Abstract
Background
Simultaneous liver-kidney (SLK) transplantation plays an important role in treating kidney failure in patients with end-stage liver disease. It used 5% of deceased donor kidney transplanted in 2015. We evaluated the utility, defined as posttransplant kidney allograft lifespan, of this practice.
Methods
Using data from the Scientific Registry of Transplant Recipients, we compared outcomes for all SLK transplants between January 1, 1995, and December 3, 2014, to their donor-matched kidney used in kidney-alone (Ki) or simultaneous pancreas kidney (SPK) transplants. Primary outcome was kidney allograft lifespan, defined as the time free from death or allograft failure. Secondary outcomes included death and death-censored allograft failure. We adjusted all analyses for donor, transplant, and recipient factors.
Results
The adjusted 10-year mean kidney allograft lifespan was higher in Ki/SPK compared with SLK transplants by 0.99 years in the Model for End-stage Liver Disease era and 1.71 years in the pre-Model for End-stage Liver Disease era. Death was higher in SLK recipients relative to Ki/SPK recipients: 10-year cumulative incidences 0.36 (95% confident interval 0.33-0.38) versus 0.19 (95% confident interval 0.17-0.21).
Conclusions
SLK transplantation exemplifies the trade-off between the principles of utility and medical urgency. With each SLK transplantation, about 1 year of allograft lifespan is traded so that sicker patients, that is, SLK transplant recipients, are afforded access to the organ. These data provide a basis against which benefits derived from urgency-based allocation can be measured.
This analysis of the UNOS data demonstrated that SLK patients had decreased long term renal graft function that SPK patients. This analysis demonstrates the difficult policy choices epitomized by prioritizing the SLK population and its impact of utility considerations. Supplemental digital content is available in the text.
Kidney transplantation is the preferred treatment for end-stage renal disease, offering clear survival and quality of life benefits relative to dialysis.1 However, there is a critical shortage of deceased donor kidneys, such that allocation is tightly constrained.
Kidney allocation is based on balancing the principles of utilitarianism (“utility”) and distributive justice (“equity”).2,3 By the utility principle, an optimal system maximizes posttransplant allograft survival to minimize retransplantation rate, thus providing more kidneys to benefit the entire end-stage renal disease population. The equity principle aims to maintain equal access for patients who might benefit from kidney transplantation, even if their posttransplant outcome may trail that of the “ideal” candidate. In contrast, liver allocation is based on medical urgency: candidates are prioritized based on waitlist mortality as a surrogate for how urgently they need a liver for survival.
In simultaneous liver-kidney (SLK) transplantation, the kidney “follows” the liver into a patient allocated a deceased donor liver based on the urgency-based allocation system. Since the advent of the Model for End-stage Liver Disease (MELD)-guided allocation era in liver transplantation, the proportion of all deceased donor kidneys allocated to SLK transplants has increased to approximately 5% as of 2015. Patients with end-stage liver disease (ESLD) and pretransplant kidney failure fare significantly worse than those without kidney failure, even with the availability of dialysis.4,5 However, kidney failure in ESLD may reverse with liver transplant and time alone.6-8 Existing clinical criteria differentiate poorly among patients who will recover and those who will not.9-11 Undertransplanting SLK may thus lead to worse survival and eventual dialysis dependence in liver transplant recipients, whereas overtransplanting SLK may lead to organ wastage and decreased access for kidney transplant candidates without liver disease.
Herein, we apply the principle of utility to the current SLK system. Most deceased donors donate 2 kidneys identical in quality to 2 different recipients: a natural experiment approximating a randomized trial of kidney allocation. Although these patient groups are profoundly different, they are similar in that they all use a deceased donor kidney allograft. From the perspective of maximizing the utility of transplanted kidneys, the comparison is therefore justified. We aimed to determine the years of allograft function gained or lost by allocating a kidney allograft to SLK candidates over kidney and kidney-pancreas transplant candidates.
MATERIALS AND METHODS
The Scientific Registry of Transplant Recipients (SRTR) contains de-identified data on all solid organ transplant donors, recipients, and recipient candidates in the United States. It also incorporates dialysis start dates from the Centers of Medicare and Medicaid Services, thus ensuring accurate ascertainment of kidney allograft failure.12-14
We identified all donor-matched kidney pairs between January 1, 1995, and December 3, 2014, where 1 kidney was used in SLK and the other in kidney-alone (Ki) or simultaneous pancreas-kidney (SPK) transplantation. We chose the study period based on availability of a full complement of donor characteristics, up to the implementation of the Kidney Allocation System (KAS). We defined SLK transplantation as deceased donor liver and kidney transplantations occurring within 2 days in the same recipient.15,16 We aimed to establish a cohort of adult SLK recipients without prolonged pretransplant dialysis, ie not on dialysis, or on dialysis for fewer than 90 days at the time of transplantation, since the SLK controversy is mostly concentrated in this patient population. We excluded recipients of pediatric transplants, concomitant multi-solid organ transplants other than SPK, SLK transplants for a metabolic disorder or familial amyloidosis, and SLK transplants with requiring pretransplant dialysis for 90 days or more.
