Supplemental Digital Content is available in the text
Keywords: HCV genotype prevalence, HCV Metavir score prevalence, HCV prevalence, HCV RNA+ prevalence, hepatitis C epidemiology, Italy, meta-prevalence
Abstract
Between western European countries, the hepatitis C virus (HCV) endemic is highest in Italy. The main objective of this paper is to estimate the endemic diffusion of hepatitis C at the national level and by geographical area, with an extrapolation at the regional level and by uniform cohorts of subjects (by sex and year of birth). The secondary objective is a stratification by gravity of the estimated statistical figures to provide an overview of possible targets of the new anti-HCV treatments.
PubMed and the Cochrane Library were searched for relevant Italian populations studies regarding HCV prevalence. Random and fixed effect models were used for pooling data. To develop the epidemiological model, a meta-analysis of studies of Italian populations and the explicit consideration of the changes in the etiology of the disease in different cohorts (by year of birth) of population and the impact of effective treatments that have been introduced since the 1990s. A Markovian transition model, which is based on the distribution of HCV+ and HCV Ribonucleic Acid (RNA)+ subjects, provides a plausible assessment of the Italian situation. The Meta-analysis of Observational Studies in Epidemiology recommendations/statements were followed.
In 2014, 1569,215 HCV+ subjects (95% credible interval [CrI]: 1202,630–2021,261) were estimated in Italy, with a 2.58% prevalence (95% CrI: 1.98%–3.33%). A total of 828,884 HCV RNA+ subjects (95% CrI: 615,892–1081,123), which is equal to a 1.36% prevalence (95% CrI: 1.01%–1.78%), is higher in southern Italy and the islands (1.9%) than in central–northern Italy (1.1%). The predominance of adult and elderly subjects, with an old or very old infection, inevitably entails a significant number of HCV RNA+ subjects in the advanced stages of the illness. According to our estimates, approximately 400,000 subjects have cirrhosis, decompensated cirrhosis, and hepatocarcinoma, with a median age of 70 years.
The model aims to support policymakers to define action plans by providing an estimate of both the emerged infected population and nonemerged infected population by age, gender, gravity, genotype, and geographical area. In the future, the model may contribute to simulation of the costs and outcome of different action strategies that can be adopted by health authorities.
1. Introduction
Hepatitis C virus (HCV) infection is currently the most frequent or concomitant cause of chronic liver disease, cirrhosis, and liver cancer in the world.
Based on a current estimate of 150 to 180 million chronic carriers of HCV worldwide,[1] a significant increase in liver complications (decompensated cirrhosis and liver cancer) is predicted within the next 10 to 20 years if effective therapeutic measures are not implemented.
In recent years, the gradual introduction of new treatments against the HCV that are capable of producing a persistently negative viremia, which is tantamount to biological healing from the infection, has introduced possible epidemiological scenarios, including the possible eradication of this disease.
The high costs associated with these treatments, however, oblige healthcare systems to define action plans and strategies that are grounded in an in-depth knowledge of the phenomenon, with regard to both quantity (prevalence) and quality, namely, its distribution in terms of age and level of liver damage (Metavir score).
As highlighted in the paper by Esteban et al,[2] a distinct north–south gradient has been observed in Europe, with endemic peaks in the Mediterranean countries, in particular Spain, southern France, Italy, and Greece, with a total prevalence between 2.5% and 3.5%. In northern and central Europe, these values range between 0.1% and 1.2%.
Considering the fact that the virus was identified in 1989 and that a gradual adoption of protection and prevention measures in at-risk groups have considerably reduced the number of new cases, especially in the western European countries, the greater prevalence of infected persons in southern Europe is likely related to a greater circulation of the virus in these regions, especially in the 1950s and 1960s.[3] In these countries, surveys detected a consistent presence of cohorts of elderly subjects who were infected in a preserological era during transfusions or unsafe medical procedures, such as the use of nondisposable syringes. We must add cohorts of subjects who were infected in the 1980s and 1990s as a result of an epidemic that was related to endovenous drug abuse; this epidemic remains problematic.
