Abstract
Objectives
We undertook a challenge to determine if one or more height-weight formula(e) can be clinically used as a surrogate for direct CT-based imaging assessment of body composition before and after radiotherapy for head and neck cancer (HNC) patients, who are at risk for cancer- and therapy-associated cachexia/sarcopenia.
Materials and Methods
This retrospective single-institution study included 215 HNC patients, treated with curative radiotherapy between 2003 and 2013. Height/weight measures were tabulated. Skeletal muscle mass was contoured on pre- and post-treatment CT at the L3 vertebral level. Three common lean body mass (LBM) formulae (Hume, Boer, and James) were calculated, and compared to CT assessment at each time point.
Results
156 patients (73%) had tumors arising in the oropharynx and 130 (61%) received concurrent chemotherapy. Mean pretreatment body mass index (BMI) was 28.5 ± 4.9 kg/m2 in men and 27.8 ± 8 kg/m2 in women. Mean post-treatment BMI were 26.2 ± 4.4 kg/m2 in men, 26 ± 7.5 kg/m2 in women. Mean CT-derived LBM decreased from 55.2±11.8 kg pre-therapy to 49.27±9.84 kg post-radiation. Methods comparison revealed 95% limit of agreement of ±12.5–13.2 kg between CT and height-weight formulae. Post-treatment LBM with the three formulae was significantly different from CT (p<0.0001). In all instances, no height-weight formula was practically equivalent to CT within ±5 kg.
Conclusion
Formulae cannot accurately substitute for direct quantitative imaging LBM measurements. We therefore recommend CT-based LBM assessment as a routine practice of head and neck cancer patient body composition.
Keywords: body composition, lean body mass, computed tomography, height- and weight-based mathematical formulas, head and neck cancer, radiotherapy
Introduction
Patients with head and neck cancer (HNC) commonly experience major weight loss, both prior to and during therapy, which is associated with poor functional and survival outcomes. Weight loss in cancer patients result from both cancer-associated metabolic states, as well as cancer treatment toxicity sequelae. For HNC, the latter are especially pronounced, and include mucositis, xerostomia, dysphagia and nausea/vomiting. These symptoms can lead to poor oral intake and weight loss, with a resultant change in body composition. The connection between weight loss and outcome largely reflects a loss in lean body mass (LBM), indicative of pathological metabolic states, such as cachexia or severe malnutrition. Recent evidence demonstrates a clear dissociation between total body weight loss and LBM loss, reflecting the increased prevalence of obesity in the population. Indeed, multiple reports show that a) the prognostic value of weight loss depends upon the patient’s body mass index (BMI)[1]; and b) elevated BMI may be associated with low LBM, and thus, decreased survival [1, 2]. Coherently, weight loss itself poorly predicts outcome in HNC patients when compared with depleted LBM [3]. Furthermore, radioactive 18Fluorodeoxyglucose uptake, the measure of metabolic activity assessed in PET scans, are traditionally normalized to patient’s body weight. However, since adipose tissue contributes very little to FDG uptake, the SUV for obese patients often underrepresents true tracer signal, and can be better estimated using LBM. [4] Together, these studies demonstrate clear value of measuring body composition, rather than just height and weight, in an HNC population.
Evaluating body composition traditionally relies upon imaging modalities, such as dual-energy x-ray absorptiometry (DEXA) abdominal CT or PET/CT scans, or whole-body MRI, which are not routinely performed in HNC patients. For those HNC patients with abdominal imaging, derivation of LBM requires the implementation of non-standard image processing and analysis into physician workflow. Methods for estimating body composition using routinely collected clinical data are then preferred. Over the past 50 years, three mathematical formulae were developed to estimate LBM using body weight (kg) and height (cm), which are routinely recorded in the clinical setting.[5–7] This method is simple, non-invasive, inexpensive, and does not involve additional radiation exposure. Therefore, formula-based LBM assessment pre- and post-therapy is a potentially useful metric for an important correlate of clinically meaningful outcomes. However, as all three methods predate CT-based lean mass measures, validation of these formulae are required prior to clinical implementation.
Consequently, in order to determine the utility of height and weight-based LBM estimation, we have sought to perform a method comparison study of these three body composition formulae, using CT body composition analysis as a gold standard reference. Our goal is to determine whether formula-based body composition assessment is sufficient as standard practice in the initial work-up and post-therapy surveillance of HNC patients, using retrospective assessment of imaging and clinical parameters from previously treated patients.