For sensitivity analysis, we assembled 2 additional cohorts identical to the primary cohort except in pretransplant dialysis duration. Cohort 1 further excluded 84 SLK recipients who were missing dialysis vintage despite being on dialysis at the time of transplantation. Cohort 2 was identical to the cohort in the primary analysis, but restricted to SLK recipients who received dialysis for 90 or more days pretransplant and their donor-paired kidney recipients.
We calculated the Kidney Donor Risk Index (KDRI), a marker of kidney quality, for each transplant kidney according to United Network of Organ Sharing publications.17 In practice, the KDRI is mapped to Kidney Donor Profile Index (KDPI), a 1- to 100-point system denoting the percentile category of the kidney in quality relative to all kidneys allocated in the previous year. We grouped kidneys into KDPI categories (≤20, 20-80, and >80), as these categories reflected allocation tiers in the KAS. The biological MELD score was calculated from the last serum creatinine, bilirubin, and international normalized ratios of prothrombin time available from the liver transplant candidate history sheet.18
The primary outcome was allograft survival, defined as freedom from death or allograft failure. Secondary outcomes were death and allograft failure, defined as dialysis initiation or kidney retransplantation.
Statistical Analysis
We stratified all analyses by MELD era and adjusted for the correlation between kidney pairs from the same donor. We compared baseline characteristics of SLK versus Ki/SPK transplants using a paired Student t test and McNemar test for continuous and categorical variables, respectively.
We censored follow-up time at 10 years posttransplant, or on December 4, 2014, whichever came first. We used flexible parametric models to estimate the mean allograft lifespan in the first 10 years posttransplant, ie., the area under the curve for allograft survival censored at 10 years.19 This model allowed transplant assignment, SLK versus Ki/SPK, to be modeled as a time-dependent covariate, since the hazards of death and allograft failure were not proportional. The fully adjusted models included adjustment for donor KDRI, donor group (kidney versus kidney-pancreas), donor sex, kidney cold ischemia time, delayed graft function, and recipient age, sex, race, diabetes status, prior kidney transplant status, dialysis status, and insurance status. For secondary outcomes, we modeled the cumulative incidence function of death and allograft failure using flexible parametric models that account for competing risk of allograft failure and patient death.20 We modeled predictions on patients 20, 40, and 60 years old who were dialysis-dependent at the time of transplant, adjusting for all other covariates. Please see Appendix 1, SDC (http://links.lww.com/TP/B351) for more complete details on the 2 models.
For sensitivity analysis, we used multiple imputation to handle missing data and ran the same analyses as detailed above.
Life Years after Transplant Subanalysis
We performed a sub-analysis to estimate the life years after transplant (LYFT) for each kidney allograft used in SLK. Conceptually, LYFT is the expected posttransplant patient survival minus expected patient survival without a transplant, ie., the survival benefit accorded by receiving the transplant. Wolfe et al21 estimated the LYFT for each Ki and SPK transplant, stratified based on recipient age and diabetes status at the time of transplantation. We therefore calculated the LYFT that would be expected if each kidney allograft used in SLK was used in Ki or SPK instead, as the age- and diabetes status-weighted average of LYFTs of the donor-matched kidney (Appendix 5, SDC, http://links.lww.com/TP/B351, for details).
Data handling and statistical analysis were conducted using SAS 9.4 (Cary, NC) and STATA 14.1 (StataCorp). Stanford University's Institutional Review Board approved this study in accordance with the Declaration of Helsinki (protocol number IRB-32753).
