investigated separately over time, roughly 80% of SZ patients experienced symptoms of this kind at index hospitalization.15 This value continually declined to approximately 30% at the 15-year follow-up, and showed only a slight increase at the last measurement point (20 years). Regarding specific hallucinations, a different pattern was found for commenting voices, which are often present in SZ. The percentage of patients experiencing them rose from 25% to 33% and 35% at first and second follow-ups (2 and 4.5 years), respectively, before declining to previous levels for the rest of the study period.14 Whereas clear conclusions about the effects of medication status are difficult to draw from these studies, a higher proportion of unmedicated subjects appears to experience longitudinally fluctuating positive symptoms (see Supplemental Table 3, http://links.lww.com/HRP/A35).
In Late Adulthood
Research on positive and negative symptom severity in senescence has produced conflicting results. In an uncontrolled study on elderly SZ patients, Harvey and colleagues16 found that, whereas positive symptomatology remained constant over the one-year follow-up, negative symptoms worsened. By contrast, McGurk and colleagues17 observed a pattern of improving positive, and stable negative, symptomatology, based on a 15-month follow-up study. These results may, in part, be explained by different subject-selection criteria. The McGurk study focused on poor-outcome SZ and excluded concurrent neurological conditions. The specific medication status of patients in the two above studies is not reported (see Supplemental Table 3, http://links.lww.com/HRP/A35). Given that patients in both studies were institutionalized long term, it is likely that most of them were treated with antipsychotic medication over the study interval. Also, considering publication dates, it can be hypothesized that most participants had been treated with typical antipsychotic medication at some time during their lifetimes.
COGNITION: LONGITUDINAL STUDIES
It is well known that SZ patients show impairments in cognitive functioning, which are thought to be rather general and not restricted to a single domain.45 Various neurocognitive phenotypes have been proposed as endophenotypes46 and been shown to predict disease outcome.47 Mojtabai and colleagues48 have shown that the neuropsychological profile of SZ patients is distinct from that of psychotic affective disorders—which further emphasizes the need for diagnostic specificity. In this section, we first consider longitudinal cognitive changes separately for short- and long-term follow-up periods, followed by an overview of schizophrenia in late adulthood. We then summarize results grouped according to broad neuropsychological domains. In the section following this one (presenting the results of longitudinal studies), we present a meta-analysis of results regardless of follow-up duration and neuropsychological domain.
Short-Term Longitudinal Follow-up Studies (∼5 years or shorter)
Albus and colleagues25 investigated cognitive function over a two-year follow-up in a sample of first-episode patients. They studied learning, memory, and executive function. While the authors showed improved performance in verbal learning, other aspects of cognitive performance remained stable. Stable performance in visual memory was attributed to the inability of SZ patients to benefit from repeated practice, as the healthy control group improved in this area. Rund and colleagues22 studied digit-span performance and found weaker results in the paranoid SZ group and decreased performance stability over time (four-year follow-up). Studying a subsample of the Chicago Follow-up Study, Burdick and colleagues27 administered a battery of cognitive tests to both SZ and bipolar patients at time points five years apart. They found a significant diagnosis-by-time interaction, with SZ patients performing worse over time in a test of executive function (verbal fluency) but with stable performance in most other domains. The digit-span test used by Rund and colleagues22 comprises distractor items that, similar to verbal fluency, also tap the executive domain. However, neuroimaging studies that also assessed executive performance10,12 have not found group-by-time interactions. This difference may be related to medication status (see Discussion): whereas high proportions of participants in neuroimaging studies were treated with atypical antipsychotics (Supplemental Table 3, http://links.lww.com/HRP/A35), patients in cognitive investigations, often with a long follow-up durations (see below), were mostly treated with typical antipsychotics.
Besides the difference concerning group-by-time interactions, neuroimaging studies8,10,12,38 have also found stable cognitive deficits of SZ patients in areas such as crystallized IQ and both episodic and working memory, thereby adding to the notion of generally stable cognitive performance (with notable exceptions). There does not appear to be a difference between first-episode and chronic patients.8 On a wider and less controlled timescale, Heaton and colleagues24 assessed a relatively large sample, at a minimum of two time points, using the comprehensive Halstead-Reitan test battery. This battery includes tests in the areas of verbal, psychomotor, abstraction/cognitive flexibility, attention, learning, delayed recall, and motor abilities. Group differences remained stable, regardless of the duration of follow-up (less or more than 36 months). Also, no differences in practice effects were identified between baseline and first assessment in either patients or controls. Nor did the authors find differences among subgroups of patients defined by various characteristics such as age, baseline level of cognitive functioning, and clinical symptomatology.
