Highlights
-
•
Ewing sarcoma/primitive neuroectodermal tumor of the kidney (ES/PNET) is a member of Ewing’s sarcoma family, occurring in young adults.
-
•
The clinical course and prognosis of ES/PNET are different from renal cell carcinoma (RCC).
-
•
For definite diagnosis, in addition to cytogenetic analysis; other techniques may be needed; such as fluorescent in situ hybridization (FISH), reverse transcriptase-polymerase chain reaction (RT-PCR) of the t (11; 22) translocation [1].
Keywords: Primitive neuroectodermal tumor (PNET), Ewing sarcoma (ES), Kidney, Radical nephrectomy, Chemotherapy, Case report
Abstract
Introduction
Ewing sarcoma/Primitive neuroectodermal tumor of the kidney (ES/PNET) is a member of Ewing’s sarcoma family, occurring in young adults and has aggressive clinical behavior and poor prognosis. However, its discrimination from the renal cell carcinoma (RCC) is very difficult preoperatively. We present three cases of this rare disease that were managed in two academic centers.
Presentation of cases
Herein we report three cases of ES/PNET of the kidney, 2 young men complaining of right flank pain and gross hematuria and one young woman complaining of left subcostal pain. In two cases computerized tomography (CT) scan revealed huge renal masses which were excised by radical nephrectomy. Microscopic examination of the nephrectomy specimen showed primitive neuroectodermal tumor features which confirmed by immunohistochemistry (IHC).
Two of 3 patients were treated with adjuvant chemotherapy and the third patient with neoadjuvant chemotherapy. They were symptom-free until now.
Discussion
The clinical course and prognosis of ES/PNET are different from renal cell carcinoma (RCC) and definite pathologic diagnosis is necessary for optimum treatment. For definite diagnosis, in addition to cytogenetic analysis; other techniques may be needed; such as fluorescent in situ hybridization (FISH), reverse transcriptase-polymerase chain reaction (RT-PCR) of the t (11; 22) translocation or the EWS-FLI and related gene fusions [1].
Conclusion
Up to our knowledge and search in English literature, this is the first case series that was reported from a major referral center from our country, Iran.
1. Case presentation
1.1. First case
A 21-year-old man was referred to our hospital in August 2010, with right flank pain and weight loss. Abdominopelvic computerized tomography (CT) scan showed a right renal mass. The patient underwent right radical nephrectomy with primary diagnosis of RCC.
Cut sections of nephrectomy sample showed variegated creamy – brown soft to rubbery mass measuring 18 × 8 × 5 cm, showing foci of necrosis, which pushed pyelocalyceal system medially. Tumor adhesion to renal capsule was identified. Ureter was free from any tumor grossly.
Microscopic examination of H&E slides showed solid sheets of rather monotonous population of small tumoral cells characterized by round nuclei; coarse chromatin pattern with indistinct cell outlines which in some areas arranged as vague pseudo-rosette fashion. Necrosis was dominated.
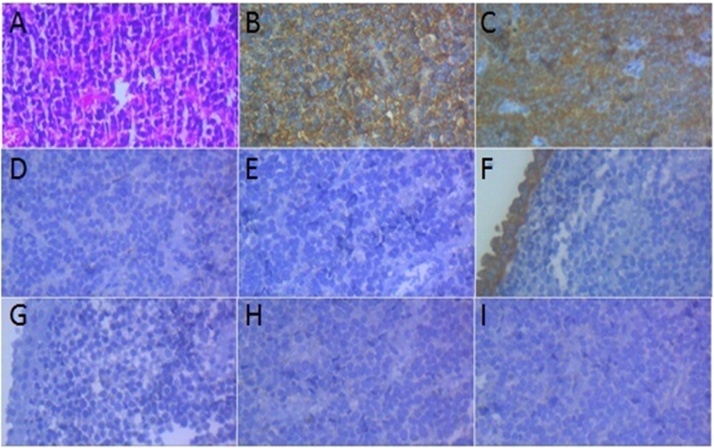
Immunohistochemical studies (IHC) showed positive immune reactions for CD99 (membranous staining in tumoral cells), NSE (cytoplasmic staining in tumoral cells) and negative reactivity for CD45, CK7, CK20, synaptophysin and chromogranin, consistent with the diagnosis of ES/PNET (Fig. 1).
