Abstract
Objectives
Gonorrhoea and MDR Neisseria gonorrhoeae remain public health concerns globally. Enhanced, quality-assured, gonococcal antimicrobial resistance (AMR) surveillance is essential worldwide. The WHO global Gonococcal Antimicrobial Surveillance Programme (GASP) was relaunched in 2009. We describe the phenotypic, genetic and reference genome characteristics of the 2016 WHO gonococcal reference strains intended for quality assurance in the WHO global GASP, other GASPs, diagnostics and research worldwide.
Methods
The 2016 WHO reference strains (n = 14) constitute the eight 2008 WHO reference strains and six novel strains. The novel strains represent low-level to high-level cephalosporin resistance, high-level azithromycin resistance and a porA mutant. All strains were comprehensively characterized for antibiogram (n = 23), serovar, prolyliminopeptidase, plasmid types, molecular AMR determinants, N. gonorrhoeae multiantigen sequence typing STs and MLST STs. Complete reference genomes were produced using single-molecule PacBio sequencing.
Results
The reference strains represented all available phenotypes, susceptible and resistant, to antimicrobials previously and currently used or considered for future use in gonorrhoea treatment. All corresponding resistance genotypes and molecular epidemiological types were described. Fully characterized, annotated and finished references genomes (n = 14) were presented.
Conclusions
The 2016 WHO gonococcal reference strains are intended for internal and external quality assurance and quality control in laboratory investigations, particularly in the WHO global GASP and other GASPs, but also in phenotypic (e.g. culture, species determination) and molecular diagnostics, molecular AMR detection, molecular epidemiology and as fully characterized, annotated and finished reference genomes in WGS analysis, transcriptomics, proteomics and other molecular technologies and data analysis.
Introduction
Gonorrhoea is a public health concern globally.1,2 The impact of antimicrobial resistance (AMR) in Neisseria gonorrhoeae (gonococci) on the treatment and control of gonorrhoea is a longstanding concern. In the last decade, while retaining AMR to previously used therapeutic drugs, gonococci have developed resistance, including high-level resistance, to the last option for empirical antimicrobial monotherapy, the extended-spectrum cephalosporin (ESC) ceftriaxone.3–10 Rare failures to treat pharyngeal gonorrhoea with ceftriaxone were verified in several countries.3,5,11–14
In response, the WHO,2 CDC15 and ECDC16 have published global and regional action plans to control the transmission and impact of MDR and XDR gonorrhoea. A key component of these plans is to enhance the surveillance of gonococcal AMR locally, nationally and internationally. The WHO global Gonococcal Antimicrobial Surveillance Programme (GASP) was relaunched in 2009.2,17 The WHO GASP works in liaison with other GASPs. For example, Euro-GASP is acting in the European Union and European Economic Area18,19 and national GASPs are active in the USA20 (http://www.cdc.gov/std/gisp), UK21 (http://www.hpa.org.uk/Publications/InfectiousDiseases/HIVAndSTIs/GRASPReports/) and many additional countries. This enhanced gonococcal AMR surveillance should monitor trends in resistance, identify newly emerging AMR and inform treatment guidelines in a timely fashion. However, reliable, quality assured and nationally and internationally comparable AMR data are essential. In the absence of uniform AMR testing methodology globally (method parameters, agar media and resistance breakpoints), inter-laboratory comparisons of AMR data are enabled through the use of international reference strains.22,23 The WHO Collaborating Centre in Örebro, Sweden and the WHO Collaborating Centre in Sydney, Australia have undertaken continuing assessments over many years to select, evaluate and nominate suitable gonococcal strains for use in the internal quality control and external quality assurance of national, WHO regional and international GASPs and for other purposes. In 2009, the 2008 WHO gonococcal reference strains were published.23 The characterizations of these reference strains (n = 8) were substantially expanded in the present study. Furthermore, due to the emergence of ESC resistance and high-level azithromycin resistance, five additional strains (with low-level to high-level resistance to ESCs, including resistance associated with ESC treatment failures, and azithromycin) have now been added to the WHO reference strain panel. In recent years, gonococcal porA mutants containing N. meningitidis porA gene sequences that result in false-negative results in porA-based gonococcal nucleic acid amplification tests (NAATs) have been described in several countries.24,25 One such gonococcal porA mutant has also been included among the 2016 WHO gonococcal reference strains (n = 14).
This study characterized the 2016 WHO N. gonorrhoeae reference strains phenotypically [antibiograms, serovars and prolyliminopeptidase (PIP) production] and genetically [AMR plasmid types, molecular resistance determinants, N. gonorrhoeae multiantigen sequence typing (NG-MAST) STs and MLST STs] and presents finished, fully characterized and annotated reference genomes, which will be exceedingly valuable for quality assurance of WGS, transcriptomics, proteomics and other molecular technologies and data analysis. The 2016 WHO gonococcal reference strains are intended for internal and external quality assurance and quality control in all types of laboratory examinations, particularly in the AMR testing (phenotypic and genetic) in GASPs, as recommended for the WHO global GASP, but also for phenotypic (e.g. culture) and genetic (e.g. NAATs) diagnostics, species determination, genetic AMR detection, molecular epidemiology and genomics.
Materials and methods
Bacterial strains
The 2016 WHO gonococcal reference strains include the previously published 2008 WHO gonococcal reference strains (WHO F, G, K, L, M, N, O and P)23 and six additional, strictly selected gonococcal strains. The new strains were designated as WHO U (Sweden, 2011),25 WHO V (Sweden, 2012),26 WHO W (Hong Kong, 2007),27 WHO X (H041; Japan, 2009),5 WHO Y (F89; France, 2010)7 and WHO Z (A8806; Australia, 2013).8 All strains were cultivated and preserved as described.28 All the 2016 WHO gonococcal reference strains (n = 14) are available at the National Collection of Type Cultures (NCTC; www.phe-culturecollections.org.uk) under the NCTC numbers 13 477–13 484, 13 817–13 822 (Table 1).
Table 1.
