Abstract
OBJECTIVE
To evaluate the environmental and ecological factors associated with Leishmania transmission and vector abundance in Chaparral, Tolima-Colombia.
METHODS
First, we compared the ecological characteristics, abundance of phlebotomies and potential reservoir hosts in the peridomestic environment (100 m radius) of randomly selected houses, between two townships with high and low cutaneous leishmaniasis incidence. Second, we examined peridomestic correlates of phlebotomine abundance in all 43 houses in the higher risk township.
RESULTS
The high transmission township had higher coverage of forest (23% vs. 8.4%) and shade coffee (30.7% vs. 11%), and less coffee monoculture (16.8% vs. 26.2%) and pasture (6.3% vs. 12.3%), compared to the low transmission township. Lutzomyia were more abundant in the high transmission township 2.5 vs. 0.2/trap/night. Lutzomyia longiflocosa was the most common species in both townships: 1021/1450 (70%) and 39/80 (49%). Numbers of potential wild mammal reservoirs were small, although four species were found to be infected with Leishmania (Viannia) spp. In the high transmission township, the overall peridomiciliary capture rate of L. longiflocosa was 1.5/trap/night, and the abundance was higher in houses located nearer to forest (ρ = −0.30, P = 0.05).
CONCLUSION
The findings are consistent with a domestic transmission cycle with the phlebotomies dependent on dense vegetation near the house.
Keywords: leishmaniasis, Lutzomyia, ecology, risk factors, reservoirs, Colombia
Introduction
The ecology of American cutaneous leishmaniasis (CL) transmission is changing as a result of expanding rural communities and the adaptation of some vector species of the subfamily Phlebotominae to disturbed ecosystems and to additional blood meal sources such as man and domestic animals. Moreover, Leishmania parasites can adapt to novel mammalian hosts (Shaw 1997; Rotureau 2006) and novel phlebotomine vectors (Bañuls et al. 2007; Rodríguez-Barraquer et al. 2008; Martínez et al. 2010; Ferro et al. 2011). These factors, together with high rates of human movement, notably in Colombia because of political instability, are expanding the distribution of different Leishmania species (Dujardin et al. 1996; Davies et al. 2000; Miranda 2007; Ferro et al. 2011). These factors have led to a significant increase in CL cases in Colombia over the last decade. While in the 1990s, an average of 6500 annual cases of CL were recorded, this rose to ~11 000 in the past decade, with 2005 and 2006 reporting the highest incidence at around 20 000 cases (Zambrano 2007, 2009). There were outbreaks in places with no previous reports, as in Chaparral (Tolima), where the largest epidemic of CL in Colombia was reported.
The municipality of Chaparral had a peak incidence of 6202 per 100 000 inhabitants in 2004 (unpublished data, Hospital San Juan Bautiste of Chaparral). A series of studies of domestic transmission of CL in Chaparral showed that the main parasite isolated from patients was Leishmania (Viannia) guyanensis (95% of 56 isolates), a species from the Amazon region not previously reported in the Andean region of Colombia (Young et al. 1987; Saravia et al. 1998, 2002; Rodríguez-Barraquer et al. 2008; Ferro et al. 2011). Transmission was confined to altitudes of 1000–2000 m, but townships (veredas) in this range showed a wide range in the prevalence of leishmaniasis cases, from 1% to 95%. Spatial analysis of variables such as land use, elevation and climatic factors showed that the incidence of CL in the townships of Chaparral was related to mean temperature (peaking at 20.6 °C), the presence of forest and low human population densities (Valderrama-Ardila et al. 2010). In turn, entomological studies implicated L. longiflocosa of the townsendi series as the main vector, based on its abundance and prevalence of infection with Leishmania parasites of the Viannia subgenus. This vector demonstrated anthropophilic and endophagic behaviour and bit overnight (Ferro et al. 2011). However, risk factors for human infection remain unknown.
The aim of the current study was (i) to compare environmental factors, abundance of phlebotomies and potential wild mammal reservoirs in two similar townships with contrasting CL incidences during the outbreak, and (ii) then to examine environmental risk factors associated with L. longiflocosa abundance in and around houses in the higher transmission township.
Methods
The study was conducted in the municipality of Chaparral, Tolima Department in the inter-Andean valley of the Magdalena River between December 2006 and September 2008. It was carried out in two townships with differing disease prevalence to seek factors associated with this difference in transmission risk. The townships were Agua Bonita, which showed a high period prevalence of CL cases reported during the outbreak (74% of 172 inhabitants), and Irco dos Aguas with a low prevalence (1.3% of 354 inhabitants) [Sistema de identificacion de potencialesbeneficiarios de programassociales, unpublished data (SISBEN)]. These previous disease prevalence data were collected by active case search during the epidemic period.
The study consisted of two phases. First, a ‘general study’ analysed potential risk variables associated with Leishmania transmission in the peridomestic environment (100 m radius around the house). Randomly selected houses were evaluated in the two townships, Agua Bonita (AB) (n = 12 of 43) and Ircodos Aguas (IdA) (n = 10 of 78). This sampling of houses was stratified by altitudinal range: 1000–1300 m (AB, n = 1; IdA, n = 3); 1300–1600 m (AB, n = 7; IdA, n = 6); and above 1600 m (AB, n = 4; IdA, n = 1). Phlebotomies and wild mammals were trapped and demographical and environmental (habitat) data were collected.
Second, a ‘focal study’ aimed to better characterise risk variables associated with L. longiflocosa abundance in the domestic environment. This study extended to all houses in Agua Bonita. Houses in Irco dos Aguas were not included in the focal study.
Data collection
A demographical questionnaire was administered to an adult present in each selected house, giving information on the number of residents, how many had active and inactive lesions, domestic animals, insecticide use and house construction.