Fig. 1.
Microscopic examination and IHC study of tumoral cells (×400).
(A) H&E: Discohesive small round cells arranged as vague rosettes.
(B) CD 99: Positive membranous staining in tumoral cells.
(C) NSE: Positive cytoplasmic staining in tumoral cells.
(D) CD 45: Negative in tumoral cells.
(E) CD 56: Negative in tumoral cells.
(F) CK 7: Positive cytoplasmic staining in surface urothelial cells and negative in tumoral cells.
G- CK 20: Negative in surface urothelial cells and tumoral cells.
H- Synaptophysin: Negative in tumoral cells.
I- Chromogranin: Negative in tumoral cells.
1.2. Second case
A 31-year-old man was referred to our center in April 2013, with the chief complaint of gross hematuria. There was no history of smoking and past medical or surgical problems in the patient. Physical examination was unremarkable.
Renal ultrasonography showed hypoechoic mass in lower pole of right kidney, measuring: 30 × 18 mm. Intravenous pyelography showed non secretion of involved right kidney. Abdominopelvic CT scan showed an exophytic mass with pressure effect over the pelvis that caused pelvic dilatation. Rigid ureterorenoscopy of right ureter was done which was unremarkable. Then open right renal biopsy was performed. Microscopic examination revealed fragments of normal urothelial mucosa underlined by a neoplastic tissue composed of diffuse growth pattern of tumoral cells, characterized by uniform hyperchromatic nuclei and scant cytoplasm, admixed with necrotic areas. The morphologic features were showed a“small round cell tumor” compatible with ES/PNET.
IHC studies showed negative immune reactions for CK7, CK20, CD56, chromogranin, synaptophysin, CD45, CD3 and CD 20 but NSE and CD99 were positive in tumoral cells. Real time PCR on paraffin embedded blocks proved the presence of t (11–22) (EWS-FL1 fusion transcript), which confirmed the diagnosis of ES/PNET. The patient underwent 9 courses of neoadjuvant chemotherapy and then right sided radical nephrectomy was performed by attending staff.
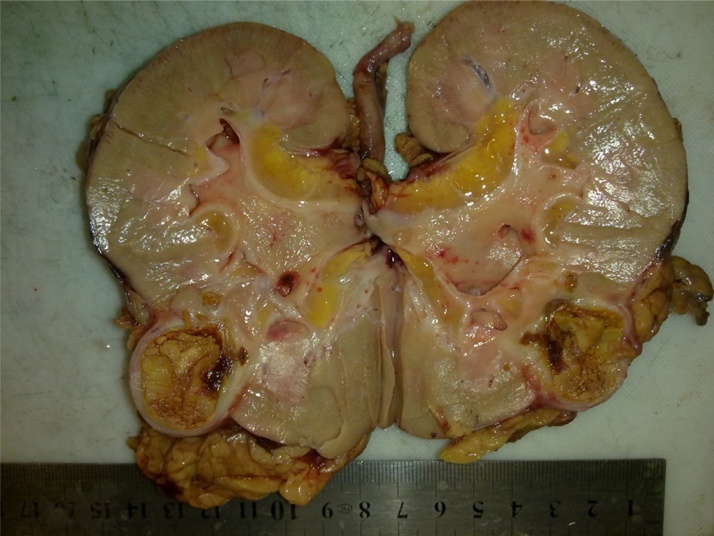
Sections of radical nephrectomy showed a yellow soft lesion resembling necrosis in lower pole measuring 2 × 2 × 2 cm (Fig. 2). Grossly no tumoral lesion was identified. Microscopic examination of H&E stained slides showed foamy macrophages, admixed necrotic areas probably due to therapy related changes. No tumoral residue was found.
Fig. 2.
Nephrectomy specimen after neoadjuvant chemotherapy. Only a necrotic area was noted in lower pole which failed to prove any residue of tumoral cells in microscopic examination.