Phenotypic characteristics of epidemiological and diagnostic relevance and antimicrobial susceptibility/resistance phenotypes displayed by the 2016 WHO N. gonorrhoeae reference strains (n = 14)
| Characteristic | WHO Fa | WHO Ga | WHO Ka | WHO La | WHO Ma | WHO Na | WHO Oa | WHO Pa | WHO U | WHO V | WHO W | WHO X | WHO Y | WHO Z |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCTC number | 13 477 | 13 478 | 13 479 | 13 480 | 13 481 | 13 482 | 13 483 | 13 484 | 13 817 | 13 818 | 13 819 | 13 820 | 13 821 | 13 822 |
| Serogroup | PorB1a | PorB1a | PorB1b | PorB1b | PorB1b | PorB1a | PorB1b | PorB1b | PorB1b | PorB1b | PorB1b | PorB1b | PorB1b | PorB1b |
| Serovar | Arst | Arst | Bpyust | Brpyust | Bpyust | Arst | Boys | Bopt | Bryust | Bropys | Bpyust | Bpyust | Bpyut | Bpyust |
| PIP productionb | Pos | — | Pos | Pos | Pos | — | Pos | Pos | Pos | — | Pos | Pos | Pos | Pos |
| β-Lactamase (PPNG)c | — | — | — | — | Pos | Pos | Pos | — | — | Pos | — | — | — | — |
| Penicillin G (0.032–>32)d | S (0.032) | I (0.5) | CMRNG (2) | CMRNG (2) | PPNGc (≥32) | PPNGc (>32) | PPNGc (>32) | I (0.25) | I (0.125) | PPNGc (>32) | CMRNG (4) | CMRNG (4) | I (1) | CMRNG (2) |
| Ampicillin (0.032–>256)d,e | 0.032 | 0.25 | 2 | 2 | PPNGc (8) | PPNGc (4) | PPNGc (24) | 0.064 | 0.125 | PPNGc (>256) | 2 | 2 | 0.5 | 2 |
| Temocillin (0.064–32)d,e | 0.064 | 1 | 16 | 4 | 1 | 1 | 4 | 1 | 0.5 | 4 | 8 | 32 | 8 | 8 |
| Cefuroxime (0.032–16)d,e | 0.064 | 0.5 | 16 | 8 | 0.5 | 0.25 | 1 | 0.125 | 0.032 | 1 | 8 | 8 | 8 | 8 |
| Cefixime (<0.016–4)d | S (<0.016) | S (<0.016) | LLR (0.25) | S (0.125) | S (<0.016) | S (<0.016) | S (0.016) | S (<0.016) | S (<0.016) | S (<0.016) | LLR (0.25) | HLR (4) | HLR (2) | HLR (2) |
| Ceftriaxone (<0.002–2)d | S (<0.002) | S (0.008) | S (0.064) | LLR (0.25) | S (0.016) | S (0.004) | S (0.032) | S (0.004) | S (0.002) | S (0.064) | S (0.064) | HLR (2) | HLR (1) | LLR (0.5) |
| Ertapenem (0.004–0.125)d,e | 0.004 | 0.008 | 0.125 | 0.032 | 0.016 | 0.008 | 0.032 | 0.008 | 0.004 | 0.012 | 0.064 | 0.064 | 0.008 | 0.032 |
| Erythromycin (0.5–>256)d,e | 0.5 | 1 | 1 | 2 | 1 | 0.5 | 1 | 4 | >256 | >256 | 2 | 2 | 2 | 4 |
| Azithromycin (0.125–>256)d | S (0.125) | S (0.25) | S (0.25) | I (0.5) | S (0.25) | S (0.25) | S (0.25) | R (4) | R (4) | HLR (>256) | I (0.5) | I (0.5) | R (1) | R (1) |
| Ciprofloxacin (0.004–>32)d | S (0.004) | LLR (0.125) | HLR (>32) | HLR (>32) | R (2) | R (4) | S (0.008) | S (0.004) | S (0.004) | HLR (>32) | HLR (>32) | HLR (>32) | HLR (>32) | HLR (>32) |
| Gemifloxacin (0.004–16)d,e | 0.004 | 0.125 | 16 | 8 | 0.5 | 1 | 0.008 | 0.016 | 0.008 | 4 | 16 | 16 | 2 | 8 |
| Moxifloxacin (0.004–16)d,e | 0.004 | 0.064 | 8 | 16 | 1 | 1 | 0.016 | 0.032 | 0.008 | 8 | 8 | 8 | 4 | 8 |
| Spectinomycin (8–>1024)d | S (16) | S (16) | S (16) | S (16) | S (16) | S (16) | HLR (>1024) | S (8) | S (8) | S (16) | S (16) | S (16) | S (16) | S (16) |
| Gentamicin (4–8)d,e | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 8 | 4 | 4 | 8 | 4 |
| Kanamycin (8–32)d,e | 16 | 16 | 16 | 32 | 16 | 16 | 16 | 8 | 8 | 16 | 16 | 16 | 16 | 8 |
| Tetracycline (0.25–32)d | S (0.25) | TRNG (32) | R (2) | R (2) | R (2) | TRNG (16) | R (2) | I (1) | I (1) | R (4) | R (4) | R (2) | R (4) | R (4) |
| Chloramphenicol (0.5–8)d,e | 0.5 | 2 | 4 | 8 | 4 | 4 | 4 | 4 | 4 | 8 | 8 | 8 | 4 | 8 |
| Thiamphenicol (0.25–4)d,e | 0.25 | 0.5 | 2 | 4 | 4 | 1 | 2 | 1 | 2 | 4 | 4 | 4 | 4 | 4 |
| Fosfomycin (8–32)d,e | 32 | 32 | 16 | 8 | 32 | 16 | 32 | 32 | 32 | 16 | 16 | 16 | 16 | 16 |
| Rifampicin (0.125–>32)d,e | 0.125 | 0.5 | 0.5 | 0.5 | >32 | >32 | 0.25 | >32 | 0.25 | 0.5 | 0.25 | 0.5 | 0.5 | 0.5 |
| Sulfamethoxazole (16–>1024)d,e | 64 | 512 | 128 | 16 | 128 | 256 | 128 | 64 | 32 | >1024 | 64 | 128 | 64 | 128 |
| Solithromycin (0.064–32)d,e | 0.064 | 0.064 | 0.064 | 0.125 | 0.064 | 0.064 | 0.125 | 0.5 | 0.25 | 32 | 0.064 | 0.064 | 0.125 | 0.125 |
| Zoliflodacin (0.032–0.125)d,e | 0.032 | 0.064 | 0.064 | 0.064 | 0.064 | 0.125 | 0.064 | 0.125 | 0.064 | 0.064 | 0.064 | 0.064 | 0.064 | 0.064 |
NCTC, National Collection of Type Cultures; S, susceptible; I, intermediate susceptible; R, resistant; LLR, low-level resistant; HLR, high-level resistant; CMRNG, chromosomally mediated resistant N. gonorrhoeae; TRNG, plasmid-mediated high-level tetracycline-resistant N. gonorrhoeae.
aIncludes some previously published results.23 However, additional antimicrobials have been examined and some consensus MICs have slightly changed when additional MIC determinations using different MIC-determining methodologies have been performed.
bPIP-negative N. gonorrhoeae strains do not produce the enzyme PIP, which can result in doubtful and/or false-negative species identification of N. gonorrhoeae using a biochemical or enzyme-substrate test. Global transmission of PIP-negative N. gonorrhoeae strains has been identified.68
cPPNG, penicillinase-producing N. gonorrhoeae (always considered resistant to all penicillins independent of identified MIC value, which might slightly vary).
dResistance phenotypes based on MIC (mg/L) using Etest or agar dilution and, where feasible, susceptibility/resistance breakpoints stated by EUCAST (www.eucast.org). The reported MIC values are mean MICs (rounded to whole MIC dilution) and the acceptable range of the MICs for each antimicrobial and the different strains is±one MIC doubling dilution, i.e. when the strains are used in quality control, for example. Note: the consensus MICs shown should be used and interpreted with caution because these were derived using one method only and, consequently, may slightly differ using other methods. However, the identified resistance phenotypes (SIR categorization) should ideally be consistent between different methods.23,59
eNo susceptibility/resistance breakpoints stated by EUCAST (www.eucast.org).
Serogroup and serovar determination
Serogroup and serovar determination using PhadeBact GC Monoclonal Serovar Test (Bactus AB, Stockholm, Sweden) were performed as described.29
Detection of PIP
PIP production was detected using API NH (bioMérieux, Marcy-l′Étoile, France), according to the manufacturer's instructions.
Antimicrobial susceptibility testing
MICs of 20 antimicrobials were determined using the Etest method (bioMérieux, Solna, Sweden), according to the manufacturer's instructions, on GCRAP agar plates [3.6% Difco GC Medium Base agar (BD Diagnostics, Sparks, MD, USA) supplemented with 1% haemoglobin (BD) and 1% IsoVitalex (BD)]. MICs of solithromycin,4,14 zoliflodacin (also known as ETX0914 and AZD0914)4,14 and thiamphenicol were determined using the agar dilution method as previously described. Where breakpoints were available, the susceptible (S), intermediate susceptibility (I) and resistance (R) categorization was based on the interpretative criteria from EUCAST (www.eucast.org). For the antimicrobials where EUCAST does not state any breakpoints, only the consensus MIC values are presented (Table 1). For all strains and antimicrobials, each determination was performed at least three times using new bacterial suspensions on separate batches of agar plates and the consensus MIC was reported. β-Lactamase production was detected using nitrocefin solution (Oxoid, Basingstoke, UK).