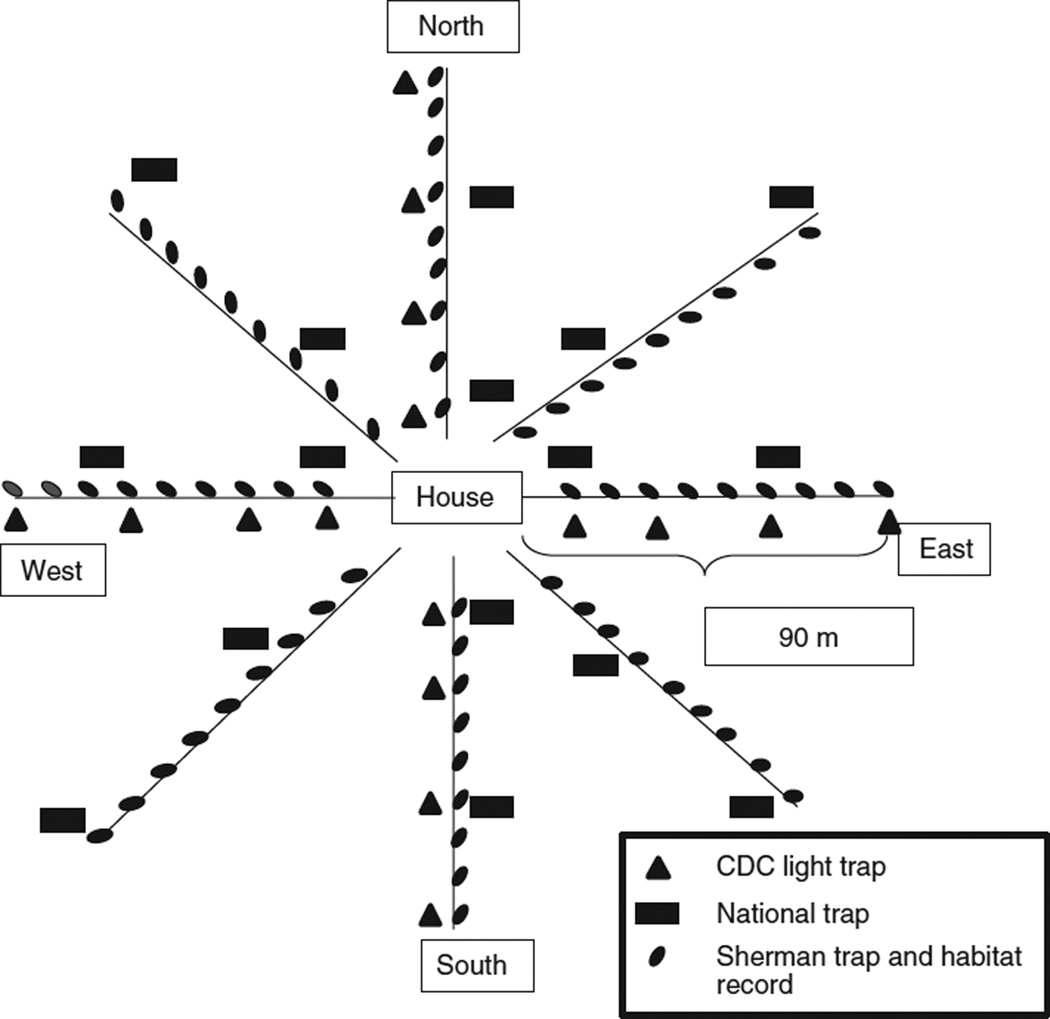
To identify the composition and abundance of phlebotomies in and around the house, extensive trapping was carried out in the randomly selected houses from each township from 2006 to 2008 during the periods of low precipitation (January–February and June–August). Phlebotomies were collected by setting up 17 CDC light traps (John W. Hock Co., model 1012 and 1212) around each of the selected houses. Sixteen traps were placed at sites located at 10, 30, 60 and 90 m from the core household in four transects of 100 m that were oriented to the cardinal points. The additional trap was set up in indoors, in an inhabited bedroom (Figure 1). Five CDC traps had incandescent light (located indoors and 10 m from the house) and 12 had UV light (located >10 m from the house). The traps were operated from 18:00 to 6:00 h for three consecutive nights. Phlebotomies were retrieved in the early morning, sorted from other insects and preserved in 70% ethanol for subsequent species identification and detection of Leishmania DNA by PCR. The identification of species of Lutzomyia was characterised by external morphological features as described previously (Ferro et al. 2011).
Figure 1.
Sampling scheme around each house where the phlebotomine and wild animals traps were set in the general study. Habitat records, in both studies, were collected every 10 m where the Sherman traps were set.
Wild mammals were trapped only for the general study simultaneously with the entomologic study. Characterisation of the peridomestic wild fauna was accomplished by live trapping over four consecutive nights. Two trap models were used: a National-type wire mesh trap (14 × 15 × 25 cm) for middle sized mammals and the Sherman trap (9 × 8 × 23 cm) (Forestry Suppliers Inc.) for small mammals. For the animal capture, with the help of a compass, 8 transects of 100 m were traced following a trap-web pattern, at 45° intervals around the house (Jones et al. 1996). Along each transect, nine Sherman traps were set at 10 m intervals and two National traps at alternate distances: either 10 and 50 m, or 30 and 90 m (Figure 1). The Sherman traps were baited with a mixture of corn, oats, sardine and vanilla extract, and the National trap with cut ripe plantain. Animals were identified to species level, sexed, weighed and classified as juvenile or adult according to the external genitalia (Adler et al. 1997). For the detection of Leishmania infection, a 3 mm2 skin sample from the animal’s ear was preserved in 70% alcohol (Travi et al. 2001). Additional liver, spleen or mandibular lymph node samples were collected from some mammals (n = 13) that were sacrificed for identification. Tissues were processed by PCR as described below. This study was performed in accordance with national guidelines and international standards for humane care and use of laboratory animals, and approved by the CIDEIM institutional review committee for research in animals of the US Department of Health and Human Services. This study also had an environmental permit from the environmental agency, CORTOLIMA (Corporación Autonoma Regional del Tolima).
