Abstract
Background
Approximately 1.5 million HIV-positive women become pregnant annually. Without treatment, up to 45% will transmit HIV to their infants, primarily through breastfeeding. These numbers highlight that HIV acquisition is a major health concern for women and children globally. They also emphasize the urgent need for novel approaches to prevent HIV acquisition that are safe, effective and convenient to use by women and children in places where they are most needed.
Methods
4′-Ethynyl-2-fluoro-2′-deoxyadenosine, a potent NRTI with low cytotoxicity, was administered orally to NOD/SCID/γc−/− mice and to bone marrow/liver/thymus (BLT) humanized mice, a preclinical model of HIV infection. HIV inhibitory activity in serum, cervicovaginal secretions and saliva was evaluated 4 h after administration. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine's ability to prevent vaginal and oral HIV transmission was evaluated using highly relevant transmitted/founder viruses in BLT mice.
Results
Strong HIV inhibitory activity in serum, cervicovaginal secretions and saliva obtained from animals after a single oral dose of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (10 mg/kg) demonstrated efficient drug penetration into relevant mucosal sites. A single daily oral dose of 4′-ethynyl-2-fluoro-2′-deoxyadenosine resulted in efficient prevention of vaginal and oral HIV transmission after multiple high-dose exposures to transmitted/founder viruses in BLT humanized mice.
Conclusions
Our data demonstrated that 4′-ethynyl-2-fluoro-2′-deoxyadenosine efficiently prevents both vaginal and oral HIV transmission. Together with 4′-ethynyl-2-fluoro-2′-deoxyadenosine's relatively low toxicity and high potency against drug-resistant HIV strains, these data support further clinical development of 4′-ethynyl-2-fluoro-2′-deoxyadenosine as a potential pre-exposure prophylaxis agent to prevent HIV transmission in women and their infants.
Introduction
HIV acquisition is a major health concern for women and children globally. Most new HIV infections occur in women living in resource-limited countries where gender, cultural and economic barriers limit the ability of women to access and use HIV prevention options.1 The majority of HIV-positive women are of reproductive age and 1.5 million HIV-positive women become pregnant every year.2 Without treatment, up to 45% will transmit HIV to their infants, mostly through breastfeeding.3 In resource-limited countries, HIV-positive women breastfeed their infants despite the risk of HIV transmission due to the health benefits of breast milk. Although paediatric HIV infection is associated with an accelerated course of disease and increased mortality, only 31% of HIV-infected children receive ART.4 Therefore, there is an urgent need for novel approaches to prevent HIV acquisition that are safe, effective and convenient to use by women and children in places where they are most needed.
Antiretroviral (ARV) pre-exposure prophylaxis (PrEP), a prevention strategy in which ARVs are administered to HIV-uninfected individuals, can significantly reduce HIV transmission.5–8 In women, PrEP can reduce vaginal HIV transmission up to 75%.7,8 Mother-to-child HIV transmission through breastfeeding is also significantly reduced by daily administration of ARVs to infants and/or their mothers.9–12 Despite the demonstrated efficacy of PrEP to reduce HIV transmission to women and infants, these strategies often require extended ARV use prompting concerns about potential drug toxicity and the emergence and transmission of drug-resistant HIV strains if PrEP fails; e.g. infants that receive daily oral nevirapine, the current first-line ARV for infant PrEP, are at a significantly increased risk of acquiring nevirapine resistance if infected.13 For these reasons, it is essential to develop novel ARVs with low toxicity and increased potency against clinically relevant HIV strains.
4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA, MK-8591), a novel NRTI, has potent anti-HIV activity several orders of magnitude higher than currently approved NRTIs14–16 and an improved intracellular half-life.16,17 Importantly, 4′-ethynyl-2-fluoro-2′-deoxyadenosine exhibits low cytotoxicity due to nearly negligible incorporation into human mitochondrial DNA by DNA polymerase γ.14,18,19 Furthermore, 4′-ethynyl-2-fluoro-2′-deoxyadenosine retains significant potency against a broad range of clinically important drug-resistant isolates, including HIV strains containing the K65R RT mutation, which is associated with resistance to tenofovir, an ARV included in all PrEP regimens evaluated in women to date.18,20 Studies on the in vivo protective efficacy of 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP are very limited21 and it is currently not known if 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP can prevent vaginal or oral HIV transmission of clinically relevant HIV-1 strains. In this study, we utilized bone marrow/liver/thymus (BLT) humanized mice22–28 individually bioengineered from NOD/SCID/γc−/− (NSG) immunodeficient mice, as a preclinical in vivo model to evaluate the efficacy of 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP to prevent vaginal and oral HIV transmission following multiple high-dose challenges with relevant transmitted/founder (T/F) viruses. Our results support further clinical development of 4′-ethynyl-2-fluoro-2′-deoxyadenosine as a potential PrEP agent to prevent HIV transmission to women and their infants.
Materials and methods
Experimental design
To evaluate penetration of 4′-ethynyl-2-fluoro-2′-deoxyadenosine into the cervicovaginal and oral mucosa, cervicovaginal secretions (CVS) and saliva were collected 4 h after a single oral administration of 10 mg/kg 4′-ethynyl-2-fluoro-2′-deoxyadenosine. In vitro HIV inhibitory activity of serum, CVS and saliva obtained from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and control untreated animals was assessed with a TZM-bl cell-based HIV infection assay.
The efficacy of oral 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP to prevent vaginal and oral HIV infection in vivo was evaluated by exposing 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and untreated BLT humanized mice vaginally (HIV-1JR-CSF, HIV-1CH040 and HIV-1RHPA) or orally (HIV-1JR-CSF, HIV-1CH4419 and HIV-1THRO) to three consecutive high-dose HIV challenges. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine was administered to BLT mice via oral gavage (10 mg/kg) once daily for 8 days and mice were challenged with HIV 3–4 h after the second, fourth and sixth 4′-ethynyl-2-fluoro-2′-deoxyadenosine doses. A power analysis was performed to determine experimental group sizes needed to achieve ∼90% power. BLT mice were randomly assigned to 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and untreated (control) HIV exposure groups and a Mann–Whitney U-test was performed to ensure that peripheral blood humanization levels (%hCD45 and %hCD4) between exposure groups were equivalent. Following HIV exposure, HIV infection was monitored longitudinally in the peripheral blood of BLT mice by measuring plasma HIV-RNA levels with real-time PCR and CD4+ T cell levels with flow cytometry. At necropsy, HIV-DNA levels in tissues were determined using real-time PCR (assay limit of detection: 10 copies). Protection in 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated BLT mice was defined by the absence of detectable HIV-RNA in peripheral blood plasma and HIV-DNA in tissues at necropsy (5–8 weeks post-4′-ethynyl-2-fluoro-2′-deoxyadenosine initiation). Amplified HIV gag was sequenced to determine the identity of the transmitting virus(es). The study was not blinded and no HIV-exposed 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated or untreated BLT mice were excluded from the data analysis and no outliers were defined.
