Abstract
Metastasis is a distinct stage of cancer progression that requires the development of angiogenic blood vessels serving as conduits for tumor cell dissemination. An accumulated body of evidence indicates that metastasis-supporting neovasculature should possess certain structural characteristics allowing for the process of tumor cell intravasation, an active entry of cancer cells into the vessel interior. It appears that the development of tumor vessels with lumens of a distinctive size and their structure supported by a discontinuous pericyte coverage, together constitute critical microarchitectural requirements to: (a) provide accessible points for vessel wall penetration by primary tumor cells; (b) provide enough lumen space for a tumor cell or cell aggregate upon intravasation; and (c) allow for sufficient rate of blood flow to carry away intravasated cells from the primary tumor to the next, proximal or distal site. This review will primarily focus on the functional roles of matrix metalloproteinases (MMPs), which catalytically trigger the development of an intravasation-sustaining neovasculature at the early stages of tumor growth and are also required for the maintenance of a metastasis-supporting state of blood vessels at later stages of cancer progression.
Keywords: Matrix metalloproteinase, Tissue inhibitor of metalloproteinases, Tumor angiogenesis, Tumor cell intravasation and metastasis, Tumor-associated neutrophils, Tumor-associated macrophages, Vascular endothelial growth factor, Epidermal growth factor receptor
1. Introduction
The establishment and progression of metastases is a complex multi-step process involving dynamic interactions between cancer cells and their microenvironment (Egeblad et al., 2010; Hanahan and Weinberg, 2011; Valastyan and Weinberg, 2011). Within the primary tumor, these reciprocal interactions initiate angiogenic vessel assembly and culminate in the formation of vascular networks contributing to both tumor growth and cancer cell spread to secondary sites (Weis and Cheresh, 2011). This metastasis-supporting vasculature is shaped in part through continuous proteolytic modifications of the tissue extracellular matrix (ECM) and various cell surface molecules, particularly by the matrix metalloproteinases (MMPs).
The notion that tumor metastasis occurs “due to the conveyance of cells from the primary tumor to some other part of the body, by the blood or lymphatic vessels, where they develop into similar growths” has been acknowledged as early as 1897 (Lovett and Councilman, 1897). However, it took almost 75 years to suggest that cancer progression could be halted by therapeutic controlling of tumor angiogenesis (Folkman, 1971), and another 15 years to conceptualize that the induction of tumor angiogenesis is a critical step in the early development of a solid tumor required for its transition from avascular premalignant to vascular neoplasic stage (Folkman et al., 1989). By 2000, the induction of tumor angiogenesis by an “angiogenic switch” has become regarded as a hallmark of cancer (Hanahan and Folkman, 1996; Hanahan and Weinberg, 2000). During that time it has been also established that this angiogenic switch requires a critical angiogenic factor, VEGF, and its proteolytic release from tumor matrix by MMPs, predominantly by the MMP-9 delivered into the tumor microenvironment by tumor-infiltrating leukocytes (Bergers et al., 2000). Nevertheless, the precise mechanisms whereby MMP-9 and other MMPs induce the development and also sustain those distinct angiogenic vessels capable of supporting tumor cell intravasation and metastatic dissemination of aggressive cancer cells, are still in the spotlight of cancer research.
Although functionally MMPs have been linked to tissue neovascularization in the early 1990s and multifaceted roles of MMPs in tumor angiogenesis since then are regarded as well established, the literature on how MMPs are mechanistically involved in angiogenic vessel development and angiogenesis-dependent metastasis is still expanding. Thus, when limited to “MMP/Tumor Angiogenesis/Metastasis” as key word criteria, more than 6,300 publications are indicated in the PubMed database since 2011 to the present versus approximately 3,000 publications in the whole decade from 2001 to 2010 and only 30 publications within the 5 years from 1991 to 1995. However, the majority of these “key-words-filtered” MMP publications is centered either on general involvement of MMPs in the neovascularization process or on various aspects of MMP-mediated activation and migration of endothelial cells and not on the unique roles of MMPs in angiogenesis-dependent metastasis. Therefore, we will review almost exclusively the original in vivo studies, which illuminate specific mechanisms underlying the MMP-mediated induction and development of a tumor angiogenic vasculature that is functionally and structurally capable of sustaining intravasation and dissemination of cancer cells.
2. Tumor angiogenic vasculature and tumor cell intravasation
2.1. Dysfunctionality of angiogenic vasculature versus its sustainability to support tumor cell intravasation and metastasis
According to a generally-accepted notion, angiogenic vessels developing in the primary tumor are structurally abnormal and functionally immature (Carmeliet and Jain, 2011). The tumor vasculature is described as chaotic and torturous, irregular in lumen diameters, dilated and highly permeable, deficient in pericyte coverage and abnormal in endothelial lining (De Bock et al., 2011; Garcia-Roman and Zentella-Dehesa, 2013; Goel et al., 2011). Nevertheless, this seemingly impaired vasculature is functional enough to provide not only the nutrients for a growing tumor, but also the conduits for metastatic dissemination of the escaping tumor cells. Whereas the leakiness and enhanced vessel permeability are compatible with the ability of angiogenic vasculature to supply nutrients to the primary tumor, the overall immature state and dysfunctionality of angiogenic vessels appear to be at odds with the capacity of the same vascular networks to sustain active intravasation of tumor cells and their dissemination to secondary sites. Exemplifying such apparent contradiction, the diminishment of pericyte recruitment to primary tumors developing in mice genetically devoid of MMP-9, a critical angiogenic enzyme, has been associated with collapsed morphology of tumor vasculature and substantially inhibited metastasis (Chantrain et al., 2006). Therefore, the pericyte-mediated vessel stabilization (Raza et al., 2010) and architectural support are essential for the functionality of tumor angiogenic vasculature and its ability to sustain tumor cell dissemination.
2.2. Tumor cell intravasation is supported by lumen-containing angiogenic vasculature
While establishing molecular pathways whereby MMPs regulate the process of tumor cell dissemination, we have noticed that high levels of tumor cell intravasation and metastasis frequently correlated with the development and enhanced density within primary tumors of lumen-containing, perfusable blood vessels. The issue of lumen-containing vessels became an important characteristic of intratumoral vasculature, particularly in the settings where tumor cell intravasation and metastasis were significantly diminished, but the overall density of microvessels or total volume of vasculature, two parameters frequently employed in cancer studies, were not affected (Bekes et al., 2011b; Juncker-Jensen et al., 2013). From a mechanistic point, lumen space and blood flow represent obvious requirements for an intravasating tumor cell to enter the angiogenic vessel and be carried away to a secondary site. Therefore, the development of a network of anastomosed angiogenic vessels containing circulating blood and possessing a certain level of blood pressure would likely be prerequisites for the process of metastatic dissemination. In this regard, the vessel-covering pericytes would structurally support the newly-developing tumor vessels and prevent their collapse, allowing for both adequate lumen space to accommodate the size and volume of an intravasating tumor cell or tumor cell aggregates, and sufficient blood flow to carry away the intravasated cells. Reflecting functional importance of these vascular characteristics, the improved pericyte coverage and blood flow are among the “normalization” responses of tumor-associated vasculature to antiangiogenic therapies (Carmeliet and Jain, 2011; Goel et al., 2011; Jain, 2005), which can lead to accelerated tumor progression and enhanced distant metastasis (Ebos et al., 2009; Loges et al., 2009; Paez-Ribes et al., 2009; Sennino et al., 2012; Stockmann et al., 2008).
3. Functional interplay of MMPs and VEGF in the induction of angiogenic vessels and their maintenance during tumor development
3.1. VEGF as a critical molecule governing the development and microarchitecture of angiogenic vessel networks
Numerous molecular pathways and systems have directly or indirectly been implicated in the induction of angiogenesis at the early stages of tumor development and in the maintenance of metastasis-supporting vascular networks during late stages of cancer progression. However, the VEGF-A (VEGF) molecule represents a critical factor that regulates practically all aspects of tumor-induced angiogenesis, including endothelial cell sprouting and assembly, lumen formation, vessel dilation and permeability, and the microarchitecture and patterning of vascular networks (Chung and Ferrara, 2011; Ellis and Hicklin, 2008; Ferrara et al., 2003; Goel and Mercurio, 2013; Welti et al., 2013). Within the tumor microenvironment, the cancer cells appear to serve as the major source of angiogenesis-inducing VEGF in vivo (Hoeben et al., 2004), although different types of stromal cells, including cancer-activated fibroblasts (De Francesco et al., 2013; Ito et al., 2007) and alternatively activated dendritic cells (Riboldi et al., 2005) and infiltrating leukocytes such as neutrophils (Jablonska et al., 2010; Scapini et al., 2000; Scapini et al., 2004; Schruefer et al., 2005), macrophages (Coffelt et al., 2010b; Kiriakidis et al., 2003) and T lymphocytes (Owen et al., 2003), can also supply VEGF.
Complex roles of VEGF in tumor angiogenesis and, by implication, in angiogenesis-dependent metastasis involve both positive and negative regulations of blood vessel development by the VEGF molecule. Whereas VEGF levels within the tumor environment appear to directly correlate with the overall microvessel density (Huss et al., 2001; Takahashi et al., 1998; Takahashi et al., 1995), VEGF also disrupts interactions between vascular pericytes and activated endothelial cells, causing incomplete pericyte coverage of angiogenic vessels (Greenberg et al., 2008). In human xenograft-mouse models, specific inhibition or trapping of VEGF produced by cancer cells results in significant diminishment of tumor angiogenesis, concomitant with reduced tumor growth and, consequently, inhibited metastasis (Byrne et al., 2003; Crawford and Ferrara, 2008; Huang et al., 2003; Kanai et al., 1998; Wang et al., 2008; Warren et al., 1995). However, in syngeneic cancer models, specific depletion of VEGF in tumor-infiltrating myeloid cells can dramatically accelerate tumor progression and normalize pericyte coverage of tumor-associated vasculature (Stockmann et al., 2008). Furthermore, the lack of substantial benefits of anti-VEGF therapies attributed to resistance, adaptation and normalization of tumor vasculature in cancer patients (Bergers and Hanahan, 2008; Claes et al., 2008; Goel et al., 2011; Mancuso et al., 2006; Shojaei et al., 2007; Welti et al., 2013), has clearly indicated that the functional activities of VEGF molecule are more broad and complex than its direct effects on the endothelium or indirect influencing of vascular pericytes and might overlap with multiple molecular networks and processes (Dawson et al., 2009; Garmy-Susini et al., 2010; Mazzone et al., 2009; Mitra and Schlaepfer, 2006; O’Connell et al., 2011).