RESULTS
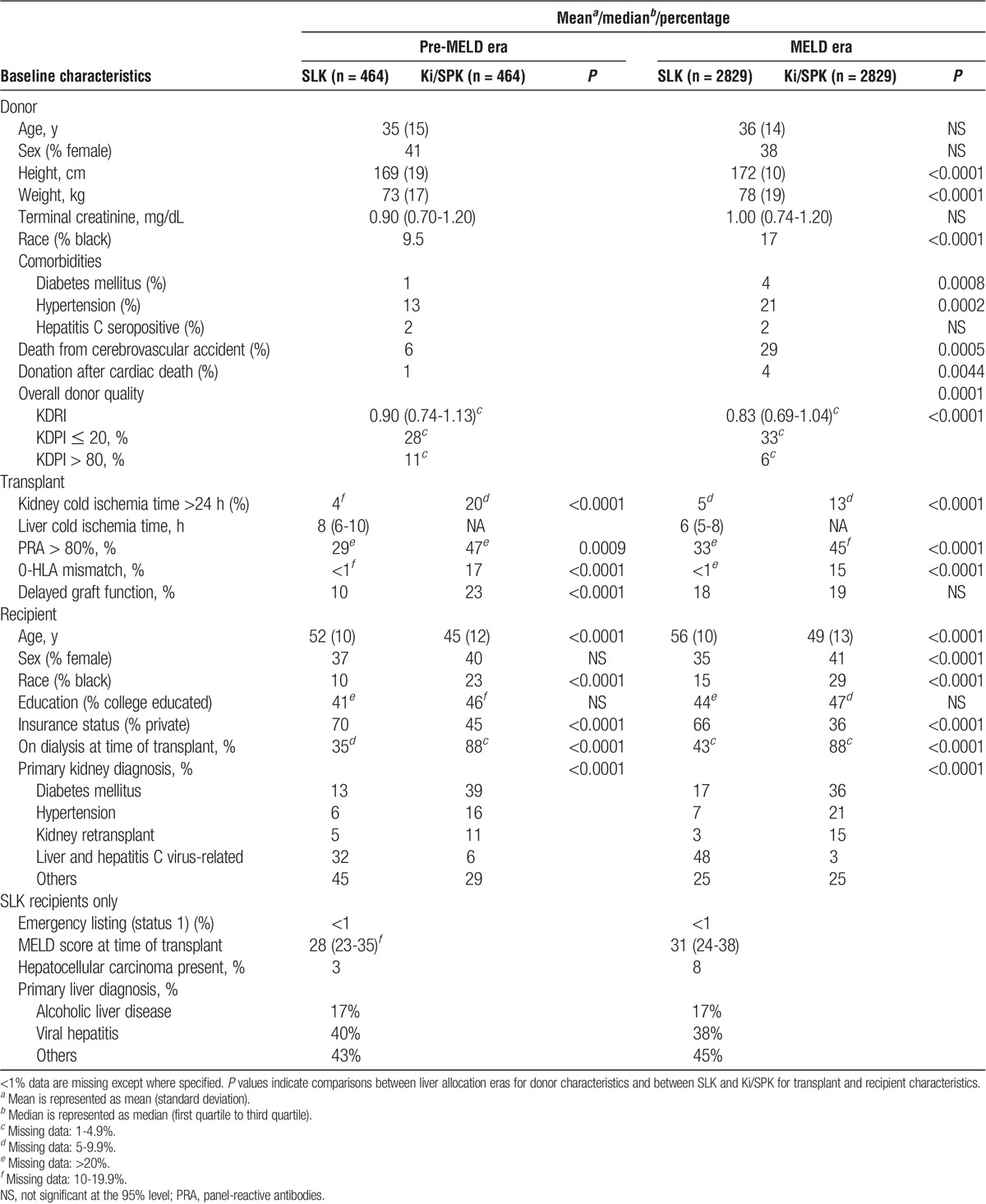
Our final study cohort consisted of 3293 SLK matched to 2614 Ki and 679 SPK transplant recipients (Figure 1). Baseline characteristics are summarized in Table 1. Kidney donor quality was generally excellent: in the MELD era, median KDRI was 0.83 and 33% of kidneys had KDPI ≤20. Kidneys allocated to SPK candidates and their SLK pairs were of higher quality than those allocated to Ki candidates and their SLK pairs (median KDRI: 0.75 vs 0.88; P < 0.001). Compared with their Ki/SPK counterparts, SLK recipients were older and less likely to be female, black, insured by public insurance, diabetic, or dialysis-dependent pretransplant.
FIGURE 1.
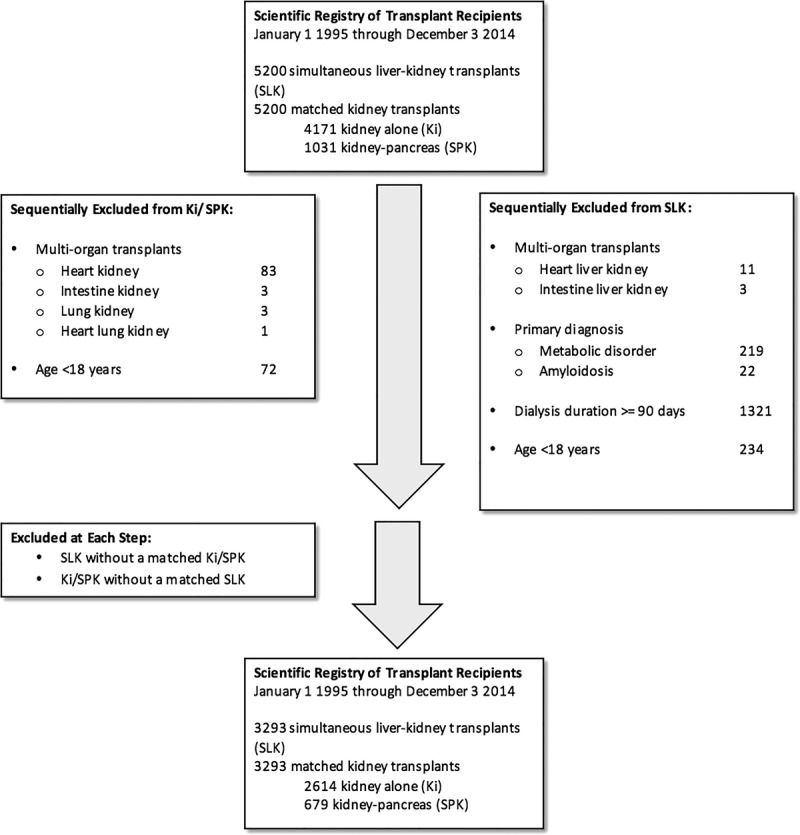
Flow diagram of cohort assembly.
