Abstract
Mechanosensitive (MS) channels are evolutionarily conserved membrane proteins that play essential roles in multiple cellular processes, including sensing mechanical forces and regulating osmotic pressure. Bacterial MscL and MscS are two prototypes of MS channels. Numerous structural studies, in combination with biochemical and cellular data, provide valuable insights into the mechanism of energy transfer from membrane tension to gating of the channel. We discuss these data in a unified two‐state model of thermodynamics. In addition, we propose a lipid diffusion‐mediated mechanism to explain the adaptation phenomenon of MscS.
Keywords: mechanosensitive channels, gating mechanism, lipid‐protein interaction, membrane tension sensing, osmoregulation
Introduction
Mechanosensitive (MS) channels are integral membrane proteins that sense mechanical forces and change permeability of the membrane in response. MS channels are ancient proteins with a footprint across the evolutionary tree of life, and possibly functioning in the first membrane covered cells.1, 2 They are involved in many biological processes such as touching, hearing, gravity sensing, and control of osmotic pressure.2, 3, 4 In prokaryotic cells, MS channels are known to utilize membrane tension to gate transmembrane channels.5 Once the surface tension reaches a threshold, the MS channel opens, allowing the efflux of cytosol content.6 Consistent with Le Châtelier's principle, the efflux results in reduction of the osmotic pressure, thus reducing the membrane tension. In animal cells, a number of MS channels are thought to use mechanical stimuli other than membrane tension for gating;2, 7 however, elucidation of their relationship with tension gating requires further investigation (see below).
Based on the homology of their primary sequences, as well as their three‐dimensional folding topologies in the transmembrane (TM) channel parts, prokaryotic MS channels are classified into two types. Functionally, these two types correspond to channels of large (∼3 nS) and small (∼1 nS) conductance, and thus they are termed MscL (mechanosensitive channel of large conductance) and MscS (for small conductance), respectively. In addition, a variant of MscS named MscM (for mini conductance) exhibits a conductance as low as ∼0.3 nS.8 Crystal structures of the two major types of prokaryotic MS channels provide crucial insights into the mechanisms of their channel gating.9, 10, 11, 12, 13, 14, 15 Furthermore, numerous techniques, such as disulfide trapping,16 spectroscopic probing,17 mutagenesis screening,18, 19, 20 and chemical modification,21 have been used to study mechanisms of the channel gating driven by membrane tension. It has been established that, upon increase of membrane tension, an MS channel changes its conformation from a closed state to an open state. Thus, the thermodynamics of an MS channel may be described as a two‐state model.7, 22, 23 The pore size of the open state of Escherichia coli MscL (Ec‐MscL) is estimated to be ∼3 nm in diameter,24 and the pore size of the open state of Ec‐MscS was measured as ∼1.3 nm in diameter.13 In this review, we will focus on two‐state models of bacterial MscL and MscS channels, which are likely to provide the basis for understanding general mechanisms of MS channels.
The question concerning the operating mechanism of MS channels remains as to how the free energy associated with membrane tension is converted to a mechanical force driving the conformational change of the channel. Intuitively, such a gating process might be analogous to that of a pinhole in a balloon, which increases its hole‐size when the balloon is filled with water. However, the energetic perspective of structural and mechanistic details of the gating process is still under debate. In the following, we will try to address the question based on a thermodynamics consideration of interactions between lipid molecules and the embedded MS channel.
Membrane Tension
It is now well established that lateral membrane tension, rather than other factors such as membrane curvature or pressure, dictates the behavior of MscL and MscS.25 Surface tension is a macroscopic description of the tendency of reducing interface between two physical phases, thus reducing the entropic cost in maintaining the interface. A characteristic of the membrane tension of biological lipid bilayer is its two aqueous‐lipid interfaces on both sides of the membrane. At the microscopic level, membrane tension not only exists between individual lipid molecules within the lipid bilayer, but also between lipid molecules and embedded membrane proteins. At the polar−nonpolar interface of a lipid bilayer, each lipid molecule experiences pulling forces from surrounding lipid molecules in all directions within the membrane plane, with a net overall force of a value near zero. At the lipid‐protein interface, both lipid molecules and the embedded membrane protein experience a pulling force between each other. Such interactions seal the interface between the lipid bilayer and proteins, in addition to promoting the folding and stabilization of the proteins within the membrane environment.26 Unlike lipid molecules, however, a membrane protein may have multiple internal states in response to different membrane tension. An integral membrane protein usually assumes an equilibrium conformation at a given membrane tension (e.g., at zero tension). Upon change of the membrane tension, however, forces applied by the lipid bilayer on the surface structural elements of the protein and forces from the interior of the protein may result in imbalance between the two forces. In response, the protein must change its conformation in order for these forces to reach a new equilibrium. The degree of the conformational changes of the embedded proteins varies from protein to protein, depending on the amplitude of the change of the membrane tension relative to the rigidity of the membrane protein. In particular, the sealing lipid molecules will pull an MS channel to its open state once the membrane tension approaches a specific threshold. This mechanism is called force‐from‐lipid principle for the operation of MS channels.27
Although membrane tension is entropic in nature (similar to stretching of a randomly coiled long DNA molecule),6 the tension‐associated forces applied on the membrane protein are mechanical in the sense that they promote more‐or‐less deterministic movements of structural components of the protein relative to each other as well as to the lipid bilayer.9 In other words, it can be phenomenologically envisioned that there exist some “hydrophobic bonds” between the membrane protein and its surrounding continuous membrane. The corresponding forces applied on the protein molecule are distributed on structural elements that are in direct contact with surrounding lipid molecules. Such a description has been used to simplify calculations in some molecular dynamic simulations.28 In general, the directions of the surface‐tension forces acting on the membrane‐embedded protein point radially away from the protein.
