Abstract
Introduction
Root canal disinfection and the establishment of an intracanal microenvironment conducive to the proliferation/differentiation of stem cells play a significant role in regenerative endodontics. This study was designed to (1) investigate the antimicrobial efficacy of triple antibiotic–containing nanofibers against a dual-species biofilm and (2) evaluate the ability of dental pulp stem cells (DPSCs) to adhere to and proliferate on dentin upon nanofiber exposure.
Methods
Seven-day-old dual-species biofilm established on dentin specimens was exposed for 3 days to the following: saline (control), antibiotic-free nanofibers (control), and triple antibiotic–containing nanofibers or a saturated triple antibiotic paste (TAP) solution (50 mg/mL in phosphate buffer solution). Bacterial viability was assessed using the LIVE/DEAD assay (Molecular Probes, Inc, Eugene, OR) and confocal laser scanning microscopy. For cyto-compatibility studies, dentin specimens after nanofiber or TAP (1 g/mL in phosphate buffer solution) exposure were evaluated for cell adhesion and spreading by actin-phalloidin staining. DPSC proliferation was assessed on days 1, 3, and 7. Statistics were performed, and significance was set at the 5% level.
Results
Confocal laser scanning microscopy showed significant bacterial death upon antibiotic-containing nanofiber exposure, differing significantly (P < .05) from antibiotic-free fibers and the control (saline). DPSCs showed enhanced adhesion/spreading on dentin specimens treated with antibiotic-containing nanofibers when compared with its TAP counterparts. The DPSC proliferation rate was similar on days 1 and 3 in antibiotic-free nanofibers, triple antibiotic–containing nanofibers, and TAP-treated dentin. Proliferation was higher (9-fold) on dentin treated with antibiotic-containing nanofibers on day 7 compared with TAP.
Conclusions
Triple antibiotic–containing polymer nanofibers led to significant bacterial death, whereas they did not affect DPSC attachment and proliferation on dentin.
Keywords: Antibiotic, disinfection, electrospinning, nanofibers, regeneration, stem cells
Over the past decade, regenerative endodontics has been deemed a clinically viable alternative to apexification in the treatment of necrotic immature permanent teeth (1). It uses the concept of tissue engineering to promote the restitution of pulpal function and subsequent root maturation. It is worth mentioning that the success of this therapy is heavily linked to 2 major clinical aspects: effective root canal disinfection and the establishment of a microenvironment conducive to the proliferation and differentiation of stem cells (1).
During infection, bacteria form biofilm on canal walls, in isthmuses and lateral canals, inside dentinal tubules, and on apical extraradicular root surfaces (2). In necrotic immature permanent teeth, root canal instrumentation must be avoided to prevent further weakening of root dentinal walls (3). Moreover, the continuous use of calcium hydroxide as an intracanal medicament has been shown to not only negatively impact the biomechanical properties of dentin (4) but also to be ineffective in cases of persistent infections (5). To date, the most widely used antibiotic mixture for disinfection is an equal blend of metronidazole, minocycline, and ciprofloxacin (ie, triple antibiotic paste [TAP]). TAP studies have shown the antimicrobial efficacy on the root canal space and deep layers of dentin (6, 7). However, it is well established that the initially recommended clinical dose of TAP (1 g/mL) is very toxic to dental stem cells (8, 9) as well as periodontal ligament fibroblasts (10). Moreover, recent findings have shown that TAP interferes with growth factor release from the dentin matrix, which, in turn, may limit regenerative outcomes (11). Several other drawbacks associated with its use include difficulty with its complete removal from the canals (12) and crown discoloration (13). Thus, there is a critical need for a clinical strategy that can decontaminate the root canal system without impairing the attachment/proliferation and differentiation of stem cells to allow for re-establishment of the pulp-dentin complex function.