TABLE 1.
Baseline donor, transplant, and recipient characteristics for SLK versus Ki and SPK transplants, stratified by liver allocation era
The bottom 2 panels of Figure 2 summarize the primary outcome for kidneys used in SLK versus Ki/SPK transplants, stratified by the liver allocation era. In the MELD era, the adjusted 10-year mean allograft lifespan, graphically presented by the area under the curve for each survival plot, was higher in Ki/SPK transplants (7.64 years) compared with SLK transplants (6.53 years) by approximately 1 year (0.99; 95% confidence interval, 0.71-1.28). In the pre-MELD era, this difference was almost 2 years (1.71; 95% confidence interval, 1.17-2.26). The top 2 panels of Figure 2 demonstrate the primary outcomes for MELD-era kidneys used in SLK versus Ki and SPK transplants separately. The differences between adjusted 10-year mean allograft lifespan for Ki versus SLK transplants was 1.25 (95% confident interval, 0.95-1.55) years and for SPK versus SLK transplants was 0.62 (95% confidence interval, 0.12-1.11) years.
FIGURE 2.
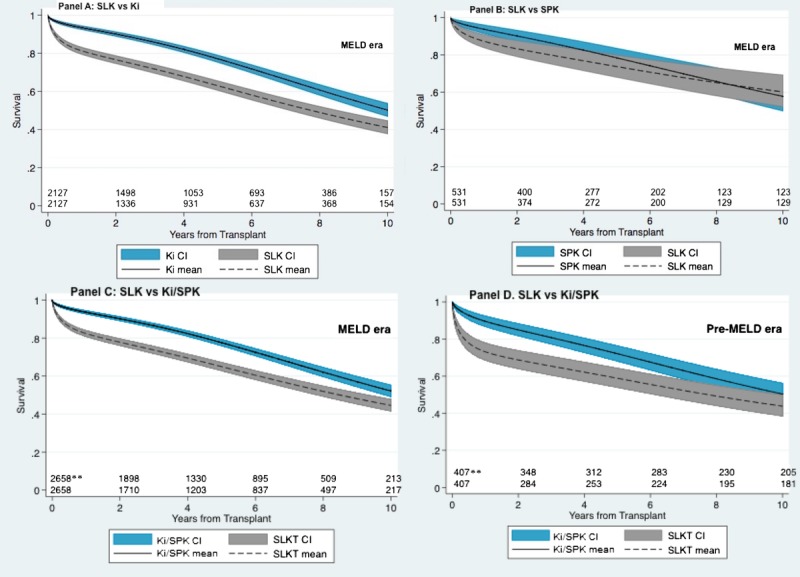
Time-to-all-cause kidney allograft loss by transplant type, wherein SLK transplants are compared to donor-paired Ki (panel A), pancreas (SPK, panel B), or Ki/SPK (panels C and D) transpalnts after multivariate adjustment*. Results for the SLK-Ki and SLK-SPK comparisons are for MELD era only, whereas results for SLK-Ki/SPK are stratified by liver allocation era. Numbers at risk are listed above the X-axis (top-row: reference group; bottom-row: SLK). * Multivariate adjustment was for kidney donor risk index, donor group (kidney versus kidney-pancreas), donor sex, kidney cold ischemia time, delayed graft function, and recipient age, sex, race, diabetes status, prior kidney transplant status, dialysis status, and insurance status. ** The initial number of pairs were 2658/407 rather than 2829/464 due to missing data.
Figure 3 is a closer examination of the cumulative incidence of posttransplant death and allograft failure in the MELD era, adjusted for age at transplantation. The 10-year cumulative incidence of death was significantly higher for SLK recipients than for Ki/SPK recipients, especially as age increased: 0.14 versus 0.07 (P = NS) for 20-year-old recipients, 0.29 versus 0.15 (P = 0.05) for 40-year-old recipients, and 0.51 versus 0.29 (P = 0.05) for 60-year-old recipients. Point estimates for the incidence of allograft failure was slightly lower in SLK compared to Ki/SPK recipients (0.48 vs 0.49, 0.33 vs 0.35 and 0.20 vs 0.23 for 20-, 40-, and 60-year-old recipients, respectively), but the 95% confidence intervals largely overlapped. Patterns for the pre-MELD era were similar.
FIGURE 3.