Isolation of bacterial DNA
Genomic DNA was isolated using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA), according to the manufacturer's instructions. Purified DNA was stored at 4°C prior to genetic analysis.
Conventional sequencing
The PCRs, purification of PCR amplicons and conventional sequencing of genes associated with diagnostics, AMR and molecular epidemiology were performed as described.23
Sequence alignments were performed using the BioEdit Sequence Alignment Editor (v. 7.0.9.0) software (Ibis Biosciences, Carlsbad, CA, USA). Determinations of NG-MAST STs and MLST STs were performed as described previously.23,29,30 All genes and determinants (Table 2) were also identified in silico from the WGS data.
Table 2.
Genetic characteristics of relevance for epidemiology, diagnostics and antimicrobial resistance in the 2016 WHO N. gonorrhoeae reference strains (n = 14)
| Characteristic | WHO Fa | WHO Ga | WHO Ka | WHO La | WHO Ma | WHO Na | WHO Oa | WHO Pa | WHO U | WHO V | WHO W | WHO X | WHO Y | WHO Z |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLST ST | ST10934 | ST1903 | ST7363 | ST1590 | ST7367 | ST1583 | ST1902 | ST8127 | ST7367 | ST10314 | ST7363 | ST7363 | ST1901 | ST7363 |
| NG-MAST ST | ST3303 | ST621 | ST1424 | ST1422 | ST3304 | ST556 | ST495 | ST3305 | ST2382 | ST8927 | ST835 | ST4220 | ST1407 | ST4015 |
| porA pseudogene mutant24,25 | — | — | — | — | — | — | — | — | yes | — | — | — | — | — |
| cppB gene65–67 | — | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| pip gene mutant68 | — | yes | — | — | — | yes | — | — | yes | — | — | — | — | — |
| penA mosaic allele3,4,69–71 | — | — | yes | — | — | — | — | — | yes | yes | yes | yes | ||
| PBP2 A311V5,70,71 | — | — | — | — | — | — | — | — | — | — | — | yes | — | yes |
| PBP2 I312M and G545S69,71 | — | — | yes | — | — | — | — | — | — | — | yes | yes | yes | yes |
| PBP2 V316T69,71 | — | — | yes | — | — | — | — | — | — | — | yes | — | yes | yes |
| PBP2 V316P5,70,71 | — | — | — | — | — | — | — | — | — | — | — | yes | — | — |
| PBP2 T483S4,70,71 | — | — | — | — | — | — | — | — | — | — | — | yes | — | yes |
| PBP2 A501V3,4,69,71 | — | — | — | yes | — | — | — | — | — | — | — | — | — | — |
| PBP2 A501P3,4,7.71 | — | — | — | — | — | — | — | — | — | — | — | — | yes | — |
| PBP2 G542S3,4,71,72 | — | — | — | yes | — | — | — | — | — | yes | — | — | — | — |
| PBP2 D345 insertion3,4,71 | — | yes | — | yes | yes | yes | yes | yes | yes | yes | — | — | — | — |
| PBP2 P551S3,4,71,72 | — | — | — | — | — | — | yes | — | — | — | — | — | — | — |
| mtrR promoter; 13 bp inverted repeat4,71,73–75 | WT | deletion of A | deletion of A | WT | deletion of A | WT | deletion of A | A → C SNP in A-repeat | WT | deletion of A | deletion of A | deletion of A | deletion of A | A → C SNP in A-repeat |
| MtrR codon G454,73–76 | WT | WT | G → D | G → D | G → D | WT | WT | NAb | WT | WT | G → D | WT | WT | WT |
| mtr12044 | — | — | — | yes | — | — | — | — | — | — | — | — | — | — |
| mtrR coding region frame shift mutation4 | — | — | — | — | — | deletion of A at bp 158b | — | insertion of T at bp 60b | — | — | — | — | — | — |
| porB1b codon G1014,71,77,78 | NAc | NAc | G → K | G → K | G → K | NAc | G → K | WT | WT | G → K | G → K | G → K | G → K | G → K |
| porB1b codon A1024,71,77,78 | NAc | NAc | A → D | A → D | A → D | NAc | A → D | A → D | WT | A → D | A → D | A → D | A → N | A → D |
| ponA1; L421 → P in PBP179 | — | yes | yes | yes | yes | yes | yes | — | yes | yes | yes | yes | yes | yes |
| gyrA codon S914,47,71 | WT | S → F | S → F | S → F | S → F | S → F | WT | WT | WT | S → F | S → F | S → F | S → F | S → F |
| gyrA codon D954,47,71 | WT | WT | D → N | D → N | D → G | D → G | WT | WT | WT | D → G | D → N | D → N | D → G | D → N |
| parC codon D864,47,71 | WT | WT | WT | D → N | WT | WT | WT | WT | WT | WT | WT | WT | WT | WT |
| parC codon S874,47,71 | WT | WT | S → R | WT | WT | S → I | WT | WT | S → W | S → R | S → R | S → R | S → R | S → R |
| parC codon S884,47,71 | WT | WT | S → P | S → P | WT | WT | WT | WT | WT | WT | S → P | S → P | WT | S → P |
| parE codon G41080 | WT | G → V | WT | WT | WT | G → V | WT | WT | WT | WT | WT | WT | WT | WT |
| 16S rRNA (bp 1192)d4,48 | WT | WT | WT | WT | WT | WT | C → T | WT | WT | WT | WT | WT | WT | WT |
| 23S rRNA (bp 2059)d4,26 | WT | WT | WT | WT | WT | WT | WT | WT | WT | A → G | WT | WT | WT | WT |
| 23S rRNA (bp 2611)d4,49 | WT | WT | WT | WT | WT | WT | WT | WT | C → T | WT | WT | WT | WT | WT |
| rpoB codon H55252,71 | WT | WT | WT | WT | H → N | H → N | WT | H → N | WT | WT | WT | WT | WT | WT |
| rpsJ codon V5754,71 | WT | V → M | V → M | V → M | V → M | V → M | V → M | V → M | V → M | V → M | V → M | V → M | V → M | V → M |
| folP codon R22855,71 | WT | R → S | R → S | WT | R → S | R → S | R → S | R → S | R → S | R → S | R → S | R → S | R → S | R → S |
| β-Lactamase plasmid type4,45,46,71 | — | — | — | — | African | Asian | African | — | — | Asian | — | — | — | — |
| blaTEM allele46 | — | — | — | — | TEM-1 | TEM-1 | TEM-1 | — | — | TEM-1 | — | — | — | — |
| tet(M) plasmid type4,53,71 | — | Dutch | — | — | — | Dutch | — | — | — | — | — | — | — | — |
aIncludes some previously published results;23 however, many additional genes and mutations, and reference genomes, have been characterized in the present paper.
bNA, not applicable due to frame-shift mutation that causes a premature stop codon and truncated peptide.
cNA, not applicable because these strains were of serogroup WI (PorB1a).
dEscherichia coli numbering used. Mutations found in all four alleles of the gene.
Genome sequencing
Single-molecule, real-time (SMRT) DNA genome sequencing was performed using the PacBio RS II (Pacific Biosciences, Menlo Park, CA, USA) with P5-C3 chemistry, resulting in average sub-read lengths of 28 476 bp. One or two SMRT cells were used for each strain to provide high coverage levels for each (average of 165×) (Table S1, available as Supplementary data at JAC Online).
De novo assembly of the genomes was conducted using the hierarchical genome assembly process (HGAP3, SMRTAnalysis 2.3.0) workflow. Full chromosome sequences were circularized using Circlator31 and then further corrected using Quiver.32 WHO F and WHO K were manually circularized and corrected with Quiver.32
Because large fragments are selected during PacBio library preparation and the longest reads are selected for assembly by the HGAP pipeline, not all plasmids were retrieved in the HGAP assemblies. The cryptic plasmid was not retrieved in six strains (WHO N, O, P, U, V and Z) and the Asian pBlaTEM plasmid in WHO N. Accordingly, Illumina sequencing was also performed on the 14 strains using the HiSeq 2000 2 × 100 bp platform. Reads were assembled using a pipeline (https://github.com/sanger-pathogens/vr-codebase) developed at the Wellcome Trust Sanger Institute.33 The missing plasmids were detected as single contigs directly from the Illumina assemblies using ACT.34 They were circularized manually using the other strains as references.