The Bottino group recently reported on the synthesis of novel and innovative, biodegradable polymer-based drug delivery systems consisting of antibiotic-containing nanofibers for root canal disinfection (1, 14–22). By tuning the antibiotics’ concentration, they showed minimal cytotoxicity and successful biofilm eradication when tested using both Actinomyces naeslundii– and Porphyromonas gingivalis–infected dentin models (1, 14–23). The present study was designed to
1. investigate the antimicrobial efficacy of triple antibiotic–containing nanofibers against a dual-species biofilm and
2. evaluate the ability of dental pulp stem cells (DPSCs) to adhere to and proliferate on dentin upon nanofiber exposure.
Materials and Methods
Nanofiber Fabrication and Confocal Laser Scanning Microscopic Evaluation of Dual-species Bacterial Biofilm Viability
Triple antibiotic (metronidazole, minocycline, and ciprofloxacin; Sigma-Aldrich, St Louis, MO)-containing polymer (polydioxanone [PDS]; Ethicon, Somerville, NJ]) fibers were fabricated via electrospinning (15). Briefly, a 10 wt% PDS polymer solution was prepared in hexafluoro-2-propanol (Sigma-Aldrich). The 3 antibiotics were added to the PDS solution at 30 wt% concentration (relative to the total PDS [600 mg] weight; ie, 180 mg of each antibiotic) and mixed together via stirring. Antibiotic-free PDS (control) and the triple antibiotic–containing polymer solutions were spun into fibers at 2 mL/h, 18-cm distance, and 15–19 kV. After processing, the fibers were dried under a vacuum for 2 days to eliminate any residual solvent and stored in the refrigerator (4°C) until used (15, 23).
A dual-species biofilm was established on dentin. Two gram-positive, facultative anaerobic bacteria, A. naeslundii and Enterococcus faecalis, were selected, because A. naeslundii, a rod-shaped bacterium, is prevalent in immature infected root canals and E. faecalis is a cocci bacterium responsible for secondary infections in necrotic teeth after treatment (24–26). This research was approved by the Indiana University Institutional Review Board (#1407656657). Twenty-four human, caries-free, nonrestored canines were used to obtain 4 × 4 × 1 mm radicular dentin specimens (15). To remove the smear layer, all the specimens were placed in an ultrasonic bath containing 2.5% sodium hypochlorite followed by 17% EDTA (InterMed, Inc, Racine, WI) solutions for 3 minutes each. All specimens were rinsed in saline solution for 10 minutes and autoclaved at 121°C. Next, the specimens were randomly placed into the wells of a 24-well plate containing 800 μL sterile brain-heart infusion broth (Difco Laboratories Inc, Detroit, MI), and bacterial suspensions (100 μL A. naeslundii [ATCC 43146] and E. faecalis [ATCC 19433]) were inoculated into each well and allowed to grow for 7 days at 37°C in an incubator for biofilm development. The broth was changed every other day. Scanning electron microscopy (SEM) (JSM-5310LV; JEOL, Tokyo, Japan) was performed to qualitatively evaluate biofilm formation. After 1 week, all specimens were rinsed for 1 minute (2×) with phosphate buffer solution (PBS, Sigma-Aldrich) to remove loosely bound bacterial cells; they were then transferred to the wells of fresh 24-well plates. In brief, ultraviolet-irradiated triple antibiotic–containing and antibiotic-free nanofibers (15 × 15 mm) were adapted to plastic inserts (CellCrown; Scaffdex Ltd, Tampere, Finland), placed into wells containing the infected dentin specimens immersed in 1 mL PBS, and then incubated at 37°C under aerobic conditions for 7 days. Similarly, a triple antibiotic solution (TAP solution, 50 mg/mL) was used as the positive control. Untreated dentin specimens with 7-day-old biofilm served as the negative control. Next, all specimens were rinsed in PBS (2×) to remove unbound bacteria followed by staining with the fluorescent LIVE/DEAD BacLight Bacterial Viability Kit L-7012 (Molecular Probes, Inc, Eugene, OR) (23). A total of 16 specimens (n = 4/group) and 5 randomly selected microscopic fields were scanned using confocal laser scanning microscopy, starting from the edges of the specimen to obtain 20 measurements per group. The images were acquired with a confocal/2-photon Leica TCS SP8 system (Leica Microsystems Inc, Buffalo Grove, IL) using the Leica HC PL APO 40 × /1.3 oil-immersion objective. A series of sections through the depth of tissue (Z stacks) were collected using optimal step size settings (0.35 μm) at a resolution of 512 × 512 pixels (221 × 221 μm2). Data quantification and 3-dimensional volume reconstruction were performed (Imaris 7.7; Bitplane USA, South Windsor, CT). Volume images were processed to extract a statistical parameter of live and dead bacteria. The data were presented as a percentage of live and dead bacterial cells (23). Statistical analysis was performed using 1-way analysis of variance at a significance level of P < .05.