In a so‐called “lipid moves first” model, it is assumed that membrane tension creates a vacuum between the protein TM helices and surrounding lipid molecules, and such a vacuum would in turn pull the MS channel to its open state.29 One assumption of such a mechanism is that the interactions between the protein and surrounding lipid molecules are distinct from (or weaker than) the interactions between lipid molecules; however, this may not necessarily be correct. For lipid molecules and an embedded protein, interactions between lipid head groups and the flanking regions of a TM helix are equivalent to the geometric fitting and electrostatic interactions among the head groups of lipid molecules, and those between lipid acyl tails and hydrophobic side chains of residues from the TM helix are equivalent to hydrophobic packing interactions among the acyl tails. In short, the lipid‐protein interactions are unlikely to be drastically distinct from the lipid‐lipid interactions. Therefore, the membrane tension can be transferred to the MS channel via interactions similar to that between lipid molecules.
The pressure profile of membrane tension (i.e., the depth‐dependent distribution of lateral pressure) is not uniform across the membrane30, 31 (Fig. 1). Instead, the profile shows (i) highest negative pressure (i.e., tension) at the two polar−nonpolar interfaces of the lipid bilayer to prevent access of water to the acyl chains and (ii) positive pressure (entropic repulsion) just beneath each of the two interfaces. The latter internal pressure gradually drops to zero in the middle of the lipid bilayer. Importantly, integration of the pressure profile along the direction of the membrane normal equals the membrane tension of the integration layer. For instance, at zero overall tension, the integration over the entire membrane thickness equals zero; in other words, the surface tension and internal pressure are in balance. In contrast, at a non‐zero tension the integration from the surface layer(s) exceeds that of internal pressure. It should be re‐emphasized that surface tension is present in a very narrow region on each of the two sides of the lipid bilayer, whereas the inner pressure is more widely spread (albeit unevenly) across the membrane. Furthermore, physical and chemical properties of lipid components affect the pressure profile of the lipid bilayer, in addition to directly affecting the properties of the membrane proteins.29, 32 Taken together, the nonuniform distribution of the pressure has a fundamental effect on the operation of an MS channel.31
Figure 1.
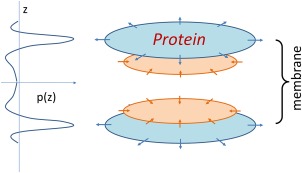
Schematic diagram of pressure profile and tension distribution. The lateral pressure (p) as a function of position z along the membrane normal is presented on the left side. Tension forces applied on the embedded membrane protein is schematically presented on the right side. Sections near the aqueous‐membrane interface are in cyan, and sections buried inside the membrane are shown in orange.
Structural Features of MS Channels
MS channels are commonly composed of symmetrical homo‐oligomers comprising four to seven subunits, with the axis of rotational symmetry being perpendicular to the membrane plane.6, 15 Whether some of the variations in oligomerization are a result of differences in sample preparation of the channel proteins remains under debate. In a typical MscL pentamer, each subunit contains two TM helices (i.e., TM1 and TM2)9, 15 [Fig. 2(A)], while in an MscS heptamer each subunit contains three TM helices (i.e., TMs 1‐3)13, 14 [Fig. 2(B)]. The central pore within both MscL and MscS channels assumes a funnel‐like shape, with the narrowest parts of the pores located at the cytosolic side and the widest opening at the periplasmic side. The pore‐forming helices are the first (TM1) and third (TM3a) transmembrane helices in MscL and MscS, respectively. At the narrowest point, the channel pore is constricted by a small number of hydrophobic residues from each of the pore‐forming helices. In the (nearly) closed form, these hydrophobic residues form a so‐called vapor plug33 or vapor bubble, to seal the channel from leaking water or ions.
Figure 2.
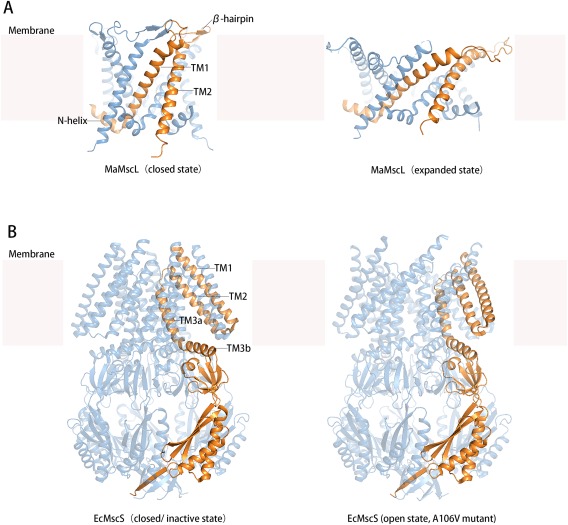
Crystal structures of Ma‐MscL and Ec‐MscS at different conformational states. A: Ma‐MscL structures in the closed state (PDB ID: 4Y7K) and expanded state (PDB ID: 4Y7J). B: Ec‐MscS structures in the closed/inactive state (PDB ID: 2OAU) and open state (obtained from A106V mutant, PDB ID: 2VV5). In each of the two panels, a representative protomer is shown in gold color.
Overall, the TM helices in the MscS complex are less tightly packed than MscL. In the crystal structures of both the closed and open forms of Ec‐MscS, the TM1 and TM2 helices form a helix hairpin, and these hairpins form a splayed layer that separates from the pore‐forming TM3a helices at their cytosolic ends [Fig. 2(B)]. Residues facing the gaps between the outer layer of TM1‐TM2 hairpins and the inner layer of TM3a helices are mostly hydrophobic, favoring interactions with the acyl chains of lipid molecules. Each helix‐hairpin in a channel complex separates from neighboring ones, leaving large surface valleys (or pockets) in between. These valley regions increase the surface area of the channel complex exposed to the lipid bilayer, and are likely to be filled with lipid molecules dynamically exchanged with the bulk membrane lipids.29 Similar valley regions are also observed on the surface of the closed form of MscL complexes. With these hydrophobic surface valleys, an MS channel is better integrated with the surrounding lipid bilayer. Presumably, the integrated interface favors transmission of membrane tension to the protein from its surrounding.