As a second check, the three types of plasmid detected (pCryptic, pBlaTEM and pConjugative) were compared with their corresponding references in public databases (pJD1, pJD4 and pEP5289, respectively) by mapping all PacBio filtered sub-reads against the reference using BWA MEM.35 Mapped reads were extracted from the bam files and assembled independently using minimap and miniasm,36 and the resulting unitigs corrected using Quiver.32 The initial, Illumina, assemblies of the WHO N and WHO V Asian pBlaTEM plasmids were found to be missing a 1.8 kb region compared with the resulting assemblies from miniasm and the Asian reference plasmid. Thus, miniasm corrected assemblies were used for subsequent steps in these two cases.
Full chromosomes and plasmids were annotated using PROKKA v. 1.1137 followed by manual curation using Artemis.38 Additional proteins annotated in N. gonorrhoeae FA1090 but not identified in the automatic annotation were checked manually and added when appropriate. InterProScan 539 was used to improve annotation of hypothetical proteins. Sequence data for each strain has been deposited in the ENA under BioProject accession no. PRJEB14020.
Genomes were compared through BLAST40 analyses and visualized using BRIG v. 0.95.41 The core genome of the 14 references was obtained using Roary.42 Pairwise core SNPs were calculated and a minimum evolution tree computed using MEGA v6.43
Results
Phenotypic characterization
Three (21%) and 11 (79%) of the strains were assigned as serogroup PorB1a (WI) and PorB1b (WII/III), respectively, and in total eight serovars were represented (Table 1). Three (21%) of the strains (WHO G, N and V) were PIP-negative. Four (29%) of the strains (WHO M, N, O and V) produced β-lactamase. The consensus MIC and, where feasible, SIR categorization for each antimicrobial and strain, and the range of MICs for all strains, as determined by the single method used, are also presented. The strains represented all important susceptible, intermediate susceptible and resistant phenotypes and the ranges of resistances seen for most antimicrobials previously or currently recommended in different guidelines, used in gonorrhoea treatment globally or considered for future use. These included strains with high-level resistance to penicillin G, ampicillin, temocillin, ceftriaxone, cefixime, cefuroxime, azithromycin, erythromycin, ciprofloxacin, moxifloxacin, gemifloxacin, tetracycline, spectinomycin, sulfamethoxazole and rifampicin (Table 1).
Genetic characterization
One of the strains (WHO F) contained a WT penA allele, five strains (WHO K, W, X, Y and Z) a mosaic penA allele (main ESC resistance determinant),3,4,14 and eight strains displayed the Asp345a alteration in the β-lactam main target penicillin-binding protein 2 (PBP2), which is observed in chromosomally mediated penicillin resistance (Table 2).3,4 WHO L and WHO Y contained a PBP2 A501V and A501P alteration, respectively, which also increase the MICs of β-lactam antimicrobials including ESCs.3,4,6,7 Other penA mutations that increase the ESC MICs are also presented. Four strains (WHO F, L, N and U) contained a WT mtrR promoter region sequence. The remaining strains displayed a deletion of a single nucleotide (A; n = 8) or an A → C substitution (n = 2) in the 13 bp inverted repeat of the mtrR promoter sequence, resulting in an increased MtrCDE efflux of substrate antimicrobials, e.g. macrolides and β-lactam antimicrobials.3,4,23 Also WHO L and WHO N had an over-expressed MtrCDE efflux pump due to the mtr120 mutation and a deletion of a single nucleotide in mtrR, respectively. These mutations result in an additional promoter for mtrCDE and a frame-shift, premature stop codon and truncated MtrR, respectively.23,44 Concerning the penB AMR determinant, among the PorB1b strains (n = 11) 10 displayed mutations in A102 [A102D (n = 9) and A102N (n = 1)] and nine additionally a G101K alteration, which mediate decreased permeability of target antimicrobials through the porin PorB1b.3,4 Twelve strains carried the ponA mutation (ponA1 allele) resulting in the L421P alteration in the second β-lactam target PBP1, which is observed in high-level chromosomally mediated penicillin resistance.4 Of the β-lactamase-producing strains (n = 4), two (WHO M and O) carried African-type plasmid and two (WHO N and V) Asian-type plasmid, which all contained blaTEM-1 resulting in high-level penicillin resistance.45,46 In regards of fluoroquinolone resistance, one strain (WHO G) displayed only an S91F mutation in GyrA, subunit A of DNA gyrase (ciprofloxacin MIC = 0.125 mg/L), one (WHO M) a GyrA S91F mutation and a GyrA D95G mutation (ciprofloxacin MIC = 2 mg/L) and the remaining eight ciprofloxacin-resistant strains contained a GyrA S91F mutation, a GyrA D95G/N mutation and one or two amino acid alterations in codons 86–88 of ParC, subunit C of DNA topoisomerase IV (ciprofloxacin MIC = 4–>32 mg/L).4,47 One strain (WHO O) had a C1192T spectinomycin target mutation in all four alleles of the 16S rRNA gene (spectinomycin MIC >1024 mg/L48). WHO U and WHO V possessed the C2611T mutation and A2059G mutation, respectively, in all four alleles of the 23S rRNA gene, which are target mutations causing low-level and high-level resistance to azithromycin.4,26,49 No azithromycin resistance mutations were found in the rplD or rplV gene (encoding ribosomal protein L4 and L22, respectively) and none of the macrolide resistance-associated genes mefA/E (encoding Mef efflux pump),50 ereA and ereB (encoding erythromycin esterase) or ermA-C and ermF (encoding RNA methylases that block macrolides from binding to the 23S subunit target)51 were identified in any of the strains. Three of the strains (WHO M, N and P) contained an H552N target mutation in RpoB (encoding RNA polymerase subunit B), resulting in high-level rifampicin resistance.52 The tet(M)-carrying conjugative plasmids, resulting in high-level tetracycline resistance, identified in WHO G and N were of the Dutch plasmid type.53 All strains except WHO F had the V57M mutation in rpsJ, encoding ribosomal protein S10 and involved in chromosomally mediated tetracycline resistance.54 All strains except WHO F and WHO L contained the R228S mutation in the sulphonamide target dihydropteroate synthase (DHPS), encoded by folP, associated with sulphonamide resistance.55 Finally, the promoter sequence for the macAB operon (encoding the MacA-MacB efflux pump) contained the −10 hexamer sequence TAGAAT in all strains. This sequence is characteristic of the macAB promoter sequence in gonococci and meningococci and has a dampening effect on the macAB transcription compared with a −10 TATAAT sequence.56 No mutations modulating transcription were found in any of the strains in the putative −35 promoter hexamer sequence (CTGACG) of the promoter sequence for the norM gene (encoding the NorM efflux pump) or in its ribosome binding site (TGAA).57