Triple Antibiotic–containing Nano?ber Effects on DPSC Function
Human mandibular incisors were longitudinally cut with a diamond disc to obtain 2 halves measuring 10 × 5 × 0.6 mm, as previously reported (15). After standard finishing procedures with SiC papers (600–1200 grit), the radicular specimens (n = 10/group) were immersed in 10 mL saline solution and placed in an ultrasonic bath (2×) for 15 minutes each (15). All specimens were placed in new glass vials containing 10 mL 17% EDTA in an ultrasonic bath for 3 minutes to remove the smear layer (15). The specimens were rinsed again in saline before disinfection and then exposed to antibiotic-free and triple antibiotic–containing nanofibers (10 × 5 mm) as well as TAP (1 g/mL) (Champs Pharmacy, San Antonio, TX) for 7 days. Specimens immersed in 1 mL saline served as the control group. After 7 days, the specimens were rinsed with 10 mL saline followed by 3 mL EDTA for 3 minutes, and a final abundant rinsing step with saline was performed before cell seeding. Human DPSCs (AllCells, LLC, Alameda, CA) were cultured in alpha-minimum essential medium (HyClone Laboratories, Inc, Logan, UT) supplemented with 10% fetal bovine serum (HyClone), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich) at 37°C with 5% CO2. In brief, 1 × 104 cells at passages 2 to 4 were seeded on the dentin-treated specimens (ie, exposure to the disinfection strategies). Cell adhesion and cytoskeletal morphology were assessed using SEM and confocal laser scanning microscopy. For SEM, the dentin specimens were gently rinsed with PBS fixed in 3.7% formaldehyde, rinsed again in PBS, dehydrated using ethanol gradients, soaked in several ethanol/hexamethyldisilazane gradients, and incubated in 100% hexamethyldisilazane. Finally, the specimens were mounted on Al stubs and sputter coated with Au-Pd before imaging. Meanwhile, for confocal laser scanning microscopic (CLSM) imaging, specimens (n = 4/group) were analyzed for actin filament assembly. After days 1 and 3 postseeding, the cells were fixed in 3.7% formaldehyde and stained using the fluorescent dye rhodamine phalloidin (Molecular Probes). The specimens were incubated with rhodamine phalloidin for 1 hours, washed in PBS (3×), counterstained with 4′, 6-diamidino-2-phenylindole dihydrochloride, and then mounted with antifading solution (Vectashield; Vector Laboratories, Inc, Burlingame, CA). Images were captured using a confocal/2-photon Olympus FV1000 MPE system (Olympus America, Center Valley, PA) using a XLUMPLFL 20XW objective with 0.95 NA.
For cell proliferation, 1 × 104 cells at passages 2 through 4 were seeded on the dentin specimens as described previously. Cell proliferation (n = 3/group) was evaluated using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI) up to 7 days. In brief, 30 μL reagent was added to 300 μL media in each well and allowed to react for 3 hours at 37°C in a humidified 5% CO2 atmosphere. After incubation, 100 mL from each well was transferred into the wells of a 96-well plate and the absorbance measured at 490 nm (Thermomax; Molecular Devices, LLC, Sunnyvale, CA). Data were expressed as the mean ± standard deviation. Differences between groups were analyzed using 1-way analysis of variance at a significance level of P < .05.