In both MscL and MscS, the cytosolic end of the pore‐forming TM helix attaches to an amphipathic helix. In MscL, this amphipathic helix is located at the N‐terminal of the subunit peptide and is thus specifically called N‐helix (also known as S1 helix). Together with TM1, this N‐helix as well as the linker between them is the most conserved region in the primary sequences of MscL proteins.15 In the crystal structures of MscL complexes, for example, that from Mycobacterium tuberculosis (Mt‐MscL, PDB ID: 2OAR), the hydrophobic surface of the N‐helix faces the putative membrane bilayer, and the helix is composed of the sequence of M1LKGFKEFLARG.13 It is noteworthy that, in crystal structures, such amphipathic helices may not always be parallel to the putative membrane plane, and sometimes may even become disordered (e.g. in 3HZQ). Since crystal structures of membrane proteins were often obtained from de‐lipidated protein samples, some native structural features may be disrupted in the purification process, especially those at the protein‐lipid interface.4 In situ, it is likely that the amphipathic helix of MscL is half‐inserted into the cellular membrane, thus anchoring the N‐terminal ends of TM1 helices to the membrane surface. As shown from molecular dynamic simulations, positively charged residues in the amphipathic helix interact with the phosphate head‐groups of surrounding lipid molecules, further suggesting functional importance of this amphipathic helix.28 In general, an amphipathic structural element may function as a sensor to detect the position of the membrane‐embedded protein relatively to the lipid bilayer, particularly to the polar−nonpolar interface. In addition to MscL, such amphipathic structural elements have also been proposed to play functional roles in transporters of the major facilitated superfamily,34 in G‐protein coupled receptors,35 and in some membrane‐integral enzymes.36 In a series of previous studies on MscL, deletion of the N‐helix abolished its channel activity.37 Furthermore, disulfide‐cross linking of N‐helices blocked the gating process, and extending the linker between N‐helix and TM1 impaired opening of the channel.14 Nevertheless, results from an experiment using a random mutagenesis approach suggested that the N‐helix is tolerant to mutations that maintain the amphipathic pattern.18 Intriguingly, mutations near the amphipathic N‐helix within TM2, namely Phe85 and Phe93, two of the most conserved residues in Ec‐MscL, yield loss‐of‐function variants whose capacity to enter their open‐state is greatly impaired.19 Together with the N‐helix, these phenylalanine residues may be involved in the formation of a lipid‐binding site. Moreover, TM1 is attached at the C‐terminal end to an amphipathic β‐hairpin.6 In the crystal structures of the closed forms of both Mt‐MscL and MscL from Methanosarcina acetivorans (Ma‐MscL; PDB ID: 4Y7K), this amphipathic β‐hairpin protrudes into the periplasmic space and covers the C‐terminal end of TM1 from a neighboring subunit. Proteolytic disruption of this loop region in Ec‐MscL resulted in increased mechanosensitivity, presumably by destabilizing the closed form of the channel.38 In addition, as suggested by the crystal structure of Ma‐MscL in an expanded conformation (PDB ID: 4Y7J), this β‐hairpin is potentially able to change its conformation during the transition from the closed state to expanded state [Fig. 2(A)]. It is likely that, in the in vivo open state, this β‐hairpin interacts with the polar−nonpolar interface of the (presumably curved) outer leaflet of the lipid bilayer. In such a scenario, this amphipathic β‐hairpin may also play an anchoring role similar to the N‐helix in stabilizing the opening state of the channel. As discussed above, the pulling force of membrane tension is concentrated in a narrow polar−nonpolar interfacial region of the lipid bilayer. Thus, amphipathic structural motifs enhance the abilities of the pore‐forming TM1 to sense and respond to change of membrane tension. In Ec‐MscS [Fig. 2(B)], an amphipathic helix connected to the C‐terminal end of the pore‐forming TM3a, termed TM3b, may function in a similar way. Like the N‐helix and TM1 in MscL, the region of TM3a and TM3b is the most conserved region among MscS homologs.8
Repacking of the pore‐forming helices is believed to be the structural basis of channel gating in both MscL and MscS. For example, an iris‐like gating mechanism was proposed earlier for MscL, whereby the opening of the channel is correlated with an increase of the tilting angle of TM1 relative to the central axis of the channel.39, 40 Recently reported crystal structures of Ma‐MscL in both closed and (partially) opened conformations support this hypothesis9 [Fig. 2(A)]. The tilting angle of the pore‐forming TM1 increases by ∼20° upon the closed‐to‐open transition, and the pivot point of the TM1 rotation appears to be in the middle of the membrane.9 Increasing tilting angles of TM helices appears to be a general strategy to increase the cross‐section of the membrane protein (i.e., the area of the TM helix bundle perpendicular to the membrane normal) in response to an increase in membrane tension.29 In the case of MscS, increasing the cross‐section of the channel complex is mainly achieved by increasing the tilting angle of the TM1‐TM2 helix hairpin, accompanied by radially outward movements of both the periplasmic end of the helix‐hairpin and the cytosolic end of TM3a.13, 14 For MscS channels, the pore‐forming helix, TM3a, is shorter than either TM1 or TM2, and at the middle level of the membrane, it is attached to a polypeptide extending from TM2. The opening of the channel pore is mainly achieved by combination of a rotation of TM3a around its own helix axis and an outward shift,13 [Fig. 3(B)] presumably by being pulled by a radial force applied through its C‐terminal amphipathic helix (TM3b). In both MscL and MscS, the conformational transition requires cooperative movements of multiple subunits. Such cooperativity is usually associated with large activation energy and is likely to be important for preventing incidental opening of the MS channel, a process potentially harmful for the cell.