Ethics
Mice were maintained under specific pathogen-free conditions by the Division of Laboratory Animal Medicine at the University of North Carolina–Chapel Hill. All animal experiments were conducted following NIH guidelines for the housing and care of laboratory animals and in accordance to protocols reviewed and approved by the Institutional Animal Care and Use Committee at the University of North Carolina–Chapel Hill (permit number 15-168).
Preparation of BLT humanized mice
BLT humanized mice were prepared as previously described.22–24,26,28 Briefly, a sandwich of human thymus–liver–thymus tissue was implanted under the kidney capsule of irradiated (200 rads) female NSG mice (The Jackson Laboratory, Bar Harbor, ME, USA). Following tissue implantation, mice received autologous CD34+ haematopoietic stem cells via tail vein injection. Starting at 8 weeks post-transplantation, human immune cell reconstitution was monitored in the peripheral blood of BLT mice longitudinally by flow cytometry as previously described.22–24,26,28 BLT mice are reconstituted with a human immune system generated de novo. The presence of human CD4+ T cells, macrophages and dendritic cells throughout the female reproductive tract (FRT), oral cavity and upper gastrointestinal tract that render BLT mice susceptible to vaginal and oral HIV transmission was previously described.23–25
4′-Ethynyl-2-fluoro-2′-deoxyadenosine preparation, administration and sample collection
4′-Ethynyl-2-fluoro-2′-deoxyadenosine (kindly provided by Dr Parniak, University of Pittsburgh School of Medicine) was reconstituted in sterile PBS at a concentration of 10 mg/mL. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (10 mg/kg) was administered to mice orally once daily by oral gavage. Peripheral blood was collected from animals with capillary tubes coated with or without EDTA to isolate plasma or serum, respectively. CVS were obtained by performing a cervicovaginal lavage with sterile PBS (three washes of 20 μL each, ∼60 μL total volume). Saliva was collected following an intraperitoneal injection of 0.1–0.3 mg pilocarpine in sterile PBS (Sigma-Aldrich, St Louis, MO, USA) to stimulate saliva production. All samples were stored at −80°C until analysis.
Generation and quantification of HIV
Stocks of HIV-1JR-CSF, HIV-1CH040, HIV-1RHPA, HIV-1CH4419 and HIV-1THRO29–33 were generated as previously described25,28 and titred on TZM-bl cells (NIH AIDS Research and Reference Reagent Program) to quantify the number of tissue culture infectious units (TCIU)/mL.
TZM-bl cell culture and in vitro HIV-1 inhibition by 4′-ethynyl-2-fluoro-2′-deoxyadenosine
We used a TZM-bl cell-based HIV infection assay to evaluate the in vitro HIV inhibitory activity of serum, CVS and saliva obtained from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated animals. TZM-bl cells were maintained in DMEM containing 10% FBS, 25 mM HEPES, 500 U/mL penicillin and 500 μg/mL streptomycin (TZM-bl medium) and cultured at 37°C and 5% CO2. TZM-bl cells were plated in 96-well plates at a density of 1 × 105 cells per well in TZM-bl medium. The next day, the medium was removed and 100 μL of diluted serum, CVS or saliva from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated animals collected 4 h after drug administration or from untreated control animals was added. Serum was diluted 1:20, 1:100, 1:500, 1:2500, 1:12 500 and 1:62 500. CVS and saliva were diluted 1:20, 1:40, 1:80, 1:160, 1:320 and 1:640. Cells were incubated for 30 min and 100 μL of TZM-bl medium containing 40 μg/mL DEAE-dextran and 3 × 103 TCIU of HIV-1JR-CSF per well was added. Approximately 48 h later, the medium was removed and the luciferase substrate One-Glo reagent (Promega, Madison, WI, USA) supplemented with 0.2%Triton X-100 was added to allow for the measurement of luciferase activity and to inactivate virus. Luciferase was measured with a SpectraMax M3 Spectrometer (Molecular Devices, Sunnyvale, CA, USA) and the results normalized to the luciferase activity of cells infected with HIV in the absence of serum, CVS or saliva and expressed as a percentage of decrease in luciferase activity.
HIV exposure of BLT mice
To evaluate the ability of 4′-ethynyl-2-fluoro-2′-deoxyadenosine to prevent vaginal HIV transmission following multiple virus challenges, BLT mice were exposed vaginally to HIV-1JR-CSF (first challenge virus), HIV-1CHO40 (second challenge virus) and HIV-1RHPA (third challenge virus) 4 h after administration of the second, fourth and sixth 4′-ethynyl-2-fluoro-2′-deoxyadenosine oral dose. Anaesthetized BLT mice were exposed vaginally to 3.0 × 105 TCIU of HIV by pipetting virus (20 μL) directly into the vaginal cavity. BLT mice were exposed orally to 1.4 × 106 TCIU of HIV-1JR-CSF (first challenge virus), HIV-1CH4419 (second challenge virus) or HIV-1THRO (third challenge virus) 3–4 h after administration of the second, fourth and sixth 4′-ethynyl-2-fluoro-2′-deoxyadenosine oral dose. Oral exposures were performed by placing anaesthetized BLT mice on their backs and pipetting virus (20 μL resuspended in RPMI medium) into their oral cavity as previously described.25,27
Analysis of HIV infection in BLT mice
HIV infection was monitored longitudinally in the peripheral blood of BLT mice by measuring HIV-RNA levels in plasma with a real-time PCR viral load assay (assay sensitivity of 750 HIV-RNA copies/mL) and by determining CD4+ T cell levels with flow cytometric analysis as previously described.22–24,26,28 The presence of HIV-DNA in tissues (spleen, lymph nodes, bone marrow, human thymus, liver, lung and FRT) collected from BLT mice at necropsy was determined by real-time PCR analysis of DNA extracted from 5 × 104–4 × 106 mononuclear cells (assay limit of detection: 10 copies). Mononuclear cells were isolated as previously described.34,35 Briefly, mononuclear cells from human thymus, lymph nodes and spleen were isolated by passing tissue through a cell strainer. Bones were homogenized with a mortar and pestle and homogenate passed through a cell strainer to isolate bone marrow cells. The lung, liver and FRT were cut into small pieces, digested in enzyme cocktail containing collagenase D and DNAse for 30 min at 37°C and then homogenized through a cell strainer. Mononuclear cells were isolated from lung and liver homogenate with a 40%–70% Percoll gradient. Red blood cells were lysed with ammonium/chloride/potassium lysis buffer. As a control for the presence of DNA extracted from human cells, all samples were tested for the presence of human gamma globin DNA by real-time PCR (range: 515–11 807 547 human gamma globin DNA copies).