Results
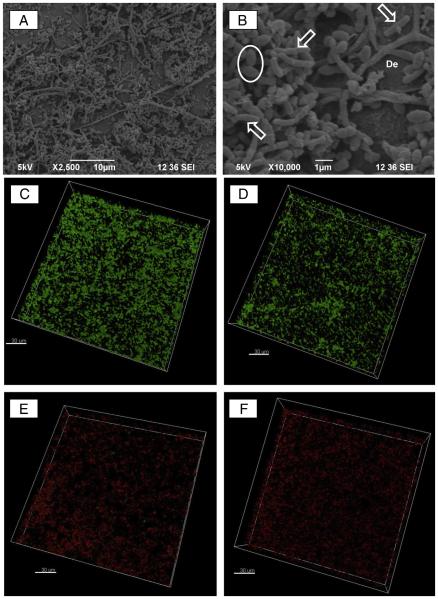
Scanning electron microscopic images reveal a homogeneous accumulation of A. naeslundii and E. faecalis as a dual-species biofilm after 7 days of culture on dentin (Fig. 1A and B). Morphologically, it is possible to identify both the cocci-shaped E. faecalis and rod-shaped A. naeslundii bacterial cells in high numbers over most of the dentin surface. CLSM analysis showed efficient and significant elimination (Table 1) of viable bacteria upon triple antibiotic–containing polymer nanofiber (Fig. 1E) exposure when compared with the control (saline, Fig. 1C) and antibiotic-free nanofibers (Fig. 1D). TAP solution was equally effective in killing bacteria as exhibited by the percentage of dead cells in CLSM images (Fig. 1F).
Figure 1.
Representative scanning electron microscopic micrographs of a 7-day biofilm formed by dual (A. naeslundii and E. faecalis) bacterial species. (A)A lower-magnification image showing a homogeneous distribution of the 2 bacterial cells; (B) a higher-magnification image of A revealing the rod-shaped A. naeslundii and cocci-shaped E. faecalis bacterial cells over the dentin (De) surface. CLSM images were collected in the sequential illumination mode by using 488-nm and 552-nm laser lines. Live bacteria presenting intact cell membranes were dyed green (SYTO 9), whereas dead bacteria with damaged membranes were stained red (propidium iodide). Fluorescent emission was collected in 2 HyD spectral detectors with a filter range set up to 500–550 nm and 590–655 nm for green (SYTO 9) and red dye (propidium iodide), respectively. CLSM images of (C) 7-day dual-species biofilm (negative control) growth inside dentinal tubules, (D) dual-species biofilm exposed to antibiotic-free nanofibers, (E) triple antibiotic-containing nanofibers, and (F) TAP solution for 7 days (bar = 30 μm).
TABLE 1.