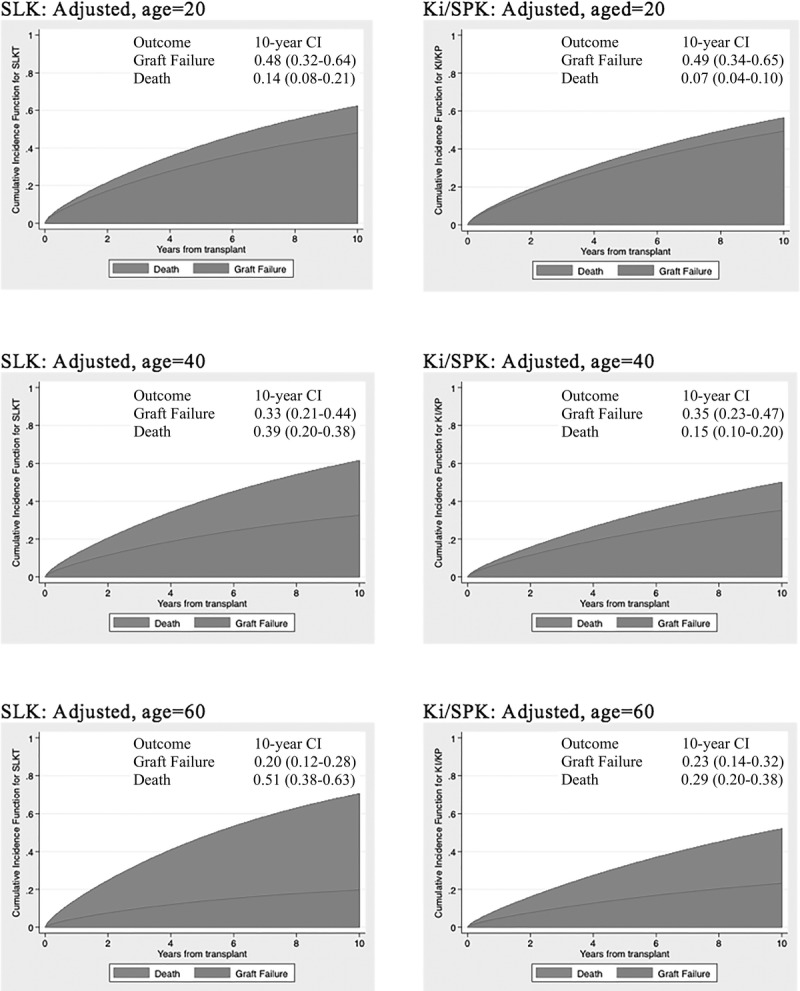
Predicted time-to-event cumulative incidence illustrating competing risk of kidney allograft failure and death in SLK transplant (left) and their donor-paired Ki/SPK (right) transplants, during the MELD era.
Figure 4 displays the population-averaged effect size of the difference in 10-year mean allograft lifespan between SLK and Ki/SPK recipients across different strata. SLK recipients experienced poorer kidney allograft survival regardless of liver allocation era, donor quality, donor sex, and whether the donor-matched kidney was used in Ki or SPK, with no significant effect modification (interaction P values all >0.05).
FIGURE 4.
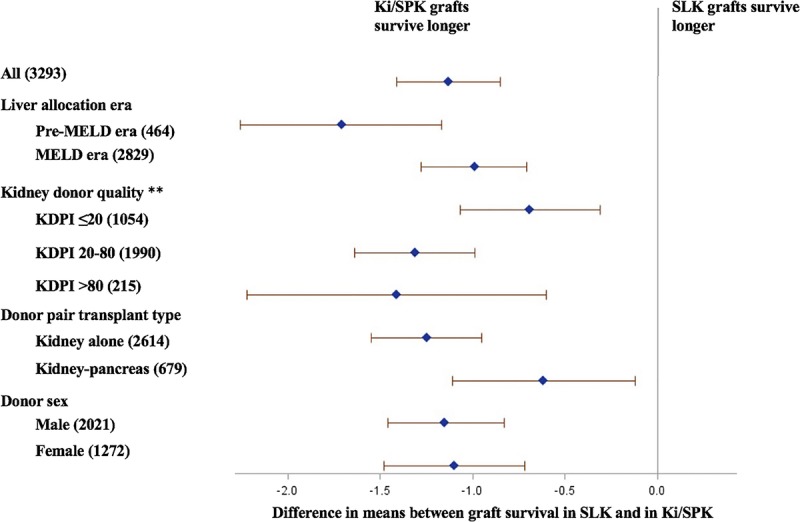
Difference in 10-year mean kidney allograft lifespan comparing SLK transplants to Ki/SPK transplants, by strata. Numbers in parenthesis represent sample size in each stratum. **The numbers in kidney donor quality strata do not add up to 3293 due to 1% missing data.
In the LYFT subset analysis, the median LYFT for an allograft used in SLK was 5.92 (25th to 75th percentile range, 5.50-6.39) years if it had been used in Ki and 9.28 (25th to 75th percentile range, 8.60-10.08) if it had been used in KP.