Of importance for phenotypic and/or molecular detection of gonococci, cppB (WHO F), pip (WHO G, N and U) and porA (WHO U) mutant strains were represented. Finally, the strains displayed 14 different porB alleles, 14 divergent NG-MAST STs and 10 different MLST STs. Notably, the MLST ST7363 and ST1901, and NG-MAST ST1407, were represented, which are internationally spread MDR clones that account for most of the ESC resistance globally (Table 2).3,4,6,7,10,11,14,19
Reference genome characterization
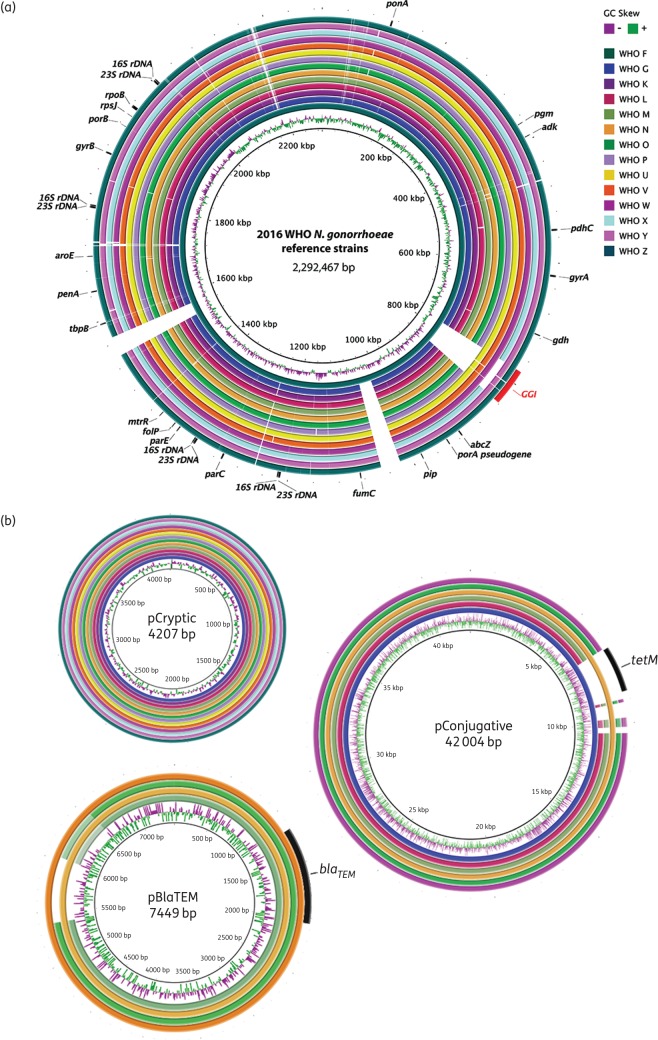
The general characteristics of the reference genomes are summarized in Table 3. The genome size ranged from 2 167 361 to 2 292 467 bp. The number of coding sequences (CDSs) after manual curation varied from 2295 to 2450 with an average CDS size of 796.6 bp. The number of core genes was 1820 and accessory genes ranged from 475 to 630. In Figure 1(a and b) BLAST atlases of the 14 reference genomes and all identified plasmid sequences, respectively, are displayed. Briefly, the reference genomes showed relatively high genomic similarity among all strains with the exception of two large insertions in WHO F and the presence or absence of the gonococcal genomic island (GGI).58 The insertions in WHO F each include 34 almost identical predicted CDSs (31 984 bp) mainly containing an apparently complete type IV secretion system, a vapD virulence gene and xerC recombinase inserted into a tRNA-Asn element, with only weak matches to other sequences in the NCBI non-redundant nt database. Other regions with low genetic conservation corresponded to mobile genetic elements and prophages (Figure 1a).
Table 3.
General characteristics of the reference genomes of the 2016 WHO N. gonorrhoeae reference strains (n = 14)
| Characteristic | WHO F | WHO G | WHO K | WHO L | WHO M | WHO N | WHO O | WHO P | WHO U | WHO V | WHO W | WHO X | WHO Y | WHO Z |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome size (bp) | 2 292 467 | 2 167 361 | 2 169 846 | 2 168 633 | 2 178 344 | 2 172 826 | 2 169 062 | 2 173 861 | 2 234 269 | 2 221 284 | 2 222 386 | 2 171 112 | 2 228 980 | 2 229 351 |
| No. of CDSs | 2450 | 2299 | 2296 | 2314 | 2305 | 2300 | 2304 | 2305 | 2378 | 2366 | 2361 | 2295 | 2380 | 2368 |
| Coding density (%) | 84.8 | 84.5 | 84.6 | 84.5 | 84.5 | 84.6 | 84.6 | 84.6 | 84.8 | 84.8 | 84.7 | 84.5 | 84.8 | 84.6 |
| Average gene size (bp) | 793.7 | 796.5 | 799.3 | 791.6 | 798.7 | 798.9 | 796.0 | 797.6 | 796.8 | 795.8 | 797.5 | 799.5 | 794.4 | 796.7 |
| GC content (%) | 52.1 | 52.6 | 52.6 | 52.6 | 52.6 | 52.6 | 52.6 | 52.6 | 52.4 | 52.4 | 52.4 | 52.6 | 52.4 | 52.4 |
| 5S rRNA | 4 | |||||||||||||
| 16S rRNA | 4 | |||||||||||||
| 23S rRNA | 4 | |||||||||||||
| tRNAs | 56 | 56 | 57 | 56 | 56 | 56 | 56 | 56 | 56 | 56 | 56 | 57 | 56 | 57 |
| ncRNAs | 16 | 15 | 15 | 15 | 15 | 15 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| tmRNA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| No. of genes in pangenome | 3478 | |||||||||||||
| No. core genes | 1820 | |||||||||||||
| Accessory genes (%) | 630 (25.7) | 479 (20.8) | 476 (20.7) | 494 (21.3) | 485 (21.0) | 480 (20.9) | 484 (21.0) | 485 (21.0) | 558 (23.5) | 546 (23.1) | 541 (22.9) | 475 (20.7) | 560 (23.5) | 548 (23.1) |
| DNA uptake sequences (DUSs)a | 1981 (1533) | 1947 (1510) | 1950 (1510) | 1956 (1518) | 1955 (1516) | 1951 (1513) | 1950 (1519) | 1959 (1517) | 1963 (1512) | 1968 (1518) | 1954 (1509) | 1949 (1510) | 1973 (1522) | 1959 (1512) |
| Number of plasmids | 0 | 2 | 1 | 2 | 3 | 3 | 3 | 1 | 1 | 2 | 2 | 1 | 1 | 1 |
aTotal of the 10-mer DUS sequence GCCGTCTGAA (no. of the 12-mer ATGCCGTCTGAA). Note: the 10-mer sequence is included in the 12-mer.
Figure 1.
BLAST atlas of the 2016 WHO N. gonorrhoeae reference genomes (n = 14). (a) A genome comparison of the 2016 WHO reference strains presented in this study using WHO F23 as reference and (b) a comparison of the up to three plasmids (named pCryptic, pBlaTEM and pConjugative65,81,82) identified in the same strains. WHO G pCryptic (cryptic plasmid), WHO M pBlaTEM (β-lactamase-producing plasmid) and WHO G pConjugative [conjugative plasmid including tet(M) in WHO G and N] were used as references, respectively. For each, GC skew is shown in the inner rings. The position in the genomes is shown for genetic resistance determinants and loci used for molecular diagnostics and epidemiological characterization, i.e. NG-MAST STs and MLST STs. The presence of the GGI58 is indicated in red. An approximately 500 bp region with lower nucleotide conservation (∼75% identity) is shown with lighter colours in WHO M and WHO O pBlaTEM plasmids corresponding to a hypothetical protein.
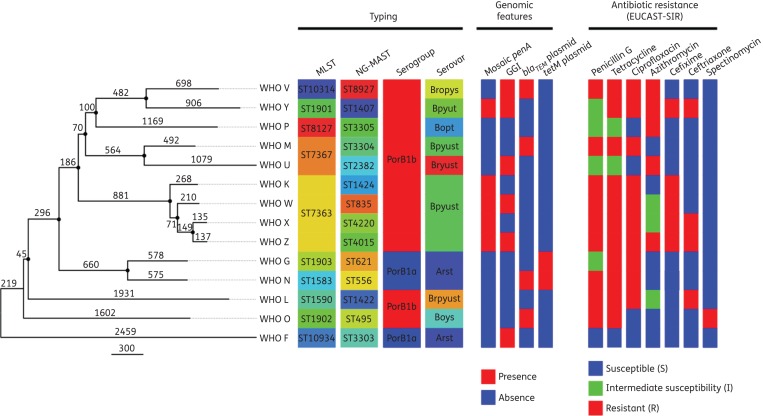
Figure 2 shows the phylogenetic relationship among all the reference core genomes (n = 14, 1820 loci). The number of SNPs between the core genomes is shown on the branches. The average pairwise SNP distance was estimated as 2962 SNPs, with WHO X and WHO Z being the most similar strains (272 SNPs) and WHO F and WHO U the most distant (4969 SNPs). Figure S1 and Table S2, show the pairwise SNP distances among all 14 reference core genomes.