Percentage of Live and Dead Bacterial Cells Cultured on Dentin after Exposure to the Triple Antibiotic–containing Nanofibers and Triple Antibiotic Paste (TAP) Solution
| Bacteria (%) ± Standard deviation (minimum-maximum) | ||
|---|---|---|
|
|
||
| Group | Live | Dead |
| Biofilm (no treatment, control) | 94.76 ± 6.92 (73.92–99.92) | 5.24 ± 6.92 (0.08–26.08) |
| Antibiotic-free nanofibers | 97.73 ± 2.94 (87.30–100.00) | 2.27 ± 2.94 (0.00–12.70) |
| Triple antibiotic–containing nanofibers | 4.68 ± 8.38 (0.01–29.76) | 95.32 ± 8.38 (81.61–99.99) |
| TAP solution (50 mg/mL) | 6.67 ± 7.93 (0.00–34.21) | 93.33 ± 7.93 (65.79–100.00) |
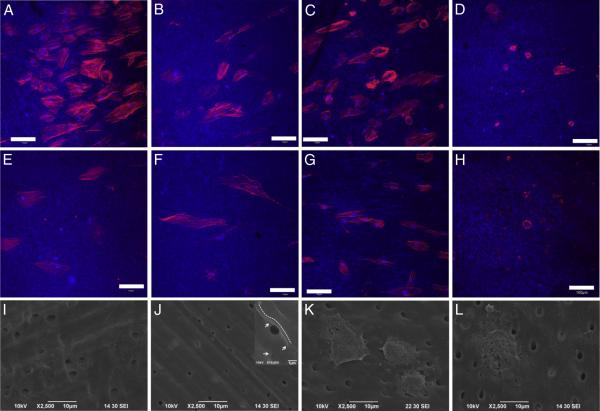
Qualitative representative CLSM images showed enhanced DPSC adhesion and spreading on triple antibiotic–containing treated dentin when compared with TAP (Fig. 2). Cell spreading was comparable among the control (Fig. 2A), antibiotic-free nanofibers (Fig. 2B), and triple antibiotic–containing nanofibers (Fig. 2C), whereas TAP-treated dentin displayed mostly round cells that did not spread (Fig. 2D) on day 1. Cells on day 3 on the control (Fig. 2E), antibiotic-free nanofibers (Fig. 2F), and triple antibiotic–containing nanofibers (Fig. 2H) spread more than on day 1. However, there was no improvement in cell spreading on TAP-treated dentin (Fig. 2G).
Figure 2.
DPSC adhesion and spreading on treated dentin on days 1, 3, and 7 after cell seeding. The images are maximum intensity projections of Z slices (depth ,~ 20 μm). Dentin was viewed using an excitation wavelength of 405 nm and is false-colored blue. Rhodamine phalloidin has excitation and emission (nm) at 540/565 nm, and the actin filaments appear as red (red: actin, blue: dentin, light blue: nucleus). Representative CLSM images showing cell adhesion on day 1 on dentin treated for 7 days (n = 4/group) with (A) saline (control), (B) antibiotic-free nanofibers, (C) triple antibiotic–containing nanofibers, and (D) TAP (1g capsule containing equal parts of ciprofloxacin, minocycline, and metronidazole in 1 mL PBS). CLSM images displaying cell adhesion on day 3 on (E) control, (F) antibiotic-free nanofibers, (G) triple antibiotic–containing nanofibers, and (H) TAP (bar = 100 μm). Representative scanning electron microscopic micrographs showing cell morphology (day 7) on dentin-treated specimens with (I) saline (control), (J) antibiotic-free nanofibers (inset [10,000× original magnification] clearly shows cells covering the dentin surface and dentinal tubules); please note the dotted white line revealing the cell’s contour and the white arrows pointing out the dentin substrate, (K) triple antibiotic–containing nanofibers, and (L) TAP.
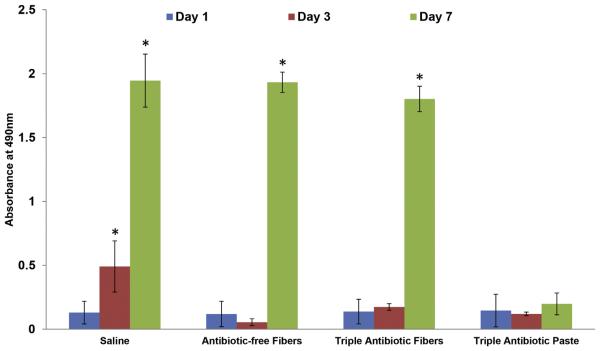
Scanning electron microscopic images showed cell-covered dentin on day 7 for the control (Fig. 2I), antibiotic-free (Fig. 2J), and triple antibiotic–containing (Fig. 2K) nanofibers. Meanwhile, cells cultured on TAP-treated dentin displayed an irregular morphology on day 7 (Fig. 2L). Although the proliferation rate was similar on days 1 and 3 in antibiotic-free nanofibers, triple antibiotic–containing nanofibers, and TAP-treated dentin specimens, cell proliferation was significantly higher (9-fold, P < .001) on dentin treated with triple antibiotic–containing nanofibers on day 7 when compared with TAP (Fig. 3).