Figure 3.
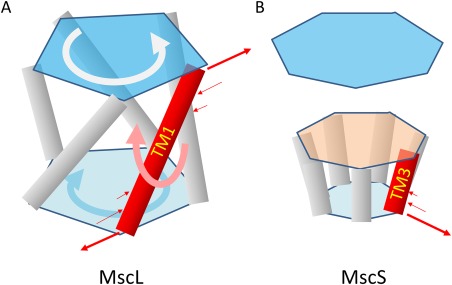
Model of putative opening mechanism of MS channels. In each of the MscL (A) and MscS (B) case, a representative pore‐forming helix is shown in red. The surface tension associated forces are indicated with thin‐line arrows. The tilting of the pore‐forming helix is indicated by the thick pink arrow. In the case of MscL, the overall rotations of top and bottom parts of the channel complex are indicated.
Energy Transmission
The two‐state model of gating of MS channels was originally proposed based on data of tension‐dependent conductance from functional studies,7 and was latter supported by crystal structures of MS channels. For an MS channel (or any systems in thermal equilibrium), the thermodynamics between two given states is solely described by the difference of their thermodynamic variables, but independent of possible paths connecting the two states. Therefore, the two‐state model that we discuss here would not conflict with other potentially more‐detailed descriptions of the same system, which may include multiple sub‐states between the two given states.
In the two‐state model of the MS channel, free‐energy ΔG associated with the gating of the channel is described as follows:
| (1) |
where ΔG 0 (>0) is the differential free energy between the two states of the channel in the absence of membrane surface tension (denoted as σ), and where ΔA is the change of the cross‐section area of the TM part of the channel complex.6 The value of ΔG 0/ΔA is defined as σ 1/2, i.e., the membrane tension at which the channel has an equal probability to be at either closed or open state. Typical values for MscL and MscS of σ 1/2 are 10 and 5 mN/m, respectively.4 Furthermore, the probability of the open state (P) as a function of σ follows the Boltzmann distribution22 (Appendix A). Mathematically, ΔA is proportional to the slope of P(σ) at σ 1/2. The larger that ΔA is, the sharper is the P(σ) curve at σ 1/2.
Activation Energy of Channel Opening
ΔG 0 is also called the “activation energy” of the channel. The positive sign of ΔG 0 indicates that the resting state of the channel (i.e., at zero tension) is its closed form. Meanwhile, a smaller (or larger) value of ΔG 0 would correspond to a softer (or more rigid) structure of the channel. In order to open the channel, work resulting from the membrane tension must be higher than ΔG 0, so that the overall free energy, ΔG, is reduced to a level comparable to or lower than that of thermal motion (i.e., RT, temperature multiplied by the universal gas constant). ΔG 0 may include energy terms from changes of hydrophobic match, of membrane deformation, of electrostatic energy, and of conformational constrains, between the closed and open states. For Ec‐MscL, ΔG 0 is in the order 120 kJ/mol (∼48 RT),6 which is so high that activation of the channel would unlikely be triggered by a single molecular event such as ATP hydrolysis (30 − 50 kJ/mol). As mentioned above, this tight control of the gating of MscL is essential for preventing accidental opening of the MscL channel, which would waste valuable resources of the cell. A mutation that reduces ΔG 0 (while keeping ΔA unchanged) will shift the P(σ) curve to the left. Thus, the channel complex will become more tension‐sensitive, and the channel pore will open at a lower membrane tension. This was shown for a Q56P mutation of Ec‐MscL, the channel of which opens at membrane tension 1/3 lower than that required for the wild type.37 Since Gln56 is located in the amphipathic β‐hairpin mentioned above, and because it is involved in forming the rim of the periplasmic ring of the complex in its closed form, the Q56P mutation enhances the tension sensitivity, presumably by destabilizing the closed state (thus reducing ΔG 0).
Hydrophobic mismatch between the protein and its surrounding lipid bilayer caused by thinning and/or bending of the membrane is sometimes considered to be a driving force for the channel gating.41 Under experimental conditions, a reduction in membrane thickness was indeed observed to induce the opening of the MS channel.42 By manipulating the lipid components of the membrane, the closed and open states of the MS channel may be affected differentially. For example, lipid (or detergent) molecules of shorter acyl chains promote the opening state, probably because such lipid molecules increase the tilting angles for TM helices, favoring the open state over the closed form (i.e. decreasing ΔG0). In fact, Ma‐MscL crystal structures showed a reduction in thickness of the TM region of the channel complex from 44 Å to 30 Å (∼30% change), upon conformational change from the closed state to an expanded state.9 However, an in vivo membrane tension cannot effectively decrease the thickness of the membrane. It is estimated that a reduction in thickness of the membrane bilayer of more than 3% would result in rupture of the lipid bilayer.43 Contrary to the model whereby thinning of the membrane induces channel opening, it is more likely that the process of channel opening induces (or increases) the in vivo hydrophobic mismatch between the channel complex and its surrounding lipid bilayer. In turn, such increased hydrophobic mismatch, which contributes to the higher free‐energy level of the open state, limits the conformational change of the channel. Such hydrophobic mismatch partially prevents the channel pore from further expanding which might have detrimental effects on the cell, such as membrane rupture. Consistent with a change in thickness upon state transition in the MscL structure9 larger than in MscS,13 MscL appears to be more sensitive to thinning of the membrane.44 In short, it is very likely that a hydrophobic mismatch is an energetic penalty imposed upon MscL during its channel opening.45