Materials and Methods
Population Cohort and Data Acquisition
This retrospective, single center study included 215 adult patients with pathologic proven diagnosis of HNC, referred to the MD Anderson Cancer Center radiation oncology clinics for radiation treatment between October 2003 and August 2013. The Institutional Review Board at MD Anderson Cancer Center approved this study. Patients were selected for analysis if they had non-contrast CT imaging of the lumbar vertebrae, including the CT-component of whole body PET-CT scans, and/or abdominal CT scans. Age, anatomic subsite of primary disease, sex, body weight (before and after radiotherapy), height, and clinical staging were obtained. Body weight was assessed at the same day of CT acquisition for all included patients. Cancer stage was based on the American Joint Committee on Cancer (7th Edition) stage groupings I, II, III, and IV. LBM and FM were assessed quantitatively using cross-sectional imaging on CT in PET/CT or CT whole abdomen before and after radiation treatment using the method proposed by Martin et al. [8] Skeletal muscle and adipose tissue were contoured by a single attending radiation oncologist [SC] with five years of clinical experience and reviewed by an attending radiation oncologist [CDF] with seven years of experience, using a commercial image processing/treatment planning platform (Pinnacle 9.6, Phillips Medical Systems, Andover, MA).
CT Image Analysis
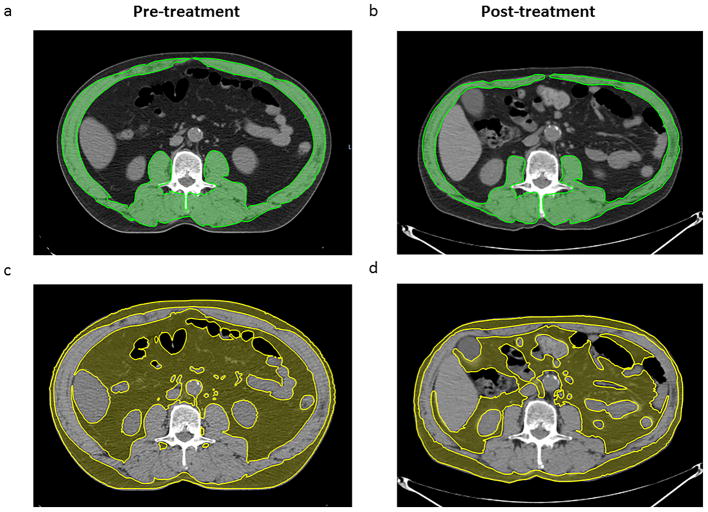
For most patients, 120 kVp, 290 mA axial CT images were obtained and reconstructed at 3.25-mm slice thickness without image enhancement. For each scan, three adjacent axial image slices were selected for each patient at the third lumbar vertebral level.[2, 8–10] Skeletal muscle and adipose tissue cross sectional area were initially auto-segmented using the following Hounsfield unit (HU) parameters: for skeletal muscle segmentation, a range of −29 to 150 HU[10] was used, while for fat tissue an HU range of −190 to −30[11, 12] was quantified. The contours were corrected manually, when appropriate, after auto-segmentation. For skeletal muscle, intra-abdominal muscles segmented included the rectus abdominis, lateral and oblique abdominal, iliopsoas, and paraspinal (quadratus lumborum, erector spinae) muscle groups. For adipose tissue, we contoured intra-abdominal and subcutaneous adipose tissue depots, again using auto-segmentation with manual correction (Figure 1). Cross-sectional area (cm2) of muscle and fat tissue were normalized for height in meters squared (m2), and reported as skeletal muscle (SMI) [2, 8, 9] and adipose tissue indices (ADI),[13] respectively.
Figure 1.
Pre- and post-treatment axial Computed Tomography (CT) image at the level of the third lumbar vertebra. The upper panel (a,b) shows the skeletal muscle mass highlighted in green (−29 to 150 Hounsfield units) while the lower panel (c,d) shows the adipose tissue mass highlighted in yellow (−190 to −30 Hounsfield units)
Body Composition Methodology
CT calculations
Gold standard reference values for pre- and post-treatment LBM and fat mass (FM) were estimated from skeletal muscle and adipose cross-sectional area (cm2) at the third lumbar vertebra using the formulae described by Mourtzakis et al. [9, 14]. This LBM calculation closely approximates total body fat-free mass (FFM), including bone, with a standard error of < 1.2 [9].