Long-Term Longitudinal Follow-up Studies
Assessing multiple time points, the Chicago Follow-up Study followed young patients with SZ and other psychotic disorders, as well as another comparison group that had nonpsychotic depression, for more than 20 years.28 The authors measured both processing speed and the ability to access general knowledge. The three groups differed significantly in processing speed at index admission, with SZ patients showing the lowest scores. All three groups had improved significantly at the first follow-up after two years, underscoring the need for the inclusion of control groups. In the remaining follow-ups, there were no changes in any group, suggesting stability of the processing-speed deficits in the SZ group. The ability to access general knowledge was lower in the SZ group at index admission, compared to the other experimental groups, but it improved significantly at follow-up—an effect not observed in both control groups. At the follow-up, SZ patients continued to perform worse than the other groups at most measuring points, adding to the notion of stable neuropsychological performance in the long term.
Schizophrenia in Late Adulthood
When subjected to a number of tests related to dementia, late-onset SZ patients show significant differences over time.26 Approximately half of the patients assessed at the five-year follow-up met DSM-IV criteria for dementia, highlighting a possible neurodegenerative aspect in late-onset SZ or hinting at comorbid dementia. Worsened cognition, however, may be a general feature of SZ in the elderly. To this end, Friedman and colleagues23 compared institutionalized older (>50 years) and institutionalized young SZ patients with older control subjects who were either healthy or suffered from Alzheimer’s disease. Progressive cognitive deterioration in the older patients measured with the Mini–Mental State Examination was observed. That pattern, which was observed in neither the younger patients nor the healthy comparison subjects, was qualitatively different from the one observed in dementia patients. Whereas the older SZ patients’ cognitive decline depended on age at follow-up, Alzheimer patients showed decline independent of age at follow-up, and healthy controls showed little decline. Neither of the above studies gives sufficient information on the medication status of participants to draw any conclusion about the influence of psychopharmacology on results.
Domain-Specific Review of Results
The above separation of results into both short- and long-term studies and SZ in late adulthood is an intuitive approach if one wants a broad overview of the effects of SZ over the lifespan. A more detailed picture emerges, however, if specific neuropsychological domains are considered apart from follow-up duration. In order to provide a more detailed view of changes in what are generally considered different abilities, Supplemental Table 4, http://links.lww.com/HRP/A36,8,10,12,22–28,38 summarizes findings in the following domains: executive functions, verbal learning, visual memory, verbal memory, episodic memory, language, processing speed, knowledge/crystallized IQ, global tests, perception, and both sensory and motor abilities.
Executive functions (for review, see Royall et al. [2002])49 are a broad set of metacognitive functions that are associated with frontal brain areas and whose borders, especially with respect to working memory,50 attention,51 and intelligence,52 are somewhat fuzzy.53 In order to account for this interdependence, we have grouped several tests and neuropsychological domains (as mentioned above; see Supplemental Table 4, http://links.lww.com/HRP/A36) under the label of executive functions. Despite the remaining variability, this approach appears to provide further evidence for a decline in executive functions after follow-up periods of approximately five years (see also preceding subsections under Cognition). It remains unclear whether verbal learning changes.25,27 The relative decline in verbal memory8 appears to be partly due to an improvement in the control group;27 it may be that unlike the controls, SZ patients have a specific inability to benefit from repeated practice.54 Visual memory appears to be stable,8,25 as does episodic memory,10,12 processing speed27,28 (see above), and knowledge/crystallized IQ10,12,28 (see above). Also, results of global tests remain unchanged24,38 except in old age23,26 (see above). There are also no changes in language, perception, and sensory or motor skills.8 Medication status appears to moderate the decline in executive functions (see above).
As discussed in next section, some evidence suggests that global cognitive function deteriorates in later life stages. And as discussed in the section after that, the measurable declines in both executive functions and verbal memory (see above) occur along with changes observed in the frontal and temporal cortices.
COGNITION: META-ANALYTIC COMPARISON OF LONGITUDINAL COGNITIVE CHANGES: MEAN OVERALL STANDARDIZED PERFORMANCE DIFFERENCES
We used meta-analytic techniques to compare global cognitive performance over time between SZ patients and controls. The analysis comparing patients to healthy controls included data from 388 SZ patients and 544 controls. When comparing SZ patients to psychiatric controls, we analyzed data from 103 SZ patients and 148 psychiatric controls.
Changes in Overall Cognition Between SZ Patients and Healthy Controls
Eight studies10,12,22–26,38 (15 comparisons) presented usable mean baseline, change, or endpoint values on cognitive tests. While patients with SZ consistently performed worse than healthy controls at both baseline (n = 7 studies; Hedges’ g = −1.43; 95% CI, −2.04 to −0.81) and endpoint (n = 7 studies; Hedges’ g = −1.5; 95% CI, −2.12 to −0.87) (see Figure 1), the magnitude of the difference between SZ patients and healthy controls showed considerable heterogeneity (I2 was 89.9% at baseline and 88.2% at endpoint). Considering the change between baseline and endpoint, the performance difference for the individual studies did not show significant increase between baseline and endpoint. That observation was also corroborated by the study of Heaton and colleagues,24 which, in contrast to the other studies, showed only the mean change and not the cognitive measures at baseline and endpoint (Hedges’ g = 0.11; 95% CI, −0.1 to 0.32).