Figure 3.
Cell proliferation on dentin specimens after exposure to the triple antibiotic–containing nanofibers and TAP. Statistical analysis compared the results of days 1, 3, and 7 of each sample (n = 3).
Discussion
In the past decade, tissue engineering strategies have been more dynamically explored as a clinical strategy to regenerate the pulp-dentin complex of immature permanent teeth with pulpal necrosis (1). It is important to bear in mind that successful regenerative endodontics require effective root canal disinfection with no or minimal harm to stem cells and growth factor release present within the dentin matrix (27). To that end, the concept of antibiotic-releasing nanofibers was recently established and broadly investigated by our group as a more cell-friendly disinfection approach (14–18, 28). Initially, our studies with single antibiotic–containing electrospun polymer nanofibers (ie, metronidazole or ciprofloxacin) showed both the inhibition and prevention of growth (16, 17) of several bacteria including Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, P. gingivalis, and E. faecalis as evidenced by numerous agar diffusion assays and spiral plating (colony-forming units [CFUs]/mL) experiments. More recently, based on the significant antimicrobial efficacy verified in the aforementioned studies, we have expanded our efforts to address the disinfection potential of antibiotic-containing polymer nanofibers using different infected dentin in vitro models. A 5-day-old E. faecalis young biofilm developed on human root fragments was exposed (ie, direct contact) to ciprofloxacin-containing (5 and 25 wt%) nanofibers for 48 hours. Antimicrobial assays involving the use of CFUs and qualitative imaging via SEM revealed maximum bacterial biofilm elimination upon 25 wt% ciprofloxacin nanofiber exposure (15). As previously mentioned, we have successfully synthesized triple antibiotic–containing polymer nanofibers with adequate mechanical and antibiotic release properties with a favorable burst in 24 hours while sustaining its effects up to 14 days (23). Of note, the antimicrobial efficacy of the triple antibiotic–containing nanofibers presented herein has been demonstrated against infected dentin A. naeslundii (23) and P. gingivalis biofilms (14) using CLSM and CFU/mL experiments, respectively.
Earlier studies have detected the presence of A. naeslundii in 10 of 15 cases (66.67%) in the root canals of infected immature teeth (25). Meanwhile, a high prevalence of E. faecalis (90%) (24) was identified in infected root canals and has been frequently associated with failed asymptomatic endodontic therapy and canals with persistent infections (26, 29). Here, as a continuation of our previous research, we aimed to evaluate the effectiveness of these unique nanofibers against a dual-species bacterial biofilm and mimic their eventual clinical use by investigating the adhesion and proliferation abilities of DPSCs on dentin after nanofiber exposure. After 7 days of exposure, no live bacteria were detected on dentin treated with triple antibiotic–containing nanofibers and TAP solution. A recent study showed that lower concentrations of TAP at 0.39 μg/mL had no cytotoxicity to DPSCs and apical papilla cells but could not completely eliminate all endodontic bacteria (9). In the present study, dentin biofilm exposure for 7 days with innovative triple antibiotic–containing polymer nanofibers was effective in promoting significant bacterial death, whereas it did not affect DPSC attachment and proliferation abilities. It is well-known that dentin is also a reservoir of bioactive growth factors, such as vascular endothelial growth factor (30) and transforming growth factor beta 1 (31), which aid in the survival, proliferation, and, more importantly, differentiation of dental stem cells. Based on our data, one could assume that leached-out morphogens might have supported the proliferation of DPSCs on dentin. The proliferation rate on dentin treated with the triple antibiotic–containing nanofibers was similar to the untreated (control), showing that the proposed intracanal drug delivery strategy had no damaging effects on stem cell attachment and viability. Conversely, recent research has shown that the use of TAP interferes with growth factor release (11) because its removal is very challenging (12), leading to the interpretation that paste blocks dentinal tubules preventing growth factors from leaching out, which might also have limited the growth of DPSCs on TAP-treated dentin. Moreover, the prolonged use of the acidic in natural TAP (pH = 2.9) has been shown to promote a significant reduction in dentin microhardness (32). Future studies are warranted to evaluate potential structural changes and demineralization of dentin upon triple antibiotic–containing nanofiber exposure.