3.2. Functional links between VEGF and MMPs during tumor angiogenesis
Specific molecular pathways governing VEGF-mediated tumor angiogenesis involve cross-talk and complex links between VEGF and MMP systems. Moreover, VEGF and certain MMPs can be regulated in a mutually coordinated manner on a transcriptional level. Thus, expression of both VEGF and MMP-9 can be induced simultaneously by a common master regulator, a hypoxia-inducible factor HIF-1 (Hoeben et al., 2004; Liao and Johnson, 2007). Counterbalancing such a mutual induction of VEGF and MMP-9 expression, secreted protein acidic and rich in cysteine (SPARC), was shown to negatively and concurrently regulate both VEGF and MMP-9 in a medulloblastoma model, resulting in development of smaller tumors with fewer blood vessels (Bhoopathi et al., 2010). In a similar manner, endogenous SPARC was shown to inhibit expression of both VEGF and MMP-7 in gastric cancer cells and therefore, SPARC silencing elevated angiogenesis, whereas SPARC overexpression diminished angiogenesis in gastric tumor xenografts in an MMP-7/VEGF-dependent manner (Zhang et al., 2012). Interestingly, protein expression of VEGF was correlated with the expression of MMP-2 and MMP-9, and expressions of both MMPs and VEGF were closely linked to metastasis and angiogenesis in gastric cancer patients (Zheng et al., 2006).
In addition to mutual regulation, MMPs and VEGF were shown to exert seemingly unilateral effects on each other’s expression and production. Tumor-produced VEGF can regulate production of distinct MMPs in stromal cells and in activated endothelial cells. Thus, VEGF produced by ovarian cancer cells enhances the expression of host MMP-9 in the ovaries of xenograft-bearing mice, in part due to the induction of neutrophil influx and delivery of neutrophil MMP-9 (Belotti et al., 2008). Furthermore, VEGF produced by distant primary tumors facilitates lung metastasis through induction of MMP-9 production in VEGF receptor (VEGFR)-positive endothelial cells and macrophages involved in the formation of premetastatic niches (Hiratsuka et al., 2002). On the other hand, via STAT activation and VEGFR2 signaling, VEGF was shown to significantly reduce MMP-9 production in B-cell leukemia cells (Ugarte-Berzal et al., 2010), indicating the complexity of VEGF-mediated regulation of MMP-9 expression.
While VEGF is involved in regulation of some MMPs and MMP-9 in particular, certain MMPs of tumor and stromal cell origin can regulate VEGF expression. Thus, the overexpression of MMP-14 in cancer cells was shown to increase VEGF production and angiogenesis in glioblastomas (Deryugina et al., 2002) and breast carcinomas (Sounni et al., 2002; Sounni et al., 2004). MMP-2 also was shown to induce VEGF expression in A549 cells through the binding to αvβ3 and subsequent integrin signaling (Chetty et al., 2010). Furthermore, cancer cell-produced MMP-13 (collagenase-3) has recently been shown to promote secretion of VEGF by fibroblasts and endothelial cells and to induce tumor angiogenesis in vivo (Kudo et al., 2012). MMP-mediated regulation of VEGF-induced tumor vascularization was demonstrated in a xenograft model in vivo, in which MMP blockade almost completely inhibited VEGF production and significantly reduced the volume of angiogenic vasculature (Woenne et al., 2010). Therefore, in multiple ways, MMPs directly and indirectly can influence the VEGF-mediated development of an angiogenic vasculature.
3.3. MMP-mediated release of matrix-sequestered VEGF in vivo
Since the majority of produced VEGF is sequestered in the ECM deposited by tumor and stromal cells, the proteolytic release of angiogenic factors from tissue matrix is regarded as a prerequisite for in vivo induced angiogenesis. Different isoforms of VEGF have been shown to bind with different affinities to heparin-binding matrix glycoproteins, with a trend that high mol. wt. forms are more tightly bound than low mol. wt. forms and the most common 165 kDa isoform, VEGF165, exhibiting an intermediate level of binding (Houck et al., 1992). In vitro, a number of recombinant MMPs, including MMP-1, 3,-7, −9, −16 and −19, were shown to cleave full-length recombinant VEGF165, generating lower mol. wt. fragments (Lee et al., 2005). Then, recombinantly-produced VEGF fragments mimicking in molecular weight those generated by MMP-3 cleavage in vitro, were demonstrated to induce either dilation or branching of angiogenic vessels in vivo, prompting a notion that MMPs release matrix-bound VEGF by direct intramolecular processing (Lee et al., 2005). However, MMP-dependent release of specific soluble fragments from VEGF that was actually sequestered in tumor matrix was not demonstrated in this study, neither for in vitro nor for in vivo conditions. Furthermore, the full-length VEGF165 was shown to be completely resistant to several MMPs, including MMP-1,-3,-7,-9, and −13 (Hashimoto et al., 2002; Hawinkels et al., 2008), therefore not supporting the intramolecular cleavage as a mechanism of VEGF release.
In contrast, the release of VEGF via MMP-mediated digestion of matrix proteins sequestering VEGF molecules is supported by several biochemical studies. In vitro, matrix-bound VEGF165, actually embedded into the ECM deposited by tumor cells, was shown to be releasable by recombinant MMP-2 and MMP-9 (Ebrahem et al., 2010; Hawinkels et al., 2008). In addition, MMP-3 (stromelysin-1) and MMP-7 (matrilysin) were capable of releasing in vitro the intact VEGF165 from its complex with connective tissue growth factor (CTGF), and this release was accompanied by digesting CTGF without any generation of low mol. wt. VEGF fragments (Hashimoto et al., 2002). Furthermore, quantitative modeling of proteolytic release of matrix-sequestered VEGF not only indicated that soluble VEGF was essentially insensitive to the cell-mediated proteolysis in vitro, but also suggested that VEGF165 conversion to lower mol. wt. form would not occur in vivo (Vempati et al., 2010). In agreement with the mechanism, whereby VEGF would be released from the matrix as intact molecule and not cleaved fragments, the full-length VEGF165 was shown in vivo to regulate different aspects of tumor angiogenesis depending on the concentration: when used at relatively low concentrations, VEGF165 increased the density of angiogenic vessels, but at a 3-fold higher concentrations, the same VEGF165 phenotypically switched normal-looking vessels to abnormal-dilated vessels typical of tumor vasculature (Parsons-Wingerter et al., 2006). Therefore, the proteolytic digestion of matrix proteins and proteoglycans by serine proteases (Houck et al., 1992) or MMPs (Hawinkels et al., 2008) appears to constitute a mechanism that is substantially supported by experimental data explaining how matrix-sequestered VEGF becomes bioavailable in vivo
MMP-mediated release of VEGF capable of inducing development of angiogenic vasculature was shown in several in vivo angiogenesis models. Importantly, VEGF-induced angiogenesis was not correlated with the levels of total VEGF present in tissue matrix, but specifically with the levels of soluble VEGF extracted from the matrix without detergents. Thus, corneal neovascularization in a micropocket model that requires the release of exogenously supplied VEGF, was stimulated by MMP-2 and MMP-9 in a VEGF-dependent manner (Ebrahem et al., 2010). Further indicating an importance of MMPs in VEGF-dependent angiogenesis, the MMP-9 produced by ovarian cancer cells released biologically active VEGF that in turn induced endothelial cell motility, enhanced endothelium permeability, and facilitated ascites formation (Belotti et al., 2003). In breast cancer xenografts engineered to express the stromal-bound form of MMP-9, the released VEGF promoted tumor angiogenesis via enhanced complexing of VEGF with endothelial cell VEGFR, the association that was dependent on MMP-9 activity (Mira et al., 2004). Stromal MMP-13 expressed by cancer-associated fibroblasts was shown to be essential for VEGF release and VEGF-dependent maintenance of angiogenesis in a skin squamous cell carcinoma model (Lederle et al., 2010).
However, as it will be shown below, the functional contribution of MMP-executed release of VEGF that triggers the angiogenic switch in vivo has been attributed almost exclusively to the proteolytic activity of host MMP-9 delivered mainly by tumor-infiltrating leukocytes.
4. Tumor-associated leukocytes and their MMP-9 in VEGF-induced angiogenesis
4.1. The role of inflammatory leukocytes in the generation of angiogenesis-inducing tumor microenvironment
The induction of tumor angiogenesis by tumor-infiltrating leukocytes involves a multi-step sequence of events, originating at the level of dysregulated tumor cells expressing and secreting various proangiogenic molecules and inflammatory cytokines, followed by a specific response of activated endothelial cells to assemble into angiogenic blood vessels, and culminating in the generation of a functional angiogenic vasculature partially covered with pericytes. The inflammatory cell influx into the tumor microenvironment is regulated by production of distinct leukocyte chemoattractants for neutrophils, circulating monocytes and monocytic precursors of macrophages, and different types of lymphocytes (Egeblad et al., 2010; Erez and Coussens, 2011; Grivennikov et al., 2010; Quail and Joyce, 2013). The relative production of lineage-specific cytokines can be tumor type-specific and/or tumor stage-specific, which would determine the preferential type of leukocytes found in a given tumor infiltrate (Coffelt et al., 2010a; Curry et al., 2014; Dumitru et al., 2012; Galdiero et al., 2013; Tazzyman et al., 2009; Tazzyman et al., 2013). In addition, different types of inflammatory leukocytes could compensate for each other and adopt angiogenesis-inducing functions of the absent leukocyte partner (Pahler et al., 2008; Sawanobori et al., 2008; Tan et al., 2013). Furthermore, depending on specific localization in the tumor area, tumor-associated leucocytes, e.g. macrophages and neutrophils, were shown to acquire either protumoral and proangiogenic characteristics or anti-tumorigenic traits (Casazza et al., 2013; Fridlender et al., 2009; Galdiero et al., 2013; Lawrence and Natoli, 2011; Mantovani et al., 2011; Piccard et al., 2011; Qian and Pollard, 2010) and to exhibit unique gene expression profiles and functions across different cancer types (Dalton et al., 2014; Elpek et al., 2014; Qian et al., 2009; Smith and Kang, 2013).
Tumor-associated leukocytes were credited with the expression of various MMPs, including MMP-1, −2, −3, −9, −10, −11, −14, −15, −16, −17, −19, −24, −26, −27, −28, mainly through the studies which demonstrated the corresponding MMP mRNA transcripts in normal leukocytes or immortal cell lines representing particular leukocyte types (Bar-Or et al., 2003; Sithu et al., 2007). Based mostly on immunohistochemical stainings, different populations of tumor-associated leukocytes have been liberally ascribed with the ability to produce and deliver angiogenesis-inducing MMP-9. However, the actual production on a protein level or functional importance in tumor development and angiogenesis was not demonstrated for the majority of these leukocyte-associated MMPs, with the exception of MMP-9. As described below, elegant experimental approaches applied to spontaneous metastasis models allowed to prove that MMP-9 produced by inflammatory leukocytes is a crucial molecule regulating tumor angiogenesis and angiogenesis-dependent tumor cell dissemination.