Using the same transects designed for wild mammal trapping, the habitat around each house was point characterised at 10-m intervals along eight transects centred on the house, in both studies. Habitats were classified according to the following categories: hen house, pigsty, horse or cow stable, forest, shaded coffee plantation, unshaded coffee plantation (monoculture), cultivation (short annual crops), shrubs, pasture and other (typically burned or bare areas). Around each house, the percentages of points in each habitat category were used to characterise the habitat.
Detection of Leishmania infections in phlebotomies and wild mammals
Phlebotomies and wild mammals were tested for Leishmania infection by PCR detection of the mini-circle kDNA of Leishmania (Viannia) species (Vergel et al. 2005; Figueroa et al. 2009). Detection of Leishmania in specimens of the most abundant species, L. longiflocosa, has been described previously (Ferro et al. 2011). Female phlebotomies were grouped in vials (no more than 10/group). The separation into pools took into account township, house and trap location (indoors, 10, and >10 m). Wild mammal tissues were processed for Leishmania infection individually. The tissues were first treated with proteinase K and then DNA extraction was carried out using the same methodology for phlebotomies. The DNA samples were amplified without dilution and with a 1:10 dilution.
DNA samples from phlebotomine and wild mammal tissues were amplified with the primers LV (5′-ATT TTT GAA CGG GGT TTC TG-3′) and B1 (5′-GGG GTT GGT GTA ATA TAG TGG-3′), which specifically amplify 700- bp mini-circle kDNA of Le. (V.) species as described (Vergel et al. 2005; Figueroa et al. 2009). PCR products were analysed by electrophoresis on 1.2% agarose gels and to improve the sensitivity, a chemiluminescent Southern blot was performed, as described by Ferro et al. (2011). Two positive controls were used to confirm the natural infection. The first was DNA extracted from a mixture of 10 female L. longiflocosa infected by xenodiagnosis from a hamster infected with Le.(V.) guyanensis isolated from a patient from Chaparral. The second was DNA extracted from 1 × 106 promastigotes of Le.(V.) panamensis (HOM/ PA/71/LS94). Additionally, two negative controls were used: DNA of 20 F1 L. longiflocosa females from an uninfected colony and PCR control without DNA.
Focal study
In the focal study, the demographical information and habitat characterisation was carried out as in the general study. No mammals were captured in this study. Phlebotomies were collected using one CDC incandescent light trap indoors and two traps placed at 10 m from the house (near to vegetation) for two consecutive nights. Trapping was performed during 2 weeks of August (2008), a period with lower rainfall corresponding to peak phlebotomies presence (Ferro et al. 2011). Traps were set at six houses simultaneously for two nights, until all houses in the township had been sampled. Phlebotomine identification was as described earlier for the general study.
Data analysis
For the general study, the numbers of captured phlebotomies, expressed as rate ratios, were compared between townships using negative binomial regression with a random effect to take account of clustering within houses. For the focal study, non-parametric methods were used because one of the 43 houses yielded more than half of the total peridomestic L. longiflocosa (132/258), and the negative binomial methods were not thought to be sufficiently robust. This observation’s standardised DFbeta influence measure for the mean equalled 0.45, greater than the 0.30 (=2/√43) threshold for ‘large influence’ (Acock 2008). Instead, house-level associations were expressed in terms of Spearman rank correlation coefficients, for continuous explanatory variables, or bootstrapped rate ratios, for categorical ones. Prevalence of infection in the phlebotomies pools was calculated assuming that at least one insect from each positive pool was infected (Katholi et al. 1995).
Results
General study
Twenty-two randomly selected houses were evaluated: 12 in Agua Bonita (high incidence) and 10 in Irco dos Aguas (low incidence). A general description of the human ecosystem in the peridomestic environment in both townships is in Table 1. There were similarities in population density per house, number of domestic animals and altitudinal distribution, although the contrasting outbreak history is reflected in the much higher prevalence of inactive lesions in Agua Bonita (44% as opposed to 0% in Irco dos Aguas, Table 1). There were clear differences between townships in terms of land use: Agua Bonita had higher coverage of forest (23% vs. 8.4%), shade coffee (30.7% vs. 11%), less coffee monoculture (16.8% vs. 26.2%) and pasture (6.3% vs. 12.3%).
Table 1.
Characteristics of the human ecosystem in the peridomestic environment from randomly selected houses in Agua Bonita and Irco dos Aguas
| Variable | Agua Bonita | Irco Dos Aguas |
|---|---|---|
| Number of houses | 12 | 10 |
| Number of people | 65 | 48 |
| Number of people per house (mean, SD) |
5.3 (2.3) | 4.8 (2.53) |
| Proportion of people with* | ||
| Active lesions | 0% (0/64) | 0 |
| Inactive lesions | 44% (28/64) | 0 |
| Altitude | ||
| Median (range) | 1552 (1290–1854) | 1427 (1068–1745) |
| Number of domestic animals per house (mean, SD) | ||
| Dog | 1.2 (1.53) | 1.5 (1.18) |
| Equine | 0.8 (1.03) | 1.0 (0.82) |
| Chicken | 0.7 (1.15) | 0.5 (0.71) |
| Rodent | 0.7 (1.15) | 0 |
| Cow | 0.08 (0.29) | 0.4 (0.51) |
| Cat | 0.08 (0.29) | 0 |
| Pig | 0 | 0.1 (0.32) |
| Insecticide use by house: n (%) | ||
| Yes | 3 (25) | 2 (20) |
| No | 9 (75) | 8 (80) |
| House construction: n (%) | ||
| Wattle & daub (bareque) |
4 (33.3) | 1 (10.0) |
| Wood | 2 (16.7) | 2 (20.0) |
| Brick | 6 (50.0) | 7 (70.0) |
| Habitat coverage percentages within 100 m of houses: mean (SD) | ||
| Forest | 23% (31.5) | 8.4% (12.6) |
| Coffee plantation with shade trees |
30.7% (26.7) | 11% (14.6) |
| Coffee plantation (monoculture) |
16.8% (21) | 26.2% (24.3) |
| Annual crops | 5.1% (6.3) | 3.7% (4.8) |
| Shrubs | 16.4% (14.1) | 15.9% (7.8) |
| Pasture | 6.3% (7.8) | 12.6% (14.6) |
| Others | 1.7% (4.8) | 23.8% (25.8) |
SD, standard deviation.