Sequence analysis of transmitted viruses
The transmitting virus(es) in HIV-infected BLT mice exposed to multiple viruses was/were identified by sequence analysis. Viral RNA was isolated from plasma using QIAamp viral RNA columns (Qiagen, Hilden, Germany) according to the manufacturer's protocol and cDNA was generated using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) with the primer 5′-CTTCCAATTATGTTGACAGGTGTAGG-3′. cDNA was amplified by nested PCR using the Expand High Fidelity PCR System (Roche, Mannheim, Germany). PCR primers were designed to anneal in regions with the fewest possible primer mismatches to HIV-1CH040, HIV-1JR-CSF, HIV-1CH4419, HIV-1RHPA and HIV-1THRO sequences. Primer sequences were as follows: outer forward primer, 5′-CTCAATAAAGCTTGCCTTGAGTGC-3′; outer reverse primer 5′-CTTCCAATTATGTTGACAGGTGTAGG-3′; inner forward primer, 5′-GTGTGGAAAATCTCTAGCAGTGGC-3′; inner reverse primer 5′-CTGTATCATCTGCTCCTGTATCTAATAGAGC-3′. The reverse inner primer 5′-CTGTATCATCTGCTCCTGTGTCTAAGAG-3′ was used to sequence HIV-1CH4419 to increase sensitivity and ensure detection. Amplified viral DNA of gag was sequenced and compared with the sequences of challenge viruses.
To identify the emergence of drug-resistant mutations in the breakthrough BLT mouse infected with HIV-1JR-CSF despite 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP, the reverse transcriptase of HIV-1JR-CSF was sequenced. Viral RNA was extracted from plasma using QIAamp viral RNA kit (Qiagen). cDNA was generated from RNA with Superscript III RT (Invitrogen) with primer 5′-GCTCTATTAGATACAGGAGC-3′ followed by nested PCR with Expand High Fidelity Kit (Roche) to amplify 1.5 kb region of reverse transcriptase. The following primers were used: forward outer primer 5′-CCTAATGCATATTGTGAGTCTG-3′, reverse outer primer 5′-GCTCTATTAGATACAGGAGC-3′, forward inner primer 5′-CCTGCAAAGCTAGGTGAATTGC-3′ reverse inner primer 5′-GTAGGACCTACACCTGTCAAC-3′. Amplified viral DNA of reverse transcriptase was sequenced and compared with the sequence of HIV-1JR-CSF.
FinchTV software (Geospiza, Seattle, WA, USA) was used to analyse sequence chromatograms, NCBI BLAST was used to identify sequences and ClustalW was used to align sequences.
Statistical analysis
A Mantel–Cox log-rank test was used to compare protection between untreated and 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated BLT mice. A two-sided Mann–Whitney U-test was used to compare the in vitro HIV inhibitory activity of serum, CVS and saliva samples obtained from untreated and 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated animals and the CD4+ T cell levels in the peripheral blood and tissues of protected and HIV-infected BLT mice. All statistical analyses were performed using GraphPad Prism software (version 6) (La Jolla, CA, USA).
Results
Efficient penetration of 4′-ethynyl-2-fluoro-2′-deoxyadenosine into the cervicovaginal and oral mucosa
ARVs must adequately penetrate into mucosal sites where HIV exposure occurs to consistently prevent HIV acquisition. The FRT is the first mucosal surface in women that HIV encounters following vaginal exposure. In infants, the oral cavity is the first mucosal surface exposed to HIV during breastfeeding. To assess the ability of 4′-ethynyl-2-fluoro-2′-deoxyadenosine to distribute systemically (peripheral blood) and to penetrate into these two different mucosal sites, we administered a single oral dose (10 mg/kg) of 4′-ethynyl-2-fluoro-2′-deoxyadenosine to NSG or BLT mice. This dose was chosen based on: (i) previously published pharmacokinetic analyses of 4′-ethynyl-2-fluoro-2′-deoxyadenosine in mice; (ii) its ability to inhibit HIV infection following an intraperitoneal HIV exposure; and (iii) its ability to suppress plasma viraemia when administered daily.21 Four hours later, we collected peripheral blood, CVS and saliva from treated and untreated (control) mice and analysed their HIV inhibitory activity using a well-established in vitro assay. Our results indicate that peripheral blood serum from both BLT and NSG mice treated with 4′-ethynyl-2-fluoro-2′-deoxyadenosine contains potent HIV inhibitory activity (Figure 1a and d) suggesting comparable distribution and penetration of active 4′-ethynyl-2-fluoro-2′-deoxyadenosine in peripheral blood from both types of mice (Figure 1a). Similarly, we observed strong HIV inhibitory activity in the CVS from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated mice (P = 0.0016) (Figure 1b and d). Consistent with the known presence of anti-HIV factors in saliva,36 HIV infection was decreased in the presence of saliva from untreated (control) animals (Figure 1c and d). Nevertheless, we were able to document strong HIV inhibitory activity in the saliva from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated animals (P = 0.0161) well above that in the saliva from untreated mice (Figure 1d). Collectively, our analysis indicates that active 4′-ethynyl-2-fluoro-2′-deoxyadenosine is systemically distributed after a single oral administration and that it efficiently penetrates into the FRT and oral cavity, the two primary sites of HIV acquisition in women and infants.
Figure 1.