Changes in Overall Cognition Between SZ Patients and Other Psychiatric Patients
Four studies22,27,28,38 (eight comparisons) had usable mean baseline and endpoint values of cognitive tests. The performance differences between patients with SZ and patients with other psychiatric disorders were smaller than when SZ patients were compared to healthy controls. These differences between SZ and other psychiatric patients were not always significant for each individual study but reached statistical significance when means of all studies per time point were pooled (Baseline: n = 4 studies; Hedges’ g = −0.48; 95% CI, −0.74 to −0.22. Endpoint: n = 4 studies; Hedges’ g = −0.65; 95% CI, −0.94 to −0.36. Heterogeneity, I2 = 0%). The differences between baseline and follow-up were not statistically significant (p = .39). The psychiatric diagnoses of the comparison groups were as follows: nonpsychotic depression and other psychotic disorders;28 bipolar disorder;27 first-episode affective psychosis;38 and a mix of borderline personality, affective, and somatization disorders.22
Exploring the Effect of Age by Meta-regression
Given the large heterogeneity of effect sizes between studies that longitudinally investigated cognitive performance, we were interested in factors accounting for this variability. We found the age of study participants at baseline to be associated with lower performance on cognitive tests (see Supplemental Figure 2, http://links.lww.com/HRP/A34). Older SZ patients had more pronounced performance differences than healthy controls (slope, −0.03; 95% CI, −0.06 to −0.01; p = .026). Regarding changes in overall cognition between patients and psychiatric controls, we did not detect any significant effect of age (slope, 0.047; 95% CI, −0.07 to 0.17; p = .239).
In essence, results of the meta-analysis show stable cognitive differences over time, both between SZ and healthy controls and between SZ and other psychiatric controls. Moreover, the age of study participants at baseline may partially explain the large heterogeneity of effect sizes between SZ and healthy controls. This age effect was not detected between SZ and psychiatric controls.
NEUROIMAGING
A multitude of cross-sectional studies support structural brain alterations in SZ patients and controls: gray matter reductions in frontal, temporal, and post-central cortical regions, and increased ventricular size and white matter reductions in the corpus callosum.55 A meta-analysis of 27 longitudinal studies56 found progressive decreases in brain volume and in gray and white matter, as well as large increases in lateral ventricular volume. Given that none of the studies reviewed here includes a follow-up interval longer than 7.5 years (see Supplemental Table 5, http://links.lww.com/HRP/A37), 8–12,29–44,57 we base the following comparison on brain structures rather than on follow-up time. In terms of imaging technique, structural magnetic resonance imaging (MRI) has been used in almost all studies investigating longitudinal volume changes. One older study,30 conducted before the widespread use of MRI in research, employed computerized tomography, an X-ray technology. Today, the relatively safe, noninvasive nature of MRI, together with its superior tissue contrast, makes it the method of choice for volumetric in vivo measurements. In functional studies, dynamic physiological differences were visualized using functional MRI (fMRI), which measures hemodynamic effects associated with neural activity, and magnetic resonance spectroscopy (MRS), which enables in vivo quantification of tissue metabolites.58
Ventricular Size
Of the eight studies29,31–33,35,38–40 on lateral ventricular size, six29,31–33,35,39 found no change in size over time. However, several studies noted heightened variability in SZ patients, both at baseline and over time. And some studies refute the notion of generally stable ventricular size. For example, males suffering from chronic SZ were found to have ventricular enlargement of the left, but not the right, lateral ventricle.31 Another study38 reported an increase of the lateral ventricles, falling short of a hemispheric effect, in first-episode patients compared to healthy controls and individuals suffering from bipolar disorder. A study of first-episode patients40 found progressive lateral ventricle enlargement in cannabis-consuming schizophrenics, but not in non-cannabis-consuming schizophrenics, compared to healthy controls. Also, poorer outcomes predicted greater enlargement of lateral ventricles.33 Likewise, a study by Davis and colleagues30 found progressive bilateral ventricle enlargement only in poor-outcome patients with no evidence of symptom remission. In summary, lateral ventricle volume generally appears to be stable, with increased variability in people suffering from SZ, possibly related to positive symptom remission.