Height- and weight-based LBM/FM calculations
Pre-treatment and post-treatment LBM were calculated using the body weight (kg) and height (cm) of each patient, measured at the time of imaging. The calculations for each formula, by sex, are as follows:
The Hume Formula: [5]
Boer Formula: [6]
James Formula [7]
Statistical and analysis
Data were tabulated and summarized by sex using standard measures of central tendency (mean and SD). Method comparison/calibration was illustrated using the Krouwer[15] variant of Bland-Altman plot[16] comparing LBM as calculated by each of the listed height-weight formulae with LBMCT, for pre- and post- radiotherapy time points. The Krouwer-variant Bland-Altman analysis illustrates measurement method agreement in the cases when an established ‘gold-standard’ has been identified (in this case, we used LBMCT as a reference standard). It consists of a graphic plot of the difference between paired measures on the ordinate axis, plotted against the reference method on the abscissa. The technique calculates a limit of agreement (LOA), (i.e. ±1.96 times the standard deviation of the differences), which when plotted relative to the mean, graphically describes the range within which 95% of 1all differences between measurement methods may be expected to fall. Simultaneously, measurement error bias components were illustrated with systematic bias estimate (SBE) (i.e. mean difference of each paired measure) and random bias estimation (RBE) (i.e. standard deviation of the difference between paired measurements).
After confirmation of distributional normality using the Shapiro-Wilks test[17], comparison of each formula data to CT data was performed using Dunnett’s test[18], in order to account for multiple comparisons of paired t-tests between each height-weight formula against a reference CT gold-standard. We performed post-hoc assessment of clinical equivalence using a two one-sided test (TOST) procedure[19, 20], whereby we compared each formula to CT-based LBM assessment to determine whether differences in LBM could be reasonably expected to fall within an arbitrarily physician-defined “clinically meaningful lean body mass measurement error difference” of ± 5 kg.
Correlation between pre- and post-treatment body weight, LBM, and BMI was assessed with linear regression. All statistical analyses were performed with JMP Pro11 software (SAS Institute, Cary, NC, USA). A non-Bonferroni corrected p-value of 0.05 was deemed statistically significant in this exploratory dataset.
Results
Patient baseline and treatment related characteristics
Data was extracted from a total of 215 HNC patients in this study. Patient and tumor characteristics are shown in Table 1. Most of the patients were men (85.5%). One hundred and fifty-six of 215 patients (73%) had tumors arising in the oropharynx, with the most common sites being the base of the tongue (51%) and tonsil (43%). One hundred and thirty patients (61%) received concurrent chemotherapy; 98% of these patients had platinum-based chemotherapy. The remaining 85 patients (39%) received radiation therapy alone. Twenty-eight patients (13%) had postoperative radiation therapy. Mean radiation dose was 68.66 Gy (range of 56–72 Gy) in 28–40 fractions.
Table 1.
Patient and Tumor Characteristics.
| Characteristics of pts. | Datum |
|---|---|
| Total no. of pts. (pts.) | 215 |
| Male | 184 (85.6%) |
| Female | 31 (14.4%) |
| Age (years) | 57.21 ± 9.79 (range 27–91) |
| Height (cm.) | 173.55 ± 8.96 (range 146–205.7) |
| Body weight (kg) | 85.7 ± 18.1 |
| Body mass index (kg/m2) | 28.41 ± 5.46 |
| Site of malignancy | |
| Oral cavity | 8 (3.7%) |
| Oropharynx | 156 (72.6%) |
| Hyopopharynx | 12 (5.6%) |
| Larynx | 24 (11.1%) |
| Nasopharynx | 6 (2.8%) |
| CUPa | 6 (2.8%) |
| Sinus | 3 (1.4%) |
| T category | |
| T0 | 6 (2.8%) |
| T1 | 38 (17.7%) |
| T2 | 65 (30.2%) |
| T3 | 61 (28.3%) |
| T4 | 45 (20.9%) |
| N category | |
| N0 | 25 (11.6%) |
| N1 | 23 (10.1%) |
| N2 | 150 (70.4%) |
| N3 | 17 (7.9%) |
| Stage | |
| I | 4 (1.9%) |
| II | 5 (2.3%) |
| III | 31 (14.4%) |
| IVA | 156 (72.6%) |
| IVB | 19 (8.8%) |
| Histology | |
| Squamous cell | 215 (100%) |
| Feeding Tube | |
| Yes | 118 (54.88%) |
| No | 95 (44.19%) |
| NA | 2 (0.93%) |
| Treatment | Datum |
| Chemotherapy | |
| Yes | 130 (60.5%) |
| No | 85 (39.5%) |
| Radiation Therapy | |
| Mean Radiation dose (Gy) | 68.66 |
| No. of fraction (F) | 33.35 |
CUP = cancer of unknown primary
Changes in body composition following radiation therapy
Mean post-treatment BW was reduced by 11% in men and 10% in women. Mean pretreatment BMI was 28.5 ± 4.9 kg/m2 in men and 27.8 ± 8 kg/m2 in women. Mean post-treatment BMI were 26.2 ± 4.4 kg/m2 in men, 26 ± 7.5 kg/m2 in women. All of the mean BMI were classified as overweight according to the International Classification of BMI from WHO[21] criteria of overweight for the United States [22]. Both of the guidelines demonstrate the principal cut-off BMI for obesity is ≥ 30 kg/m2. Seventy-four patients (34%) in the pre-treatment population had obesity while only 44 patients (20%) remained obese post-treatment. Quantitative body composition measurements of pre- and post-radiation therapy from CT image analysis are shown in Table 2 and from the height-/weight-based mathematical formulae are shown in Table 3.