1.3. Third case
The patient was a 34 years old female who was referred to our center with abdominal swelling associated with left lower chest pain since one month before admission.
In physical examination a large abdominal mass filling the left side of abdomen was detected.
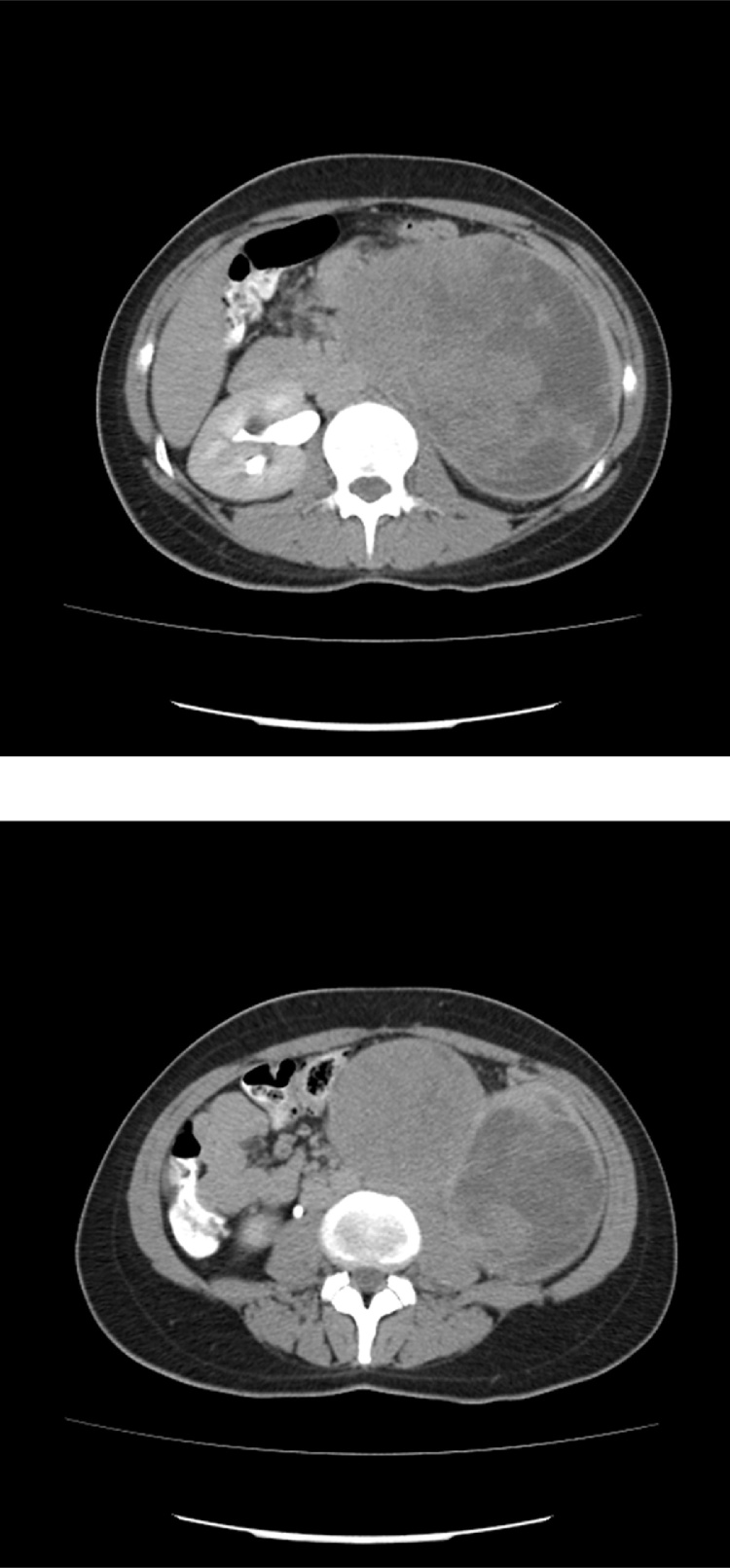
In abdominopelvic ultrasonography, a huge abdominal mass was reported. In abdominopelvic contrast enhanced CT scan (Fig. 3) a large left renal mass was confirmed. Metastatic work up was negative. The patient underwent left radical nephrectomy with midline laparotomy incision. Severe adhesions were present without gross lymph nodes enlargement. The pathology diagnosis was in favor of ES/PNET of the kidney (Fig. 4, Fig. 5). The patient underwent adjuvant chemotherapy.