These diverse epidemiological models, which entail different genotype compositions between the older cohorts and the more recent infected cohorts,[4] require more complex treatment strategies that must consider non-neutral consequences on public health.
Against this scenario, the situation of Italy—where the HCV virus is endemic—is particularly significant. A predominance of subjects in Italy who were born prior to the 1950s and were infected many years ago has generated the assumption that the number of cases of cirrhosis and its complications peaked in 2008.[5]
Various mathematical models have been employed to estimate the Italian prevalence. Although these models produced significantly different estimates, they highlight the alarming diffusion of the infection in the country and rank Italy as the European country with the highest number of infected people. The estimated prevalence over the general population ranges from 5.1%, as estimated by the European Centre for Disease Prevention and Control,[6] which has calculated over 3 million HCV-positive subjects, to 3.0%,[7] 2.0% as estimated by Gower et al,[1] and 1.42%.[8]
National surveys do not provide a stratification of the phenomenon by age group or level of gravity.
The main objective of this paper, which is based on all epidemiological surveys of the general population that have been conducted in Italy since the identification of the virus, is to estimate the endemic diffusion of hepatitis at the national level and by geographical area, with an extrapolation to the regional level and by uniform cohorts of subjects (by sex and year of birth). Based on these results, the secondary objective is a stratification by level of gravity (according to the Metavir score) of the estimated statistical figures to provide an overview of the possible targets of new anti-HCV treatments—by age and gravity of the disease, that highlights a particularly high level of sustained viral response (SVR), which will enable the planning of healthcare policies and treatment strategies that are aimed at the (possible) eradication of the disease.
2. Materials and methods
This systematic review and meta-analysis of available nationally and internationally published literature and gray literature was performed according to the Meta-analysis of Observational Studies in Epidemiology Guidelines 13. Ethical approval was not required considering the nature of the study.
With regard to the published literature, the following databases were consulted:
MEDLINE;
Cochrane Library.
The research strategy was consistent with the terminology of the National Library of Medicine's Medical Subject Headings and specific keywords. The main research concepts were as follows: hepatitis C, hepacivirus, hepatitis C antibodies, epidemiology, incidence, prevalence, and Italy, including all Italian regions.
The research was limited to studies in English and Italian that were published after 1989 (excluding conference abstracts).
To obtain unpublished records, such as guidelines and reviews, which can provide useful information, the following websites and databases were consulted:
the websites of the major regulatory agencies (Food and Drug Administration, Canadian Agency for Drugs and Technologies in Health, and National Institute for Health and Clinical Excellence);
the websites of the major Health Technology Assessment agencies;
the database of the University of York Centre for Reviews and Dissemination.
The research was completed on October 25, 2014 (refer to Supplementary materials SM1).
A total of 358 records were identified (343 published literature publications and 15 gray publications). The diagram in Fig. 1 shows the literature review and selection process.
Figure 1.
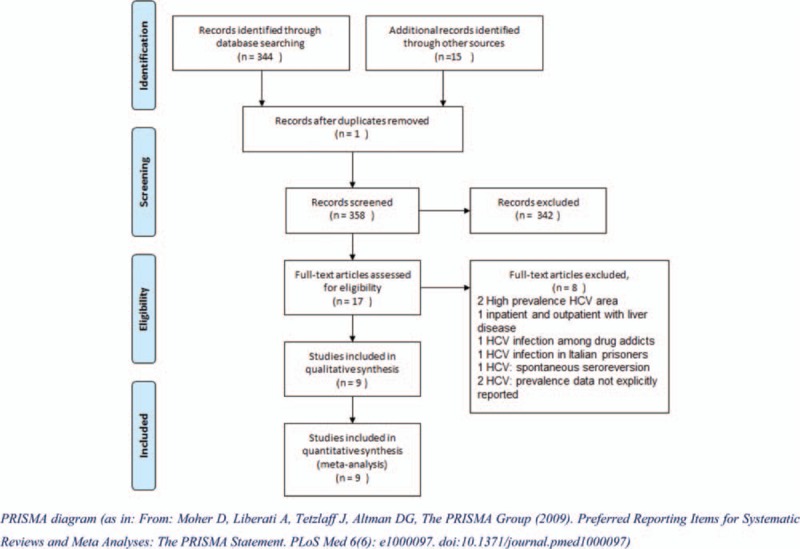
Hepatitis C virus prevalence studies: literature identification, review, and selection process.