4.2. Tumor-infiltrating leukocytes deliver angiogenesis-inducing MMP-9
Genetic ablation of MMP-9 in tumor recipients, resulting in reduced microvessel density in developing tumors and even preventing the angiogenic switch during cancer progression, provided original evidence for the functional involvement of host MMP-9 in tumor angiogenesis (Bergers et al., 2000). Nevertheless, it was the reconstitution of tumor-bearing Mmp9-knockout (KO) mice by transplantation of wild-type, MMP-9-competent hematopoietic cells that provided a direct proof that tumor angiogenesis-inducing MMP-9 was delivered by tumor-infiltrating myeloid cells (Bergers et al., 2000; Chantrain et al., 2006; Jodele et al., 2005). Depending on the prevalence of a specific type of MMP-9-positive leukocytes in a particular tumor infiltrate, different tumor-associated leukocytes have been ascribed with the capacity of MMP-9 delivery. In general, the “reappearance” of MMP-9-positive tumor-associated macrophages (TAMs) concomitant with the “restoration” of tumor angiogenesis and metastasis in genetically-deficient tumor bearers transplanted with wild-type hematopoietic cells led to a prevailing conclusion that TAMs are critical for MMP-9-mediated tumor angiogenesis (Huang et al., 2002), even though other types of MMP-9-positive myeloid cells were not rigorously screened or investigated. Thus, when tumors genetically lacking TAMs but manifesting high levels of MMP-9 were searched for the cell source of host MMP-9, an important compensatory mechanism evoking neutrophil infiltration was established (Pahler et al., 2008), highlighting the functional difference between the MMP-9-expressing macrophages versus MMP-9-delivering tumor-infiltrating neutrophils. Further complicating the mechanisms underlying the functions of leukocyte-produced MMP-9 in tumor angiogenesis, genetic ablation of MMP-9 not only decreased microvessel density in ovarian carcinomas in vivo, but also decreased macrophage infiltration into primary tumors (Huang et al., 2002). Similar dependence of infiltrating macrophages on MMP-9 and its proteolytic activity is manifested in plasminogen-knockout mice, where supplementation of active MMP-9 was shown to restore impaired macrophage influx (Gong et al., 2008). In support of the notion that intratumoral MMP-9 can locally regulate influx and presentation of leukocytes in developing tumors, it was demonstrated that the adenovirus-mediated induction of MMP-9 in breast cancer xenografts dramatically altered their cytokine profile and enhanced production of neutrophil chemoattractants, KC and MIP-2, leading to a massive neutrophil infiltration (Leifler et al., 2013).
Pharmacological or genetic depletion of a specific hematopoietic lineage in tumor-bearing mice allowed for more precise attributing of MMP-9 delivery and MMP-9-dependent angiogenesis to a particular category of MMP-9-positive tumor-associated leukocytes. By using mast cell-deficient transgenic mice, the infiltration by mast cells delivering MMP-9-activating proteases was linked to the MMP-9-mediated angiogenic switch and resulting tumor angiogenesis in the skin carcinogenesis model (Coussens et al., 1999). Specific ablation of MMP-9-positive TAMs with zoledronic acid resulted in reduced tumor angiogenesis, leading to a conclusion that TAMs deliver angiogenesis-inducing MMP-9 (Giraudo et al., 2004; Rogers and Holen, 2011). It appears that tumor infiltration by inflammatory leukocytes belonging to a particular hematopoietic lineage can depend on specific tumor microenvironments, which also can change with the stage of tumor development.
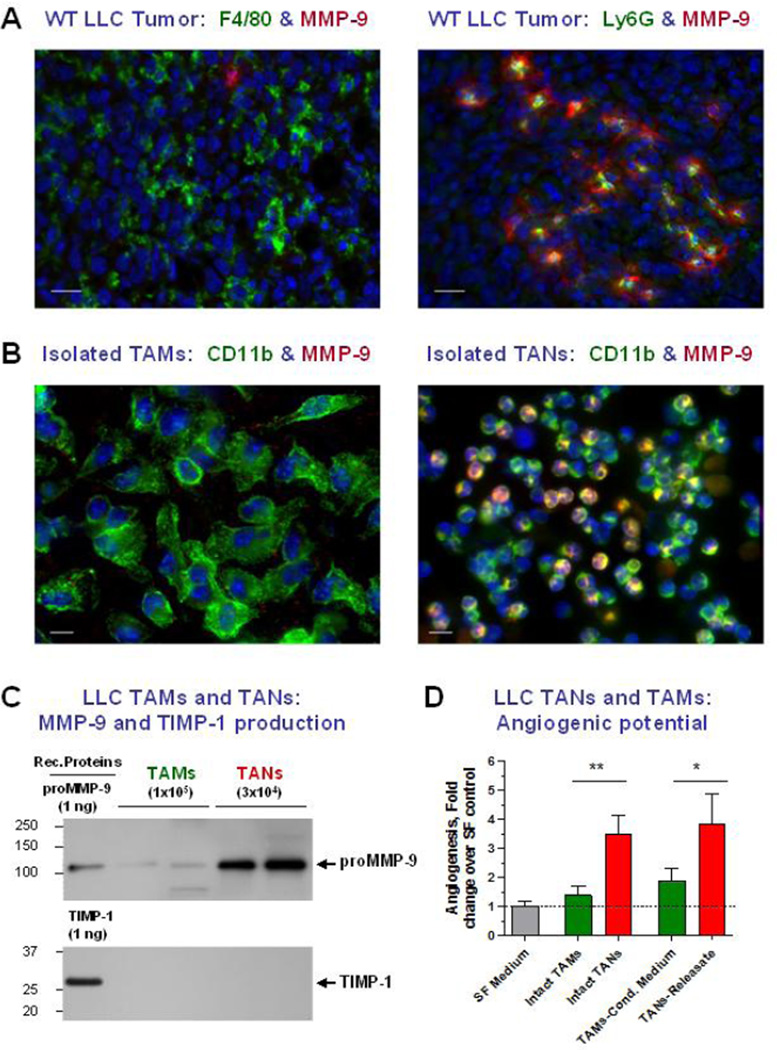
Several independent studies repeatedly demonstrated that the elimination of MMP-9-bearing neutrophils with a granulocyte-specific Gr-1 antibody (Fleming et al., 1993) or prevention of neutrophil infiltration with anti-inflammatory agents consistently caused inhibition of tissue neovascularization (Shaw et al., 2003) and tumor angiogenesis concomitant with a significant reduction of intravasation and metastasis (Bekes et al., 2011b; Jablonska et al., 2010; Pekarek et al., 1995; Tazawa et al., 2003). Neutrophil-depletion studies also allowed to link tumor-infiltrating neutrophils specifically with the initial angiogenic switch in the genetic multistage model of pancreatic β-cell cancer (Nozawa et al., 2006; Shojaei et al., 2008). In addition to lineage depletion studies, abundant immunohistochemical and protein array data clearly link infiltration of primary tumors with MMP-9-bearing neutrophils with angiogenic progression, metastatic disease, poor prognosis and survival in patients with several cancer types (Dumitru et al., 2012; Gregory and McGarry Houghton, 2011; Houghton, 2010; Kuang et al., 2011; Tazzyman et al., 2009; Tazzyman et al., 2013). Furthermore, a comparative analysis of TAMs and tumor-associated neutrophils (TANs) isolated from syngeneic mouse tumors demonstrated that only TANs showed high levels of MMP-9 mRNA expression and intracellular MMP-9 protein (Sawanobori et al., 2008). That inflammatory neutrophils constitute the major tumor-associated leukocyte type expressing abundant MMP-9, has also been demonstrated in our recent study employing human xenografts or syngeneic murine tumors (Deryugina et al., 2014). Specifically, when tumors or isolated TAMs and TANs were double-stained for MMP-9 and respective macrophage- or neutrophil-specific antigens, only TANs were emitting strong immunofluorescent signal for MMP-9 (Figure 1, A and B). Together, the majority of functional and morphological data point to tumor-infiltrating neutrophils as a critical tumor-associated leukocyte type that is responsible for delivery of angiogenesis-inducing MMP-9.
Figure 1. TANs constitute the major cellular source of angiogenic proMMP-9.
(A) LLC tumor was stained for MMP-9 (red) and either macrophage-specific antigen F4/80 or neutrophil-specific antigen Ly6G (green). Note the MMP-9-positive fibrillar matrix surrounding the MMP-9/Ly6G–positive neutrophils that appear yellow due to the overlap of red and green immunofluorescent signals. Bars, 20 µm.
(B) Isolated TAMs and TANs were stained for common cell myeloid marker CD11b (green) and MMP-9 (red). Only isolated TANs are positively stained for MMP-9, producing yellow signal indicating the overlap of MMP-9 (red) and lineage-specific (green) signals. Bars, 10 µm.
(C) Western blot analysis of MMP-9 and TIMP-1 produced by isolated TAMs and TANs. Whereas both cell types do not produce detectable TIMP-1, only TANs release substantial quantities of proMMP-9.
(D) Angiogenic potential of isolated TAMs and TANs and their respective products, TAMs’ conditioned medium and TANs’ releasate, as measured in the in vivo angiogenesis assay in live chick embryos. Note that TANs and TANs releasate induce higher levels of angiogenesis compared to low angiogenic TAMs and their conditioned medium.