Brain Volume
Investigations of whole brain volume have produced clear results. With a few exceptions,39 studies have not detected differences over time.8,33,35,36,40 Price and colleagues36 used magnetization transfer imaging, an MRI technique sensitive to subtle anomalies, to longitudinally investigate progression of potential whole-brain neuropathological changes. They found no evidence of exaggerated progression in patients. Regarding volume changes of specific brain areas, progressive frontal volume changes were noted8 in one study, and another study33 observed a similar effect. Differences in temporal lobe volume were found in neither study. Further evidence for a lack of progressive loss in temporal lobe volume comes from two other studies investigating the superior temporal gyrus42 and the amygdala-hippocampal complex.35,42 The shape (but not volume) of the hippocampus changes over time in SZ patients.10 The parietal lobe was also investigated, but no progressive changes, compared to controls, were detected.33
Gray Matter, Sulcal Cerebrospinal Fluid, and White Matter
Several fine-grained investigations of gray matter changes have found progressive decreases in both first-episode34,37,38,40,43,57 and chronic31,39 patients. Whereas two of these studies observed a decline in total cerebral gray matter,39,40 other investigations found specific regional gray matter reductions in frontal8,31,37,38,43 and temporal34,37,38 cortices. Notably, an inverse relationship between gray matter volume and symptom severity has been reported.38 Complementing studies on gray matter changes, some studies have found increases in sulcal cerebrospinal fluid over time,31,33,38 and faster rates of increase have been correlated with positive symptoms scores.31 Some evidence suggests exaggerated longitudinal progression of cortical thinning of frontal and temporal areas in SZ patients, which is correlated with deficits in several cognitive domains.12 Cortical surface contraction, observed in both SZ patients and control subjects, is exaggerated in SZ, resembling accelerated normal neurodevelopmental processes.41 Regarding white matter changes, some evidence suggests differential changes over time in patients and controls. In one study, white matter volume increases were less in patients than in controls,39 and in another study a small white matter volume reduction was detected in patients, whereas white matter increased in controls over the same study period.33 More severe negative symptoms were correlated with a greater decline of frontal white matter.33
Subcortical Brain Structures
Although most research focuses on cortical regions, a few studies investigate deeper brain structures. Apart from the hippocampal surface changes mentioned above, evidence indicates a deformation (exaggerated surface changes over time) of the caudate nucleus and thalamus in SZ, with a volume decline occurring only in the caudate nucleus.10 Putative progressive changes in two neurodevelopmental alterations, the cavum septum pellucidum and a reduced adhesio interthalamica, have also been investigated, though with no significant differences over time in the SZ group.44
fMRI and MRS
Only a few studies have investigated longitudinal functional changes. Théberge and colleagues57 described elevated glutamine levels in the left thalamus in never-treated, first-episode SZ patients that progressively normalized over the 10- and 30-month follow-ups. This finding suggests abnormalities of the brain glutamate system in untreated first-episode patients, progressively normalizing over time; it may therefore be a treatment effect. In an fMRI study of first-episode patients, positive and negative mood induction was studied over a period of six months.9 Results show frontal, temporal, and occipital, as well as both pre- and postcentral, group-by-time effects during negative mood induction. As positive symptomatology was reduced at follow-up, these findings were also interpreted as treatment effects.9 Finally, one study investigated the reliability of functional activations induced by listening to a factual story.11 It found, both globally and locally, that controls and chronic SZ individuals had a similar reproducibility over the 21-month period. In that study the authors found no change in symptomatology over the follow-up period. Taken together, the few available functional-imaging studies suggest changes following initial treatment but stability in chronic SZ.
DISCUSSION
The goal of our review and meta-analysis was to identify hallmarks of the course of SZ. We aimed at reducing heterogeneity across studies by applying a stringent methodology. First, we applied a narrow diagnostic definition of SZ, thereby excluding all studies with schizo-affective subjects. Second, we concentrated on repeated measurements rather than on outcomes, with the aim of capturing the effects of time more directly. Third, we focused on studies that included control groups. Although our approach has led to a concise description of the course of SZ and to novel concepts of the etiology of cognitive dysfunction (see below), these three restrictive factors may result in a few limitations that need to be discussed. It could be argued that, taken together, these factors considerably reduced the body of available studies and that our approach therefore ignores a large body of important work accumulated over the last decades. Even so, our approach enabled us to substantially reduce heterogeneity of individual trajectories by narrowing our diagnostic focus. Such an approach is in line with current research work on classification, as the poor reliability and therefore questionable validity of schizo-affective disorder in DSM-IV led to new diagnostic criteria in DSM-5,59 which emphasizes the need for diagnostic specificity. It is well known, however, that in clinical reality, symptom clusters such as psychosis often cross (artificial) diagnostic boundaries. An alternative approach to reduce heterogeneity would thus be to investigate specific symptom clusters only, regardless of DSM diagnosis. The National Institute of Mental Health Research Domain Criteria project spearheads such investigations, exchanging diagnostic categories for dimensional biobehavioral approaches.60 While we strongly believe that this approach will prove fruitful in the future, we think that if the aim is to summarize findings of past longitudinal studies, relying on specific DSM categories provides a good framework.