Fig. 3.
CT scan of the third case revealing a large inhomogenous left renal mass occupying the left side of abdomen.
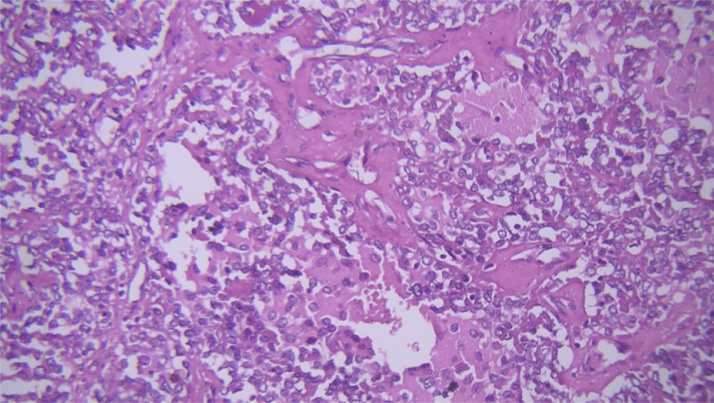
Fig. 4.
Small round cells with high N/C ratio, rather round monotonous nuclei and scanty cytoplasm (×400).
Fig. 5.
CD 99 was diffusely positive in tumoral cells (×20).
2. Discussions
Ewing sarcoma/Primary neuroectodermal tumor (ES/PNET) of the kidney as a primary renal tumor is a rare entity; firstly described in 1975 [1] . Fewer than 150 cases were reported in the literature till now [2] . It is a member of Ewing’s sarcoma family, with aggressive clinical behavior which usually occurs in young adults most often between 20 and 30 years. The male: female ratio is about 3:1 [3].
The major differential diagnosis of small-round cell tumors of the kidney includes: malignant lymphoma, embryonal rhabdomyosarcoma, renal neuroblastoma, Wilms tumor, small cell osteosarcoma, and Ewing sarcoma (ES) or its variant with neural differentiation, primary neuroectodermal tumor (PNET) [4].
ES/PNET of the kidney usually reaches huge size and is almost always more than 10 cm in maximum diameter [4], [5], [6].The largest case series including 146 patients with ES/PNET was described in 2001 by Parham et al. [6]. The age of patients ranging from 2 months to 73 years (median: 20 years). Another large series reported by Zollner et al. [4]. They found 24 cases in German database from 1980 to 2009. The median age was 24.9 years (range 11–60 years). In 37.5%, patients presented with metastases. In 90% rearrangements of t (11, 22) were found.
Clinical symptoms and para-clinical data are similar to renal cell carcinoma; therefore definitive diagnosis needs pathologic confirmation with immunohistochemical (IHC) studies. IHC studies showed more than 90% positivity for CD99, but CD99 is not specific and cannot be used as an absolute biomarker for its diagnosis [7], [8].
Cytogenetic analyses can be helpful in the diagnosis of ES/PENT showing Ewing sarcoma (EWS) gene and ETS translocation.The translocation of t (11:22) (q24:q12) have been detected in more than 90% of the ES/PNET of the kidney [5].
ES/PNET of the kidney has more aggressive behavior than PNET of other sites [6].
Macroscopically many of the cases are located in the medullary/pelvic region of the kidney. Distant metastases occur in about 20 to 50% of patients; most commonly to regional lymph nodes. Metastases to the bone, bone marrow, lung, and liver are not uncommon [3].
The 5-year survival of ES/PNET of the kidney is about 45–55% [9], [10].
Metastases to the regional nodes or distant metastases decrease overall survival in comparison with localized ES/PENT of the kidney [11] .
The main differential diagnosis is with blastema-predominant Wilms tumor.