4.3. Dependence of tumor angiogenesis on the bioavailability of VEGF released by inflammatory cell MMP-9
The actual functional link between the MMP-9 delivered by tumor-infiltrating leukocytes and endothelial cells responding to angiogenic induction has been established in studies demonstrating that the levels of tumor angiogenesis are proportional to the levels of VEGF liberated by the activity of MMP-9. The initial evidence was provided in the transgenic model of pancreatic cancer, where MMP-9-mediated liberation of matrix-sequestered VEGF induced the angiogenic switching in avascular pre-malignant tumors (Bergers et al., 2000). The overall involvement of bone marrow-derived myeloid cells in the delivery of VEGF-releasing MMP-9 and initiation of VEGF-dependent tumor angiogenesis was also shown in a glioblastoma model (Du et al., 2008a). In a cervical cancer model, reduced tumor angiogenesis due to diminished levels of mobilized VEGF was associated with MMP-9 produced by TAMs (Giraudo et al., 2004). In prostate cancer bone xenografts, where MMP-9 expression was immunohistochemically linked to the osteoclast cell population, significantly fewer and smaller blood vessels were observed in MMP-9 null mice, concurrent with lower levels of soluble VEGF produced by MMP-9-negative osteoclasts in vitro (Bruni-Cardoso et al., 2010). However, although many macrophage-depleting and macrophage-inducing studies have emphasized an overall importance of tumor-infiltrating monocytes and TAMs in tumor angiogenesis through VEGF-involving mechanisms (Green et al., 2009; Halin et al., 2009; Zaynagetdinov et al., 2011; Zeisberger et al., 2006; Zhang et al., 2010), these TAM-centric studies did not directly or conclusively implicate MMP-9 produced by TAMs neither in MMP-9-mediated VEGF mobilization in vivo, nor in the induction of metastasis-supporting tumor vasculature. In contrast, MMP-9 produced by tumor-infiltrating neutrophils and TANs has been repeatedly shown to release matrix-bound VEGF during in vivo angiogenesis. Thus, VEGF-induced angiogenesis in the mouse brain specifically required neutrophil infiltration and delivery of neutrophil MMP-9 to produce functionally bioavailable VEGF (Hao et al., 2007). In the genetic model of pancreatic cancer progression, neutrophil MMP-9 was demonstrated to act as a critical contributor to the bioavailability of VEGF and induction of the angiogenic switch (Nozawa et al., 2006). By cleaving heparan sulphates in the matrix deposited by colon carcinoma cells, neutrophil MMP-9 was specifically implicated in a colorectal cancer model in liberation of actual matrix-sequestered VEGF and induction of VEGF-dependent angiogenesis (Hawinkels et al., 2008). Recently, neutrophils and their TIMP-free MMP-9 have also been shown to increase VEGF bioavailability during inflammatory lymphangiogenesis (Tan et al., 2013). In addition to release of VEGF, our studies on physiological and tumor-induced angiogenesis highlighted the importance of neutrophil MMP-9-mediated release of another angiogenic factor, basic fibroblast growth factor, or FGF-2 (Ardi et al., 2009).
4.4. Possible VEGF-independent functions of neutrophil MMP-9
Neutrophil-derived MMP-9 has also been ascribed with direct, VEGF-independent functions during tumor angiogenesis. In a 3-dimensional in vitro assay, sprouting from endothelial cell spheroids could be 2-fold enhanced by either exogenous VEGF or neutrophil MMP-9, but VEGF-induced angiogenesis was insensitive to MMP-9 inhibition and vice versa, MMP-9-induced angiogenesis was insensitive to VEGF blockade (Bausch et al., 2011). Surprisingly, the combined use of VEGF and MMP-9 produced a synergistic, 6-fold enhancement and full ablation of this enhancement required simultaneous inhibition of VEGF and MMP-9. Based on these in vitro data, the in vivo findings demonstrating that the inhibition of MMP-9 activity causing a reduction of vascular density in tumors was not accompanied by significant changes in total VEGF, were interpreted as evidence against MMP-9-mediated release of VEGF and for VEGF-independent functions of MMP-9 in the angiogenic switch (Bausch et al., 2011). However, since the total, detergent-extracted VEGF rather than soluble VEGF was measured in this study, it is still possible that MMP-9 activity was involved in liberation of matrix-sequestered VEGF in the tumor. It is also possible that neutrophil MMP-9 is capable of catalytically activating the angiogenic process independent of VEGF release, e.g. by a proteolytic digest of various ECM proteins, including collagenase-cleaved collagen fibrils, resulting in less stiff matrices more amenable for endothelial cell invasion.
5. Specific roles of neutrophils and neutrophil MMP-9 in the induction of intravasation-and metastasis-sustaining vasculature
5.1. Differential ability of tumor variants to intravasate depends on inflammatory neutrophil influx
To investigate the MMP-involving mechanisms governing the process of tumor cell intravasation, we have employed a number of congenic tumor cell variants selected in vivo from human cancer cell lines, including HT-1080 fibrosarcoma, PC-3 prostate carcinoma and HEp-3 epidermoid carcinoma, for low and high levels of dissemination (i.e., lo/diss and hi/diss variants). The respective pairs generate primary tumors of comparable sizes, but hi/diss and lo/diss variants display up to 100-fold differentials in the levels of vascular intravasation in the live chick embryo model and up to 25-fold difference in spontaneous lung metastasis in various mouse models, including orthotopic implantation models (Bekes et al., 2011a; Deryugina et al., 2005; Juncker-Jensen et al., 2013; Partridge et al., 2007). Furthermore, the HT-hi/diss and PC3-hi/diss cells are approximately 2–3-fold more angiogenic than their respective lo/diss counterparts in the chick embryo onplant and mouse angiotube models (Bekes et al., 2011b), suggesting that different levels of angiogenesis in the primary tumors might determine different levels of intravasation. Histological and immunohistochemical analyses of primary tumors, however, indicated that intravasation differential between hi/diss and lo/diss variants directly correlated with the density of lumen-containing vessels rather than with the overall density of the tumor-associated angiogenic vasculature (Bekes et al., 2011b; Deryugina et al., 2014; Juncker-Jensen et al., 2013). Therefore, the distinct ability to induce and sustain the development of angiogenic vessels possessing specific-sized lumens has been linked for the first time to the capacity of tumor cells to complete the process of vascular intravasation.
The intravasation differential of our tumor variants as it relates to the intratumoral networks of lumen-containing angiogenic blood vessels and tumor cell dissemination has, in turn, been linked to the differential ability of these tumor variants to induce inflammatory cell influx. Specifically, the density of TANs and the rates of neutrophil influx were substantially different between the hi/diss and lo/diss pairs of fibrosarcoma and prostate carcinoma variants whereas the levels of TAMs were similar (Bekes et al., 2011b). Furthermore, the close association between the levels of neutrophil infiltration and tumor cell intravasation was linked directly to the levels of neutrophil proMMP-9 delivered by tumor-infiltrating neutrophils into the primary tumor site. Thus, inhibition of neutrophil infiltration into primary HT-hi/diss and PC-hi/diss tumors by functionally blocking tumor cell-produced IL-8, a potent and specific neutrophil chemoattractant, resulted in a substantially inhibited development of a lumen-containing intratumoral vasculature concomitantly with significantly reduced levels of intravasation. Importantly, these effects of inflammatory neutrophil deficit were reversed by the supplementation of developing tumors with purified neutrophil proMMP-9, which in the HT-hi/diss in vivo metastasis model restored in a coordinated fashion the levels of intravasation and the density of lumen-containing vasculature. Further illuminating the functional role of neutrophil proMMP-9 in tumor-induced angiogenesis, supplementation of HT-lo/diss and PC-lo/diss cells with purified neutrophil proMMP-9, increased their low angiogenic potentials in the collagen onplant model, bringing angiogenesis to hi/diss levels. Reciprocally, the use of an anti-inflammatory drug, ibuprofen, inhibited hi/diss-induced angiogenesis, and these ibuprofen-mediated effects were also reversed and brought back to control levels by exogenous delivery of purified neutrophil MMP-9 (Bekes et al., 2011b).
5.2. Unique angiogenesis-inducing potency of TIMP-free MMP-9 produced by neutrophils and neutrophil-like MMP-9 produced M2-polarized macrophages
Compared to tumor-infiltrating monocytes or TAMs and other types of tumor-associated inflammatory leukocytes, inflammatory neutrophils appear to be much better equipped to deliver angiogenesis-inducing quantities of MMP-9, capable of liberating VEGF and bFGF and executing other matrix-modifying activities (Owen and Campbell, 1999). Our initial studies have demonstrated that neutrophil MMP-9 is exceptionally powerful in the induction of tumor angiogenesis, functioning as a pro-angiogenic factor at low nanomolar concentrations, comparable with the angiogenesis-inducing concentrations of VEGF and bFGF (Ardi et al., 2007; Ardi et al., 2009). This unusual angiogenic potency of neutrophil MMP-9 was linked first, to the unique form of the zymogen produced by neutrophils as an inactive proenzyme unencumbered by the natural MMP-9 inhibitor, TIMP-1, and second, to the catalytic action of an activated enzyme. In other cell types, secreted proMMP-9 is bound to TIMP-1, which not only significantly slows the activation of the MMP-9 zymogen, but also can immediately inhibit the proteolytic activity of the once activated enzyme (Ogata et al., 1995; Okada et al., 1992). Therefore, a TIMP-1-free form of proMMP-9 has a much higher probability for in vivo activation and execution of its enzymatic activity before either of these biochemical processes would be inhibited by endogenous TIMP-1 or TIMP-2, the latter a potent and abundant natural inhibitor of many activated MMPs.
In contrast to neutrophils, monocytic cell lines, like most cells, produce their proMMP-9 in a conventional stoichiometric 1:1 complex with TIMP-1 rendering it with low angiogenesis-inducing activity (Ardi et al., 2007), suggesting that monocyte-derived macrophages would also produce proMMP-9 complexed with TIMP-1. The production of proMMP-9/TIMP-1 complex was indeed demonstrated for normal human and mouse monocytes differentiated in vitro from either peripheral blood or bone marrow precursors in response to M-CSF (Zajac et al., 2013). Similarly, a TIMP-1-encumbered status of MMP-9 was also characteristic of proMMP-9 produced by mouse and human macrophages polarized into “classic” M1 phenotype. Remarkably, when in vitro-generated macrophages were polarized towards an “alternative” M2 phenotype, they shut down their TIMP-1 expression and initiated the production of a “neutrophil-like”, TIMP-1-free proMMP-9 (Zajac et al., 2013). Furthermore, we have recently demonstrated that TAMs isolated from murine tumors and manifesting specific characteristics of M2-skewed macrophages, such as production of arginase and the lack of iNOS production, also did not express TIMP-1 and therefore produced TIMP-1-free proMMP-9 (Deryugina et al., 2014).
5.3. Inflammatory neutrophils and TANs constitute the major source of angiogenesis-inducing MMP-9
Despite compelling evidence for the delivery of angiogenesis-inducing MMP-9 to the tumor microenvironment by bone marrow-derived myeloid cells, the majority of the studies were not accompanied by a quantitative analysis of MMP-9 production or comparison of the angiogenic potency of the MMP-9 produced by various tumor-associated leukocytes. In attempt to fill this apparent gap in the biology of MMP-9-mediated tumor angiogenesis and to clarify the controversy in the accumulated evidence regarding the leukocyte delivery of MMP-9, we recently have conducted a quantitative investigation and compared MMP-9-producing capacities of isolated neutrophils and monocyte-derived macrophages of human and murine origin as well as TANs and TAMs isolated from syngeneic murine tumors (Deryugina et al., 2014; Zajac et al., 2013). In addition, a special focus was placed on quantification of MMP-9 produced by monocytes and different types of macrophages, namely mature macrophages versus macrophages polarized towards M1 or M2 phenotypes. Consistent with its TIMP-free status, MMP-9 purified from M2-macrophages and TAMs, has been shown to be as angiogenic as proMMP-9 from neutrophils or TANs, when compared on a molar basis as purified zymogens. However, on a cell-to-cell level, murine macrophages and TAMs were shown to produce 40–50 times less proMMP-9 in 48 hours compared to the intracellular MMP-9 load of blood-circulating neutrophils and TANs, which can release their cargo on demand. Our quantification indicated that while 1×106 neutrophils and TANs can release approximately 100–200 ng proMMP-9 within 1–2 hr of incubation, 1×106 macrophages and TAMs would require at least 10 weeks to synthesize and secrete the same amount of proMMP-9 [Figure 1C and (Deryugina et al., 2014)]. Thus, it appears that inflammatory neutrophils constitute the major source of MMP-9 capable of matrix remodeling, liberation of VEGF, and induction of angiogenesis in primary tumors. Consistent with this notion, the isolated TANs and their released cellular contents but not TAMs and their conditioned medium were capable of inducing high levels of in vivo angiogenesis (Figure 1D).