It may further be cautioned that in determining the course of illness, our review does not primarily focus on two important variables: treatment, and treatment adherence. Most of the studies reviewed here have, of necessity, been conducted in patients receiving treatment (see Supplemental Table 3, http://links.lww.com/HRP/A35). Apart from progressive pathological processes, the observed alterations may thus also be ascribed to effects of antipsychotic medication or other treatment. To acknowledge this feature of the studies, we have reported treatment information along with the results of interest. Nevertheless, the studies differ considerably not only in the proportion of participants treated with antipsychotic drugs but also in the type of antipsychotic medication—which makes it difficult to distinguish drug from illness effects. Keeping these restrictions in mind, results suggest that medication is an important modulator of the short-term decline of executive functions—which appears to be absent in patients treated with atypical antipsychotic medication. However, as important as this finding may be, we need to emphasize that the focus of our review was on change over time compared to controls, and not a comparison of treatments or adherence within the SZ group. Also, Olabi and colleagues56 highlight the methodological challenge of adequately distinguishing between illness and long-term treatment effects in longitudinal neuroimaging studies. In the present review, neuroimaging studies were mostly conducted in patients that received atypical antipsychotics. The neurobiological alterations observed in most of these studies12,32–34,43 did not show a correlation with medication dose. Exceptions include both protective38 and detrimental8 effects of antipsychotics on neocortical gray matter and frontal lobe volume, respectively, in first-episode patients. In contrast to other findings in patients mainly treated with typical antipsychotic medication,61 the neurobiological alterations reviewed here thus appear to reflect the disorder rather than the treatment, at least in chronic patients.
Our focus on controlled studies and repeated measures was intended to identify methodologically sound properties of SZ over the lifespan—rather than correlative results. To that end, it is noteworthy that only four studies followed patients over one or more decades.14,15,24,28 Particularly in the field of neuroimaging, follow-up durations are limited to only a few years, and rarely beyond six years. Importantly, longer follow-up durations may allow for the investigation of long-term effects of antipsychotics on brain structure, given that their long-term antipsychotic efficacy has recently been challenged.62 In line with this observation, we found varying degrees of symptomatic impairment over longer time intervals.
In conclusion, given these limitations, we think that the following observations may be considered robust (see also Text Box 1):
– While positive and negative symptoms decrease or stay stable in the short term, studies that have longer observation times and more measuring points suggest fluctuating positive and somewhat stable negative symptoms, possibly related to medication intake.
– The deficits across cognitive domains are relatively stable, both short and long term, with executive functions showing short-term variability in some studies,22,27 putatively related to typical antipsychotic medication. Meta-analysis identifies age at baseline as a predictor of the magnitude of cognitive change. In a recent communication, Zipursky and colleagues63 noted that the accelerated cognitive decline in elderly SZ patients demanded further attention by scientists (see also below).
– Brain-imaging studies in young adults show overall stable whole-brain and lateral ventricle volume. In line with the meta-analysis of Olabi and colleagues,56 there is progressive frontal, but not temporal, lobe volume reduction. A progressive reduction in gray matter is well established in first-episode patients, and white matter changes are also apparent over time. In general, the few functional studies suggest changes after initial treatment but stability in chronic SZ.
Text Box 1.
Main Findings over Time in the Different Areas Under Study*
-
Symptomatology
– Decreasing or stable positive and negative symptoms (short term)
– In the long term, positive symptoms vary, whereas negative symptoms remain relatively constant
– Conflicting findings concerning later life
-
Cognition
– Generally stable impairments, both short and long term, in all domains
– Age modulates the magnitude of differences between patients and controls
– Putative short-term variation in the executive domain, possibly related to medication status
– Worsening of cognition in later life, associated with neurodegeneration
-
Neuroimaging (short follow-up periods)
– Overall stable whole-brain and lateral ventricle volume, despite varying results
– Ongoing frontal, but not temporal, lobe volume reduction
– Progressive gray and white matter changes
– Surface changes of deep brain nuclei
– Functional changes covary with symptomatology
*In all but the first area, results are in comparison to control groups.
More than a century after Kraepelin’s seminal work, our study underscores the need for stringent studies of the longitudinal course of SZ (see also Text Box 2).
Text box 2.
Suggestions for Future Longitudinal Research Studies
– Longer follow-up intervals and more measurement points
– Diagnostic consistency
– A focus on SZ progression in later life stages
– A combination of cross-sectional and longitudinal research to distinguish between age and birth-cohort effects.