Figure 2.
Phylogenetic tree of the 2016 WHO N. gonorrhoeae reference core genomes (n = 14). The tree is rooted using an N. meningitidis genome as outgroup (not shown). The number of SNPs is shown on each branch. Highlighted nodes show bootstrap supports higher than 80%. Typing, genomic features and antibiotic resistance patterns of the 2016 WHO reference strains are shown alongside the tree. Only antimicrobials with a SIR categorization assigned are displayed.
Between none and three plasmids were detected in each strain (Table 3), either from the PacBio or Illumina assemblies. The cryptic plasmid was found in all strains except WHO F, and the β-lactamase plasmid containing a blaTEM-1 gene was found in four of the isolates (WHO M, N, O and V; Figure 1b), producing plasmid-mediated high-level penicillin resistance (Tables 1 and 2). The tet(M)-carrying conjugative plasmid causing high-level tetracycline resistance through the tet(M) gene was found in WHO G and WHO N (Tables 1 and 2). However, WGS also identified the conjugative plasmid in four additional strains (WHO L, M, O and W), although these were lacking the tet(M) resistance gene (Figure 1b).
Discussion
In this study, the 2016 WHO N. gonorrhoeae reference strains and their detailed phenotypic, genetic and reference genome characteristics are reported. The utility of these strains includes quality control and quality assurance practices in the WHO global and other GASPs. Comprehensive description regarding applications and use of WHO reference strains in GASPs has previously been published.23,59 The strains include all important susceptible, intermediate susceptible and resistant phenotypes and the ranges of resistances seen for most antimicrobials previously or currently recommended in different guidelines and/or used in the gonorrhoea treatment globally. However, the consensus MIC values (Table 1) were determined using one AMR method only. Accordingly, these MIC values may differ slightly using other AMR methods, though the resistance phenotypes should be consistent. It is strongly recommended that laboratories using the superseded WHO A–E reference strains or the 2008 WHO gonococcal reference strains23 update to the current 2016 panel. The 2016 WHO gonococcal reference strains will be available through WHO sources and the NCTC (www.phe-culturecollections.org.uk).
In many countries, NAATs are replacing culture for gonococcal detection and, accordingly, genetic detection of AMR determinants to predict resistance or susceptibility to antimicrobials has become of increased interest, both for future AMR surveillance and, ideally, also to guide individually tailored treatment.4,60,61 Thus, the genetic AMR determinants, acting singly or collaboratively, that mediate the different AMR phenotypes in the 2016 WHO gonococcal reference strains were characterized in detail and included most known gonococcal AMR determinants. These reference strains are designed for internal and external quality assurance and quality control components of both gonococcal phenotypic AMR surveillance and future surveillance using molecular AMR prediction. Molecular AMR methods can never entirely replace phenotypic AMR testing because they only detect identified AMR determinants and new ones will continue to evolve. However, the molecular prediction of AMR or susceptibility can supplement the conventional culture-based phenotypic AMR surveillance. For example, ciprofloxacin susceptibility is relatively straightforward to predict, azithromycin resistance can be indicated and detection of a mosaic penA gene can predict decreased susceptibility or resistance to ESCs.4,60–62 However, due to the many different genes, mutations and accumulation of mutations causing, for example, ESC resistance the molecular methods will not be able to predict an exact MIC of the antimicrobials. However, this is not essential if the susceptibility/resistance phenotypes can be predicted by targeting the main AMR determinants. The sensitivity and/or specificity of the AMR prediction will also vary in different settings due to the myriad of gonococcal strains circulating and some cross-reactivity with non-gonococcal Neisseria species, particularly in pharyngeal specimens, might be unavoidable.3,4,60–62 Despite these limitations, molecular prediction of AMR or susceptibility enables testing of substantially more gonococcal samples (including NAAT samples), assessing the spread of genetic potential for AMR development and identifying settings where targeted culture-based phenotypic AMR testing should be initiated. WGS and other novel molecular technologies will likely revolutionize the molecular AMR prediction in gonococci. Ultimately, point-of-care (POC) genetic AMR methods, combined with gonococcal detection, might be used to guide individually tailored treatment of gonorrhoea, which can ensure rational use of antimicrobials (including sparing last-line antimicrobials) and affect the control of both gonorrhoea and AMR.
In recent years, WGS, providing a dramatic increase in resolution, has become more cost-effective and user-friendly. WGS has the potential to revolutionize investigations into gonococcal evolution and population genetics, to identify and track specific strains spreading globally in particular populations and/or in core groups, to identify temporal and geographical changes in strain types as well as the emergence and transmission of individual strains (e.g. MDR ones), to investigate strain identity in contact tracing, test-of-cure and suspected treatment failures, to confirm presumed epidemiological connections or discount isolates from suspected clusters and outbreaks, and to predict AMR or susceptibility in future AMR surveillance.63 However, when WGS becomes widely used internationally it is crucial that appropriate, validated and finished gonococcal reference genomes are available. Consequently, we present the fully characterized, annotated and finished reference genomes of the 2016 WHO gonococcal reference strains, to enable quality assurance of N. gonorrhoeae WGS analysis.
The 2016 WHO N. gonorrhoeae reference strain panel includes the previously published 2008 WHO gonococcal reference strains (n = 8).23 However, in the present study these strains were subjected to further exceedingly detailed analyses. For example, the susceptibility/resistance to additional antimicrobials (sulfamethoxazole, chloramphenicol, gemifloxacin, moxifloxacin, solithromycin, zoliflodacin, fosfomycin, temocillin and thiamphenicol), additional molecular AMR or diagnostic determinants (mutations in the rpsJ,54 folP,55 23S rRNA,4,26,49 rplD, rplV and blaTEM genes4,46 and additional penA mutations of interest for ESC resistance,3,4 as well as the presence of macAB56 and norM57 promoter mutations, the mtr120 mutation,44 ermA, ermB, ermC and ermF genes,51,64 ereA and ereB genes, mefA/E genes50 that can cause macrolide resistance, the cppB gene,65–67 the mutated porA pseudogene,24,25 the mutated pip gene68 and the GGI58) and further molecular epidemiological characteristics (MLST STs) were investigated. Furthermore, finished reference genomes were produced, fully annotated and characterized. The six novel WHO reference strains (WHO U, V, W, X, Y and Z) represent phenotypes and genotypes not available when the earlier reference strain panel23 was developed. Now included are gonococcal strains with low-level to high-level ESC resistance due to different ESC resistance penA mutations and associated with both cefixime and ceftriaxone treatment failures. These will be of particular value for enhanced validation of the phenotypic AMR testing, especially monitoring ESC MIC drifts over time. The 2016 WHO reference strains also include a gonococcal strain with high-level azithromycin resistance, due to the A2059G mutation in all four alleles of the 23S rRNA gene,26 and one with a mutated porA pseudogene (N. meningitidis porA gene sequences resulting in false-negative results in porA-based gonococcal NAATs).24,25
In conclusion, the 2016 WHO N. gonorrhoeae reference strains were extensively characterized both phenotypically and genetically, including characterizing the reference genomes, and are intended for internal and external quality assurance and quality control purposes in laboratory investigations. These strains should prove particularly useful in WHO global and other GASPs (to allow valid intra- and inter-laboratory comparisons of AMR data derived by different methods in various countries), but also in phenotypic (e.g. culture, species determination) and molecular diagnostics, genetic AMR detection, molecular epidemiology and as fully characterized, annotated and finished reference genomes in WGS analysis, transcriptomics, proteomics and other molecular technologies and data analysis. When additional resistant phenotypes and/or genotypes emerge, novel WHO gonococcal reference strains will be selected, characterized and added to the panel of existing strains.