Collectively, the findings from this study further support our working hypothesis (ie, nanofiber-based drug delivery systems are a more cell-friendly disinfection strategy than the currently used TAP, which, in turn, may contribute to better and more predictable regenerative outcomes). In summary, the results of the present study, combined with groundwork research on the synthesis of 3-dimensional tubular drug delivery constructs recently reported by our group (17, 21), place us in an ideal position to explore its clinical potential using a preclinical in vivo model of apical periodontitis.
Highlights.
Antibiotic nanofibers led to significant biofilm death.
Antibiotic nanofibers are a more cell-friendly disinfection strategy than triple antibiotic paste (TAP).
Dental pulp stem cell proliferation was higher (day 7) on fiber-treated dentin compared with TAP.
Significance.
Nanofiber-based drug delivery systems are a more cell-friendly disinfection strategy than the currently used triple antibiotic paste, which, in turn, may contribute to better and more predictable regenerative outcomes.
Acknowledgments
Supported by start-up funds from Indiana University School of Dentistry, an International Development Funds (IDF) Grant from Indiana University Purdue University (IUPUI/OVCR), and the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) (Grant #DE023552) (M.C.B.). Supported by the Indiana Clinical and Translational Sciences Institute, funded in part by grant #UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors deny any conflicts of interest related to this study.
References
- 1.Albuquerque MT, Valera MC, Nakashima M, et al. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res. 2014;93:1222–31. doi: 10.1177/0022034514549809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricucci D, Loghin S, Siqueira JF., Jr Exuberant biofilm infection in a lateral canal as the cause of short-term endodontic treatment failure: report of a case. J Endod. 2013;39:712–8. doi: 10.1016/j.joen.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Endod Dent Traumatol. 1992;8:45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 5.Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endod. 2002;28:689–93. doi: 10.1097/00004770-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino E, Kurihara-Ando N, Sato I, et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–30. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 7.Sato I, Ando-Kurihara N, Kota K, et al. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118–24. doi: 10.1111/j.1365-2591.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–5. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Chuensombat S, Khemaleelakul S, Chattipakorn S, Srisuwan T. Cytotoxic effects and antibacterial efficacy of a 3-antibiotic combination: an in vitro study. J Endod. 2013;39:813–9. doi: 10.1016/j.joen.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Yadlapati M, Souza LC, Dorn S, et al. Deleterious effect of triple antibiotic paste on human periodontal ligament fibroblasts. Int Endod J. 2014;47:769–75. doi: 10.1111/iej.12216. [DOI] [PubMed] [Google Scholar]
- 11.Galler KM, Buchalla W, Hiller KA, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41:363–8. doi: 10.1016/j.joen.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Berkhoff JA, Chen PB, Teixeira FB, Diogenes A. Evaluation of triple antibiotic paste removal by different irrigation procedures. J Endod. 2014;40:1172–7. doi: 10.1016/j.joen.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Kim Y, Shin SJ, et al. Tooth discoloration of immature permanent incisor associated with triple antibiotic therapy: a case report. J Endod. 2010;36:1086–91. doi: 10.1016/j.joen.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Albuquerque MT, Evans JD, Gregory RL, et al. Antibacterial TAP-mimic electrospun polymer scaffold: effects on P. gingivalis-infected dentin biofilm. Clin Oral Investig. 