In functional studies of MS channels, patch clamp electrophysiology represents the “gold standard” for monitoring tension‐activated channel current, and provides quantitative information on the relationship between membrane tension and conductance.46 However, it is noteworthy that if an MS channel carries electrical charges, the membrane potential (ΔΨ) exerts electrostatic forces on the channel complex and thus affects the equilibrium distribution of the channel between the two states (i.e., ΔG0). Such ΔΨ‐dependency of mechanosensitivity has been implied in earlier reports,5 and this phenomenon is commonly referred to as rectification. Those reports showed for membrane containing MS channels, that under the condition of purification, σ1/2 (reciprocal to tension sensitivity) dropped from 30 mm Hg at ΔΨ of −30 mV to 0 mm Hg at ΔΨ of +20 mV. (At that time, the exact nature of the MS channels had not been defined.) This measurement suggested that some of the tension‐associated input energy was spent to compensate electrostatic cost during the channel opening. In other words, during the process of channel opening, overall electric charges move against the ΔΨ (−30 mV). Under physiological conditions, the E. coli inner membrane carries a negative‐inside membrane potential of typically −100 mV. In addition, the crystal structure of Ec‐MscS revealed a highly positively charged TM domain containing several basic residues.14 Thus, under normal cellular conditions, it is anticipated that an inward electrostatic force perpendicular to the membrane plane is exerted on the MscS channel complex, and this force may differentially affect the properties of the channel at closed and open states. In agreement with this argument, the activity (i.e., pore size and conductance) of MscS exhibits strong ΔΨ‐dependence, as shown in patch‐clamp experiments.47 Similarly, in another report Ec‐MscL also showed weak ΔΨ‐dependence of mechanosensitivity.48 Furthermore, the membrane potential may dynamically regulate the channel gating, since efflux of cytosol content may transiently diminish local ΔΨ and adjust ΔG0 in response. The phenomenon of ΔΨ‐dependence of mechanosensitivity may be conceptually analogous to the equilibrium position of a boat (and thus its hydrodynamic properties) which is affected by the effect of gravity on its loaded cargo. In some (eukaryotic) MS channels, the gating is regulated by tethering the channel complex with extracellular matrixes and/or cytoskeletons.2 Such a gating mechanism (the so‐called “elevator” model) may be considered as an alternative way to adjust the perpendicular positions (thus ΔG0) of the channels relative to the membrane. The vertical movement of the channel complex would generate hydrophobic mismatch and asymmetry in the channel‐lipid bilayer system and result in channel gating, similar to what a change of ΔΨ may do.
Change in Cross‐Section of the Channel Complex during Gating
ΔA is an intrinsic property for a given channel. A constant ΔA is critically related to the two‐state model of an MS channel, in contrast to a pinhole in a rubber balloon in which the hole‐size may change continuously in direct correlation to the membrane tension. ΔA is estimated to be ∼20 and 10 nm2 for MscL and MscS, respectively.24 Mechanisms of expanding the cross‐section of an MS channel include (i) tilting of the TM helices and (ii) rotation of TM helices so that large side chains of amino acid residues are moved from the central channel to previously lipid‐occupied space.12, 13 Whilst there is a positive correlation between ΔA and the pore size of the channel, it is important to differentiate these two terms. While ΔA determines the tension threshold for gating, the pore size of the channel determines the conductance of the open state. Interestingly, proteolytic digestion of the cytosolic side of the Ec‐MscL complex increases the slope of P(σ), but reduces σ1/2,38 indicating that ΔA is increased in the cleaved channel complex. This observation might be due to the fact that the cytosol‐located C‐terminal peptide is involved in restricting the expansion of the channel complex. Thus, cleavage of the peptide alleviates this restriction on ΔA, whereas the conductance (thus the pore size) of the channel remains unchanged. Moreover, in agreement with the two‐state model, TRAAK and TREK, eukaryotic mechanosensitive K+ channels possessing considerably smaller ΔA values (2.7–4.7 nm2), are activated at a much wider range of tension values than MscL and MscS.45
For an MS channel to alter its cross‐section, tilting of TM helices is required. For titling to occur, torque is essential, which in turn is created by the membrane tension. Here, we discuss the relationship between the membrane tension profile and torque generation. At zero tension, the pressures at different levels of the membrane are balanced with each other as well as with the internal forces of the protein. When the membrane tension is increased, the profile of the pressure changes its shape. In a simplified model, the pressure profile assumes a zero value outside of the polar−nonpolar interface regions. Thus, each of the two interfaces of the lipid bilayer holds half of the overall tension. Given that the typical diameter of a TM helix is approx. 10 Å, and provided that the membrane tension is approx. 10 mN/m, the corresponding force at each end of the helix is estimated as ∼5 pN. The amplitude of this force is in the typical range of biologically meaningful forces operating on macromolecules, for example that of an electrostatic force applied on a proton‐driven transporter.34 In comparison, in a real lipid bilayer, the tension at the polar−nonpolar interface of the lipid bilayer may reach levels of ∼50 mN/m per monolayer, because of the non‐uniform profile of the membrane tension. This tension value is an order of magnitude larger than the apparent overall membrane tension.31 Such strong forces associated with the tension at the two surface regions are partially compensated by opposite, more evenly distributed pressure in the intermediate layer, in a way that the mass center of a given TM helix remains unchanged. However, the arm of force for the surface tension is often longer than those of compensating forces. Thus, the torque induced by the surface tension plays a dominant role in tilting the TM helix. As shown in the diagram in Figure 3(A), for the pore‐forming TM1 helix in MscL, each of its two ends sense membrane tensions from the periphery of the channel complex. As a net result of the tension‐associated torque on TM1, an increase of the tilting angle of TM1 is expected upon an increase of the surface tension.