5.4. Microarchitecture and pericyte coverage of intravasation-sustaining vasculature depends on neutrophil influx and delivery of angiogenic MMP-9
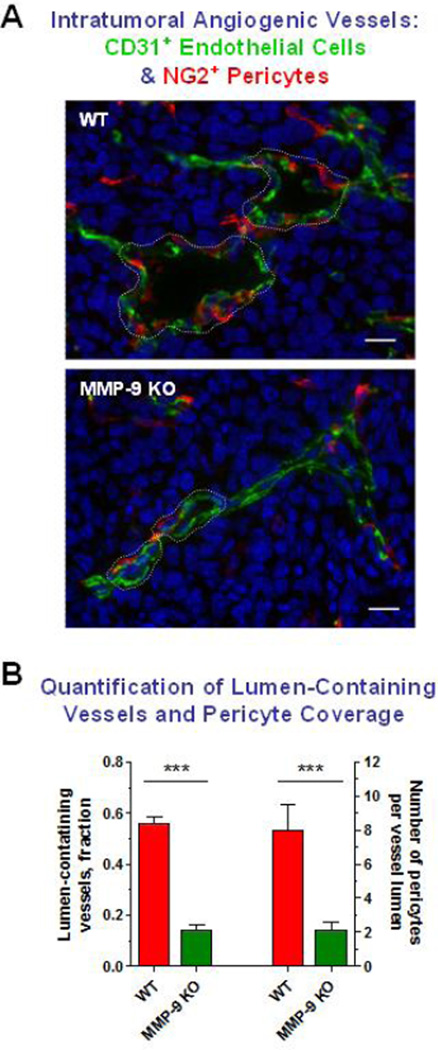
MMP-9-dependent tumor angiogenesis especially at the early stages of primary tumor development appear to be determined specifically by the rates of neutrophil influx (Bekes et al., 2011b; Deryugina et al., 2014; Nozawa et al., 2006; Shojaei et al., 2008; Zajac et al., 2013; Zijlstra et al., 2006), which, in turn, is regulated by the levels of neutrophil attractants expressed by tumor cells. Thus, in several cancer models enhanced IL-8 production has specifically been linked to high levels of tumor infiltration by neutrophils and tumor angiogenesis (Benelli et al., 2002; Brat et al., 2005; De Larco et al., 2004; Haqqani et al., 2000; Kim et al., 2001; Sparmann and Bar-Sagi, 2004; Waugh and Wilson, 2008; Yao et al., 2007). In a positive feed-back loop, neutrophil MMP-9 also has been shown to significantly potentiate the functional activity of IL-8 (Van den Steen et al., 2000). By using our hi/diss variants in a spontaneous metastasis avian model, in which neutrophil infiltration and therefore the delivery of neutrophil MMP-9 were inhibited by IL-8 blockade, we functionally linked the levels of intravasation and metastasis with the development of an angiogenic, lumen-containing vasculature (Bekes et al., 2011b). Histological examinations of angiogenic vasculature in syngeneic tumors developing in wild-type versus MMP-9-KO mice also indicates that the lumen-containing vasculature was fully developed only when tumors were infiltrated with MMP-9-positive leukocytes (Chantrain et al., 2004). Our recent detailed analyses of syngeneic tumors developing in MMP-9 null and wild-type mice confirmed that the majority of influxing MMP-9-positive leukocytes were mature neutrophils and that the lack of neutrophil MMP-9 delivery in Mmp9-KO hosts resulted in the development of collapsed, lumen-devoid vasculature severely deficient in pericyte coverage [Figure 2A and (Deryugina et al., 2014)]. Thus, while in a MMP-9-competent microenvironment almost 60% of tumor vessels developed lumens of >11 µm in diameter, less than 20% of all tumor vessels presented with lumens of this size in Mmp9-KO hosts (Figure 2B). Furthermore, 4-times more pericytes were associated with blood vessel lumens in WT tumors compared with MMP-9-deficient tumors (Figure 2B). It would appear that in wild type animals, the distinctive albeit discontinuous pericyte coverage apparently allows for enough structural support of lumen-containing vessels, but also for their sufficient penetrability by intravasating tumor cells. Therefore, neutrophil MMP-9 constitutes a critical microenvironmental contributor to the development of an architecturally sound intratumoral vasculature capable of sustaining maximal tumor cell intravasation and metastatic dissemination.
Figure 2. Development of intratumoral lumen-containing angiogenic vessels and their partial coverage with pericytes depend on the host MMP-9.
(A) LLC tumors grown in wild-type (WT) or MMP-9 knockout (KO) mice were immunostained for the endothelial cell marker CD31 (green) and pericyte marker NG2 (red). Blood vessels in WT tumors exhibit enlarged lumens (outlined) associated with more pericytes than the vessels in MMP-9-deficient tumors. Bars, 20 µm.
(B) Lumen-containing vessels (Y axis on the left) and average number of lumen-associated pericytes (Y axis on the right) were quantified in WT tumors versus MMP-9-deficient LLC tumors. Note a 4-fold differential in both parameters in favor of angiogenic vessels in MMP-9-competent tumors.
In addition to MMP-9 delivered to the tumor microenvironment by neutrophils, other MMPs and non-neutrophilic cells have also been implicated in pericyte coverage of angiogenic vasculature. Thus, complete genetic ablation of MMP-2 in a glioblastoma model resulted in the development of impaired and dysfunctional tumor vasculature that surprisingly, provided a attractive scaffold for tumor cell invasion along these poorly perfused vessels (Du et al., 2008b). Recently, pericyte coverage of tumor-associated vasculature and its metastasis-supporting ability has also been linked to a heterogeneous population of CD13-positive bone marrow-derived myeloid cells, represented by tumor-associated monocytes, macrophages, granulocytes, dendritic cells and T lymphocytes, implicated in production of a variety of proangiogenic factors, including MMP-9 (Dondossola et al., 2013). However, it remains unclear how much of the MMP-9 is produced by different types of CD13-positive leukocytes and whether this specific MMP-9 regulates pericyte coverage of tumor vasculature.
6. EGFR-mediated regulation of tumor cell intravasation through an MMP-9/VEGF-involving mechanism
Some recent findings suggest that MMP-9-dependent regulation of tumor angiogenesis can also be governed by specific tumor cell receptors, which regulate MMP-9 expression at the transcriptional level. Thus, activation of the pathways downstream of epidermal growth factor receptor (EGFR) increases MMP-9 expression and activity in many cancer cell types, including breast carcinomas, lung carcinomas, head and neck squamous carcinomas and glioblastomas (Choe et al., 2002; Ellerbroek et al., 2001; Kim et al., 2009; O-Charoenrat et al., 2000; Zhao et al., 2010). Reciprocally, anti-EGFR agents, which can directly effect EGFR-overexpressing tumor and endothelial cells, also inhibit expression of MMP-9 and reduce tumor growth indirectly by blocking VEGF production (Normanno and Gullick, 2006; Pore et al., 2006; Ratushny et al., 2009). Whereas EGF/EGFR-regulated MMP-9 functions have mostly been attributed to well-established roles of tumor cell-produced MMP-9 in matrix remodeling and tumor cell stromal invasion (Hwang et al., 2011; Zhao et al., 2010), downregulation of VEGF production in tumor xenografts treated with an EGFR inhibitor was linked to normalization of morphology and permeability of tumor-associated vessels (Cerniglia et al., 2009).
Providing novel insights to the role of tumor cell-produced MMP-9 to tumor angiogenesis and angiogenesis-dependent tumor cell dissemination, we have recently linked the expression and activity of tumor cell EGFR to development of an intravasation-supporting vasculature (P. Minder and EID, unpublished data). First evidence has come from the finding that all our hi/diss variants, independent of tissue origin, i.e. fibrosarcoma, prostate carcinoma and epidermoid carcinoma, express significantly higher levels of EGFR compared to their lo/diss counterparts. Interestingly, increased expression of EGFR in breast cancer cells has also been linked to enhanced intravasation and metastasis in a breast cancer mouse model, but these higher rates of cell dissemination were linked to increased cell motility of EGFR-overexpressing tumor cells (Xue et al., 2006). In contrast, we found no correlation between the motility of tumor cells in our HT-1080 and HEp-3 lo/diss and hi/diss variants (Deryugina et al., 2005; Juncker-Jensen et al., 2013), indicating that additional mechanisms or changes in tumor microenvironment other than overall EGFR levels could indirectly regulate EGFR-mediated tumor cell intravasation.