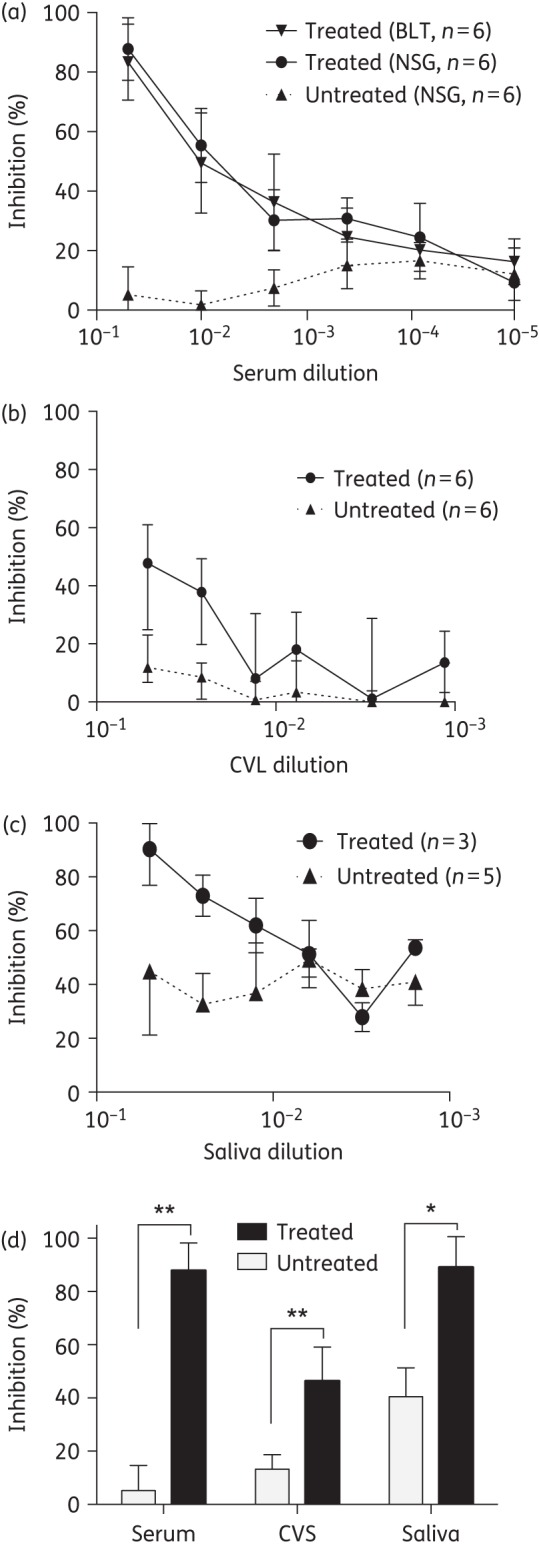
Orally administered 4′-ethynyl-2-fluoro-2′-deoxyadenosine distributes and penetrates into relevant mucosal sites of vaginal and oral HIV transmission. BLT and NSG mice were dosed orally with 10 mg/kg 4′-ethynyl-2-fluoro-2′-deoxyadenosine or left untreated. Four hours later, (a) serum (six untreated mice and six 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated NSG and BLT mice), (b) CVS (six 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and six untreated NSG mice) and (c) saliva (three 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and five untreated NSG mice) were collected and analysed for their ability to inhibit in vitro HIV-1JR-CSF infection of TZM-bl cells. Inhibition is expressed as the percentage decrease in luciferase activity. Results are normalized to the luciferase activity of cells infected with HIV in the absence of serum, CVS or saliva. (d) In vitro HIV inhibitory activity of serum, CVS and saliva from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and untreated mice (diluted 1:20). The HIV inhibitory activity of serum, CVS and saliva from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated and untreated mice was compared using a two-sided Mann–Whitney U-test (*P < 0.05, **P < 0.01).
4′-Ethynyl-2-fluoro-2′-deoxyadenosine PrEP prevents vaginal HIV transmission following multiple high-dose challenges with T/F viruses
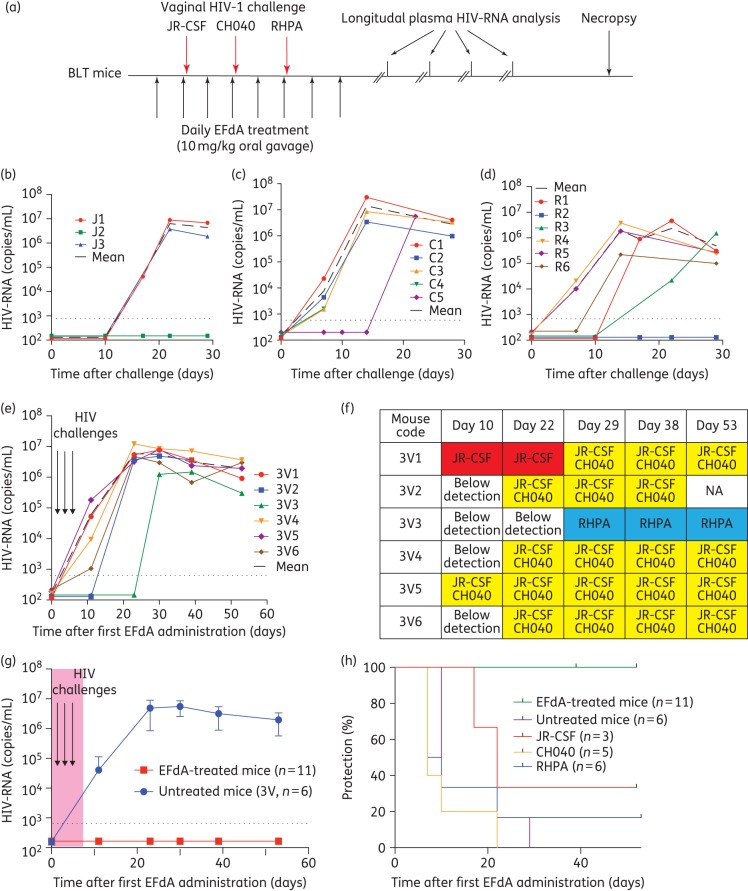
To assess the ability of 4′-ethynyl-2-fluoro-2′-deoxyadenosine to prevent vaginal HIV transmission, we administered 4′-ethynyl-2-fluoro-2′-deoxyadenosine (10 mg/kg) orally to BLT mice (n = 11) once daily for 8 days. Six untreated BLT mice served as positive controls. Mice were exposed vaginally to a high dose of HIV-1JR-CSF (first challenge), HIV-1CH040 (second challenge) and HIV-1RHPA (third challenge) 4 h after the second, fourth and sixth 4′-ethynyl-2-fluoro-2′-deoxyadenosine administration, respectively (Figure 2a). We then monitored the levels of HIV-RNA and CD4+ T cells in peripheral blood longitudinally. Protection from HIV infection was confirmed at necropsy and was defined as: (i) absence of detectable HIV-RNA in plasma; (ii) absence of HIV-DNA in tissues; and (iii) lack of CD4+ T cell depletion.