Table 2.
Quantitative body composition measurements by CT using of study patients. SKI = skeletal muscle cross sectional area (cm2)/(height (m))2. ADI = adipose tissue cross sectional area (cm2)/(height (m))2. Estimated skeletal muscle mass (kg) = 0.3 x [skeletal muscle at L3 (cm2)] + 6.06. Estimated fat mass (kg) = 0.042 x [total adipose tissue at L3 (cm2)] + 11.2.
| Data | Pretreatment | Post-treatment | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Men (184 pts.) | Women (31 pts.) | Total (215 pts.) | p value | Men (184 pts.) | Women (31 pts.) | Total (215 pts.) | p value | |
|
|
||||||||
| Lumbar Total muscle cross- sectional area (cm2) | 173.43 ± 32.32 | 105.02 ± 23.25 | 163.84 ± 39.48 | < 0.0001 | 151.54 ± 27.70 | 100.47 ± 23.77 | 144.02 ± 32.81 | < 0.0001 |
|
| ||||||||
| Lumbar Total fat cross- sectional area (cm2) | 393.57 ± 174.60 | 371.27 ± 234.44 | 390.81 ± 182.63 | 0.8819 | 278.76 ± 142.19 | 295.97 ± 219.65 | 280.90 ± 154.67 | 0.6771 |
|
| ||||||||
| Estimated skeletal muscle mass (kg) | 58.09 ± 9.69 | 37.56 ± 7.00 | 55.21 ± 11.84 | < 0.0001 | 51.52 ± 8.31 | 36.20 ± 7.13 | 49.27 ± 9.84 | < 0.0001 |
|
| ||||||||
| Estimated fat mass (kg) | 18.48 ± 1.36 | 15.61 ± 0.98 | 27.61 ± 7.67 | 0.8819 | 17.56 ± 1.16 | 15.42 ± 1.00 | 23.00 ± 6.50 | 0.6771 |
|
| ||||||||
| Skeletal muscle Index (SMI) (cm2/m2) | 56.19 ± 9.61 | 40.29 ± 8.64 | 53.99 ± 10.95 | < 0.0001 | 49.08 ± 7.98 | 28.59 ± 8.89 | 47.51 ± 8.95 | < 0.0001 |
|
| ||||||||
| Adipose tissue index (ADI) (cm2/m2) | 128.66 ± 58.25 | 143.62 ± 89.73 | 130.82 ± 63.71 | 0.3745 | 91.06 ± 46.99 | 111.50 ± 81.59 | 94.01 ± 53.60 | 0.2082 |
Table 3.
Estimated Lean Body Mass (eLBM) by Composition Methodology.
| Data | Pretreatment (kg) | Post-treatment (kg) | ||
|---|---|---|---|---|
|
|
||||
| Mean | Range | Mean | Range | |
|
|
||||
| LBMCT | 55.21 ± 11.84 | 26.70–84.07 | 49.27 ± 9.84 | 25.87–77.38 |
|
| ||||
| eLBMHume | 57.00 ± 8.33 | 32.42–80.81 | 54.76 ± 7.72 | 31.77–70.17 |
|
| ||||
| eLBMBoer | 61.02 ± 9.69 | 33.25–89.21 | 58.32 ± 8.79 | 32.70–76.98 |
|
| ||||
| eLBMJames | 61.10 ± 9.65 | 35.99–80.86 | 58.65 ± 9.29 | 34.40–76.47 |
SMI post-treatment was reduced 12.65% and 4.22% in men and women, respectively (p<.0001 for men and p=0.3 for women). Post-treatment mean ADI was reduced by 29.24% in men and by 20.64% in women (p<.0001 for men and p=0.1 for women). Mean LBM dropped significantly post-treatment compared to pre-treatment for men (58.1 ± 9.8 to 51.5 ± 8.4; p<0.0001) but didn’t reach statistical significance in women (37.8 ± 6.35 to 36.0 ± 6.9; p=0.3). Additionally, mean FM dropped in both men and women after treatment (27.7 ± 7.3 to 22.9 ± 5.9; p<0.0001 for men) and (26.8±9.7 to 23.4±9.1; p=0.1 for women).