Kinetics of the Conformational Transition
According to the two‐state model, the parameters ΔG 0 and ΔA determine the thermodynamic equilibrium of the channel at a given membrane tension σ (which is a variable of the two‐state model). However, such thermodynamic parameters do not solely determine the kinetic behavior of the channel. Instead, the kinetic parameters of the system include k on and k off of the transition state between the closed and open states (Appendix B), which are related with each other by the equation ΔG =RT·ln(k off/k on). The k on and k off parameters are the reciprocal of the average dwelling times of the corresponding states, which may be determined experimentally with single‐molecule techniques. According to the Arrhenius equation, k on and k off are further related to the heights of the front‐ and back‐side of the transition‐state energy barrier (ΔG ‡ and ΔG’‡), respectively.
The energy barrier of the transition state may include energy terms required (i) to transiently enlarge the vapor plug in the hydrophobic channel pore and (ii) to overcome structural constrains in the transition path. A vapor plug prevents flux of water and other hydrophilic molecules, as long as the inner diameter of the hydrophobic pore remains smaller than the critical radius of wetting (typically ∼7 Å).33, 49 Stability of the vapor plug is determined by two opposite factors. First, on the two sides of the vapor plug, there are aqueous‐vapor interfaces which have a large surface tension estimated to be ∼70 mN/m, based on a description of continuum mechanics.49 The energy cost of increasing such an interface is roughly proportional to the square of the pore radius. Thus, expanding the vapor bubble requires increasingly large energy input, which is a significant part of the energy barrier of the transition state of the MS channel. Second, the energy term associated with wetting the hydrophobic pore would roughly be linearly proportional to the pore radius. At the critical radius, these two energy terms are equal to each other. With an increasing membrane tension, the pore switches from the high energy, vapor‐plugged mode to the low energy, hydrated mode, thus breaking the vapor bubble. Rendering the pore more hydrophilic, for example by introducing a point‐mutation, reduces the energetic cost to hydrate the channel pore,21, 50 allowing the vapor bubble to break more easily. In addition, in the open state, the lining of the pore may become more hydrophilic (e.g., by exposing some main‐chain polar groups) than the closed state due to local rotations of the TM helices, thus reducing the hydration cost in the open form.49 Once the bubble breaks apart, the free energy stored in the vapor bubble will be partially released to drive the state transition, and the remaining energy is incorporated into ΔG 0, part of which is used to wet the hydrophobic pore. Experimentally, most Ec‐MscL mutations introducing a hydrophilic residue in the hydrophobic pore result in both reduced ΔG 0 (gain‐of‐function mutations) and reduced transition barriers ΔG ‡, i.e., the channels flicker during gating.19, 21 Taken together, both chemical properties and physical shape of the pore are likely to affect the overall kinetics of the gating process.
In addition to those residues located inside the channel pore, amino acid residues located away from the pore may also affect the kinetic parameters of the transition‐state energy barrier. For example, Ec‐MscS contains an “Ala110‐Leu115 switch”, whereby Ala100 acts as a “bump,” hindering Leu115 from moving during the transition. Mutation of either Ala110 or Leu115 had a drastic effect on the gating kinetics of Ec‐MscS,13 presumably because of changing the energy barrier of the transition state. In addition, the pore‐forming TM helices often contain Ala and Gly residues. It has been shown that, in Ec‐MscS, such residues with small or no side chains are important for maintaining a proper energy barrier.51 For instance, the A102G variant exhibits a flickering channel phenotype, probably induced by an “over”‐reduced energy barrier of the transition state.
Adaptation
Adaptation is a peculiar phenomenon observed exclusively for MscS channels.47, 52 Following channel opening upon abrupt application of membrane tension, the channel becomes gradually closed. The inactivated MscS channel can be re‐activated after resting in the absence of membrane tension (e.g., for 3 min). Furthermore, MscS was shown to lose its channel activity when membrane tension was slowly raised using patch clamp.47 Adaptation is believed to help bacteria survive during exposure to prolonged osmotic shock, and was taken as evidence that an inactive state exists in addition to the close and open states.47 The mechanism for this phenomenon, however, remains under debate.
Here, we propose a simple mechanism for the adaptation phenomenon based on the two‐state model: Adaption of MscS is caused by slow, lateral diffusion of lipid molecules [Fig. 4(A)]. A necessary condition for an MS channel to function properly is that the pore formed during the state transition is not occupied by lipid molecules. However, the MscS structure in its open state may not make such a condition sustainable. The pore‐forming TM3a helices of the MscS complex become less tightly packed in the open state than in the closed state.13 Thus, expanding the inner layer composed of TM3a helices generates multiple small clefts connecting the above‐mentioned surface valleys to the central pore [Fig. 4(B)], and the sizes of these clefts may fluctuate because of thermal motion. As a consequence, lipid molecules located within the inter‐subunit valleys will slowly penetrate into the central pore of the channel through these fluctuating inter‐subunit clefts existing only in the open or partially opened form of the channel complex. These penetrated lipid molecules will gradually decrease the effective size of the pore and re‐establish the hydrophobic vapor plug. The lateral diffusion movement of the lipid molecules is driven by the concentration difference of the lipid molecules between outside and inside of the channel pore. The penetration of the lipid molecules is not necessarily a cooperative process, and may dynamically break the rotational symmetry of the channel complex. Furthermore, the inner surface of the pore is mainly assembled from hydrophobic residues (PDB ID: 2VV5), thus enabling favorable interactions with the invading lipid molecules. Once the membrane tension is removed, the channel complex will relax and return to the closed resting state. Releasing of the “activation energy” ΔG0 will force the lipid molecules out of the central pore. Since the lateral diffusion of the lipid molecules is likely to be slow, both the desensitization and reactivation are delayed relative to the change of membrane tension. Thus, if the relaxation time is not sufficiently long, some lipid molecules may remain inside the pore, resulting in the corresponding state(s) deviating from the bona fide closed state. Such partially lipid‐filled, inactive states, once being re‐activated, would show a smaller ΔA (i.e. a shallower slope of P(σ) at σ1/2) and probably a smaller ΔG0, with σ1/2 being maintained approximately constant, as exemplified before.47 Such (partially) inactive states will also show reduced activities (conductance) upon reactivation, because of a smaller effective size of the final pore resulting from remaining lipid molecules. In contrast to MscS, the open form of MscL does not contain inter‐subunit gaps, since its subunits have tighter contacts with each other. In addition, while MscS and MscK share sequence homology in the channel forming region, MscK is characterized by more sustained activities under constant stimulation.53 The lack of adaptation in MscK is likely to be related to its extra TM helices which better seal the channel against lateral lipid penetration. Interestingly, in a eukaryotic mechanosensitive K+ channel, TRAAK, which is evolutionarily unrelated to either MscL or MscS, the channel gating is also mediated by removing blockage of lipid molecules from the conductance channel.45 Taken together, the two‐state model provides a reasonable explanation for the adaptation phenomenon of MscS. A testable prediction based on our hypothesis would be that short‐acyl chain lipid molecules (if their effect on ΔG 0 can be ignored) enhance the adaptation of MscS compared with long‐acyl chain lipids because of the relative ease with which they enter into the channel pore.