One missing data.
Phlebotomies were much more abundant in Agua Bonita than Irco dos Aguas: 2.5 vs. 0.2/trap/night (Table 2). L. longiflocosa was the most common species in both townships: 1.8/trap/night (70% of all Lutzomyia) in Agua Bonita and 0.08/trap/night (49%) in Irco dos Aguas. Other species caught at a rate of more than 0.05/trap/night in one or both townships were L. trinidadensis, L. columbiana and L. (Helcocyrtomyia) (Table 2). Infection prevalence in pools of L. longiflocosa from Agua Bonita was the highest indoors (prevalence = 5%; n = 233; 95% CI = 2–8%) followed by 10 m (prevalence = 4%; n = 197; 95% CI = 2–19%) and >10 m from the house (prevalence = 3%; n = 156; 95% CI = 0.7–7%).
Table 2.
Phlebotomines composition and capture rates observed in the peridomestic environment from randomly selected houses in Agua Bonita and Irco dos Aguas
| Agua Bonita (12 houses, 574 trap-nights) |
Irco Dos Aguas (10 houses, 467 trap-nights) |
|||
|---|---|---|---|---|
| Species | n | L/t/n | n | L/t/n |
| L. longiflocosa | 1021 | 1.8 | 39 | 0.08 |
| L. trinidadensis | 237 | 0.4 | 1 | 0.002 |
| L. columbiana | 54 | 0.1 | 0 | 0 |
| L. (Helcocyrtomyia) spp. | 104 | 0.2 | 34 | 0.07 |
| L. nuneztovari | 6 | 0.01 | 0 | 0 |
| L. carpentieri | 2 | 0.003 | 3 | 0.006 |
| L. shannoni | 1 | 0.002 | 3 | 0.006 |
| Lutzomyia sp. | 25 | 0.04 | 0 | 0 |
| Total | 1450 | 2.5 | 80 | 0.20 |
L/t/n, Lutzomyia caught per trap per night.
The L. longiflocosa trapping success was evaluated in terms of environmental variables (Table 3). Female flies were caught more frequently than males in both townships. In Agua Bonita, traps located in forest and coffee monoculture were the most productive (2.2 and 1.6/trap/night, respectively). In Irco dos Aguas, no L. longiflocosa were caught in forest, although few traps were located there (n = 7). In Agua Bonita, the capture success was higher inside the houses (8.8/trap/night) than other zones (<2.7/trap/night). In Agua Bonita, L. longiflocosa captures were higher at altitudes higher than 1300 m. The small numbers of L. longiflocosa caught in Irco dos Aguas limits this analysis.
Table 3.
Lutzomyia longiflocosa capture success in the peridomestic environment of the randomly collected houses in Agua Bonita and Irco dos Aguas
| Variable | Agua Bonita n/trap-nights (L/t/n) |
Rate ratio (95% CI, P) | Ircos Dos Aguas n/trap-nights (L/t/n) |
|---|---|---|---|
| Total captures of L. longiflocosa | 1021/574 (1.8) | 39/467 (0.08) | |
| Males | 272/574 (0.5) | 1.16 (0.33–4.07, 0.814) | 7/467 (0.01) |
| Females | 749/574 (1.3) | 1.72 (0.75–3.96, 0.200) | 32/467 (0.07) |
| Land use | |||
| Forest | 232/106 (2.2) | – | 0/7 (0) |
| Coffee with shade trees | 36/104 (0.3) | – | 0/7 (0) |
| Coffee monoculture | 260/161 (1.6) | 3.56 (1.31–9.65, 0.013) | 13/132 (0.1) |
| Cultivation | 63/21 (3) | 2.00 (0.14–29.1, 0.611 | 1/9 (0.11) |
| Shrub | 142/115 (1.2) | 2.70 (0.60–12.2, 0.196) | 7/95 (0.07) |
| Pasture (potrero) | 4/24 (0.16) | 7.15 (0.38–134.2, 0.188) | 3/99 (0.03) |
| Others | 1/11 (0.09) | 0.77 (0.10–6.01, 0.803) | 13/90 (0.14) |
| Zone | |||
| Indoors | 283/32 (8.8) | 3.86 (0.71–21.0, 0.118) | 2/28 (0.07) |
| Outdoors | |||
| Within 10 m | 350/131 (2.7) | 2.44 (0.82–7.25, 0.109) | 16/109 (0.1) |
| ≥10 m | 388/411 (0.9) | 2.97 (1.30–6.79, 0.010) | 21/330 (0.06) |
| Attitude (n houses in Agua Bonita: Irco dos Aguas): | |||
| 1000–1300 (1:3) | 5/48 (0.1) | 1.67 (0.49–5.70, 0.410) | 9/139 (0.06) |
| 1301–1600 (7:6) | 418/333 (1.25) | 1.06 (0.42–2.68, 0.897) | 27/279 (0.9) |
| 1601–1854 (4:1) | 598/193 (3.1) | 2.69 (0.24–30.2, 0.424) | 3/49 (0.1) |
L/t/n, Lutzomyia/trap/night.