Comparison of body composition estimates
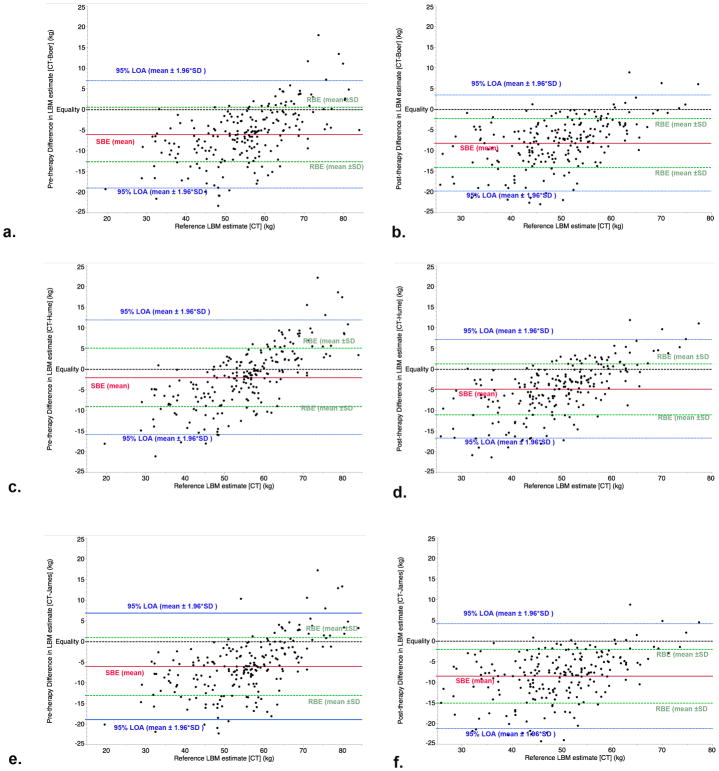
Bland-Altman assessment is shown graphically in Figure 2, for both pre- and post-therapy LBM. Cumulative measurement error, described via calculated 95% LOA method. The overall 95% LOA for the Boer, Hume, and James was ±12.5, ±13.2, and ±13.0, respectively. The 95% LOA for pre-therapy assessment was fairly consistent at ±13.0, ±13.9, and ±12.9 kg, while the post-therapy assessment was ±11.7, ±12.0, and ±12.7 kg for the Boer, Hume, and James formulae, respectively. Over the course of therapy, systematic measurement error increased, as measured by systematic bias estimate; details for all calculated Bland-Altman analyses are included in Table 4.
Figure 2.
Method calibration plots of pre- and post-therapy Boer (a,b), Hume (c,d), and James (e,f) plots of lean body mass (LBM), in kg. Shown are systematic bias estimate (SBE, solid red line), random bias estimate (RBE, dashed green line), 95% limits of agreement (LOA, dashed blue line) and identity line (dashed black line).
Table 4.
Bland-Altman calculated systematic (SBE), random (RBE), and cumulative error estimators (95% LOA), for each height-weight formula, compared to CT, by time cohort.