Figure 4.
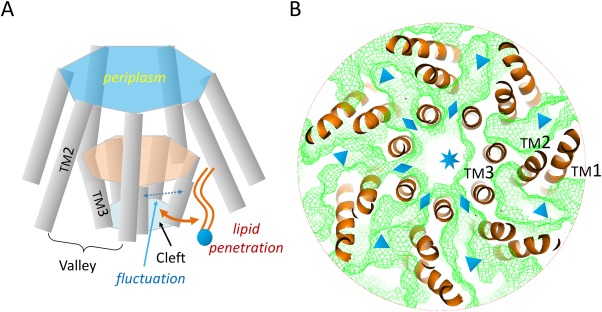
Putative mechanism of adaptation of MscS. A: Schematics of lipid penetration. B: Channel connections within the Ec‐MscS complex. TM helices (orange ribbons) are obtained from the crystal structure of the open form (PDB ID: 2VV5). The complex is viewed from the cytosolic side, with cytosolic domains removed for clarity. Channels within the TM complex were calculated with probes of 1.4 Å radius and illustrated with green meshes. The central pore, clefts between TM3a helices, and valleys between TM1‐TM2 hairpins are marked with a star, diamond symbols, and triangle symbols, respectively.
Interaction between MS channels and their surrounding lipid molecules is a key to our understanding on how membrane tension drives the gating of the channels. The two‐state model of bacterial MS channels reviewed here likely provides a theoretical basis for future studies on other MS channels that may have more sophisticated regulation mechanisms.
Supporting information
Supporting Information
Acknowledgment
The authors thank Dr. Torsten Juelich for linguistic assistance during the preparation of this manuscript.
References
- 1. Martinac B, Kloda A (2003) Evolutionary origins of mechanosensitive ion channels. Prog Biophys Mol Biol 82:11–24. [DOI] [PubMed] [Google Scholar]
- 2. Kung C (2005) A possible unifying principle for mechanosensation. Nature 436:647–654. [DOI] [PubMed] [Google Scholar]
- 3. Sukharev S, Sachs F (2012) Molecular force transduction by ion channels: diversity and unifying principles. J Cell Sci 125:3075–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kung C, Martinac B, Sukharev S (2010) Mechanosensitive channels in microbes. Ann Rev Microbiol 64:313–329. [DOI] [PubMed] [Google Scholar]
- 5. Martinac B, Buechner M, Delcour AH, Adler J, Kung C (1987) Pressure‐sensitive ion channel in Escherichia coli . Proc Natl Acad Sci USA 84:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haswell ES, Phillips R, Rees DC (2011) Mechanosensitive channels: what can they do and how do they do it? Structure 19:1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard J, Roberts WM, Hudspeth AJ (1988) Mechanoelectrical transduction by hair cells. Ann Rev Biophys Biophys Chem 17:99–124. [DOI] [PubMed] [Google Scholar]
- 8. Naismith JH, Booth IR (2012) Bacterial mechanosensitive channels–MscS: evolution's solution to creating sensitivity in function. Ann Rev Biophys 41:157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Guo J, Ou X, Zhang M, Li Y, Liu Z (2015) Mechanical coupling of the multiple structural elements of the large‐conductance mechanosensitive channel during expansion. Proc Natl Acad Sci USA 112:10726–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai JY, Poon YS, Kaiser JT, Rees DC (2013) Open and shut: crystal structures of the dodecylmaltoside solubilized mechanosensitive channel of small conductance from Escherichia coli and Helicobacter pylori at 4.4 Å and 4.1 Å resolutions. Protein Sci 22:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Wang J, Feng Y, Ge J, Li W, Sun W, Iscla I, Yu J, Blount P, Li Y, Yang M (2012) Structure and molecular mechanism of an anion‐selective mechanosensitive channel of small conductance. Proc Natl Acad Sci USA 109:18180–18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Z, Gandhi CS, Rees DC (2009) Structure of a tetrameric MscL in an expanded intermediate state. Nature 461:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR (2008) The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science 321:1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bass RB, Strop P, Barclay M, Rees DC (2002) Crystal structure of Escherichia coli MscS, a voltage‐modulated and mechanosensitive channel. Science 298:1582–1587. [DOI] [PubMed] [Google Scholar]
- 15. Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC (1998) Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- 16. Sukharev S, Betanzos M, Chiang CS, Guy HR (2001) The gating mechanism of the large mechanosensitive channel MscL. Nature 409:720–724. [DOI] [PubMed] [Google Scholar]
- 17. Vasquez V, Sotomayor M, Cordero‐Morales J, Schulten K, Perozo E (2008) A structural mechanism for MscS gating in lipid bilayers. Science 321:1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maurer JA, Dougherty DA (2003) Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. J Biol Chem 278:21076–21082. [DOI] [PubMed] [Google Scholar]
- 19. Levin G, Blount P (2004) Cysteine scanning of MscL transmembrane domains reveals residues critical for mechanosensitive channel gating. Biophys J 86:2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okada K, Moe PC, Blount P (2002) Functional design of bacterial mechanosensitive channels. Comparisons and contrasts illuminated by random mutagenesis. J Biol Chem 277:27682–27688. [DOI] [PubMed] [Google Scholar]
- 21. Bartlett JL, Li Y, Blount P (2006) Mechanosensitive channel gating transitions resolved by functional changes upon pore modification. Biophys J 91:3684–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sukharev SI, Blount P, Martinac B, Kung C (1997) Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Ann Rev Physiol 59:633–657. [DOI] [PubMed] [Google Scholar]
- 23. Phillips R, Kondev J, Theriot J. 2009. Physical biology of the cell. New York: Garland Science. [Google Scholar]