The conclusions of our review are in line with the work of Kurtz,64 as they also show stability of deficits in young SZ adults and a decline in elderly SZ individuals. A major finding of the present work, however, is that age at baseline acts as an important modulator of the magnitude of the mostly stable cognitive differences between patients and controls. This finding indicates that interactions of time and disease progression need to be further investigated. More specifically, it is unclear if patients show an insidious decline in cognitive performance, not reliably captured by studies using relatively short follow-up intervals, or whether observed cognitive changes are due, instead, to birth-cohort effects or the effects of differential disease onset. Birth-cohort effects include, for example, effects of different medication or other environmental variables that are differentially effective in particular age groups. In other words, it is unclear whether the observed differences between age groups are due to the endogenous disease process or not. Research on normal human cognitive aging benefited enormously from the combination of longitudinal and cross-sectional research,65–67 demonstrating that cross-sectional differences between birth cohorts may be larger than longitudinal changes within birth cohorts.68 Such an approach has the potential to explain how SZ can be both a neurodevelopmental disorder inducing static encephalopathy69 and a disorder with a neurodegenerative course.2,70
Apart from time effects discussed above, an important goal of the present work has been to integrate different fields of study. The synthesis of findings from studies on symptoms, neuroimaging, and neuropsychology reveals a combination of pathological events apparent in studies with a short (<5 years) follow-up period. The progressive decline in executive functions proceeds in tandem with progressive frontal volume reductions, increased frontal cortical thinning, and declines in both frontal gray matter and white matter. Taken together, these results suggest both frontal atrophy and decline in behavioral processes thought to be mediated by the frontal lobes. Despite a general symptom reduction over this time period, the subgroup of patients with decreased gray matter and white matter experience greater positive and negative symptoms, respectively.
The following results are also worth noting. First, a subtle reduction in verbal memory over time, only indirectly detected via an improvement in the control group, may indicate progressive gray matter alterations in temporal lobe structures, such as the hippocampus, responsible for memory formation. Second, despite the general finding of overall constant ventricle volume over time, some studies associate increasing ventricle volume in a subset of patients with poor outcome, pointing to hemispheric lateralization as an important mediating factor. Third, although antipsychotic medication appears to normalize disturbed metabolism in first-episode patients, this process is halted in chronic patients. Later in disease, cognitive function remains stable, but neuroanatomical changes continue. The processes mediating the magnitude of this stable cognitive impairment require careful investigation. In later life stages, however, the continuous changes in the brain may culminate in a global deterioration of cognitive function.
By integrating results from various sources, this review has provided a concise summary of the course of SZ over the lifespan. While the approach taken here may have helped to reduce the effect of heterogeneity across studies, only well-designed longitudinal studies with adequate power will eventually pave the way for a more detailed and robust understanding of the disease processes underlying SZ. Such advances can be expected, in turn, to lead to the more individualized treatment that Kapur and colleagues71 refer to as “stratified psychiatry.”
Supplementary Material
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Footnotes
Original manuscript submitted 31 August 2014; revised manuscript received 25 November 2014, accepted for publication subject to revision 12 January 2015; revised manuscript received 16 February 2015.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.harvardreviewofpsychiatry.org).
REFERENCES
- 1. Tandon R, Nasrallah HA, Keshavan MS. “Just the facts”: meandering in schizophrenia’s many forests. Schizophr Res 2011; 128: 5– 6. [DOI] [PubMed] [Google Scholar]
- 2. Kraepelin E. Die Erscheinungsformen des Irreseins. Ztg Für Gesamte Neurol Psychiatr 1920; 62: 1– 29. [Google Scholar]
- 3. Bleuler M. A 23-year longitudinal study of 208 schizophrenics and impressions in regard to the nature of schizophrenia. J Psychiatr Res 1968; 6: 3– 12. [Google Scholar]
- 4. Häfner H, an der Heiden W. Course and outcome of schizophrenia. In: Hirsch SR, Weinberger DR, eds. Schizophrenia. Oxford: Blackwell Science, 2007; 101– 41. [Google Scholar]
- 5. Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health longitudinal study of chronic schizophrenia. Prognosis and predictors of outcome. Arch Gen Psychiatry 1991; 48: 239– 46. [DOI] [PubMed] [Google Scholar]
- 6. Häfner H, an der Heiden W. Methodological problems of longitudinal studies on schizophrenia. Fortschr Neurol Psychiatr 2000; 68: 193– 205. [DOI] [PubMed] [Google Scholar]