| Measurement method | Cohort | SBE (Mean of difference vs. CT) |
RBE (±SD of difference vs. CT) |
95% LOA (±1.96xSD of difference vs. CT) |
|---|---|---|---|---|
| Boer | All | −7.1 | ±6.4 | ±12.5 |
| Pre-therapy | −6.0 | ±6.6 | ±13.0 | |
| Post-therapy | −8.2 | ±5.9 | ±11.7 | |
| Hume | All | −3.4 | ±6.7 | ±13.2 |
| Pre-therapy | −2.0 | ±7.1 | ±13.9 | |
| Post-therapy | −4.8 | ±6.1 | ±12.0 | |
| James | All | −7.2 | ±6.6 | ±13.0 |
| Pre-therapy | −6.0 | ±6.6 | ±12.9 | |
| Post-therapy | −8.5 | ±6.5 | ±12.7 |
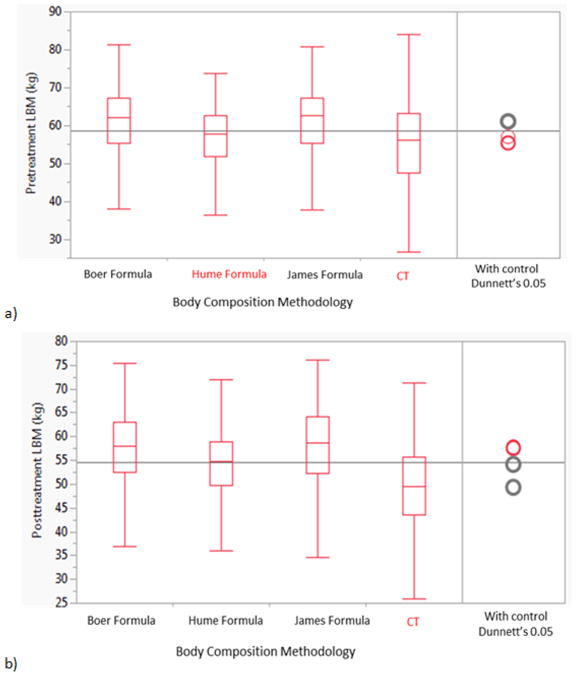
Table 3 shows pre- and post-treatment LBM formula estimates compared to CT. All pre- and post-treatment LBM calculated using the three different tested formulae were significantly higher than calculated with CT (p<0.0001) as shown in Figure 3; except for the pre-treatment eLBMHume that was not statistically different LBMCT (p<0.15).
Figure 3.
Comparison box plots between the three mathematical formula and CT analysis using Dunnett’s test for controls with CT as a gold-standard reference a) Pretreatment LBM b) Post-treatment LBM.
In order to ensure that the difference between height-weight formulae was clinically meaningful, (in addition to statistically meaningful), we confirmed our findings using post hoc practical equivalence testing. We determined that a difference of ±5 kg from the gold standard might be considered clinically acceptable for use of a height-weight formula. As with our initial assessment using Dunnett’s test, only the Hume formula pre-therapy was found to be functionally equivalent, with an observed mean difference of 1.8 kg, compared to 5.9 kg for both the James and Boer formulae; no formula could be considered practically equivalent within a clinically usable range post-therapy.
Discussion
While evaluation using DEXA scan or CT-based methods remains the reference objective standard for body composition assessment, several widely used population-based estimators (e.g. the Boer, James, and Hume formulae) present attractive and simple alternatives when access or costs prohibit direct imaging assessment. We sought to determine whether any of the widely used population-based body composition estimators could approximate CT-based “gold standard” estimates of LBM. [8, 23] Although many patients have staging whole body PET-CT, routine abdominal imaging is not performed as a part of NCCN Guidelines [24], and is not part of clinical practice at our facility. Furthermore, post-therapy, imaging is only performed as clinically indicated. However, cachexia assessment and clinical nutrition management could benefit greatly from routine body composition monitoring, and it would be especially convenient if this could be done using a simple formula. Unfortunately, our study demonstrates that the tested estimates of LBM using BW and height formulae fail to adequately approximate objective CT-based assessment in our head and neck population. Consequently, while these formulae may perform accurately in other populations, for head and neck cancer patients direct body composition measurement of LBM should be performed.
That height- and weight-based estimators cannot substitute for imaging assessment of body composition is not surprising. The prevalence of obesity has been steadily rising since the 1960s, when the first of the three evaluated formulae was developed. [25, 26] In the context of changing population demographics and anthropometrics, the relationship between height, weight, muscle mass and adiposity will not remain constant. Several recent studies have shown that BMI, an aggregate measure of height and weight, is a poor predictor of underlying muscle mass, particularly in obese populations. [8] Indeed, these studies also warn against using high BMI as a reassuring factor regarding nutritional status, as patients with obesity and low muscle mass (sarcopenic obesity) exhibit worsened survival compared to non-obese or non-sarcopenic cohorts. [2] In this study 34% of the patients met criteria for obesity at the time of pre-treatment imaging, whereas only 20% met criteria after treatment. This drop in obesity rate under the conditions of curative-intent oncologic treatment clearly does not indicate improved metabolic health, again illustrating the inadequacy of BMI to adequately reflect nutritional status. Although each of these studies uses SMI (normalized cross-sectional area) to identify patients with low skeletal muscle, LBM estimation is proportional to SMI and provides additional information that can be used for both medication dosing and PET interpretation.