Funding
This study was supported by grants from: the Department of Reproductive Health and Research, WHO Headquarters, Geneva, Switzerland; the Australian Government Department of Health and the Department of Microbiology, South Eastern Area Laboratory Services, New South Wales, Australia; and Örebro County Council Research Committee and Foundation for Medical Research at Örebro University Hospital, Sweden. WGS and analysis at the Wellcome Trust Sanger Institute was supported through Wellcome Trust grant number 098051. Y. G. was supported by K08-AI104767 from the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We are very grateful to Janice Lo and Patrice Sednaoui for providing the WHO W strain and the WHO Y strain, respectively.
References
- 1.Newman L, Rowley J, Vander Hoorn S et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10: e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria gonorrhoeae. 2012. http://whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf?ua=1.
- 3.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012; 7: 1401–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27: 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnishi M, Golparian D, Shimuta K et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 2011; 55: 3538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cámara J, Serra J, Ayats J et al. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 2012; 67: 1858–60. [DOI] [PubMed] [Google Scholar]
- 7.Unemo M, Golparian D, Nicholas R et al. High-level cefixime-and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012; 56: 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahra MM, Ryder N, Whiley DM. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med 2014; 371: 1850–1. [DOI] [PubMed] [Google Scholar]
- 9.Chen SC, Yin YP, Dai XQ et al. Antimicrobial resistance, genetic resistance determinants for ceftriaxone and molecular epidemiology of Neisseria gonorrhoeae isolates in Nanjing, China. J Antimicrob Chemother 2014; 69: 2959–65. [DOI] [PubMed] [Google Scholar]
- 10.Unemo M, Golparian D, Stary A et al. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill 2011; 16: pii=19998. [PubMed] [Google Scholar]
- 11.Golparian D, Ohlsson A, Janson H et al. Four treatment failures of pharyngeal gonorrhoea with ceftriaxone (500 mg) or cefotaxime (500 mg), Sweden, 2013 and 2014. Euro Surveill 2014; 19: pii=20862. [DOI] [PubMed] [Google Scholar]
- 12.Chen YM, Stevens K, Tideman R et al. Failure of ceftriaxone 500 mg to eradicate pharyngeal gonorrhoea, Australia. J Antimicrob Chemother 2013; 68: 1445–7. [DOI] [PubMed] [Google Scholar]
- 13.Read PJ, Limnios EA, McNulty A et al. One confirmed and one suspected case of pharyngeal gonorrhoea treatment failure following 500 mg ceftriaxone in Sydney, Australia. Sex Health 2013; 10: 460–2. [DOI] [PubMed] [Google Scholar]
- 14.Unemo M. Current and future antimicrobial treatment of gonorrhoea—the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis 2015; 15: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Cephalosporin-Resistant Neisseria gonorrhoeae Public Health Response Plan. 2012; 1–43. http://www.cdc.gov/std/gonorrhea/default.htm.
- 16.ECDC. Response Plan to Control and Manage the Threat of Multidrug-Resistant Gonorrhoea in Europe. 2012; 1–23. www.ecdc.europa.eu/en/publications/Publications/1206-ECDC-MDR-gonorrhoea-response-plan.pdf.
- 17.Ndowa F, Lusti-Narasimhan M, Unemo M. The serious threat of multidrug-resistant and untreatable gonorrhoea: the pressing need for global action to control the spread of antimicrobial resistance, and mitigate the impact on sexual and reproductive health. Sex Transm Infect 2012; 88: 317–8. [DOI] [PubMed] [Google Scholar]
- 18.Cole MJ, Spiteri G, Jacobsson S et al. Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in Europe? Results from the 2013 European surveillance. BMC Infect Dis 2015; 15: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chisholm SA, Unemo M, Quaye N et al. Molecular epidemiological typing within the European Gonococcal Antimicrobial Resistance Surveillance Programme reveals predominance of a multidrug-resistant clone. Euro Surveill 2013; 18: pii=20358. [PubMed] [Google Scholar]
- 20.Kirkcaldy RD, Hook EW 3rd, Soge OO et al. Trends in Neisseria gonorrhoeae susceptibility to cephalosporins in the United States, 2006–2014. JAMA 2015; 314: 1869–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ison CA, Town K, Obi C et al. Decreased susceptibility to cephalosporins among gonococci: data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. Lancet Infect Dis 2013; 13: 762–8. [DOI] [PubMed] [Google Scholar]
- 22.Reyn A, Bentzon MW, Thayer JD et al. Results of comparative experiments using different methods for determining the sensitivity of Neisseria gonorrhoeae. Bull WHO 1965; 32: 477–502. [PMC free article] [PubMed] [Google Scholar]
- 23.Unemo M, Fasth O, Fredlund H et al. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J Antimicrob Chemother 2009; 63: 1142–51. [DOI] [PubMed] [Google Scholar]
- 24.Ison C, Golparian D, Saunders P et al. Evolution of Neisseria gonorrhoeae is a continuing challenge for molecular detection of gonorrhoea—false negative gonococcal porA mutants are spreading internationally. Sex Transm Infect 2013; 89: 197–201. [DOI] [PubMed] [Google Scholar]
- 25.Golparian D, Johansson E, Unemo M. Clinical Neisseria gonorrhoeae isolate with a N. meningitidis porA gene and no prolyliminopeptidase activity, Sweden, 2011—danger of false-negative genetic and culture diagnostic results. Euro Surveill 2012; 17: pii=20102. [PubMed] [Google Scholar]
- 26.Unemo M, Golparian D, Hellmark B. First three Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Sweden: a threat to currently available dual-antimicrobial regimens for treatment of gonorrhea? Antimicrob Agents Chemother 2013; 58: 624–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo JYC, Ho KM, Leung AOC et al. Ceftibuten resistance and treatment failure in gonococcal infection. Antimicrob Agent Chemother 2008; 52: 3564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unemo M, Olcén P, Berglund T et al. Molecular epidemiology of Neisseria gonorrhoeae: sequence analysis of the porB gene confirms presence of two circulating strains. J Clin Microbiol 2002; 40: 3741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unemo M, Sjöstrand A, Akhras M et al. Molecular characterisation of Neisseria gonorrhoeae identifies transmission and resistance of one ciprofloxacin resistant strain. APMIS 2007; 115: 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolley KA. Multi-locus sequence typing. In: Pollard AJ, Maiden MC, eds. Meningococcal Disease: Methods and Protocols. Totowa, NJ: Humana Press, 2001; 173–86. [DOI] [PubMed] [Google Scholar]
- 31.Hunt M, Silva ND, Otto TD et al. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol 2015; 16: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin CS, Alexander DH, Marks P et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 2013; 10: 563–9. [DOI] [PubMed] [Google Scholar]
- 33.Makendi C, Page AJ, Wren BW et al. A phylogenetic and phenotypic analysis of Salmonella enterica serovar weltevreden, an emerging agent of diarrheal disease in tropical regions. PLoS Negl Trop Dis 2016; 10: e0004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carver TJ, Rutherford KM, Berriman M et al. ACT: the Artemis Comparison Tool. Bioinformatics 2005; 21: 3422–3. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010; 26: 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H. Minimap and miniasm: fast mapping and de novo assembly for noisy long sequences. Bioinformatics 2016; doi:10.1093/bioinformatics/btw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30: 2068–9. [DOI] [PubMed] [Google Scholar]
- 38.Rutherford K, Parkhill J, Crook J et al. Artemis: sequence visualization and annotation. Bioinformatics 2000; 16: 944–5. [DOI] [PubMed] [Google Scholar]