With regard to the 17 applicable records, the following records were discarded:
Epidemiological surveys of populations in specific areas that featured high rates of HCV prevalence[9,10];
Epidemiological surveys of hospitalized subjects and/or healthcare facilities[11];
Epidemiological surveys of specific population subgroups, in particular drug addicts and the prison population[12,13];
-
Surveys that did not specifically report the distribution of the prevalence by age group.[14–16]
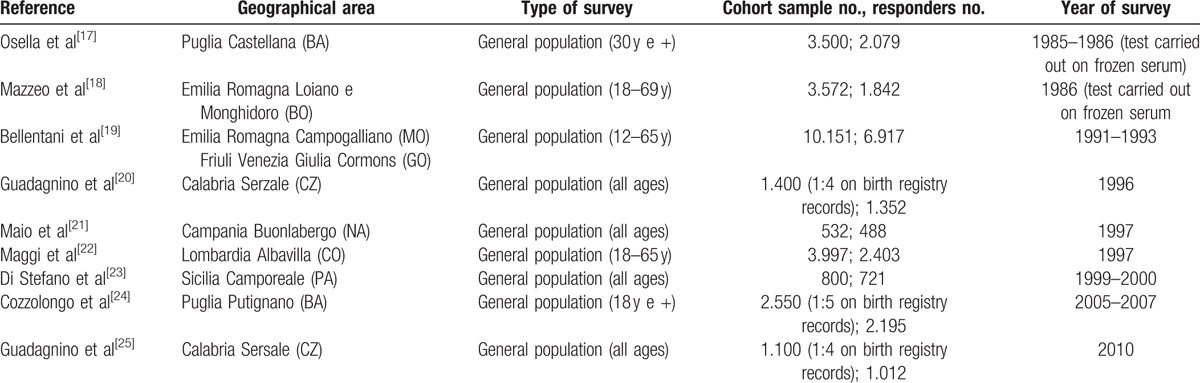
For prevalence estimation purposes, therefore, 9 records were selected, which refer to a total population of 20,654 subjects who were screened for HCV, the principal characteristics of which are listed in Table 1.
Table 1.
Principal characteristics of the studies included in the analysis.
To define a strategy for estimating the prevalence of HCV in the Italian population, 2 main factors of the dynamics of diffusion of the virus were considered: time and geographical location.
Regarding the former, the infection risk has significantly changed over the years. From 1989 to 1990, the HCV virus was identified and the first ad hoc virological tests were developed. This period may be considered as a watershed period between 2 eras that features vastly different methods of transmission and intensity of infection. In the preserological era, the risk of infection was much more widespread in terms of population, primarily due to the use of nondisposable syringes, which highlights a greater diffusion in earlier periods. In addition, the inability to identify the virus has also prompted widespread medical practices, such as blood transfusions and dialysis, which can have grave outcomes. Although the implementation of adequate prevention and protection measures since the early 1990s had the initial effect of limiting and deleting these methods of infection and substantially restricting new infections to specific population groups, such as drug addicts and the prison population. For prevalence estimation purposes, the risk of mother–child or sexual infection is considered to be minimal. A higher prevalence of HCV is observed in the southern regions of Italy and the islands probably due to the delayed subsequent scrapping of nondisposable syringes. As a result, the population samples of the selected studies have not been analyzed by age but separately by year of birth for 2 macroareas (north and central Italy and southern Italy and the islands). Regarding the years of birth, we have identified 5 cohorts that are characterized by uniform risk factors.
With regard to the 9 selected studies, Fig. 2 shows the population sample ranges by year of birth; the vertical bars indicate the 5 cohorts of the population.
Figure 2.