- 7. Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P. A longitudinal study of symptom dimensions in schizophrenia. Prediction and patterns of change. Arch Gen Psychiatry 1995; 52: 352– 60. [DOI] [PubMed] [Google Scholar]
- 8. Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 1998; 55: 145– 52. [DOI] [PubMed] [Google Scholar]
- 9. Reske M, Kellermann T, Habel U, et al. Stability of emotional dysfunctions? A long-term fMRI study in first-episode schizophrenia. J Psychiatr Res 2007; 41: 918– 27. [DOI] [PubMed] [Google Scholar]
- 10. Wang L, Mamah D, Harms MP, et al. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol Psychiatry 2008; 64: 1060– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maïza O, Mazoyer B, Hervé PY, Razafimandimby A, Dollfus S, Tzourio-Mazoyer N. Reproducibility of fMRI activations during a story listening task in patients with schizophrenia. Schizophr Res 2011; 128: 98– 101. [DOI] [PubMed] [Google Scholar]
- 12. Cobia DJ, Smith MJ, Wang L, Csernansky JG. Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr Res 2012; 139: 1– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Möller H-J, Jäger M, Riedel M, Obermeier M, Strauss A, Bottlender R. The Munich 15-year follow-up study (MUFUSSAD) on first-hospitalized patients with schizophrenic or affective disorders: comparison of psychopathological and psychosocial course and outcome and prediction of chronicity. Eur Arch Psychiatry Clin Neurosci 2010; 260: 367– 84. [DOI] [PubMed] [Google Scholar]
- 14. Rosen C, Grossman LS, Harrow M, Bonner-Jackson A, Faull R. Diagnostic and prognostic significance of Schneiderian first-rank symptoms: a 20-year longitudinal study of schizophrenia and bipolar disorder. Compr Psychiatry 2011; 52: 126– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goghari VM, Harrow M, Grossman LS, Rosen C. A 20-year multi-follow-up of hallucinations in schizophrenia, other psychotic, and mood disorders. Psychol Med 2012; 1: 1– 10. [DOI] [PubMed] [Google Scholar]
- 16. Harvey PD, Lombardi J, Leibman M, et al. Cognitive impairment and negative symptoms in geriatric chronic schizophrenic patients: a follow-up study. Schizophr Res 1996; 22: 223– 31. [DOI] [PubMed] [Google Scholar]
- 17. McGurk SR, Moriarty PJ, Harvey PD, Parrella M, White L, Davis KL. The longitudinal relationship of clinical symptoms, cognitive functioning, and adaptive life in geriatric schizophrenia. Schizophr Res 2000; 42: 47– 55. [DOI] [PubMed] [Google Scholar]
- 18. Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35: 773– 82. [DOI] [PubMed] [Google Scholar]
- 19. Kendler KS. The super-normal control group in psychiatric genetics: possible artifactual evidence for coaggregation. Psychiatr Genet 1990; 1: 45– 53. [Google Scholar]
- 20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177– 88. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration, 2011.
- 22. Rund BR. Distractibility and recall capability in schizophrenics. A 4 year longitudinal study of stability in cognitive performance. Schizophr Res 1989; 2: 265– 75. [DOI] [PubMed] [Google Scholar]
- 23. Friedman JI, Harvey PD, Coleman T, et al. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. Am J Psychiatry 2001; 158: 1441– 8. [DOI] [PubMed] [Google Scholar]
- 24. Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry 2001; 58: 24– 32. [DOI] [PubMed] [Google Scholar]
- 25. Albus M, Hubmann W, Scherer J, et al. A prospective 2-year follow-up study of neurocognitive functioning in patients with first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci 2002; 252: 262– 7. [DOI] [PubMed] [Google Scholar]
- 26. Brodaty H, Sachdev P, Koschera A, Monk Dorothy, Cullen B. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry 2003; 183: 213– 9. [DOI] [PubMed] [Google Scholar]
- 27. Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis 2006; 194: 255– 60. [DOI] [PubMed] [Google Scholar]
- 28. Bonner-Jackson A, Grossman LS, Harrow M, Rosen Cherise. Neurocognition in schizophrenia: a 20-year multi-follow-up of the course of processing speed and stored knowledge. Compr Psychiatry 2010; 51: 471– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Degreef G, Ashtari M, Wu HW, Borenstein M, Geisler S, Lieberman J. Follow up MRI study in first episode schizophrenia. Schizophr Res 1991; 5: 204– 6. [DOI] [PubMed] [Google Scholar]
- 30. Davis KL, Buchsbaum MS, Shihabuddin L, et al. Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry 1998; 43: 783– 93. [DOI] [PubMed] [Google Scholar]
- 31. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 2001; 58: 148– 57. [DOI] [PubMed] [Google Scholar]
- 32. Puri BK, Hutton SB, Saeed N, et al. A serial longitudinal quantitative MRI study of cerebral changes in first-episode schizophrenia using image segmentation and subvoxel registration. Psychiatry Res 2001; 106: 141– 50. [DOI] [PubMed] [Google Scholar]
- 33. Ho B-C, Andreasen NC, Nopoulos P, Arndt Stephan, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 2003; 60: 585– 94. [DOI] [PubMed] [Google Scholar]
- 34. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry 2003; 60: 766– 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitworth AB, Kemmler G, Honeder M, et al. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res 2005; 140: 225– 37. [DOI] [PubMed] [Google Scholar]