Figure 2.
Oral EFdA PrEP significantly reduces HIV transmission following multiple high-dose vaginal HIV challenges. (a) EFdA (10 mg/kg) was administered to BLT mice orally for 8 days. Untreated (n = 6) and EFdA-treated (n = 11) BLT mice were exposed vaginally to a high dose (3.0 × 105 TCIU) of HIV-1JR-CSF, HIV-1CH040 and HIV-1RHPA 4 h after administration of the second, fourth and sixth EFdA doses, respectively. Plasma HIV-RNA levels were monitored longitudinally and tissue HIV-DNA levels were analysed at necropsy. Plasma HIV-RNA levels of untreated BLT mice exposed vaginally to a single high dose of HIV-1JR-CSF (n = 3, J1–J3) (b), HIV-1CH040 (n = 5, C1–C5) (c) and HIV-1RHPA (n = 6, R1–R6) (d). (e) Plasma HIV-RNA levels of untreated BLT mice exposed vaginally to high doses of HIV-1JR-CSF, HIV-1CH040 and HIV-1RHPA (n = 6, 3V1–3V6). (f) Sequence analysis indicating the identity of the virus(es) detected in the plasma of HIV-infected BLT mice. (g) Plasma HIV-RNA levels of EFdA-treated (n = 11) and untreated (n = 6) BLT mice. Shown is the mean viral load (±SEM). Dashed line indicates the assay limit of detection. (h) Kaplan–Meier plot depicts the percentage of BLT mice protected from vaginal HIV transmission. Mantel–Cox log-rank test was used to compare protection between EFdA-treated and untreated BLT mice. EFdA, 4′-ethynyl-2-fluoro-2′-deoxyadenosine; NA, not applicable.
HIV transmission was observed in control BLT mice following a single individual challenge to HIV-1JR-CSF, HIV-1CH040 or HIV-1RHPA (Figure 2b to d). We also detected HIV-RNA in the plasma of all control mice challenged vaginally after repeated single exposures to the three different viruses (Figure 2e). Sequence analysis of plasma HIV-RNA revealed that one or more viruses were transmitted in BLT mice challenged with three different viruses. The majority of BLT mice (five of six) were co-infected with HIV-1JR-CSF and HIV-1CH040 the first and second challenge viruses, respectively (Figure 2f). One animal was infected with HIV-1RHPA only, the third challenge virus (Figure 2f).
In stark contrast, we did not detect HIV-RNA in any of the 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated BLT mice exposed to three consecutive high-dose vaginal challenges, once each with HIV-1JR-CSF, HIV-1CH040 and HIV-1RHPA (Figure 2g). We also did not detect HIV-DNA in any of the tissues analysed from 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated mice at necropsy (Table S1, available as Supplementary data at JAC Online). Longitudinal analysis of CD4+ T cell levels in peripheral blood and in tissues at necropsy revealed that they were preserved in 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated BLT mice (Figure S1). Collectively, these data show that oral administration of 4′-ethynyl-2-fluoro-2′-deoxyadenosine protects BLT mice from HIV infection following multiple high-dose vaginal HIV challenges (P = 0.0002) (Figure 2h).
4′-Ethynyl-2-fluoro-2′-deoxyadenosine PrEP prevents oral HIV transmission following multiple high-dose challenges with T/F viruses
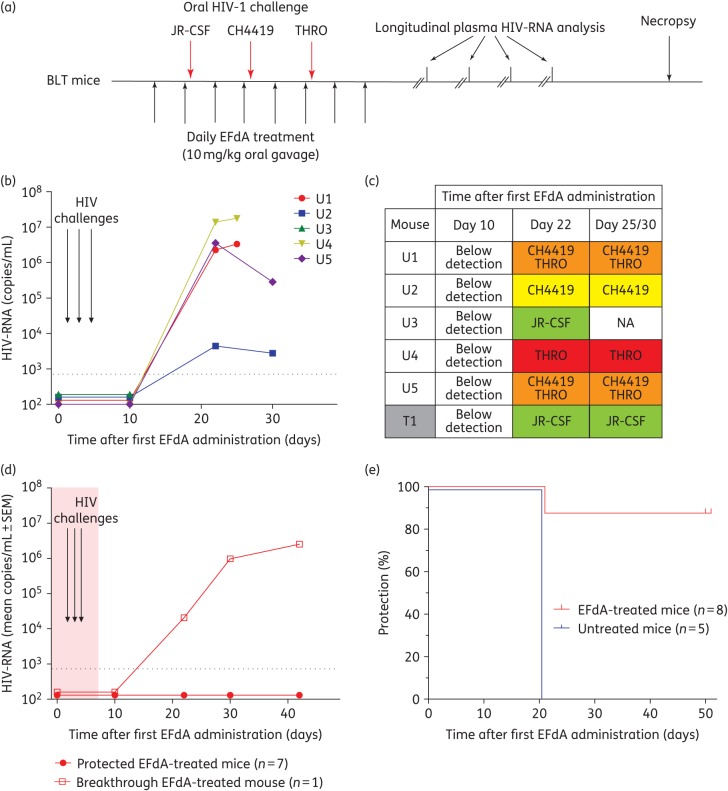
Breastfed infants are exposed to HIV in their mother's breast milk several times per day for periods that can extend over 1 year. Infant HIV PrEP strategies must be able to protect against oral HIV challenges under these extreme circumstances. Having demonstrated strong in vitro anti-HIV activity in saliva after oral administration of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (Figure 1c and d), we utilized a BLT mouse model of oral HIV transmission25,27 to evaluate the ability of 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP to prevent HIV infection following multiple high-dose oral HIV challenges. We administered 4′-ethynyl-2-fluoro-2′-deoxyadenosine (10 mg/kg) orally to BLT mice (n = 8) once daily for 8 days and challenged these mice orally to HIV-1JR-CSF (first exposure), HIV-1CH4419 (second exposure) and HIV-1THRO (third exposure) 3–4 h after the second, fourth and sixth 4′-ethynyl-2-fluoro-2′-deoxyadenosine doses, respectively (Figure 3a). Untreated BLT mice (n = 5) exposed orally to HIV-1JR-CSF, HIV-1CH4419 and HIV-1THRO served as positive controls.
Figure 3.