Thirty individual wild mammals of eight species were trapped around the houses, with similar numbers in both townships (Table 4). Infection with Le.(V.) spp. was observed in four wild mammals from Agua Bonita and one from Irco dos Aguas (Table 4). No Leishmania parasites were detected in the ear tissues analysed. Le.(V.) guyanensis was detected in one sample of liver from Sigmodomhispidus, which was identified to the species level through sequencing a7SLRNA fragment (data not shown) (Ferro et al. 2011).
Table 4.
General Study. Wild mammal composition and capture rates observed in the peridomestic environment from the randomly collected houses in Agua Bonita (n = 8) and Irco dos Aguas (n = 6). (Agua Bonita: National = 128 trap-nights; Sherman = 576 and Irco dos Aguas: National = 96; Sherman = 432). The number of infected mammals with Leishmania (Viannia) detected by PCR-Southern blot are indicated
| Agua Bonita (eight houses) |
Irco Dos Aguas (six houses) |
|||
|---|---|---|---|---|
| Species | n (m/t/n) | Infected | n (m/t/n) | Infected |
| Marsupialia: Didelphidae | ||||
| Didelphis marsupialis | 3 (0.02) | 1 | 8 (0.08) | |
| Marmosops impavidus | 1 (0.002) | 0 | ||
| Micoureus cf. demererae | 1 (0.002) | 0 | ||
| Rodentia: Heteromyidae | ||||
| Heteromys anomalus | 5 (0.009) | 1 (0.002) | ||
| Rodentia: Muridae | ||||
| Oecomys trinitatus | 1 (0.002) | 1 | 0 | |
| Melanomys caliginosus | 2 (0.003) | 1 (0.002) | ||
| Zygodontomys brunneus | 1 (0.002) | 1 | 2 (0.005) | 1 |
| Sigmodon hispidus | 1 (0.002) | 1 | 3 (0.007) | |
| Total | 15 | 4 (27%) | 15 | 1 (6.7%) |
m/t/n, mammal per trap per night.
Focal study
The results of the first (general) study strongly suggested much higher transmission in Agua Bonita than in Irco dos Aguas. To better characterise environmental variables associated with L. longiflocosa in the domestic environment, a second study was extended to all the houses in Agua Bonita (n = 43). In the focal study, L. longiflocosa was again the dominant species (Table S1). Similar trapping success of L. longiflocosa was observed indoors and in the peridomiciliary area (1.4/trap/night for both). Aggregating both locations, L. longiflocosa was observed in or around 48.8% of 43 houses sampled. As in the previous study, the other phlebotomine species were captured in low numbers (Table S1).
Lutzomyia longiflocosa was more abundant in the peridomiciliary area of houses located nearer to forest (≤30 m) (Spearman correlation coefficient ρ = −0.30, P = 0.05) and intradomiciliary captures were higher in house closer to coffee (ρ = −0.30, P = 0.05, Table 5). Similar tendencies were seen for intradomiciliary catches in relation to forest, and for both types of catches in relation to coffee cultivation near the house. Additional information on the characteristics of the houses, and the trap densities, is shown in the online-only Table S2.
Table 5.
Lutzomyia longiflocosa capture success in the peridomiciliary and intradomiciliary environments of all 43 Agua Bonita houses
| Variable | Numbers of houses |
Peri-domiciliary catches (two traps per house) n caught/ trap-nights (rate) |
Rate ratio |
Spearman correlation coefficient (P value) |
Intra-domiciliary catches (one trap per house) n caught/ trap-nights (rate) |
Rate ratio |
Spearman correlation coefficient (P value) |
|---|---|---|---|---|---|---|---|
| Total | 43 | 258/172 (1.50) | 125/86 (1.45) | ||||
| Altitude | |||||||
| 1000–1300 | 3 | 0/12 (0) | 0 | 0.22 (0.16) | 0/6 (0) | 0 | 0.15 (0.33) |
| 1301–1600 | 20 | 37/80 (0.46) | 1 | 64/40 (1.6) | 1 | ||
| ≥1601 | 20 | 221/80 (2.8) | 6.0 | 61/40 (1.5) | 1.0 | ||
| Forest: coverage | |||||||
| ≤10% | 22 | 51/88 (0.58) | 1 | 0.18 (0.26) | 15/44 (0.34) | 1 | 0.17 (0.29) |
| 10.01–20% | 10 | 62/40 (1.55) | 2.7 | 14/20 (0.70) | 2.1 | ||
| >20% | 11 | 145/44 (3.30) | 5.7 | 96/22 (4.36) | 12.8 | ||
| Forest: nearest distance from house | |||||||
| ≤30 m | 15 | 200/60 (3.33) | 1 | −0.30 (0.05) | 113/30 (3.8) | 1 | −0.22 (0.15) |
| 30.1–50 m | 13 | 32/52 (0.62) | 0.18 | 2/26 (0.1) | 0.02 | ||
| >50 m | 15 | 26/60 (0.43) | 0.13 | 10/30 (0.1) | 0.09 | ||
| Coffee: coverage | |||||||
| ≤20% | 13 | 15/52 (0.29) | 1 | 0.09 (0.59) | 4/26 (0.15) | 1 | 0.19 (0.22) |
| >20% | 30 | 243/120 (2.0) | 7.0 | 121/160 (2.0) | 13.1 | ||
| Coffee: nearest distance from house | |||||||
| ≤10 m | 30 | 245/120 (2.0) | 1 | −0.25 (0.10) | 119/60 (2.0) | 1 | −0.30 (0.05) |
| >10 m | 13 | 13/52 (0.25) | 0.12 | 6/26 (0.23) | 0.12 | ||
Discussion
These findings are further evidence for domestic transmission of Le.(V.) parasites by L. longiflocosa. This was the main phlebotomine species collected and was infected with Le.(V.) parasites in Agua Bonita. These results are in accordance with previous data from our group (Ferro et al. 2011), indicating that this species was the principal vector in the outbreak and that the parasite still persists in the area. L. longiflocosa was more abundant indoors than outdoors in Agua Bonita: such differences were not observed in Irco dos Aguas, although phlebotomine here were rare. These data, as well as the high level of human blood-feeding (Ferro et al. 2011), indicate that L. longiflocosa is endophagic. However, although commonly entering houses to feed, the vector appears to be dependent on forest habitats, and not to have adapted to peridomestic ones. This study demonstrates a significant negative correlation between L. longiflocosa abundance inside and around the house and distance of the house from dense vegetation, suggesting that such