We performed the sensitivity analyses in 2 additional cohorts with different pretransplant dialysis vintage cutoffs (see Materials and Methods) and using multiple imputation for missing variables. The results for these cohorts were virtually identical to that of the primary analysis cohort (Appendix 2 and 3, SDC, http://links.lww.com/TP/B351). Multiple imputation for missing variables did not materially alter our results (Appendix 4, SDC, http://links.lww.com/TP/B351).
DISCUSSION
In this era of organ shortage, fair and equitable allocation of deceased donor organs is a key objective of the transplant community. We sought to address the SLK system from the utility perspective. We focused on the use of SLK transplantation to treat kidney failure of relatively short duration in ESLD, excluding patients with metabolic disorders and patients on dialysis for 90 days or more, among whom the benefit of SLK transplantation is virtually undisputed.
We have shown that, in the current liver allocation era (the MELD era), use of kidneys in SLK transplants for kidney failure of short duration currently results in an average loss of one year of function per allograft, compared to their alternative uses in Ki/SPK transplantation. This estimate, derived from data censored at 10 years posttransplant, is a conservative estimate of the net effect across the allograft's whole lifespan. A recent publication by Choudhury et al,22 also using a mate-kidney design, has similarly concluded that kidney allograft survival is inferior when kidneys are used in SLK rather than kidney-alone transplantation. Our studies differ most by the metric in which we assessed allograft survival. Rather than using 5-year kidney graft survival as a binary outcome, we chose to directly estimate the expected allograft lifespan over the first 10 years of transplantation, therefore arriving at a result that is more precisely quantitative and more intuitive to interpret.
We chose to focus on allograft survival, encompassing both patient death and allograft failure, as the primary outcome. Although this is the traditional metric of utility in kidney transplantation, there is an important caveat. Allograft failure is defined as retransplantation or dialysis reinitiation. Thus, transplant recipients with residual (native) kidney function will appear to have a lower allograft failure rates than transplant recipients without, even if their allograft function were identical. In limiting our SLK cohort to patients with pretransplant dialysis of short duration, we would expect a bias toward lower rates of allograft failure and allograft loss in the SLK group, again suggesting that our estimate on the loss of functioning allograft years associated with SLK transplantation is an underestimate of the true effect.
We found comparable allograft failure rates in SLK recipients and Ki/SPK recipients when death was accounted for in a competing risk framework. In contrast, Fong et al23 mate-kidney study, using data from the pre-MELD era, concluded a kidney allograft-enhancing effect conferred by the liver. An intriguing hypothesis is whether the liver, in acting as an antibody “sink,” can “protect” a kidney allograft immunologically in a highly sensitized patient with preformed donor-specific antibodies.24,25 We suspect that the difference in our finding stems from our use of a competing risk framework and adjustment for additional recipient covariates known to modify long-term kidney graft outcome, eg diabetes status, prior kidney transplantation, and insurance status, which were not balanced in the SLK and Ki/SPK groups in either Fong et al or our study. Furthermore, as discussed above, generally preserved residual kidney function in the SLK group will bias result toward an apparent improvement in kidney allograft survival, independent of any immunologic factors that might be in play. We therefore do not find evidence to support the contention that the liver exerts a clinically significant salutary effect on kidney allograft survival. Whether such an effect exists in a subset of highly sensitized patients remains an open question for future investigation.
We included SPK transplantation in the reference group alongside Ki transplantation, against which SLK transplantation was compared, despite the similarity between SPK and SLK in that both bypasses the KAS in determining kidney allocation. This was done for several reasons. The renal indication for SPK transplantation, advanced diabetic nephropathy, is biologically irreversible, whereas kidney failure in ESLD is often reversible, albeit difficult to predict. The concern over whether SLK transplantation may be causing organ wastage is therefore not relevant to SPK transplantation. SPK transplantation also lends itself to excellent long-term patient and allograft outcomes26,27; therefore, we believe that inclusion of both Ki and SPK transplantation in our reference group is justified. This comparison between SPK and SLK transplantation does highlight 2 key challenges that frame the controversy over SLK allocation: (1) the difficulty in determining the biological indication for kidney transplant, ie. whether the kidney failure is truly reversible and (2) the inferior posttransplant outcomes which we have highlighted. To address any residual concern regarding inclusion of SPK recipients in our referent group, we also conducted the analysis including only Ki recipients as reference (Figure 2). Results were qualitatively unchanged, and quantitatively very similar.
Brought into focus by our findings is the discrepancy between the current state of SLK transplantation and the KAS. The KAS upholds as 1 of its tenets “mak[ing] better use of available kidneys,”28 which entails some degree of matching between projected recipient survival and allograft survival. SLK transplantation contradicts this tenet in matching kidneys of excellent quality to recipients with a high posttransplant mortality. In our analysis, while kidney allograft survival is comparable between SLK and Ki/SPK recipients, the higher relative mortality in SLK recipients persists beyond the first year, suggesting a sustained negative effect of ESLD despite transplantation. The mortality difference is especially meaningful for older SLK recipients. Given that the median age for SLK recipients is 56, the implication is that much of the utility decrement we have observed is attributable to the allocation of kidneys to elder SLK recipients with relatively high posttransplant mortality.