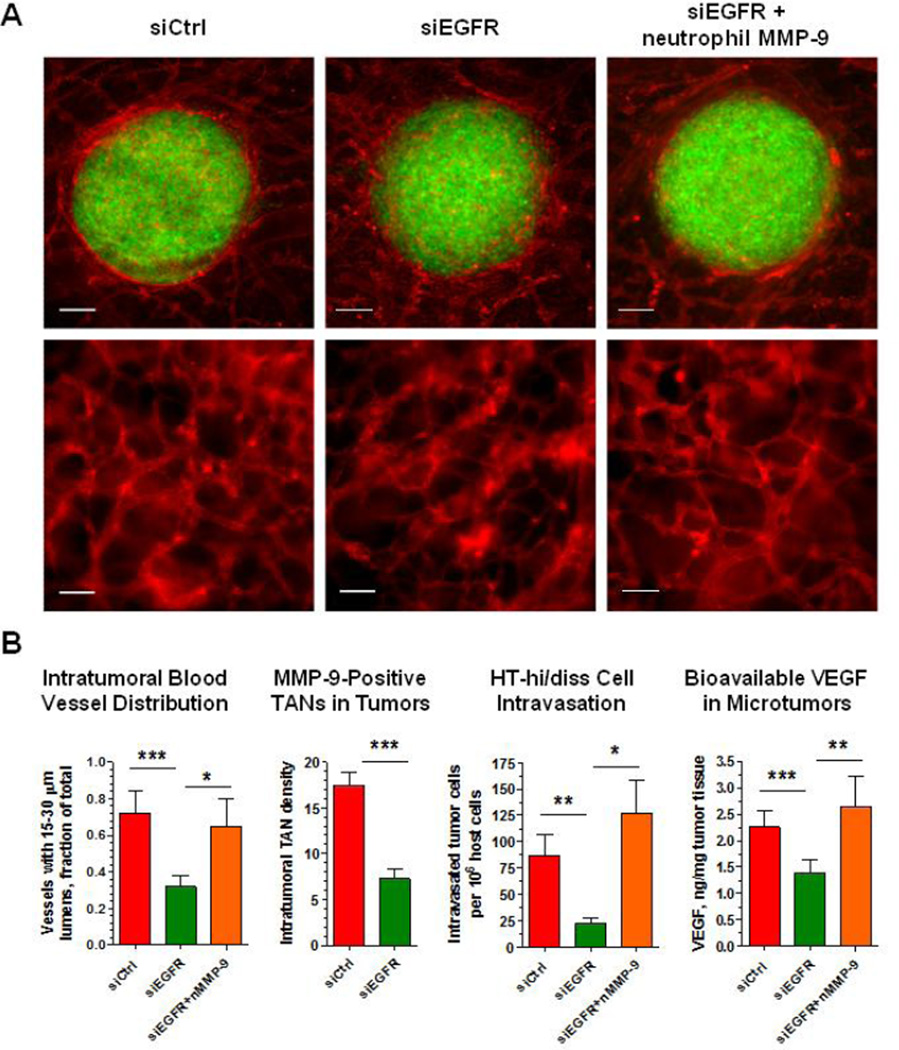
To investigate mechanistic links between EGFR and EGFR-dependent tumor cell dissemination, we employed our recently-established microtumor model, allowing for simultaneous measurements of tumor cell intravasation, tumor angiogenesis, vascular permeability and vascular microarchitecture of the intratumoral vasculature (Deryugina, in press). As an initial approach, the levels of EGFR were downregulated in HT-hi/diss cells by siRNA interference. It is important that in our model, down-regulation of EGFR significantly reduced HT-hi/diss intravasation independent of EGFR effects on tumor growth and overall levels of angiogenesis (Figure 3A), prompting the analysis of specific EGFR-dependent attributes of the intratumoral vasculature. Thus, EGFR silencing was associated with a severe underrepresentation of lumen-containing vessels, reduction in the density of TANs and bioavailable VEGF (Figure 3B). Similar effects were observed when EGFR function was inhibited with the EGFR-specific tyrosine kinase inhibitor, erlotinib, implicating EGFR activity and signaling in the development of intravasation-sustaining blood vessels. However, supplementation of purified neutrophil MMP-9 to EGFR-silenced tumors, fully restored the levels of intravasation along with the restoration of 15–30 µm-sized vessels and bioavailable VEGF (Figure 3B), thereby confirming functional regulation of intravasation-sustaining vascular development by EGFR via the MMP-9/VEGF pathway,
Figure 3. Tumor cell EGFR regulates the development of angiogenic vessels in the HT-hi/diss microtumors in a neutrophil MMP-9/VEGF-dependent manner.
(A) GFP-tagged HT-hi/diss cells were treated with control siRNA (siCtrl) or EGFR siRNA (siEGFR) and grafted on the CAM of live chick embryos. After 5 days, intratumoral blood vessels within developed microtumors were highlighted in vivo with red-fluorescent lectin. Despite the similar size of HT-hi/diss microtumors (upper panels; bars, 100 µm), EGFR silencing resulted in the development of thin and collapsed blood vessels with reduced size lumens compared to control (lower panels; bars, 50 µm). Note that treatment of siEGFR tumors with purified neutrophil proMMP-9 restored the appearance of intratumoral blood vessels.
(B) Quantification of intratumoral angiogenic vessels with 15–30 µm-lumens, the density of intratumoral MMP-9-positive TANs, the levels of intravasation and the levels of bioavailable VEGF in tumors. Downregulation of EGFR resulted in a significant underrepresentation of angiogenic vessels with the lumens of specific 15–30 µm size, concomitant with the reduction of neutrophil influx and tumor cell intravasation. Both blood vessel development and intravasation rates were restored by supplementation of siEGFR-silenced tumors with purified neutrophil MMP-9 (nMMP-9), which also fully restored the levels of bioavailable VEGF.
7. VEGF-induced tumor angiogenesis mediated by MMP-9 expressed by cancer cells and cancer-associated fibroblasts
The role of tumor cell MMP-9 in the overall process of tumor metastasis has been associated mainly with the regulation of tumor growth through the mechanisms involving matrix remodeling and stromal invasion by tumor and endothelial cells. In these processes, tumor cell-produced MMP-9 was shown to function through cooperation with complementary molecules such as uPA, uPAR, and cathepsin B (Bjorklund and Koivunen, 2005; Deryugina and Quigley, 2006; Farina and Mackay, 2014). Since the expression of MMP-9 and VEGF in tumor cells appears to be coordinately regulated on the transcriptional level, this particular mutual connection has been implicated in tumor angiogenesis and metastasis models where MMP-9 and VEGF were either enhanced or downregulated in a tandem fashion. Thus, the simultaneously enhanced production of MMP-9 and VEGF in A549 lung carcinoma cells in response to cytochrome P450 omega-hydroxylase resulted in a 2-fold increase in microvessel density in primary tumors and a similar 2-fold increase in metastasis from lung tumors to distant organs (Yu et al., 2011). Reciprocally, downregulation of MT1-MMP (MMP-14) in human ovarian cancer OVCAR-4 cells by RNA interference resulted in a significant diminishment of MMP-9 and VEGF production, concomitant with a dramatic reduction in tumor cell-induced angiogenesis in matrigel plugs (Kaimal et al., 2013).
Some agents appear to regulate levels of MMP-9 expression independently of the VEGF gene. Thus, late SV40 factor (LSF), which directly targets the MMP-9 gene and induces MMP-9 production, was shown to enhance tumor angiogenesis in an MMP-9-dependent manner (Santhekadur et al., 2012). In an orthotopic implantation model, MMP-9 produced by breast cancer cells regulated spontaneous lung metastasis via facilitating the motility-dependent crossing of the endothelial cell barrier by cancer cells (Kim et al., 2011). A recent study also re-confirmed overall dependence of tumor angiogenesis on the stromal MMP-9 produced by cancer-associated fibroblasts activated by specific interactions with tumor cells (Taguchi et al., 2014).
8. Tumor angiogenesis and metastasis regulated by tumor cell-produced MMP-1 inducing an intravasation-supporting vasculature
A critical example of a tumor cell-produced MMP regulating the development of intravasation-sustaining vasculature is represented by secreted MMP-1 in the head and neck cancer model described in our recent publication (Juncker-Jensen et al., 2013). By using our HEp-3 dissemination variants in a microtumor model, we have linked MMP-1 secreted by HEp3-hi/diss carcinoma cells to their high levels of intravasation into CAM vasculature and metastasis to the liver. Similar to EGFR-dependent intravasation-sustaining blood vessels, MMP-1-dependent intravasation also required the presence of lumens of 15–30 µm and increased levels of vascular permeability as measured by high mol. wt. Dextran exudation. However, in the case of tumor-produced MMP-1 it appeared to be a proteolytic activation of the endothelial cell membrane-tethered receptor, PAR1, which ultimately facilitated HEp3-hi/diss intravasation (Juncker-Jensen et al., 2013). These in vivo data corroborate a MMP-1/PAR1-involving mechanism previously demonstrated in vitro for endothelial cells and for tumor cell-endothelial cell settings (Boire et al., 2005; Brinckerhoff et al., 2000; Tressel et al., 2011; Yang et al., 2009). Furthermore, it has recently been demonstrated that MMP-1/PAR1-pathway is involved in the up-regulation of VEGFR in the endothelial cells (Mazor et al., 2013).
An additional mechanism whereby tumor cell-produced MMP-1 could regulate the development of metastasis-supporting vasculature was underscored in our recently-established orthotopic mouse model of head and neck cancer (Anna Juncker-Jensen, EID and JPQ, unpublished data). Similar to the chick embryo model, the tumor-associated vasculature in MMP-1-competent primary tumors developing in the buccal mucosa of immunodeficient mice manifesting high rates of spontaneous lung metastasis has been characterized by the presence of lumens of ∼11–15 µm. Furthermore, MMP-1 deficiency in HEp3-hi/diss tumors caused the development of dense but lumen-deficient intratumoral vasculature, reduced neutrophil influx and significant inhibition of spontaneous metastasis to the lungs. This MMP-1 repression in HEp-hi/diss cells also resulted in downregulation of IL-8 production in primary tumors, suggesting that MMP-1-dependent angiogenesis involves IL-8-induced neutrophil inflammatory response and a delivery of neutrophil MMP-9 into the tumor microenvironment. This putative tumor MMP-1/tumor IL-8/neutrophil MMP-9 axis might represent yet another example of complex and diversified pathways whereby MMPs of tumor and host origin directly, via matrix degradation, or indirectly, via angiogenic factor release and activation, regulate the quality of metastasis-sustaining angiogenic vasculature.
9. Conclusions
The overall dependence of tumor angiogenesis on the expression of various MMPs is still in the spotlight of experimental and clinical cancer research, deeply focusing on unraveling the precise interrelations between different regulatory molecules and proteolytic activities of specific MMPs (Al Rawashdeh et al., 2014; Barillari et al., 2014; Depner et al., 2014; Gong et al., 2014; Kahlert et al., 2014; Zhang et al., 2014). In this review, however, we attempted to summarize the data on the mechanisms whereby various MMPs, characterized by different substrate specificities, e.g. collagenases and gelatinases, and originating from different cell types, e.g. tumor cells, inflammatory leukocytes and cancer-associated fibroblasts, induce within primary tumors the development of a distinct angiogenic vasculature capable of sustaining tumor cell intravasation and intravasation-dependent metastasis. As indicated by in vivo imaging and quantitative measurements in our studies, this intravasation- and metastasis-sustaining vasculature should possess specific microarchitectural characteristics such as partial but distinctive pericyte coverage, vessel lumens of ∼10–30 µm in diameter, and elevated vascular permeability, all of which are required for optimal support of active tumor cell intravasation and dissemination of metastatic cells via vascular routes. It would appear that the major biochemical mechanisms that operate in MMP-mediated development of the intravasation-sustaining vasculature are proteolytic remodeling of the tumor matrix and the linked release of angiogenic factors like VEGF and bFGF. In this regard, the majority of accumulated evidence indicates that the proMMP-9 delivered by tumor-influxing neutrophils possesses the highest biochemical and physiological potentials, such as rapid release, high local concentration, TIMP-free status, high rates of activation and efficiency of substrate catalysis, to digest tumor matrix and release matrix-sequestered VEGF and bFGF into the tumor microenvironment. In turn, the released and functionally induced VEGF and bFGF directly regulate the microarchitecture and functions of the intratumoral vasculature, including its ability to sustain tumor cell intravasation and metastasis.