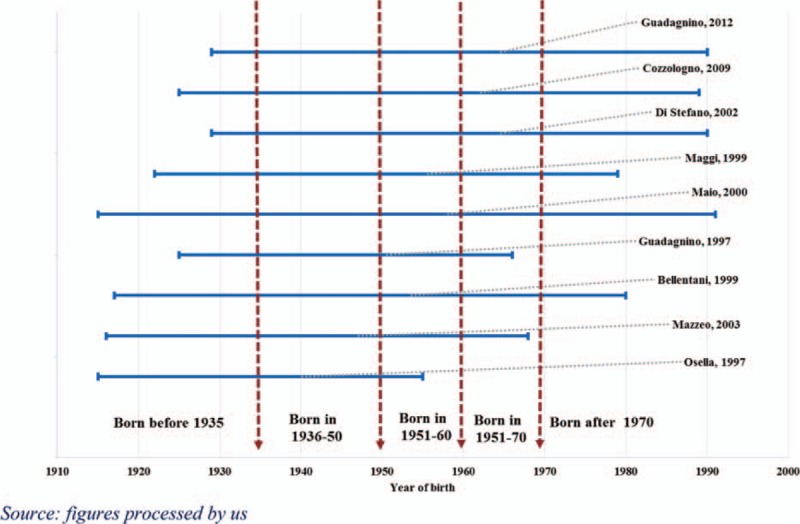
Population samples in the 9 selected studies. Range by year of birth.
Supplementary materials provide the distribution of the cases by uniform cohort in each of the 9 studies (refer to Supplementary materials SM2).
The percentage of HCV RNA positivity was separately estimated by macroarea with reference to the same studies.
The meta-prevalences were estimated with the MetaXL ver. 2.0 package (© EpiGear International Pty Ltd ABN 51 134 897 411 Brisbane, Australia, 2011-2014)[26] (refer to Supplementary materials SM3).
A Markovian transition model, which is based on the distribution of HCV+ and HCV RNA+ subjects and provides a plausible assessment of the Italian situation (Fig. 3), was constructed. Table 2 lists the clinical parameters and sources.
Figure 3.
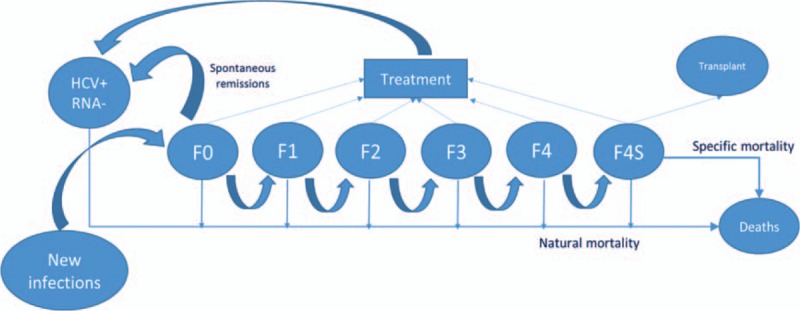
Diagram of the Markov model.
Table 2.
HCV: likelihood of annual transition.