These findings highlight the fundamental tension between utility-based allocation, aimed at maximizing posttransplant survival as in KAS, and urgency-based allocation, aimed at minimizing waitlist mortality as in liver allocation. When the urgency principle is applied to kidney allocation, as in the case of SLK, kidneys are preferentially allocated to patients with higher medical urgency with the resultant decrement in posttransplant kidney allograft survival. A natural compromise between the 2 competing principles is benefit-based allocation, in which access to organs is in part determined by the expected benefit.21,29 Benefit is the incremental reduction in mortality attributable to the kidney allograft. In the case of Ki and SPK, the best quantification of benefit is life-years after transplant, calculated in our cohort to be approximately 6 years for Ki and 9 years for SPK. As attractive as benefit-based allocation might be, accurately quantifying the net benefit from each organ transplant is challenging.29 A 2014 study of patients waitlisted for SLK but who received liver-only transplant reported 5-year survival of 73% versus 52% in favor of SLK transplantation.30 The survival curves showed a sharp drop in survival within 2 days of transplantation in the liver-only group, beyond which they were parallel. It is unclear whether there is an immediate benefit of SLK, or whether these findings simply reflect confounding by indication, that is, the tenuous condition of the recipient during or soon after the liver transplant operation precluded the kidney operation. A propensity score-matched analysis of Scientific Registry of Transplant Recipient data31 found a modest 3.7-month expected gain in survival time with SLK during the first 5 years posttransplant. The latter study is limited by residual confounding from unobserved confounders not adequately captured by SRTR. Our study was not designed to answer the question of the incremental benefit of the kidney to liver transplant recipients, but our findings support its central importance to the controversy of liver-kidney allocation as discussed above.
The SLK allocation system is expected to undergo major changes in upcoming years. The Organ Procurement and Transplant Network, the national organization responsible for establishing transplant criteria in the United States, has put forth a proposal for standardizing SLK listing criteria on a national level.32,33 It is unclear how this proposed policy change, if implemented, might affect the number of SLK transplants. Meanwhile, the number of deceased donor kidneys available for transplantation has remained flat in the past 5 years. It is thus likely that we will need to continue to balance between utility- and urgency-based allocation for the foreseeable future. We think a reasonable first step may be to explicitly introduce the measure of utility into the SLK allocation algorithm and reconsider SLK allocation in cases of a low-quality kidney with high KDPI score, where the expected utility decrement is high (~1.5 years in our analysis, Figure 4) and expected benefit is low.31,34 Ongoing efforts, including discovering better tools to predict kidney failure reversibility after liver transplantation and exploring alternative SLK allocation algorithms (such as the new Safety Net proposal), need to continue.
There are several important limitations to our analysis. As with most studies using registry data, we have limited granularity. For instance, we have data on the presence or absence of comorbid conditions, but not on severity or duration. We are unable to distinguish between CKD and AKI, and between functional impairment of kidney function, that is, hepatorenal syndrome, and intrinsic kidney disease. We addressed the issue of severity and duration of kidney disease by restricting our analysis to SLK recipients on dialysis for fewer than 90 days. The amount of missing data, especially in immunologic covariates including PRA and HLA mismatch (more likely to be missing in SLK than Ki/SPK transplants, as liver transplantation does not require reactive antibody screening and crossmatching), is another limitation, which we had sought to circumvent partly by our sensitivity analysis using multiple imputation.
In summary, we quantified the decrement in 10-year mean kidney allograft lifespan in SLK to be approximately 1 year compared with Ki/SPK transplantation. This decrement is driven by higher posttransplant mortality of SLK recipients across all age groups. Although this represents only the utility side of the equation, in consideration of the kidney allocation policy, results derived from this analysis of paired recipients provide the least biased estimates by which the benefits stemming from urgency-based allocation could be informed. The incremental benefit of SLK over liver transplant alone, or liver transplant followed by kidney transplant should be quantified to further inform SLK allocation.
Supplementary Material
ACKNOWLEDGMENTS
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR, the Department of Health and Human Services, the National Institutes of Health, or the United States Government.
Findings in this manuscript were reported in abstract form at the American Society of Transplantation Cutting Edge of Transplantation conference in February, 2016 and American Transplant Congress in June, 2016.
Footnotes
Research reported here was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K24DK092336 (W.R.K.) and K24 DK085446 (G.M.C.). Data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C.
The authors declare no conflicts of interest.