3. Conclusions
Like other case reports of ES/PNET of the kidney our cases were manifested by renal masses which treated by radical nephrectomy in combination with chemotherapy. Microscopic features were in favor of small round cell tumor and final diagnosis was confirmed by IHC staining. Up to our knowledge and search in English literature, only one case was previously reported from Iran [9].
It's noteworthy that our work has been reported in line with the SCARE criteria of case reports [12].
Conflict of interest
None declared.
Author contributions
Mariam abolhasani and Sareh Salarinejad are corresponding pathologists and Mohammad kazem Moslemi is the Corresponding Author and Corresponding Surgeon of the third case.
Funding
None.
Ethical approval
We have obtained written consent from the patients and we can provide this consent upon editor request.
Contributor Information
Maryam Abolhasani, Email: mar.abolhasani@gmail.com.
Sareh Salarinejad, Email: Shelman3206000@yahoo.com.
Mohammad Kazem Moslemi, Email: mkmoslemi@gmail.com, mkmoslemi@gmail.com.
References
- 1.Seemayer T.A., Thelmo W.L., Bolande R.P., Wiglesworth F.W. Peripheral neuroectodermal tumors. Perspect. Pediatr. Pathol. 1975;2:151–172. [PubMed] [Google Scholar]
- 2.Parham D.M., Roloson G.J., Feely M., Green D.M., Bridge J.A., Beckwith J.B. Primary malignant neuroepithelial tumors of the kidney: a clinicopathologic analysis of 146 adult and pediatric cases from the National Wilms Tumor Study Group Pathology Center. Am. J. Surg. Pathol. 2001;25(2):133–146. doi: 10.1097/00000478-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Pakravan A., Vo T., Sandomirsky M., Bastani B. Primary Ewing sarcoma of kidney in an elderly. Iran. J. Kidney Dis. 2012;6:307–310. [PubMed] [Google Scholar]
- 4.Zollner S., Dirksen U., Jurgens H., Ranft A. Renal Ewing tumors. Ann. Oncol. 2013 Sep;24(9):2455–2461. doi: 10.1093/annonc/mdt215. [DOI] [PubMed] [Google Scholar]
- 5.Su J.R., Yang S., Lo K.Y. Primitive neuroectodermal tumor (PNET) of the kidney. J. Urol. Roc. 2001;12(December 4) [Google Scholar]
- 6.Sun C., Du Z., Tong S., Xu K., Ding W., Sun J., Ding Q. Primitive neuroectodermal tumor of the kidney: case report and review of literature. Sun et al. World J. Surg. Oncol. 2012;10:279. doi: 10.1186/1477-7819-10-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli R., Cai G. Ewing sarcoma/primitive neuroectodermal tumor of the kidney. A rare and lethal entity. Arch. Pathol. Lab. Med. 2016;140:281–285. doi: 10.5858/arpa.2014-0367-RS. [DOI] [PubMed] [Google Scholar]
- 8.Antonescu C. Round Cell sarcomas beyond Ewing: emerging entities. Histopathology. 2013;64(1):26–37. doi: 10.1111/his.12281. [DOI] [PubMed] [Google Scholar]
- 9.Basiri A., Parvin M., Simaei N.E. Haji- Mohammad mehdi-Arbab A. The role of surgery for local recurrence of renal Ewing’s Sarcoma. Urol. J. 2006;4:250–252. [PubMed] [Google Scholar]
- 10.Chakrabarti I., De A., Giri A. Primitive neuroectodermal tumor (PNET) of kidney – a rare entity. Iran. J. Pathol. 2011;6(3):147–152. [Google Scholar]
- 11.Teegavarapu P.S., Rao P., Matrana M.R., Cauley D.H., Wood C.G., Patel S., Tannir N.M. Outcomes of adults with Ewing sarcoma family of tumors (ESFT) of the kidney: a single – institution experience. Am. J. Clin. Oncol. 2014;(September 12) doi: 10.1097/COC.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.for the SCARE Group. Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;(September 6) doi: 10.1016/j.ijsu.2016.08.014. Article in press. [DOI] [PubMed] [Google Scholar]