Highlights.
MMPs are involved in angiogenesis-dependent intravasation and metastasis
Inflammatory cell MMP-9 triggers the onset of tumor neovascularization
IL-8-responding neutrophils are the major source of angiogenesis-inducing MMP-9
Neutrophil MMP-9 catalytically releases angiogenesis factor VEGF from tumor matrix
MMP-9/VEGF axis regulates intravasation- and metastasis-sustaining neovasculature
Acknowledgments
Cited work from our laboratory was supported by grants from National Institutes of Health (NIH) R01CA55852, R01CA105412, R01CA129484; NIH Training grants HL07695, 5T32 HL07195-31, 5T32CA077109; American Heart Association Fellowship 0225103Y; Human Diversity and Re-Entry Award from NIH/NCI; NIH/National Center for Research Resources/Scripps Translational Science Institute grants UL1 RR025774 and UL1 TR000109-05; and by grants and fellowships from the Danish National Research Foundation, the Danish Cancer Society, the University of Aarhus, the Max Kade Foundation, the Fund for Scientific Research of Flanders, the University of Zurich, and the Swiss National Science Foundation for Prospective Researches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Rawashdeh W, Arns S, Gremse F, Ehling J, Knuchel-Clarke R, Kray S, Spoler F, Kiessling F, Lederle W. Optical tomography of MMP activity allows a sensitive noninvasive characterization of the invasiveness and angiogenesis of SCC xenografts. Neoplasia. 2014;16:235–246. doi: 10.1016/j.neo.2014.03.005. 246 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. PCMID2154419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284:25854–25866. doi: 10.1074/jbc.M109.033472. PCMID2757987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- Barillari G, Iovane A, Bacigalupo I, Labbaye C, Chiozzini C, Sernicola L, Quaranta MT, Falchi M, Sgadari C, Ensoli B. The HIV protease inhibitor indinavir down-regulates the expression of the pro-angiogenic MT1-MMP by human endothelial cells. Angiogenesis. 2014;17:831–838. doi: 10.1007/s10456-014-9430-9. [DOI] [PubMed] [Google Scholar]
- Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, Thayer SP, Keck T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14:235–243. doi: 10.1007/s10456-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekes EM, Deryugina EI, Kupriyanova TA, Zajac E, Botkjaer KA, Andreasen PA, Quigley JP. Activation of pro-uPA is critical for initial escape from the primary tumor and hematogenous dissemination of human carcinoma cells. Neoplasia. 2011a;13:806–821. doi: 10.1593/neo.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011b;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotti D, Calcagno C, Garofalo A, Caronia D, Riccardi E, Giavazzi R, Taraboletti G. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol Cancer Res. 2008;6:525–534. doi: 10.1158/1541-7786.MCR-07-0366. [DOI] [PubMed] [Google Scholar]
- Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–269. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoopathi P, Chetty C, Gujrati M, Dinh DH, Rao JS, Lakka SS. The role of MMP-9 in the anti-angiogenic effect of secreted protein acidic and rich in cysteine. Br J Cancer. 2010;102:530–540. doi: 10.1038/sj.bjc.6605538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- Bruni-Cardoso A, Johnson LC, Vessella RL, Peterson TE, Lynch CC. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol Cancer Res. 2010;8:459–470. doi: 10.1158/1541-7786.MCR-09-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, Yancopoulos GD, Jaffe RB. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Cerniglia GJ, Pore N, Tsai JH, Schultz S, Mick R, Choe R, Xing X, Durduran T, Yodh AG, Evans SM, Koch CJ, Hahn SM, Quon H, Sehgal CM, Lee WM, Maity A. Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS One. 2009;4:e6539. doi: 10.1371/journal.pone.0006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantrain CF, Henriet P, Jodele S, Emonard H, Feron O, Courtoy PJ, DeClerck YA, Marbaix E. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer. 2010;127:1081–1095. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe G, Park JK, Jouben-Steele L, Kremen TJ, Liau LM, Vinters HV, Cloughesy TF, Mischel PS. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res. 2002;8:2894–2901. [PubMed] [Google Scholar]
- Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- Claes A, Wesseling P, Jeuken J, Maass C, Heerschap A, Leenders WP. Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization. Mol Cancer Ther. 2008;7:71–78. doi: 10.1158/1535-7163.MCT-07-0552. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010a;176:1564–1576. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010b;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford Y, Ferrara N. Chapter 6. Mouse models to investigate anti-cancer effects of VEGF inhibitors. Methods Enzymol. 2008;445:125–139. doi: 10.1016/S0076-6879(08)03006-1. [DOI] [PubMed] [Google Scholar]
- Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, Tuluc M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41:217–234. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Dalton HJ, Armaiz-Pena GN, Gonzalez-Villasana V, Lopez-Berestein G, Bar-Eli M, Sood AK. Monocyte subpopulations in angiogenesis. Cancer Res. 2014;74:1287–1293. doi: 10.1158/0008-5472.CAN-13-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:E4. doi: 10.1038/nature08254. discussion E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1alpha/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast Cancer Res. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- Depner S, Lederle W, Gutschalk C, Linde N, Zajonz A, Mueller MM. Cell type specific interleukin-6 induced responses in tumor keratinocytes and stromal fibroblasts are essential for invasive growth. Int J Cancer. 2014;135:551–562. doi: 10.1002/ijc.27951. [DOI] [PubMed] [Google Scholar]
- Deryugina E. Martin SG, Hewett P, editors. Chorioallantoic membrane microtumor model to study the mechanisms of tumor angiogenesis, vascular permeability and tumor cell intravasation. Angiogenesis. doi: 10.1007/978-1-4939-3628-1_19. (in press). Vol. Angiogenesis. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62:580–588. [PubMed] [Google Scholar]
- Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing mmp-9 in the tumor microenvironment. Neoplasia. 2014;16:771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryugina EI, Zijlstra A, Partridge JJ, Kupriyanova TA, Madsen MA, Papagiannakopoulos T, Quigley JP. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res. 2005;65:10959–10969. doi: 10.1158/0008-5472.CAN-05-2228. [DOI] [PubMed] [Google Scholar]
- Dondossola E, Rangel R, Guzman-Rojas L, Barbu EM, Hosoya H, St John LS, Molldrem JJ, Corti A, Sidman RL, Arap W, Pasqualini R. CD13-positive bone marrow-derived myeloid cells promote angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:20717–20722. doi: 10.1073/pnas.1321139110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008a;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Petritsch C, Lu K, Liu P, Haller A, Ganss R, Song H, Vandenberg S, Bergers G. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008b;10:254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, Asosingh K, Erzurum S, Anand-Apte B. Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol. 2010;176:496–503. doi: 10.2353/ajpath.2010.080642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek SM, Halbleib JM, Benavidez M, Warmka JK, Wattenberg EV, Stack MS, Hudson LG. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res. 2001;61:1855–1861. [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- Elpek KG, Cremasco V, Shen H, Harvey CJ, Wucherpfennig KW, Goldstein DR, Monach PA, Turley SJ. The tumor microenvironment shapes lineage, transcriptional, and functional diversity of infiltrating myeloid cells. Cancer Immunol Res. 2014;2:655–667. doi: 10.1158/2326-6066.CIR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Coussens LM. Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization. Int J Cancer. 2011;128:2536–2544. doi: 10.1002/ijc.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina AR, Mackay AR. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers (Basel) 2014;6:240–296. doi: 10.3390/cancers6010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404–1412. doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- Garcia-Roman J, Zentella-Dehesa A. Vascular permeability changes involved in tumor metastasis. Cancer Lett. 2013;335:259–269. doi: 10.1016/j.canlet.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL, Cheresh D, Ginsberg M, Varner JA. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–3051. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel) 2014;6:1298–1327. doi: 10.3390/cancers6031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, Liu T, Montel V, Hsiao G, Lester RD, Subramaniam S, Gonias SL, Klemke RL. Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One. 2009;4:e6713. doi: 10.1371/journal.pone.0006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AD, McGarry Houghton A. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halin S, Rudolfsson SH, Van Rooijen N, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- Haqqani AS, Sandhu JK, Birnboim HC. Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia. 2000;2:561–568. doi: 10.1038/sj.neo.7900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB, Sier CF. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44:1904–1913. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- Houghton AM. The paradox of tumor-associated neutrophils: Fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9 doi: 10.4161/cc.9.9.11297. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, New T, O’Toole K, Zabski S, Rudge JS, Holash J, Yancopoulos GD, Yamashiro DJ, Kandel JJ. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci U S A. 2003;100:7785–7790. doi: 10.1073/pnas.1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- Huss WJ, Hanrahan CF, Barrios RJ, Simons JW, Greenberg NM. Angiogenesis and prostate cancer: identification of a molecular progression switch. Cancer Res. 2001;61:2736–2743. [PubMed] [Google Scholar]
- Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, Kwon KI, Jeong TC, Jeong HG. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011;55:594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]
- Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–7203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Jodele S, Chantrain CF, Blavier L, Lutzko C, Crooks GM, Shimada H, Coussens LM, Declerck YA. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- Juncker-Jensen A, Deryugina EI, Rimann I, Zajac E, Kupriyanova TA, Engelholm LH, Quigley JP. Tumor MMP-1 activates endothelial PAR1 to facilitate vascular intravasation and metastatic dissemination. Cancer Res. 2013;73:4196–4211. doi: 10.1158/0008-5472.CAN-12-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert C, Pecqueux M, Halama N, Dienemann H, Muley T, Pfannschmidt J, Lasitschka F, Klupp F, Schmidt T, Rahbari N, Reissfelder C, Kunz C, Benner A, Falk C, Weitz J, Koch M. Tumour-site-dependent expression profile of angiogenic factors in tumour-associated stroma of primary colorectal cancer and metastases. Br J Cancer. 2014;110:441–449. doi: 10.1038/bjc.2013.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimal R, Aljumaily R, Tressel SL, Pradhan RV, Covic L, Kuliopulos A, Zarwan C, Kim YB, Sharifi S, Agarwal A. Selective blockade of matrix metalloprotease-14 with a monoclonal antibody abrogates invasion, angiogenesis, and tumor growth in ovarian cancer. Cancer Res. 2013;73:2457–2467. doi: 10.1158/0008-5472.CAN-12-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T, Konno H, Tanaka T, Baba M, Matsumoto K, Nakamura S, Yukita A, Asano M, Suzuki H, Baba S. Anti-tumor and anti-metastatic effects of human-vascular-endothelial-growth-factor-neutralizing antibody on human colon and gastric carcinoma xenotransplanted orthotopically into nude mice. Int J Cancer. 1998;77:933–936. doi: 10.1002/(sici)1097-0215(19980911)77:6<933::aid-ijc23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kim J, Thorne SH, Sun L, Huang B, Mochly-Rosen D. Sustained inhibition of PKCalpha reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene. 2011;30:323–333. doi: 10.1038/onc.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Cho S, Kim JS, Kim JH, Choe JH, Nam SJ, Lee JE, Yang JH. EGF-induced MMP-9 expression is mediated by the JAK3/ERK pathway, but not by the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cell Signal. 2009;21:892–898. doi: 10.1016/j.cellsig.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3:33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J Cell Sci. 2003;116:665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Iizuka S, Yoshida M, Tsunematsu T, Kondo T, Subarnbhesaj A, Deraz EM, Siriwardena SB, Tahara H, Ishimaru N, Ogawa I, Takata T. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J Biol Chem. 2012;287:38716–38728. doi: 10.1074/jbc.M112.373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- Lederle W, Hartenstein B, Meides A, Kunzelmann H, Werb Z, Angel P, Mueller MM. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31:1175–1184. doi: 10.1093/carcin/bgp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifler KS, Svensson S, Abrahamsson A, Bendrik C, Robertson J, Gauldie J, Olsson AK, Dabrosin C. Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. J Immunol. 2013;190:4420–4430. doi: 10.4049/jimmunol.1202610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Lovett RW, Councilman WT. A Case of Double Teratoma. J Exp Med. 1897;2:427–438. doi: 10.1084/jem.2.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Mazor R, Alsaigh T, Shaked H, Altshuler AE, Pocock ES, Kistler EB, Karin M, Schmid-Schonbein GW. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem. 2013;288:598–607. doi: 10.1074/jbc.M112.417451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jimenez-Baranda S, Gomez-Mouton C, Martinez AC, Manes S. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Normanno N, Gullick WJ. Epidermal growth factor receptor tyrosine kinase inhibitors and bone metastases: different mechanisms of action for a novel therapeutic application? Endocr Relat Cancer. 2006;13:3–6. doi: 10.1677/erc.1.01185. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, Neilson EG, Zeisberg M, Kalluri R. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O-Charoenrat P, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, Eccles SA. Epidermal growth factor-like ligands differentially up-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res. 2000;60:1121–1128. [PubMed] [Google Scholar]
- Ogata Y, Itoh Y, Nagase H. Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinases-1 complex by 4-aminophenylmercuric acetate and proteinases. J Biol Chem. 1995;270:18506–18511. doi: 10.1074/jbc.270.31.18506. [DOI] [PubMed] [Google Scholar]
- Okada Y, Gonoji Y, Naka K, Tomita K, Nakanishi I, Iwata K, Yamashita K, Hayakawa T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J Biol Chem. 1992;267:21712–21719. [PubMed] [Google Scholar]
- Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol. 1999;65:137–150. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- Owen JL, Iragavarapu-Charyulu V, Gunja-Smith Z, Herbert LM, Grosso JF, Lopez DM. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol. 2003;171:4340–4351. doi: 10.4049/jimmunol.171.8.4340. [DOI] [PubMed] [Google Scholar]
- Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons-Wingerter P, Chandrasekharan UM, McKay TL, Radhakrishnan K, DiCorleto PE, Albarran B, Farr AG. A VEGF165-induced phenotypic switch from increased vessel density to increased vessel diameter and increased endothelial NOS activity. Microvasc Res. 2006;72:91–100. doi: 10.1016/j.mvr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Partridge JJ, Madsen MA, Ardi VC, Papagiannakopoulos T, Kupriyanova TA, Quigley JP, Deryugina EI. Functional analysis of matrix metalloproteinases and tissue inhibitors of metalloproteinases differentially expressed by variants of human HT-1080 fibrosarcoma exhibiting high and low levels of intravasation and metastasis. J Biol Chem. 2007;282:35964–35977. doi: 10.1074/jbc.M705993200. [DOI] [PubMed] [Google Scholar]
- Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2011 doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res. 2006;66:3197–3204. doi: 10.1158/0008-5472.CAN-05-3090. [DOI] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratushny V, Astsaturov I, Burtness BA, Golemis EA, Silverman JS. Targeting EGFR resistance networks in head and neck cancer. Cell Signal. 2009;21:1255–1268. doi: 10.1016/j.cellsig.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–2792. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhekadur PK, Gredler R, Chen D, Siddiq A, Shen XN, Das SK, Emdad L, Fisher PB, Sarkar D. Late SV40 factor (LSF) enhances angiogenesis by transcriptionally up-regulating matrix metalloproteinase-9 (MMP-9) J Biol Chem. 2012;287:3425–3432. doi: 10.1074/jbc.M111.298976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, Cassatella MA. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. 2004;172:5034–5040. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- Schruefer R, Lutze N, Schymeinsky J, Walzog B. Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. Am J Physiol Heart Circ Physiol. 2005;288:H1186–H1192. doi: 10.1152/ajpheart.00237.2004. [DOI] [PubMed] [Google Scholar]
- Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DT, McDonald DM. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Chuang N, Yee H, Shamamian P. Polymorphonuclear neutrophils promote rFGF-2-induced angiogenesis in vivo. J Surg Res. 2003;109:37–42. doi: 10.1016/s0022-4804(02)00020-3. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Sithu SD, English WR, Olson P, Krubasik D, Baker AH, Murphy G, D’Souza SE. Membrane-type 1-matrix metalloproteinase regulates intracellular adhesion molecule-1 (ICAM-1)-mediated monocyte transmigration. J Biol Chem. 2007;282:25010–25019. doi: 10.1074/jbc.M611273200. [DOI] [PubMed] [Google Scholar]
- Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91:411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Roghi C, Chabottaux V, Janssen M, Munaut C, Maquoi E, Galvez BG, Gilles C, Frankenne F, Murphy G, Foidart JM, Noel A. Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J Biol Chem. 2004;279:13564–13574. doi: 10.1074/jbc.M307688200. [DOI] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Kawana K, Tomio K, Yamashita A, Isobe Y, Nagasaka K, Koga K, Inoue T, Nishida H, Kojima S, Adachi K, Matsumoto Y, Arimoto T, Wada-Hiraike O, Oda K, Kang JX, Arai H, Arita M, Osuga Y, Fujii T. Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts (CAFs) is suppressed by omega-3 polyunsaturated fatty acids in vitro and in vivo. PLoS One. 2014;9:e89605. doi: 10.1371/journal.pone.0089605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Bucana CD, Cleary KR, Ellis LM. p53, vessel count, and vascular endothelial growth factor expression in human colon cancer. Int J Cancer. 1998;79:34–38. doi: 10.1002/(sici)1097-0215(19980220)79:1<34::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- Tan KW, Chong SZ, Wong FH, Evrard M, Tan SM, Keeble J, Kemeny DM, Ng LG, Abastado JP, Angeli V. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122:3666–3677. doi: 10.1182/blood-2012-11-466532. [DOI] [PubMed] [Google Scholar]
- Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90:222–231. doi: 10.1111/j.1365-2613.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23:149–158. doi: 10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Tressel SL, Kaneider NC, Kasuda S, Foley C, Koukos G, Austin K, Agarwal A, Covic L, Opal SM, Kuliopulos A. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med. 2011;3:370–384. doi: 10.1002/emmm.201100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte-Berzal E, Redondo-Munoz J, Eroles P, Del Cerro MH, Garcia-Marco JA, Terol MJ, Garcia-Pardo A. VEGF/VEGFR2 interaction down-regulates matrix metalloproteinase-9 via STAT1 activation and inhibits B chronic lymphocytic leukemia cell migration. Blood. 2010;115:846–849. doi: 10.1182/blood-2009-08-239426. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- Vempati P, Mac Gabhann F, Popel AS. Quantifying the proteolytic release of extracellular matrix-sequestered VEGF with a computational model. PLoS One. 2010;5:e11860. doi: 10.1371/journal.pone.0011860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu H, Ren L, Pan Y, Zhang Y. Inhibiting colorectal carcinoma growth and metastasis by blocking the expression of VEGF using RNA interference. Neoplasia. 2008;10:399–407. doi: 10.1593/neo.07613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woenne EC, Lederle W, Zwick S, Palmowski M, Krell H, Semmler W, Mueller MM, Kiessling F. MMP inhibition blocks fibroblast-dependent skin cancer invasion, reduces vascularization and alters VEGF-A and PDGF-BB expression. Anticancer Res. 2010;30:703–711. [PubMed] [Google Scholar]
- Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai KL, Zhang ZY, Sahai E, Condeelis J, Segall JE. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66:192–197. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- Yang E, Boire A, Agarwal A, Nguyen N, O’Callaghan K, Tu P, Kuliopulos A, Covic L. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69:6223–6231. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–1957. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- Yu W, Chen L, Yang YQ, Falck JR, Guo AM, Li Y, Yang J. Cytochrome P450 omega-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancer. Cancer Chemother Pharmacol. 2011;68:619–629. doi: 10.1007/s00280-010-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, Deryugina EI. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood. 2013;122:4054–4067. doi: 10.1182/blood-2013-05-501494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R, Sherrill TP, Polosukhin VV, Han W, Ausborn JA, McLoed AG, McMahon FB, Gleaves LA, Degryse AL, Stathopoulos GT, Yull FE, Blackwell TS. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J Immunol. 2011;187:5703–5711. doi: 10.4049/jimmunol.1100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14:310. doi: 10.1186/1471-2407-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JL, Chen GW, Liu YC, Wang PY, Wang X, Wan YL, Zhu J, Gao HQ, Yin J, Wang W, Tian ML. Secreted protein acidic and rich in cysteine (SPARC) suppresses angiogenesis by down-regulating the expression of VEGF and MMP-7 in gastric cancer. PLoS One. 2012;7:e44618. doi: 10.1371/journal.pone.0044618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xiao A, diPierro CG, Carpenter JE, Abdel-Fattah R, Redpath GT, Lopes MB, Hussaini IM. An extensive invasive intracranial human glioblastoma xenograft model: role of high level matrix metalloproteinase 9. Am J Pathol. 2010;176:3032–3049. doi: 10.2353/ajpath.2010.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, Tsuneyama K, Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–3583. [PubMed] [Google Scholar]
- Zijlstra A, Seandel M, Kupriyanova TA, Partridge JJ, Madsen MA, Hahn-Dantona EA, Quigley JP, Deryugina EI. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006;107:317–327. doi: 10.1182/blood-2005-04-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]