Oral EFdA PrEP significantly reduces HIV transmission following multiple high-dose oral HIV challenges. (a) EFdA (10 mg/kg) was administered to BLT mice orally for 8 days. Untreated (n = 5) and EFdA-treated (n = 8) BLT mice were exposed orally to a high dose (1.4 × 106 TCIU) of HIV-1JR-CSF, HIV-1CH4419 and HIV-1THRO 3–4 h after administration of the second, fourth and sixth EFdA doses, respectively. Plasma HIV-RNA levels were monitored longitudinally and tissue HIV-DNA levels were analysed at necropsy. (b) Plasma HIV-RNA levels of untreated BLT mice (U1–U5). (c) Identity of the virus(es) detected in the plasma of HIV-infected untreated (U1–U5) and EFdA-treated (T1) BLT mice. (d) Mean plasma HIV-RNA levels (±SEM) of protected EFdA-treated BLT mice (n = 7, T2–T8) and the breakthrough EFdA-treated BLT mouse (T1). Also shown are the results of the sequence analysis of HIV-1JR-CSF RT in the plasma of the breakthrough EFdA-treated mouse at the timepoints indicated (WT = WT amino acid sequence). Dashed line indicates the assay limit of detection. (e) Kaplan–Meier plot illustrates the percentage of EFdA-treated and untreated mice protected from oral HIV transmission. Mantel–Cox log-rank test was used to compare protection between EFdA and untreated BLT mice (P = 0.0031). EFdA, 4′-ethynyl-2-fluoro-2′-deoxyadenosine; NA, not applicable.
HIV-RNA was readily detected in the plasma of all positive control BLT mice (Figure 3b). Longitudinal sequence analysis of plasma HIV-RNA revealed that all three viruses were orally transmitted in untreated BLT mice, albeit at different frequencies (Figure 3c). The majority of mice (four of five) were infected with the T/F viruses HIV-1CH4419 and/or HIV-1THRO. Two mice were co-infected with HIV-1CH4419 and HIV-1THRO. HIV-1JR-CSF alone was identified in the plasma of one control mouse.
In contrast, we did not detect HIV-RNA in the plasma or HIV-DNA in the tissues of seven of eight 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated BLT mice (Figure 3d and Table S2). Longitudinal sequence analysis of plasma HIV-RNA in the HIV-infected 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated mouse demonstrated transmission of the first challenge virus, HIV-1JR-CSF (Figure 3c). Sequence analysis also revealed that although 4′-ethynyl-2-fluoro-2′-deoxyadenosine treatment continued for six additional days after HIV-1JR-CSF exposure, no RT mutations associated with 4′-ethynyl-2-fluoro-2′-deoxyadenosine resistance emerged (Figure 3d).20 Analysis of CD4+ T cell levels in peripheral blood longitudinally and in tissues at necropsy also revealed that CD4+ T cell levels were significantly higher in protected 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated mice (Figure S2). Collectively these data demonstrate that oral 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP significantly reduced oral HIV transmission following multiple high-dose oral HIV challenges with relevant T/F viruses (P = 0.0031) (Figure 3e).
Discussion
Globally, adolescent girls and young women (15–24 years) are twice as likely to be at risk of HIV infection compared with boys and young men in the same age group. This higher risk of HIV is associated with unsafe and often unwanted and forced sexual activity and lack of access to information and health services.37 In areas such as sub-Saharan Africa, the region with the highest incidence of HIV, the majority of new HIV infections occur in women of reproductive age, which contributes to the increased incidence of paediatric HIV infection.1,2 The vast majority of HIV-infected children (∼90%) acquire HIV from their mothers and mostly through breastfeeding.38 Given the urgent need for novel HIV prevention approaches for women and their infants, we utilized a preclinical BLT humanized mouse model of HIV infection22–28 to evaluate the efficacy of 4′-ethynyl-2-fluoro-2′-deoxyadenosine, a novel NRTI that has the highest potency to date and low toxicity profiles to prevent vaginal and oral HIV transmission.
Effective ARVs for PrEP in women and infants must be able to penetrate efficiently into the FRT and oral mucosa, the first mucosal surfaces that contact HIV following vaginal and oral exposure, respectively. ARV distribution into mucosal tissues and secretions has been correlated with an ARV's affinity to bind proteins in plasma and/or mucosal tissues. The antiviral activity of NRTIs is also reliant on their cellular uptake and phosphorylation into an active form that can inhibit the RT enzyme.39 HIV target cells (CD4+ T cells, dendritic cells and macrophages) are present throughout the FRT, which consists of two different types of mucosa, i.e. a monolayer of columnar epithelial cells with tight junctions in the endocervix, endometrium and fallopian tubes (type I mucosa), and a multilayer of squamous epithelial cells in the vagina and ectocervix (type II mucosa).40,41 HIV-susceptible cells have also been demonstrated in the oral cavity, which is protected by multiple layers of stratified epithelial cells. However, in comparison to adults, the oral mucosa of infants is incompletely stratified and contains lower levels of innate anti-HIV factors rendering it much more susceptible to HIV infection.42,43 Our results showing strong in vitro HIV inhibitory activity in serum, CVS and saliva after a single oral dose of 4′-ethynyl-2-fluoro-2′-deoxyadenosine demonstrates efficient penetration of active 4′-ethynyl-2-fluoro-2′-deoxyadenosine into highly relevant mucosal sites of transmission following a vaginal or oral HIV challenge. Even after a 20-fold dilution, the CVS and saliva of 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated animals significantly inhibited HIV infection in vitro (50% inhibition CVS, 90% inhibition saliva). It should be noted that in vitro antiviral activity data provide evidence for efficient drug penetration in CVS and saliva. However, it does not offer information regarding the levels of phosphorylated drug in cells. Therefore, further analysis will be necessary to evaluate 4′-ethynyl-2-fluoro-2′-deoxyadenosine intracellular levels, of its active intracellular phosphorylated form, in target tissues.