In head and neck oncology, body weight, weight loss, and body composition have a unique role. This is because the local toxicities of treatment and tumor can dramatically impact nutritional status in the pre-treatment and therapeutic contexts, causing flux in what is often a stable anthropometric variable. Weight is often followed throughout therapy, as a proxy of nutritional status. However, the prognostic value of weight loss during treatment is equivocal, at best, in light of contrasting reports. [3, 27, 28] Although these differences may reflect the populations studied, there remains no clear association between weight loss and survival. The pharmacodynamics, and thus, toxicities, of systemic agents used in HNC is generally based on weight, or, more commonly, surface area. Although each may change significantly during treatment, FFM may vary considerably less, leading to changes in effective drug distribution. Furthermore, recent data from our group found that patients with low SMI on presentation who required a feeding tube during radiation treatment were at greater risk of death than patients with normal muscle mass, identifying a uniquely high risk population. [3] Therefore, to reveal significant malnutrition, appropriately dose chemotherapy, and identify high risk patients, a feasible and reliable LBM assessment method for rapid clinical implementation is of significant interest.
A recent study reported that bioelectrical impedance analysis (BIA) can be used to assess body composition in head and neck cancer patients. This method, which is widely-used, non-invasive, inexpensive, and feasible, is based on impedance of a low-voltage current passing through the body which can then be used to calculate an estimate of total body water (TBW). TBW can then be used to estimate FFM, by comparison with BW and body fat. Thus BIA may provide an acceptable alternative tool for assessment of FFM in clinical practice, but must first be validated longitudinally and within dehydrated populations.[29] Our group is currently prospectively evaluating this approach.
Despite these findings, our study presents several limitations. Due to its retrospective nature and single institution design, the standard caveats apply. This study applied to images acquired between 2003 and 2013, so there is variability in quality of the imaging, which may affect skeletal muscle mass contouring and adipose tissue mass segmentation. Moreover, the pre- and post-treatment cross-sectional imaging at the same level (mid- L3 vertebral) showed differing locations of the intra-abdominal organs (e.g. kidney, bowel) on different scans based on patient position. Thus, skeletal muscle and adipose tissue were also contoured at the L4-L5 vertebral level for an internal quality control comparison, with approximately equivalent results. Also, the formulae used in this study to estimate LBM and FM are not the only ones that have been generated in the literature. For example, Shen et al. describe equations based on a larger, albeit non-oncologic, study cohort. [30]
Our study, however, is the first, to our knowledge, to rigorously evaluate the utility of height- and weight-based formulae for assessment of LBM against a quantitative imaging gold-standard using CT data. Additionally, our data represents the single largest quantitative imaging evaluation of pre-and post-radiotherapy LBM alteration in head and neck cancer patients. Consequently, this study offers important inferences. Namely, LBM estimation for head and neck cancer patients, especially post-therapy, should be performed using image-based assessment, otherwise measurement error of >10 kg should be presupposed (Figure 2; Table 3). Our data also show substantial differentials in body composition pre- and post-therapy (Table 2), and serve as a benchmark for future efforts to assess and address nutritional and disease-associated processes contributing to these alterations.
In conclusion, in this study we demonstrated that HNC patients lose a significant amount of LBM and adipose tissue mass while undergoing radiation therapy. Furthermore, clinical height- and weight-based formulae are not sufficient for body mass quantification and should not substitute CT-based assessment in head and neck cancer patients undergoing radiotherapy. Therefore, we recommend routine use of quantitative imaging (e.g. CT body composition or DEXA analysis) in head and neck cancer patients, especially in those prone to changes in nutritional status, as opposed to general population-based height-weight formulae.
Highlights.
Head and neck cancer (HNC) patients lose a significant amount of lean body mass and adipose tissue mass while undergoing radiation therapy (P<0.0001 for both).
All pre- and post-treatment lean body mass calculated using the three different tested formulae were significantly higher than calculated with CT (p<0.0001); except for the pre-treatment Hume formula was not statistically different than CT (p<0.15).
Clinical height- and weight-based formulae are not sufficient for the evaluation of body composition and should not substitute CT-based assessment in HNC patients undergoing radiotherapy.