- 36. Price G, Cercignani M, Bagary MS, et al. A volumetric MRI and magnetization transfer imaging follow-up study of patients with first-episode schizophrenia. Schizophr Res 2006; 87: 100– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitford TJ, Grieve SM, Farrow TFD, et al. Progressive grey matter atrophy over the first 2–3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage 2006; 32: 511– 9. [DOI] [PubMed] [Google Scholar]
- 38. Nakamura M, Salisbury DF, Hirayasu Y, et al. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry 2007; 62: 773– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brans RGH, van Haren NEM, van Baal GCM, et al. Longitudinal MRI study in schizophrenia patients and their healthy siblings. Br J Psychiatry 2008; 193: 422– 3. [DOI] [PubMed] [Google Scholar]
- 40. Rais M, Cahn W, Haren NV, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry 2008; 165: 490– 6. [DOI] [PubMed] [Google Scholar]
- 41. Sun D, Stuart GW, Jenkinson M, et al. Brain surface contraction mapped in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Mol Psychiatry 2009; 14: 976– 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida T, McCarley RW, Nakamura M, et al. A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala-hippocampal complex in chronic schizophrenia. Schizophr Res 2009; 113: 84– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koo M-S, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW. A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry 2008; 65: 746– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trzesniak C, Schaufelberger MS, Duran FLS, et al. Longitudinal follow-up of cavum septum pellucidum and adhesio interthalamica alterations in first-episode psychosis: a population-based MRI study. Psychol Med 2012; 42: 1– 12. [DOI] [PubMed] [Google Scholar]
- 45. Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev 2009; 19: 365– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szöke A, Trandafir A, Dupont M-E, Méary Alexandre, Schürhoff F, Leboyer M. Longitudinal studies of cognition in schizophrenia: meta-analysis. Br J Psychiatry 2008; 192: 248– 57. [DOI] [PubMed] [Google Scholar]
- 47. Wölwer W, Brinkmeyer J, Riesbeck M, et al. Neuropsychological impairments predict the clinical course in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2008; 258: 28– 34. [DOI] [PubMed] [Google Scholar]
- 48. Mojtabai R, Bromet EJ, Harvey PD, Carlson GA, Craig TJ, Fennig S. Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. Am J Psychiatry 2000; 157: 1453– 60. [DOI] [PubMed] [Google Scholar]
- 49. Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci 2002; 14: 377– 405. [DOI] [PubMed] [Google Scholar]
- 50. Baddeley AD. Working memory. New York: Oxford University Press, 1986. [Google Scholar]
- 51. Norman D, Shallice T. Attention to action. In: Davidson R, Schwartz G, Shapiro D, eds. Consciousness and self-regulation. New York: Springer, 1986: 1– 18. [Google Scholar]
- 52. Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci 2006; 17: 172– 9. [DOI] [PubMed] [Google Scholar]
- 53. Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology 2005; 19: 532– 45. [DOI] [PubMed] [Google Scholar]
- 54. Granholm E, Link P, Fish S, Kraemer H, Jeste D. Age-related practice effects across longitudinal neuropsychological assessments in older people with schizophrenia. Neuropsychology 2010; 24: 616– 24. [DOI] [PubMed] [Google Scholar]
- 55. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev 2012; 36: 1342– 56. [DOI] [PubMed] [Google Scholar]
- 56. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry 2011; 70: 88– 96. [DOI] [PubMed] [Google Scholar]
- 57. Théberge J, Williamson KE, Aoyama N, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry J Ment Sci 2007; 191: 325– 34. [DOI] [PubMed] [Google Scholar]
- 58. Jäncke L. Methoden der Bildgebung in der Psychologie und den kognitiven Neurowissenschaften. Stuttgart, Germany: Kohlhammer, 2005. [Google Scholar]
- 59. Malaspina D, Owen MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res 2013; 150: 21– 5. [DOI] [PubMed] [Google Scholar]
- 60. Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull 2010; 36: 1061– 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011; 68: 128– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harrow M, Jobe TH, Faull RN. Does treatment of schizophrenia with antipsychotic medications eliminate or reduce psychosis? A 20-year multi-follow-up study. Psychol Med 2014; 44: 3007– 16. [DOI] [PubMed] [Google Scholar]
- 63. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull 2012; 39: 1363– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res 2005; 74: 15– 26. [DOI] [PubMed] [Google Scholar]
- 65. Schaie KW. A general model for the study of developmental problems. Psychol Bull 1965; 64: 92– 107. [DOI] [PubMed] [Google Scholar]
- 66. Baltes PB. Longitudinal and cross-sectional sequences in the study of age and generation effects. Hum Dev 1968; 11: 145– 71. [DOI] [PubMed] [Google Scholar]
- 67. Schaie KW, Baltes PB. On sequential strategies in developmental research: description or explanation. Hum Dev 1975; 18: 384– 90. [Google Scholar]
- 68. Schaie KW, Parham IA. Cohort-sequential analyses of adult intellectual development. Dev Psychol 1977; 13: 649. [Google Scholar]
- 69. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44: 660– 9. [DOI] [PubMed] [Google Scholar]
- 70. Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull 1992; 18: 437– 48. [DOI] [PubMed] [Google Scholar]
- 71. Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012; 17: 1174– 9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