- 39.Jones P, Binns D, Chang HY et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 2014; 30: 1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camacho C, Coulouris G, Avagyan V et al. BLAST+: architecture and applications. BMC Bioinformatics 2009; 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alikhan NF, Petty NK, Ben Zakour NL et al. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 2011; 12: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Page AJ, Cummins CA, Hunt M et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015; 31: 3691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura K, Stecher G, Peterson D et al. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30: 2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohneck EA, Zalucki YM, Johnson PJ et al. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. MBio 2011; 2: e00187–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer HM, Leeming JP, Turner A. A multiplex polymerase chain reaction to differentiate β-lactamase plasmids of Neisseria gonorrhoeae. J Antimicrob Chemother 2000; 45: 777–82. [DOI] [PubMed] [Google Scholar]
- 46.Muhammad I, Golparian D, Dillon JA et al. Characterisation of blaTEM genes and types of β-lactamase plasmids in Neisseria gonorrhoeae—the prevalent and conserved blaTEM-135 has not recently evolved and existed in the Toronto plasmid from the origin. BMC Infect Dis 2014; 14: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trees DL, Sandul AL, Peto-Mesola V et al. Alterations within the quinolone resistance-determining regions of GyrA and ParC of Neisseria gonorrhoeae isolated in the Far East and the United States. Int J Antimicrob Agents 1999; 12: 325–32. [DOI] [PubMed] [Google Scholar]
- 48.Galimand M, Gerbaud G, Courvalin P. Spectinomycin resistance in Neisseria spp. due to mutations in 16S rRNA. Antimicrob Agents Chemother 2000; 44: 1365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng L-K, Martin I, Liu G et al. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 2002; 46: 3020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luna VA, Cousin S Jr, Whittington WL et al. Identification of the conjugative mef gene in clinical Acinetobacter junii and Neisseria gonorrhoeae isolates. Antimicrob Agents Chemother 2000; 44: 2503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cousin SL Jr, Whittington WL, Roberts MC. Acquired macrolide resistance genes and the 1 bp deletion in the mtrR promoter in Neisseria gonorrhoeae. J Antimicrob Chemother 2003; 51: 131–3. [DOI] [PubMed] [Google Scholar]
- 52.Nolte O, Müller M, Reitz S et al. Description of new mutations in the rpoB gene in rifampicin-resistant Neisseria meningitidis selected in vitro in a stepwise manner. J Med Microbiol 2003; 52: 1077–81. [DOI] [PubMed] [Google Scholar]
- 53.Turner A, Gough KR, Leeming JP. Molecular epidemiology of tetM genes in Neisseria gonorrhoeae. Sex Transm Infect 1999; 75: 60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu M, Nandi S, Davies C et al. High-level chromosomally mediated tetracycline resistance in Neisseria gonorrhoeae results from a point mutation in the rpsJ gene encoding ribosomal protein S10 in combination with the mtrR and penB resistance determinants. Antimicrob Agents Chemother 2005; 49: 4327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiebelkorn KR, Crawford SA, Jorgensen JH. Mutations in folP associated with elevated sulfonamide MICs for Neisseria meningitidis clinical isolates from five continents. Antimicrob Agents Chemother 2005; 49: 536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouquette-Loughlin CE, Balthazar JT, Shafer WM. Characterization of the MacA-MacB efflux system in Neisseria gonorrhoeae. J Antimicrob Chemother 2005; 56: 856–80. [DOI] [PubMed] [Google Scholar]
- 57.Rouquette-Loughlin CE, Dunham SA, Kuhn M et al. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J Bacteriol 2003; 185: 1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton HL, Domínguez NM, Schwartz KJ et al. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol 2005; 55: 1704–21. [DOI] [PubMed] [Google Scholar]
- 59.WHO GASP Document ‘Rationale and Applications for the Current (2008) WHO Panel of Neisseria gonorrhoeae for Antimicrobial Resistance Surveillance for Public Health Purposes, and Instructions for Their Use’. Technical document D007-0408-1#1 Sydney: WHO Collaborating Centre for STD, 2008. [Google Scholar]
- 60.Low N, Unemo M. Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: when, where, and how to use? Curr Opin Infect Dis 2016; 29: 45–51. [DOI] [PubMed] [Google Scholar]
- 61.Goire N, Lahra MM, Chen M et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol 2014; 12: 223–9. [DOI] [PubMed] [Google Scholar]
- 62.Whiley DM, Lahra MM, Unemo M. Prospects of untreatable gonorrhea and ways forward. Future Microbiol 2015; 10: 313–6. [DOI] [PubMed] [Google Scholar]
- 63.Unemo M, Dillon JA. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev 2011; 24: 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts MC, Chung WO, Roe D et al. Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob Agents Chemother 1999; 43: 1367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korch C, Hagblom P, Ohman H et al. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol 1985; 163: 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ho BS, Feng WG, Wong BK et al. Polymerase chain reaction for the detection of Neisseria gonorrhoeae in clinical samples. J Clin Pathol 1992; 45: 439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lum G, Freeman K, Nguyen NL et al. A cluster of culture positive gonococcal infections but with false negative cppB gene based PCR. Sex Transm Infect 2005; 81: 400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Unemo M, Palmer HM, Blackmore T et al. Global transmission of prolyliminopeptidase-negative Neisseria gonorrhoeae strains: implications for changes in diagnostic strategies. Sex Transm Infect 2007; 83: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tomberg J, Unemo M, Davies C et al. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 2010; 49: 8062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tomberg J, Unemo M, Ohnishi M et al. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob Agents Chemother 2013; 57: 3029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Unemo M, Nicholas RA, Jerse AE et al. Molecular mechanisms of antibiotic resistance expressed by the pathogenic Neisseria. In: Davies JK, Kahler CM, eds. Pathogenic Neisseria: Genomics, Molecular Biology and Disease Intervention. London: Caister Academic Press, 2014; 161–92. [Google Scholar]
- 72.Whiley DM, Goire N, Lambert SB et al. Reduced susceptibility to ceftriaxone in Neisseria gonorrhoeae is associated with mutations G542S, P551S and P551L in the gonococcal penicillin-binding protein 2. J Antimicrob Chemother 2010; 65: 1615–8. [DOI] [PubMed] [Google Scholar]
- 73.Folster JP, Johnson PJ, Jackson L et al. MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae. J Bacteriol 2009; 191: 287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veal WL, Nicholas RA, Shafer WM. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J Bacteriol 2002; 184: 5619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zarantonelli L, Borthagaray G, Lee EH et al. Decreased azithromycin susceptibility of Neisseria gonorrhoeae due to mtrR mutations. Antimicrob Agents Chemother 1999; 43: 2468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hagman KE, Pan W, Spratt BG et al. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995; 141: 611–22. [DOI] [PubMed] [Google Scholar]
- 77.Olesky M, Hobbs M, Nicholas RA. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob Agents Chemother 2002; 46: 2811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olesky M, Zhao S, Rosenberg RL et al. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J Bacteriol 2006; 188: 2300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ropp PA, Hu M, Olesky M et al. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 2002; 46: 769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindbäck E, Rahman M, Jalal S et al. Mutations in gyrA, gyrB, parC, and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS 2002; 110: 651–7. [DOI] [PubMed] [Google Scholar]
- 81.Pachulec E, van der Does C. Conjugative plasmids of Neisseria gonorrhoeae. PLoS One 2010; 5: e9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pagotto F, Aman AT, Ng LK et al. Sequence analysis of the family of penicillinase-producing plasmids of Neisseria gonorrhoeae . Plasmid 2000; 43: 23–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.