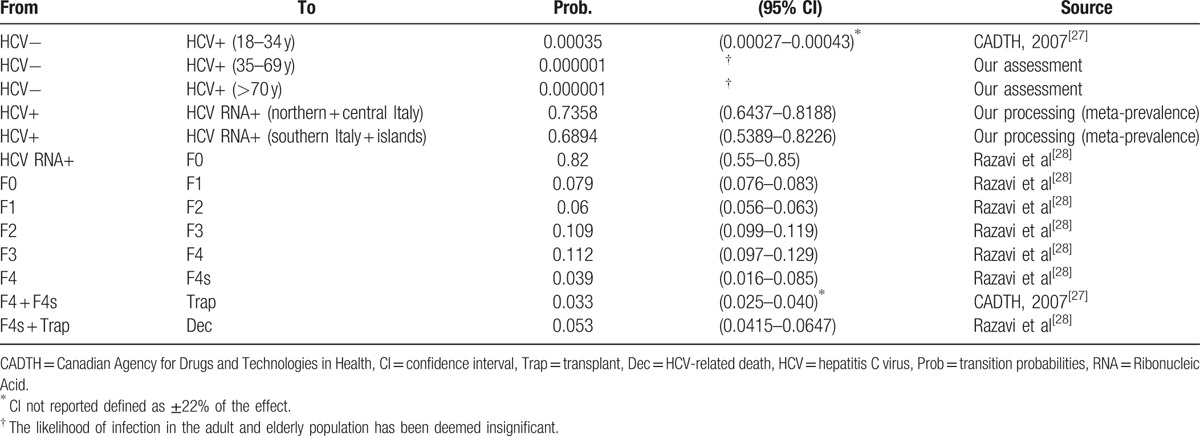
As the majority of the epidemiological surveys of the surveyed populations were conducted in the 1990s, the projection of the meta-prevalences on the resident population on January 1, 2014 may overestimate the phenomenon, especially in the case of the elderly population. Although the risk of new infections among the elderly is considered to be minimal, we can presume that the endemic diffusion has been reduced due to the new specific treatments that have been introduced over the years and with regard to the specific mortality for decompensated cirrhosis and liver cancer. Therefore, we have proceeded as follows:
We have considered the basic population as the resident population on January 1, 1995 by sex, age, and region of residence (we have assumed that the proportion of adult/elderly subjects who were infected as a result of transfusions and dialysis since 1995 is negligible, and therefore, significantly stable with regard to the endemic diffusion among the adult population).
- We projected the model to 10 years (2005) based on the following assumptions:
- Likelihood of transition, per the model (Table 2).
- Likelihood of specific death, 5.3% (Table 2).
- Likelihood of transplant has not been considered.
- Percentage of emergence (our assessment).
- F0 = 0%
- F1 = 0%
- F2 = 20%
- F3 = 40%
- F4 = 40%
- F4s = 40%
- Percentage of treated (subjects); only F4 and F4s 100% of the emerged (subjects) (our assessment).
- SVR% of 30.7%: simple average of the response rate to the introduction of pegylated interferon (PegIFN)[29] (Adapted from the US Food and Drug Administration, Antiviral Drugs Advisory Committee Meeting, April 27–28, 2011, Silver Spring, MD).
- Spontaneous remission rate of 25%.[5]
We calculated the specific outgoing weights (as a result of death, healing by remission, and SVR%) for each age between 28 and 90 years at 2005 (national weights).
The model was projected from 2005 to 2014 based on the same assumptions, with the exception of SVR% assumed equal to 62.5%: average response between pegylated interferon/ribavirin (PegIFN/RBV) and PegIFN/RBV/direct-acting antiviral drugs.[29]
We calculated the specific outgoing weights for each age between 38 and 90 years at 2014 (national weights).
Regarding the estimate of emerged patients, that is, all subjects known to and treated by the National Health Service (NHS), we have assumed the following emergence rates per stage, with respect to the model at 2014 (our assessment):
F0 = 10%
F1 = 15%
F2 = 20%
F3 = 50%
F4 = 60%
F4s = 80%
The implemented model enables a punctual estimate per age of the surveyed parameters (HCV+, HCV RNA+, genotype of total and emerged population); in the analysis of the results, the data have been grouped into 3 age groups (youths: 18–34, adults: 35–69, and elderly: 70+).
The distribution by genotype has been estimated according to a 2-stage procedure based on the 9 population studies that are included in the estimate of the HCV meta-prevalences, which are employed to identify the geographical trends (northern Italy vs central–southern Italy). We applied Italian studies of hospital and outpatient populations to estimate the genotype distribution.[30–33]
3. Results
Based on the studies that are deemed eligible for the purposes of the survey (refer to Table 3 and the supporting materials for details) to determine the relevant meta-analysis of prevalence and considering that the analyzed studies predominantly date to the period from 1991 to 2000 (some were previous studies were conducted using frozen serum), these estimates can be considered to be valid for the mid-1990s.
Table 3.
Estimated prevalence for HCV+ and HCV RNA+ pooling analysis by cohort.