habitats are important for daytime resting and/or breeding sites. This study demonstrates that altitude (>1300 m) and land use (forest coverage) are risk factors for the abundance of this species at the local scale and agrees with our previous study at landscape scale (Valderrama-Ardila et al. 2010). The results from the present study endorse the use of geographical variables such as altitude and land use as proxy measures of vector abundance, to identify areas at high risk of Leishmania transmission. Additionally, the method used in this study – radial transects around each house – proved to be easy and fast for measuring land use in the peridomestic area, and hence identifying variables of ecological and behavioural importance to the vector. Our sampling occurred after the period of peak incidence (December 2006–September 2008 compared to 2003– 2006), but examination of remote-sensed images from 1989, 2002 and 2007 revealed no major land use changes over this period (Valderrama-Ardila et al. 2010).
Our results are in agreement with other studies of leishmaniasis outbreaks in the upper Magdalena River valley where L. longiflocosa is the most abundant species (Pardo et al. 1999; Cardenas et al. 1999, 2005; Pardo et al. 2006). All the outbreaks have occurred in areas of coffee cultivation (900–2000 m) and, as in Chaparral, were not disseminated over all the coffee plantations. From these studies, only Pardo et al. (1999) suggest that the incidence was associated with forest coverage. Other leishmaniasis outbreaks reported in Colombia and Venezuela, with different phlebotomine species incriminated as vectors, suggested association with coffee cultivation, but did not evaluate other environmental factors (Scorza & Rojas 1988; Montoya et al. 1990; Alexander et al. 1992, 1995).
The reservoir hosts of Le.(V.) guyanensis in the study area are unknown. The very rapid spread of the epidemic, and the high rate of human infection, suggests that anthroponotic transmission may have had a major role during the epidemic. However, the incidence of human disease is now very low, consistent with acquired immunity to re-infection (Muñoz & Davies 2006).Sloths are thought to be the reservoir hosts in primary forest in the Amazon region, but the opossum Didelphismarsupialis and rodents may be important in disturbed habitats (Arias et al. 1981; Rotureau 2006). In this study, no major differences were observed in mammal abundance between the two townships, although Agua Bonita presented more species associated with forested habitats, such as the marsupials Marmosopsimpavidus and Micoureus cf. demererae, and rodents of the genera Heteromys, Melanomys and Oecomys (Eisenberg 1989; Emmons 1990; Nowak 1991). Infection with Le.(V). spp was reported more frequently in Agua Bonita, although sample sizes were low. Infection with Le.(V.) guyanensis was confirmed in one Sigmodonhispidus individual, representing a new host record. Infection with Le.(V.) spp. is reported in Oecomystrinitatus and Zygodontomysbrunneus. These parasites are likely to be Le.(V.) guyanensis, but Le.(V.) panamensis and Le.(V.) brasiliensis have also been reported from the study area (Rodríguez-Barraquer et al. 2008). In addition, Melanomyscaliginosus, Micoureus cf. demererae and Heteromys species have been reported as reservoirs for Le.(V.) elsewhere (Scorza et al. 1984; Chable-Santos et al. 1995; Alexander et al. 1998; De Lima et al. 2002).
These results suggest that the transmission in the study area could be maintained in one or more wild mammal reservoirs. Sample sizes were too low to estimate the prevalence of infection in individual species, but the overall prevalence in Agua Bonita was moderate (18%, Table 4). However, the observation that the animals were asymptomatic and infections were only detected in internal tissues and not in ear skin may suggest that the infectiousness of the infected wild mammals was low. In contrast, parasites were readily detected in ear biopsies of domestic dogs in the study area, which represent a possible domestic reservoir (Santaella et al. 2011).
The post-outbreak presence of the parasite in phlebotomies and wild animals suggests that the parasite has become endemic in the area. The association of L. longiflocosa with variables such as altitude and land use has public health importance in terms of identifying leishmaniasis transmission risk and ultimately for the development of interventions.
Supplementary Material
Acknowledgments
We acknowledge all the research team that were involved and supported the field data collection and laboratory standardisation for this study: Dairo Marín, Jorge E. Trujillo, Karina Rueda, María C. Carrasquilla, Leyder Lozano, César Ramirez, James Montoya-Lerma and Leonard Munstermann. We also acknowledge the cooperation of Department of Public Health of Tolima, and the Hospital San Juan Bautista de Chaparral. We are grateful to Dr. Gustavo Adolfo Vallejo for his support with the students and the Tolima University in Chaparral where we established the field laboratory. We are also grateful to James Becerra for creating the entomological database. The study was supported by the United States National Institutes of Health, Division of Microbiology and Infectious Diseases, International Collaboration in Infectious Disease Research Programme and John E. Fogarty International.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Phlebotomine composition and capture rates observed at indoors and 10 m from the house in all the houses from Agua Bonita.