X.S.C. participated in research design, data acquisition, data analysis, results interpretation, and article writing. M.R.S. participated in data analysis, results interpretation, and article writing. G.M.C. participated in results interpretation and article writing. W.R.K. participated in results interpretation and article writing. J.C.T. participated in research design, data acquisition, results interpretation, and article writing.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 2.Reese PP, Veatch RM, Abt PL, et al. Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant. 2014;14:21–26. [DOI] [PubMed] [Google Scholar]
- 3.Courtney AE, Maxwell AP. The challenge of doing what is right in renal transplantation: balancing equity and utility. Nephron Clin Pract. 2009;111:c62–67; discussion c68. [DOI] [PubMed] [Google Scholar]
- 4.Gonwa TA, McBride MA, Anderson K, et al. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651–2659. [DOI] [PubMed] [Google Scholar]
- 5.Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901–2908. [DOI] [PubMed] [Google Scholar]
- 6.Iwatsuki S, Popovtzer MM, Corman JL, et al. Recovery from “hepatorenal syndrome” after orthotopic liver transplantation. N Engl J Med. 1973;289:1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koppel MH, Coburn JW, Mims MM, et al. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367–1371. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Goodrich NP, Zhang M, et al. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vagefi PA, Qian JJ, Carlson DM, et al. Native renal function after combined liver-kidney transplant for type 1 hepatorenal syndrome: initial report on the use of postoperative Technetium-99 m-mercaptoacetyltriglycine scans. Transpl Int. 2013;26:471–476. [DOI] [PubMed] [Google Scholar]
- 10.Levitsky J, Baker T, Ahya SN, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12:2949–2957. [DOI] [PubMed] [Google Scholar]
- 11.Francis JM, Palmer MR, Donohoe K, et al. Evaluation of native kidney recovery after simultaneous liver-kidney transplantation. Transplantation. 2012;93:530–535. [DOI] [PubMed] [Google Scholar]
- 12.Hanto DW. Reliability of voluntary and compulsory databases and registries in the United States. Transplantation. 2003;75:2162–2164. [DOI] [PubMed] [Google Scholar]
- 13.Leppke S, Leighton T, Zaun D, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando). 2013;27:50–56. [DOI] [PubMed] [Google Scholar]
- 14.Massie AB, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin EF, Huang J, Xiang Q, et al. Recipient survival and graft survival are not diminished by simultaneous liver-kidney transplantation: an analysis of the united network for organ sharing database. Liver Transpl. 2012;18:914–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong TL, Khemichian S, Shah T, et al. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation. 2012;94:411–416. [DOI] [PubMed] [Google Scholar]
- 17.United Network of Organ Sharing. A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI). 2014; https://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf. Accessed November 19, 2014.
- 18.Kamath PS, Kim WR, Group ALDS. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797–805. [DOI] [PubMed] [Google Scholar]
- 19.Lambert PC, Patrick R. Further development of flexible parametric models for survival analysis. The Stata Journal. 2009;9:265–290. [Google Scholar]
- 20.Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol. 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant. 2008;8(4 Pt 2):997–1011. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury RA, Reese PP, Goldberg DS, et al. A paired kidney analysis of multiorgan transplantation: implications for allograft survival. Transplantation. 2016. [DOI] [PubMed] [Google Scholar]
- 23.Fong TL, Bunnapradist S, Jordan SC, et al. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76:348–353. [DOI] [PubMed] [Google Scholar]
- 24.Olausson M, Mjörnstedt L, Nordén G, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7:130–136. [DOI] [PubMed] [Google Scholar]
- 25.Dar W, Agarwal A, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. [DOI] [PubMed] [Google Scholar]
- 26.Waki K, Terasaki PI. Kidney graft and patient survival with and without a simultaneous pancreas utilizing contralateral kidneys from the same donor. Diabetes Care. 2006;29:1670–1672. [DOI] [PubMed] [Google Scholar]
- 27.Morath C, Zeier M, Döhler B, et al. Metabolic control improves long-term renal allograft and patient survival in type 1 diabetes. J Am Soc Nephrol. 2008;19:1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UNOS/OPTN Kidney Transplantation Committee. The New Kidney Allocation System (KAS): The First Year. 2016; https://www.transplantpro.org/wp-content/uploads/sites/3/KAS_12month_analysis.pdf. Accessed July 5, 2016.
- 29.Kim WR, Kremers WK. Benefits of “the benefit model” in liver transplantation. Hepatology. 2008;48:697–698. [DOI] [PubMed] [Google Scholar]
- 30.Hmoud B, Kuo YF, Wiesner RH, et al. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation. 2015;99:823–828. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Shu X, Schaubel DE, et al. Propensity Score-Based Survival Benefit of Simultaneous Liver-Kidney Transplant over Liver Transplant Alone for Recipients with Pre-Transplant Renal Dysfunction. Liver Transpl. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UNOS/OPTN Kidney Transplantation Committee. Simultaneous liver kidney (SLK) allocation policy. 2015; http://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf. Accessed October 20, 2015.
- 33.Formica RN, Aeder M, Boyle G, et al. Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of scarce resources. Am J Transplant. 2015. [DOI] [PubMed] [Google Scholar]
- 34.Wadei HM, Bulatao IG, Gonwa TA, et al. Inferior long-term outcomes of liver-kidney transplantation using donation after cardiac death donors: single-center and organ procurement and transplantation network analyses. Liver Transpl. 2014;20:728–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