2016;20:387–93. doi: 10.1007/s00784-015-1577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albuquerque MT, Valera MC, Moreira CS, et al. Effects of ciprofloxacin-containing scaffolds on enterococcus faecalis biofilms. J Endod. 2015;41:710–4. doi: 10.1016/j.joen.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 16.Bottino MC, Arthur RA, Waeiss RA, et al. Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig. 2014;18:2151–8. doi: 10.1007/s00784-014-1201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bottino MC, Kamocki K, Yassen GH, et al. Bioactive nanofibrous scaffolds for regenerative endodontics. J Dent Res. 2013;92:963–9. doi: 10.1177/0022034513505770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottino MC, Yassen GH, Platt JA, et al. A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J Tissue Eng Regen Med. 2015;9:E116–23. doi: 10.1002/term.1712. [DOI] [PubMed] [Google Scholar]
- 19.Kamocki K, Nor JE, Bottino MC. Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability-in vitro studies. Arch Oral Biol. 2015;60:1131–7. doi: 10.1016/j.archoralbio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamocki K, Nor JE, Bottino MC. Dental pulp stem cell responses to novel antibioticcontaining scaffolds for regenerative endodontics. Int Endod J. 2015;48:1147–56. doi: 10.1111/iej.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palasuk J, Kamocki K, Hippenmeyer L, et al. Bimix antimicrobial scaffolds for regenerative endodontics. J Endod. 2014;40:1879–84. doi: 10.1016/j.joen.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter ML, Munchow EA, Albuquerque MT, et al. Effects of novel 3-dimensional antibiotic-containing electrospun scaffolds on dentin discoloration. J Endod. 2016;42:106–12. doi: 10.1016/j.joen.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albuquerque MT, Ryan SJ, Munchow EA, et al. Antimicrobial effects of novel triple antibiotic paste-mimic scaffolds on Actinomyces naeslundii biofilm. J Endod. 2015;41:1337–43. doi: 10.1016/j.joen.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sassone LM, Fidel R, Faveri M, et al. Microbiological evaluation of primary endodontic infections in teeth with and without sinus tract. Int Endod J. 2008;41:508–15. doi: 10.1111/j.1365-2591.2008.01397.x. [DOI] [PubMed] [Google Scholar]
- 25.Nagata JY, Soares AJ, Souza-Filho FJ, et al. Microbial evaluation of traumatized teeth treated with triple antibiotic paste or calcium hydroxide with 2% chlorhexidine gel in pulp revascularization. J Endod. 2014;40:778–83. doi: 10.1016/j.joen.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 27.Diogenes AR, Ruparel NB, Teixeira FB, Hargreaves KM. Translational science in disinfection for regenerative endodontics. J Endod. 2014;40:S52–7. doi: 10.1016/j.joen.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Waeiss RA, Negrini TC, Arthur RA, Bottino MC. Antimicrobial effects of drug-containing electrospun matrices on osteomyelitis-associated pathogens. J Oral Maxillofac Surg. 2014;72:1310–9. doi: 10.1016/j.joms.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Gomes BP, Pinheiro ET, Sousa EL, et al. Enterococcus faecalis in dental root canals detected by culture and by polymerase chain reaction analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:247–53. doi: 10.1016/j.tripleo.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 2000;45:1013–6. doi: 10.1016/s0003-9969(00)00075-3. [DOI] [PubMed] [Google Scholar]
- 31.Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42:219–23. doi: 10.1016/S0003-9969(96)00115-X. [DOI] [PubMed] [Google Scholar]
- 32.Yassen GH, Eckert GJ, Platt JA. Effect of intracanal medicaments used in endodontic regeneration procedures on microhardness and chemical structure of dentin. Restor Dent Endod. 2015;40:104–12. doi: 10.5395/rde.2015.40.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]