Table S2. Focal study. Lutzomyia longiflocosa capture success in the peridomiciliary and intradomiciliary environments of all 43 Agua Bonita houses.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Acock A. A Gentle Introduction to Stata. 2nd. Stata Press; College Station: 2008. p. 33. [Google Scholar]
- Adler G, Arboleda J, Travi B. Population dynamics of the common opossum (Didelphis marsupialis) in agricultural habitats of northern Colombia. Studies on Neotropical Fauna and Environment. 1997;32:7–11. [Google Scholar]
- Alexander B, Ferro C, Young DG, Morales A, Tesh RB. Ecology of phlebotomine sand flies (Diptera: Psychodidae) in a focus of Leishmania (Viannia) braziliensis in northeastern Colombia. Memorias do Instituto Oswaldo Cruz. 1992;87:387–395. doi: 10.1590/s0074-02761992000300009. [DOI] [PubMed] [Google Scholar]
- Alexander B, Usma MC, Cadena H, et al. Phlebotomine sandflies associated with a focus of cutaneous leishmaniasis in Valle del Cauca, Colombia. Medical and Veterinary Entomology. 1995;9:273–278. doi: 10.1111/j.1365-2915.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Alexander B, Lozano C, Barker DC, McCann SH, Adler GH. Detection of Leishmania (Viannia) braziliensis complex in wild mammals from Colombian coffee plantations by PCR and DNA hybridization. Acta Tropica. 1998;69:41–50. doi: 10.1016/s0001-706x(97)00114-9. [DOI] [PubMed] [Google Scholar]
- Arias JR, Naif RD, Miles MA, de Souza AA. The opossum, Didelphis marsupialis (Marsupialia: Didelphidae), as a reservoir host of Leishmania braziliensis guyanensis in the Amazon Basin of Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75:537–541. doi: 10.1016/0035-9203(81)90194-2. [DOI] [PubMed] [Google Scholar]
- Bañuls AL, Hide M, Prugnolle F. Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Advances in Parasitology. 2007;64:1–109. doi: 10.1016/S0065-308X(06)64001-3. [DOI] [PubMed] [Google Scholar]
- Cardenas R, Romo G, Santamaria E, Bello F, Ferro C. Presencia de Lutzomyia longiflocosa (Diptera: Psychodidae) posible vector en el foco de leishmaniasis cutanea del municipio de Planadas, zona cafetera del Tolima. Biomedica. 1999;19:239–244. [Google Scholar]
- Cardenas R, Pabon E, Anaya H, Sandoval C. Presencia de Lutzomyia longiflocosa (Diptera: Psychodidae) en el foco de leishmaniasis tegumentaria americana del municipio de Abrego, Norte de Santander. Primer registro para el departamento. Clone. 2005;3:7–14. [Google Scholar]
- Chable-Santos JB, Van Wynsberghe NR, Canto-Lara SB, Andrade-Narvaez FJ. Isolation of Leishmania (L.) mexicana from wild rodents and their possible role in the transmission of localized cutaneous leishmaniasis in the state of Campeche, Mexico. American Journal of Tropical Medicine & Hygiene. 1995;53:141–145. doi: 10.4269/ajtmh.1995.53.141. [DOI] [PubMed] [Google Scholar]
- Davies CR, Reithinger R, Campbell-Lendrum D, Feliciangeli D, Borges R, Rodriguez N. The epidemiology and control of leishmaniasis in Andean countries. Cadernos de Saude Publica. 2000;16:925–950. doi: 10.1590/s0102-311x2000000400013. [DOI] [PubMed] [Google Scholar]
- De Lima H, De Guglielmo Z, Rodríguez A, Convit J, Rodriguez N. Cotton rats (Sigmodon hispidus) and black rats (Rattus rattus) as possible reservoirs of Leishmania spp. in Lara State, Venezuela. Memorias do Instituto Oswaldo Cruz. 2002;97:169–174. doi: 10.1590/s0074-02762002000200004. [DOI] [PubMed] [Google Scholar]
- Dujardin JP, Le Pont F, Cruz M, et al. Cryptic speciation in Lutzomyia (Nyssomyia) trapidoi (Fairchild & Hertig) (Diptera: Psychodidae) detected by multilocus enzyme electrophoresis. American Journal of Tropical Medicine & Hygiene. 1996;54:42–45. doi: 10.4269/ajtmh.1996.54.42. [DOI] [PubMed] [Google Scholar]
- Eisenberg J. Mammals of the Neotropics: The Northern Neotropics. Vol. 1. Chicago, IL: The University of Chicago Press; 1989. [Google Scholar]
- Emmons L. Neotropical Rainforest Mammals. Chicago, IL: The University of Chicago Press; 1990. [Google Scholar]
- Ferro C, Marín D, Góngora R, et al. Phlebotomine vector ecology in the domestic transmission of American cutaneous leishmaniasis in Chaparral, Colombia. American Journal of Tropical Medicine & Hygiene. 2011;85:847–856. doi: 10.4269/ajtmh.2011.10-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa RA, Lozano LE, Romero IC, et al. Detection of Leishmania in unaffected mucosal tissues of patients with cutaneous leishmaniasis caused by Leishmania (Viannia) species. Journal of Infectious Diseases. 2009;200:638–646. doi: 10.1086/600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, McShea W, Coroy M, Kunz T. Capturing Mammals. In: Wilson D, Russell F, Nichols J, Rudran R, Foster M, editors. Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Smithsonnian Institution Press; Washington, DC: 1996. pp. 115–155. [Google Scholar]
- Katholi CR, Toé L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. Journal of Infectious Diseases. 1995;172:1414–1417. doi: 10.1093/infdis/172.5.1414. [DOI] [PubMed] [Google Scholar]