- 24. Cruickshank CC, Minchin RF, Le Dain AC, Martinac B (1997) Estimation of the pore size of the large‐conductance mechanosensitive ion channel of Escherichia coli . Biophys J 73:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moe P, Blount P (2005) Assessment of potential stimuli for mechano‐dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry 44:12239–12244. [DOI] [PubMed] [Google Scholar]
- 26. Cymer F, von Heijne G, White SH (2015) Mechanisms of integral membrane protein insertion and folding. J Mol Biol 427:999–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kocer A (2015) Mechanisms of mechanosensing—mechanosensitive channels, function and re‐engineering. Curr Opin Chem Biol 29:120–127. [DOI] [PubMed] [Google Scholar]
- 28. Vanegas JM, Arroyo M (2014) Force transduction and lipid binding in MscL: a continuum‐molecular approach. PLoS One 9:e113947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pliotas C, Dahl AC, Rasmussen T, Mahendran KR, Smith TK, Marius P, Gault J, Banda T, Rasmussen A, Miller S, Robinson CV, Bayley H, Sansom MS, Booth IR, Naismith JH (2015) The role of lipids in mechanosensation. Nat Struct Mol Biol 22:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andersen OS, Koeppe RE (2007) Bilayer thickness and membrane protein function: an energetic perspective. Ann Rev Biophys Biomol Struct 36:107–130. [DOI] [PubMed] [Google Scholar]
- 31. Gullingsrud J, Schulten K (2004) Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys J 86:3496–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perozo E, Kloda A, Cortes DM, Martinac B (2002) Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol 9:696–703. [DOI] [PubMed] [Google Scholar]
- 33. Anishkin A, Sukharev S (2004) Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J 86:2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang XC, Zhao Y, Heng J, Jiang D (2015) Energy coupling mechanisms of MFS transporters. Protein Sci 24:1560–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang XC, Cao C, Zhou Y, Zhao Y (2014) Proton transfer‐mediated GPCR activation. Protein Cell 6:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mao G, Zhao Y, Kang X, Li Z, Zhang Y, Wang X, Sun F, Sankaran K, Zhang XC (2016) Crystal structure of E. coli lipoprotein diacylglyceryl transferase. Nat Commun 7:10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blount P, Sukharev SI, Schroeder MJ, Nagle SK, Kung C (1996) Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli . Proc Natl Acad Sci USA 93:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ajouz B, Berrier C, Besnard M, Martinac B, Ghazi A (2000) Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J Biol Chem 275:1015–1022. [DOI] [PubMed] [Google Scholar]
- 39. Spencer RH, Rees DC (2002) The alpha‐helix and the organization and gating of channels. Ann Rev Biophys Biomol Struct 31:207–233. [DOI] [PubMed] [Google Scholar]
- 40. Sukharev S, Durell SR, Guy HR (2001) Structural models of the MscL gating mechanism. Biophys J 81:917–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinac B, Hamill OP (2002) Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc Natl Acad Sci USA 99:4308–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Powl AM, East JM, Lee AG (2003) Lipid‐protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry 42:14306–14317. [DOI] [PubMed] [Google Scholar]
- 43. Bloom M, Evans E, Mouritsen OG (1991) Physical properties of the fluid lipid‐bilayer component of cell membranes: a perspective. Q Rev Biophys 24:293–397. [DOI] [PubMed] [Google Scholar]
- 44. Balleza D (2012) Mechanical properties of lipid bilayers and regulation of mechanosensitive function: from biological to biomimetic channels. Channels 6:220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brohawn SG (2015) How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann NY Acad Sci 1352:20–32. [DOI] [PubMed] [Google Scholar]
- 46. Guharay F, Sachs F (1984) Stretch‐activated single ion channel currents in tissue‐cultured embryonic chick skeletal muscle. J Physiol 352:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akitake B, Anishkin A, Sukharev S (2005) The “dashpot” mechanism of stretch‐dependent gating in MscS. J Gen Physiol 125:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A (1996) Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol 151:175–187. [DOI] [PubMed] [Google Scholar]
- 49. Anishkin A, Akitake B, Kamaraju K, Chiang CS, Sukharev S (2010) Hydration properties of mechanosensitive channel pores define the energetics of gating. J Phys Condens Matt 22:454120. [DOI] [PubMed] [Google Scholar]
- 50. Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C (1999) Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys J 77:1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edwards MD, Li Y, Kim S, Miller S, Bartlett W, Black S, Dennison S, Iscla I, Blount P, Bowie JU, Booth IR (2005) Pivotal role of the glycine‐rich TM3 helix in gating the MscS mechanosensitive channel. Nat Struct Mol Biol 12:113–119. [DOI] [PubMed] [Google Scholar]
- 52. Koprowski P, Kubalski A (1998) Voltage‐independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J Membr Biol 164:253–262. [DOI] [PubMed] [Google Scholar]
- 53. Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P (2002) Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli . Embo J 21:5323–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