Acknowledgments
Funding sources: Dr. Fuller received/receives grant support from the National Institutes of Health/National Cancer Institute Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG Hope Foundation Dr. Charles A. Coltman, Jr. Fellowship in Clinical Trials; Elekta AB/MD Anderson Consortium Seed Grant; GE Medical Systems/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; the MD Anderson Center for Radiation Oncology Research Seed Grant; and the MD Anderson Institutional Research Grant Program Award. Dr. Mohamed received support via the Union for International Cancer Control American Cancer Society International Fellowships for Beginning Investigators. Dr. Heukelom received support from the Koningin Wilhemina Fonds/René Vogels Foundation grant. These listed funders/supporters played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33:90–9. doi: 10.1200/JCO.2014.56.1894. [DOI] [PubMed] [Google Scholar]
- 2.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 3.Grossberg AJ, Chamchod S, Fuller CD, Mohamed AS, Heukelom J, Eichelberger H, et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahari AK, Chien D, Azadi JR, Wahl RL. Optimum lean body formulation for correction of standardized uptake value in PET imaging. J Nucl Med. 2014;55:1481–4. doi: 10.2967/jnumed.113.136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19:389–91. doi: 10.1136/jcp.19.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:F632–6. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 7.James WPT, Waterlow JC. DHSS/MRC Group on Obesity Research. Research on obesity : a report of the DHSS/MRC group. London: H.M.S.O; 1976. [Google Scholar]
- 8.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 9.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 10.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10:53–67. [PubMed] [Google Scholar]
- 12.Heymsfield SB, McManus CB. Tissue components of weight loss in cancer patients. A new method of study and preliminary observations. Cancer. 1985;55:238–49. doi: 10.1002/1097-0142(19850101)55:1+<238::aid-cncr2820551306>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–9. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 14.Holt DQ, Strauss BJ, Lau KK, Moore GT. Body composition analysis using abdominal scans from routine clinical care in patients with Crohn's Disease. Scand J Gastroenterol. 2016;51:842–7. doi: 10.3109/00365521.2016.1161069. [DOI] [PubMed] [Google Scholar]
- 15.Krouwer JS. Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Statistics in medicine. 2008;27:778–80. doi: 10.1002/sim.3086. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 17.Shapiro SS, Wilk MB. An Analysis of Variance Test for Normality (Complete Samples) Biometrika. 1965;52:591-&. [Google Scholar]
- 18.Dunnett CW. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. Journal of the American Statistical Association. 1955;50:1096–121. [Google Scholar]
- 19.Walker E, Nowacki AS. Understanding Equivalence and Noninferiority Testing. Journal of General Internal Medicine. 2011;26:192–6. doi: 10.1007/s11606-010-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. Journal of pharmacokinetics and biopharmaceutics. 1987;15:657–80. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 21.Sumi M, Nakamura T. Head and neck tumours: combined MRI assessment based on IVIM and TIC analyses for the differentiation of tumors of different histological types. Eur Radiol. 2014;24:223–31. doi: 10.1007/s00330-013-3002-z. [DOI] [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72:1074–81. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 23.Vickie Baracos P. Measurement of LBM using CT scan.pdf [Google Scholar]
- 24.Veiga C, McClelland J, Moinuddin S, Lourenco A, Ricketts K, Annkah J, et al. Toward adaptive radiotherapy for head and neck patients: Feasibility study on using CT-to-CBCT deformable registration for "dose of the day" calculations. Med Phys. 2014;41:031703. doi: 10.1118/1.4864240. [DOI] [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD. Statistics NCfH, editor. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007–2008. Hyattsville, MD: NCHS Health E-Stat; 2010. [Google Scholar]
- 27.Ghadjar P, Hayoz S, Zimmermann F, Bodis S, Kaul D, Badakhshi H, et al. Impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer: secondary results of a randomized phase III trial (SAKK 10/94) Radiat Oncol. 2015;10:21. doi: 10.1186/s13014-014-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langius JA, Bakker S, Rietveld DH, Kruizenga HM, Langendijk JA, Weijs PJ, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013;109:1093–9. doi: 10.1038/bjc.2013.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jager-Wittenaar H, Dijkstra PU, Earthman CP, Krijnen WP, Langendijk JA, van der Laan BF, et al. Validity of bioelectrical impedance analysis to assess fat-free mass in patients with head and neck cancer: an exploratory study. Head Neck. 2014;36:585–91. doi: 10.1002/hed.23336. [DOI] [PubMed] [Google Scholar]
- 30.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]