- Martínez LP, Rebollo JA, Luna AL, Cochero S, Bejarano EE. Molecular identification of the parasites causing cutaneous leishmaniasis on the Caribbean coast of Colombia. Parasitology Research. 2010;106:647–652. doi: 10.1007/s00436-009-1712-6. [DOI] [PubMed] [Google Scholar]
- Miranda M. El conflicto colombiano y la leishmaniasis. Biomedica. 2007;27:29. [Google Scholar]
- Montoya J, Jaramillo C, Palma G, Gómez T, Segura I, Travi B. Report of an epidemic outbreak of tegumentary leishmaniasis in a coffee-growing area of Colombia. Memorias do Instituto Oswaldo Cruz. 1990;85:119–121. doi: 10.1590/s0074-02761990000100022. [DOI] [PubMed] [Google Scholar]
- Muñoz G, Davies CR. Leishmania panamensis transmission in the domestic environment: the results of a prospective epidemiological survey in Santander, Colombia. Biomedica. 2006;26(Suppl. 1):131–144. [PubMed] [Google Scholar]
- Nowak R. Walker’s Mammals of the World. 1 and 2. Baltimore, MD: The John Hopkins University Press; 1991. [Google Scholar]
- Pardo R, Ferro C, Lozano G, Lozano C, Cabrera O, Davies C. Flebotomos (Diptera: Psychodidae) vectores de leishmaniasis cutanea y sus determinantes ecologicos en la zona cafetera del departamento del Huila. Memorias XXVI Congreso de la Sociedad Colombiana de Entomologia. 1999:147–163. [Google Scholar]
- Pardo RH, Cabrera OL, Becerra J, Fuya P, Ferro C. Lutzomyia longiflocosa as suspected vector of cutaneous leishmaniasis in a focus of cutaneous leishmaniasis on the sub-andean region of Tolima department, Colombia, and the knowledge on sandflies by the inhabitants. Biomedica. 2006;26(Suppl. 1):95–108. [PubMed] [Google Scholar]
- Rodríguez-Barraquer I, Góngora R, Prager M, et al. Etiologic agent of an epidemic of cutaneous leishmaniasis in Tolima, Colombia. American Journal of Tropical Medicine & Hygiene. 2008;78:276–282. [PubMed] [Google Scholar]
- Rotureau B. Ecology of the leishmania species in the Guianan ecoregion complex. American Journal of Tropical Medicine & Hygiene. 2006;74:81–96. [PubMed] [Google Scholar]
- Santaella J, Ocampo CB, Saravia NG, et al. Leishmania (Viannia) infection in the domestic dog in Chaparral, Colombia. American Journal of Tropical Medicine&Hygiene. 2011;84:674–680. doi: 10.4269/ajtmh.2011.10-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravia NG, Segura I, Holguin AF, Santrich C, Valderrama L, Ocampo C. Epidemiologic, genetic, and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. American Journal of Tropical Medicine & Hygiene. 1998;59:86–94. doi: 10.4269/ajtmh.1998.59.86. [DOI] [PubMed] [Google Scholar]
- Saravia NG, Weigle K, Navas C, et al. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania viannia in Colombia. American Journal of Tropical Medicine & Hygiene. 2002;66:738–744. doi: 10.4269/ajtmh.2002.66.738. [DOI] [PubMed] [Google Scholar]
- Scorza J, Rojas E. Caficultura y leishmaniasis tegumentaria en Venezuela. Boletin de la direccion de malariologia y saneamiento ambiental. 1988;28:114–127. [Google Scholar]
- Scorza JV, Rezzano S, César Márquez J. Didelphis marsupialis: principal reservoir of Leishmania spp. in the city of Trujillo, Venezuela. Revista Cubana de Medicina Tropical. 1984;36:194–200. [PubMed] [Google Scholar]
- Shaw J. Ecological and evolutionary pressures on leishmanial parasites. Brazilian Journal of Genetics. 1997;20 ISSN 0100-8455. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S010084551997000100021&lng=en&nrm=iso, http://dx.doi.org/10.1590/S0100 84551997000100021. [Google Scholar]
- Travi BL, Tabares CJ, Cadena H, Ferro C, Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. American Journal of Tropical Medicine & Hygiene. 2001;64:119–124. doi: 10.4269/ajtmh.2001.64.119. [DOI] [PubMed] [Google Scholar]
- Valderrama-Ardila C, Alexander N, Ferro C, et al. Environmental risk factors for the incidence of American cutaneous leishmaniasis in a sub-Andean zone of Colombia (Chaparral, Tolima) American Journal of Tropical Medicine & Hygiene. 2010;82:243–250. doi: 10.4269/ajtmh.2010.09-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergel C, Walker J, Saravia NG. Amplification of human DNA by primers targeted to Leishmania kinetoplast DNA and post-genome considerations in the detection of parasites by a polymerase chain reaction. American Journal of Tropical Medicine & Hygiene. 2005;72:423–429. [PubMed] [Google Scholar]
- Young DG, Morales A, Kreutzer RD, et al. Isolations of Leishmania braziliensis (Kinetoplastida: Trypanosomatidae) from cryopreserved Colombian sand flies (Diptera: Psychodidae) Journal of Medical Entomology. 1987;24:587–589. doi: 10.1093/jmedent/24.5.587. [DOI] [PubMed] [Google Scholar]
- Zambrano P. Comportamiento de la leishmaniasis en Colombia. Biomedica. 2007;27:83–84. [Google Scholar]
- Zambrano P. Comportamiento de los casos de leishmania notificados al sivigila hasta el año 2009. Informe epidemiologico de leishmanisis; Período epidemiologico XIII, actualización de casos a; Marzo 31 de 2009; Instituto Nacional de salud-Colombia. 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.