BLT mice have been extensively utilized as an in vivo preclinical tool to evaluate systemic and topical ARV PrEP strategies.22–24,26,28 The presence of human CD4+ T cells, macrophages and dendritic cells at important mucosal sites of HIV acquisition including the FRT, oral cavity and upper gastrointestinal tract render BLT mice susceptible to vaginal and oral HIV transmission.24,25 Here, we utilized BLT mice to evaluate the protective efficacy of 4′-ethynyl-2-fluoro-2′-deoxyadenosine against repeated high-dose vaginal and oral HIV challenges mimicking multiple high-risk exposures. Breastfed infants in particular are challenged multiple times per day with HIV as they ingest their mother's breast milk. Our results indicate that oral administration of 4′-ethynyl-2-fluoro-2′-deoxyadenosine provided sufficient distribution of 4′-ethynyl-2-fluoro-2′-deoxyadenosine into relevant mucosal tissues to prevent vaginal and oral HIV transmission after multiple high-dose exposures. Importantly, 4′-ethynyl-2-fluoro-2′-deoxyadenosine prevented HIV infection after exposure to highly relevant T/F viruses (Table 1) demonstrating that 4′-ethynyl-2-fluoro-2′-deoxyadenosine has potent in vivo antiviral activity against the viruses that are responsible for establishing infection. Recently, it was reported that the active intracellular tri-phosphorylated form of 4′-ethynyl-2-fluoro-2′-deoxyadenosine has a long intracellular half-life in humans;44 therefore, it will be important in future experiments to evaluate lower doses of 4′-ethynyl-2-fluoro-2′-deoxyadenosine in BLT mice and to determine whether a single dose of 4′-ethynyl-2-fluoro-2′-deoxyadenosine can prevent vaginal and oral HIV acquisition and what is the duration of protection.
Table 1.
4′-Ethynyl-2-fluoro-2′-deoxyadenosine prevents vaginal and oral transmission in BLT mice challenged with multiple high doses of HIV-1 T/F viruses
| Treatment | At the time of HIV exposure |
Transmission rate | ||||
|---|---|---|---|---|---|---|
| challenge virus(es) | exposure route | number of mice | peripheral blood humanization |
|||
| %hCD45 ± SD | %hCD4 ± SD | |||||
| None | JR-CSF | vaginal | 3 | 76.7 ± 1.2 | 80.4 ± 2.2 | 2/3 |
| None | CH040 | vaginal | 5 | 71.1 ± 15.7 | 72.9 ± 12.6 | 5/5 |
| None | RHPA | vaginal | 6 | 64.3 ± 10.2 | 70.4 ± 5.8 | 5/6 |
| None | JR-CSF, CH040, RHPA | vaginal | 6 | 73.9 ± 12.8 | 76.7 ± 9.2 | 6/6 |
| EFdA | JR-CSF, CH040, RHPA | vaginal | 11 | 71.3 ± 9.9 | 75.4 ± 9.4 | 0/11 |
| None | JR-CSF, CH4419, THRO | oral | 5 | 66.4 ± 14.3 | 82.7 ± 5.0 | 5/5 |
| EFdA | JR-CSF, CH4419, THRO | oral | 8 | 65 ± 9.7 | 80.2 ± 4.7 | 1/8 |
EFdA, 4′-ethynyl-2-fluoro-2′-deoxyadenosine.
BLT mice were dosed orally with 10 mg/kg EFdA daily for 8 days and challenged vaginally or orally with the indicated HIV-1 isolates 3–4 h after the second, fourth and sixth EFdA doses. Untreated mice challenged with a single dose or multiple doses of HIV-1 served as positive controls for transmission. Peripheral blood humanization levels at the time of HIV exposure indicate the percentage of human CD45+ (hCD45+) of live cells and the percentage of human CD4+ (hCD4+) of live hCD45+ CD3+ cells. HIV transmission was defined by the presence of HIV-RNA in plasma and/or HIV-DNA in tissues at necropsy.
Although multiple clinical trials have indicated that ARV PrEP can prevent HIV acquisition, protection is not 100%, raising concerns about the emergence of drug resistance in individuals that become infected with HIV despite PrEP. In clinical trials of PrEP in serodiscordant couples, PrEP-selected drug resistance is rare when infection occurs in the presence of PrEP.45 In contrast, infants that become infected with HIV while receiving nevirapine PrEP are at a significantly increased risk of developing nevirapine resistance.13 Drug resistance can emerge in infants who receive a single dose of nevirapine.46 In our study, we observed one breakthrough infection in a 4′-ethynyl-2-fluoro-2′-deoxyadenosine-treated BLT mouse exposed orally to HIV. Sequence analysis of plasma HIV-RNA indicated that HIV-1JR-CSF, an early passage isolate and the first challenge virus, was transmitted despite 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP. In this study, HIV-1JR-CSF was transmitted in only one of five control animals despite our previously published results, which demonstrate faithful transmission of HIV-1JR-CSF in BLT mice challenged orally with a single high virus dose.25,27
The majority of control animals were infected with the T/F virus(es) HIV-1THRO and/or HIV-1CH4419. Our results here support the concept that molecular and biological determinants bestow a selective advantage for mucosal transmission of T/F viruses. Since HIV-1JR-CSF was the first challenge virus, our results could also indicate that drug absorption in target tissue might be low and that allowing for greater drug accumulation at sites of exposure the effectiveness of 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP may increase over time. Although we did not detect the emergence of RT resistance mutations following oral HIV acquisition in the presence of 4′-ethynyl-2-fluoro-2′-deoxyadenosine PrEP, it is important to note that 4′-ethynyl-2-fluoro-2′-deoxyadenosine was only administered for an additional 6 days after the first HIV challenge. Infants that acquire HIV while receiving ARV PrEP may continue to receive ARVs for several weeks before infection is confirmed. Therefore, future studies may be needed to examine the emergence of 4′-ethynyl-2-fluoro-2′-deoxyadenosine resistance upon extended 4′-ethynyl-2-fluoro-2′-deoxyadenosine administration in the context of HIV infection. Nonetheless, our results combined with those of others demonstrating 4′-ethynyl-2-fluoro-2′-deoxyadenosine's high genetic barrier to resistance20 indicate that the risk of developing 4′-ethynyl-2-fluoro-2′-deoxyadenosine-resistant HIV strains in vivo is lower compared with nevirapine, the current first-line ARV for PrEP in infants.
Significant gender inequalities limit the ability of women to access and exercise HIV prevention options resulting in earlier acquisition of infection, higher transmission rates and increased mother-to-child transmission of HIV. Collectively, our in vivo results demonstrating 4′-ethynyl-2-fluoro-2′-deoxyadenosine's excellent efficacy in preventing both vaginal and oral HIV transmission together with its relatively low toxicity and high potency against drug-resistant HIV strains support 4′-ethynyl-2-fluoro-2′-deoxyadenosine as a potential PrEP agent for use in women and infants, two of the most vulnerable populations at risk for acquiring HIV.
Funding
This work was supported by National Institutes of Health grants AI073146 (J. V. G.), AI096138 (J. V. G.) and UNC Center for AIDS Research grant P30 AI50410.
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We thank M. Parniak for providing 4′-ethynyl-2-fluoro-2′-deoxyadenosine. We thank I. Chen for providing pYK-JR-CSF and J. Kappes and C. Ochsenbauer for providing pCH040.c/2625, pTHRO.c/2626 and pRHPA.c/2635 via the AIDS Research and Reference Reagent Program. We thank F. Gao for providing pCH4419. We also thank Garcia laboratory members and UNC Division of Laboratory Animal Medicine technicians for their assistance.
References
- 1.UNAIDS. Fact Sheet 2016. http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet.
- 2.UNAIDS. The Gap Report. 2014. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
- 3.John GC, Kreiss J. Mother-to-child transmission of human immunodeficiency virus type 1. Epidemiol Rev 1996; 18: 149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS. 2015 Progress Report on the Global Plan: Towards the Elimination of New HIV Infections Among Children and Keeping Their Mothers Alive. http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf.
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329: 1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RM, Lama JR, Anderson PL et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Donnell D, Ndase P et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Paxton LA et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–34. [DOI] [PubMed] [Google Scholar]
- 9.de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 2011; 11: 171–80. [DOI] [PubMed] [Google Scholar]
- 10.Marazzi MC, Nielsen-Saines K, Buonomo E et al. Increased infant human immunodeficiency virus-type one free survival at one year of age in Sub-Saharan Africa with maternal use of highly active antiretroviral therapy during breast-feeding. Pediatr Infect Dis J 2009; 28: 483–7. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro RL, Hughes MD, Ogwu A et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010; 362: 2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chasela CS, Hudgens MG, Jamieson DJ et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med 2010; 362: 2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JA, Fokar A, Hudgens MG et al. Frequent nevirapine resistance in infants infected by HIV-1 via breastfeeding while on nevirapine prophylaxis. AIDS 2015; 29: 2131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakata H, Amano M, Koh Y et al. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother 2007; 51: 2701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muftuoglu Y, Sohl CD, Mislak AC et al. Probing the molecular mechanism of action of the HIV-1 reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) using pre-steady-state kinetics. Antiviral Res 2014; 106: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michailidis E, Marchand B, Kodama EN et al. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J Biol Chem 2009; 284: 35681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby KA, Michailidis E, Fetterly TL et al. Effects of substitutions at the 4′ and 2′ positions on the bioactivity of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother 2013; 57: 6254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohrui H, Kohgo S, Hayakawa H et al. 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine: a nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma. Nucleosides Nucleotides Nucleic Acids 2007; 26: 1543–6. [DOI] [PubMed] [Google Scholar]
- 19.Sohl CD, Singh K, Kasiviswanathan R et al. Mechanism of interaction of human mitochondrial DNA polymerase gamma with the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine indicates a low potential for host toxicity. Antimicrob Agents Chemother 2012; 56: 1630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamoto A, Kodama E, Sarafianos SG et al. 2′-deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol 2008; 40: 2410–20. [DOI] [PubMed] [Google Scholar]
- 21.Stoddart CA, Galkina SA, Joshi P et al. Oral administration of the nucleoside EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque. Antimicrob Agents Chemother 2015; 59: 4190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denton PW, Estes JD, Sun Z et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 2008; 5: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denton PW, Othieno F, Martinez-Torres F et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol 2011; 85: 7582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesen R, Wahl A, Denton PW et al. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol 2011; 88: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahl A, Swanson MD, Nochi T et al. Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog 2012; 8: e1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Council OD, Swanson MD, Spagnuolo RA et al. Role of semen on vaginal HIV-1 transmission and maraviroc protection. Antimicrob Agents Chemother 2015; 59: 7847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahl A, Baker C, Spagnuolo RA et al. Breast milk of HIV-positive mothers has potent and species-specific in vivo HIV-inhibitory activity. J Virol 2015; 89: 10868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovarova M, Council OD, Date AA et al. Nanoformulations of rilpivirine for topical pericoital and systemic coitus-independent administration efficiently prevent HIV transmission. PLoS Pathog 2015; 11: e1005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Permar SR, Salazar MG, Gao F et al. Clonal amplification and maternal-infant transmission of nevirapine-resistant HIV-1 variants in breast milk following single-dose nevirapine prophylaxis. Retrovirology 2013; 10: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keele BF, Giorgi EE, Salazar-Gonzalez JF et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA 2008; 105: 7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochsenbauer C, Edmonds TG, Ding H et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol 2012; 86: 2715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salazar-Gonzalez JF, Salazar MG, Keele BF et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 2009; 206: 1273–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyanagi Y, Miles S, Mitsuyasu RT et al. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 1987; 236: 819–22. [DOI] [PubMed] [Google Scholar]
- 34.Melkus MW, Estes JD, Padgett-Thomas A et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 2006; 12: 1316–22. [DOI] [PubMed] [Google Scholar]
- 35.Sun Z, Denton PW, Estes JD et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med 2007; 204: 705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malamud D, Wahl SM. The mouth: a gateway or a trap for HIV? AIDS 2010; 24: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO. Women's Health. http://www.who.int/mediacentre/factsheets/fs334/en/.
- 38.UNAIDS. Prevention of Mother-To-Child Transmission of HIV. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/programmes/programmeeffectivenessandcountrysupportdepartment/gfresourcekit/20110927_Technical_brief_PMTCT.pdf.
- 39.Heneine W, Kashuba A. HIV prevention by oral preexposure prophylaxis. Cold Spring Harb Perspect Med 2012; 2: a007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eid SG, Mangan NE, Hertzog PJ et al. Blocking HIV-1 transmission in the female reproductive tract: from microbicide development to exploring local antiviral responses. Clin Transl Immunology 2015; 4: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumamoto Y, Iwasaki A. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol 2012; 24: 411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tugizov SM, Herrera R, Veluppillai P et al. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 2011; 409: 211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tugizov SM, Herrera R, Veluppillai P et al. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J Virol 2012; 86: 2556–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman E, Schuermann D, Rudd DJ et al. A single monotherapy dose of MK-8591, a novel NRTI, suppresses HIV for 10 days. In: Abstracts of the Twenty-third Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2016 Abstract 437LB Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 45.Lehman DA, Baeten JM, McCoy CO et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis 2015; 211: 1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eshleman SH, Hoover DR, Hudelson SE et al. Development of nevirapine resistance in infants is reduced by use of infant-only single-dose nevirapine plus zidovudine postexposure prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Infect Dis 2006; 193: 479–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.