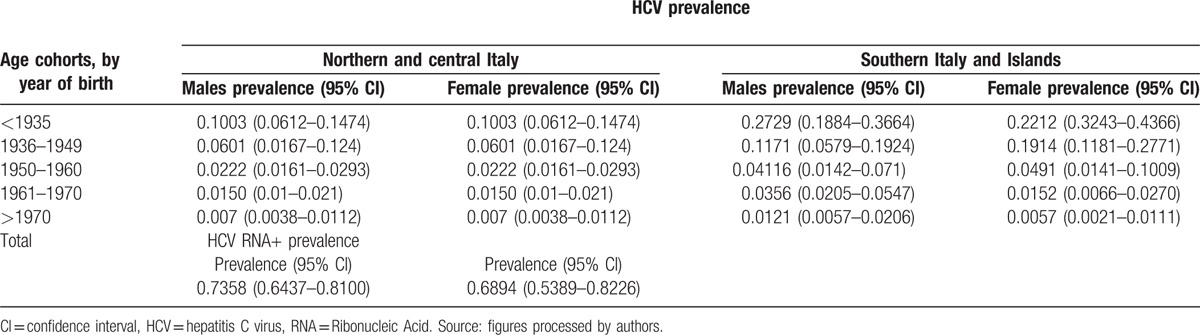
In 1995, 3166,393 HCV+ subjects (95% credible interval [CrI]: 2527,484–3887,328)—1428,674 males and 1737,719 females—were estimated in Italy, with a prevalence of 5.57% (95% CrI: 4.45%–6.84%); this prevalence is considerably to be higher in southern Italy and the islands (8.1%) than in central–northern Italy (4.1%). In the same year, 2284,286 million HCV RNA+ subjects (95% CrI: 1829,944–2807,443)—1030,175 million men and 1254,110 million women—were estimated, with a prevalence of 4.02% (95% CrI: 3.22%–4.94%); this prevalence is considerably higher in southern Italy and the islands (5.7%) than in central–northern Italy (3.1%).
Due to the lack of recent populations, a Markovian model has been employed, as described in Section 2. We have estimated the effect of the introduction of infection contrast policies in the 1990s and the gradual appearance of increasingly effective treatments.
The endemic load of HCV in the Italian population considerably decreased in 2014: the number of HCV+ subjects decreased to 1569,215 (95% CrI: 1202,630–2021,261) with a 2.58% prevalence (95% CrI: 1.98%–3.33%). The contraction of RNA+ subjects over the years is significant: it decreased to 828,884 (95% CrI: 615,892–1081,123) with a 1.36% prevalence (95% CrI: 1.01%–1.78%) (refer to Table 4).
Table 4.
Estimated endemic load of HCV infection (1995–2005–2014).
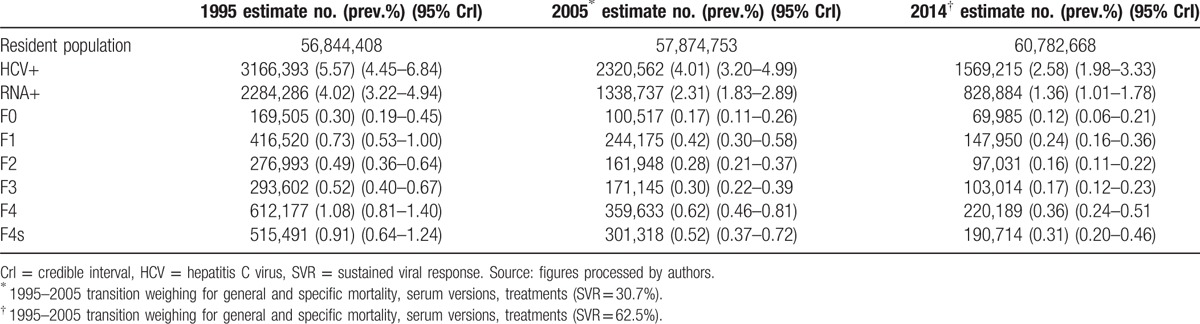
Table 4 also enables an analysis of the different impacts of the effectiveness of the treatments before and after 2005.
The total distribution by sex remains approximately constant over time, with a higher female component (54.4% of cases). If we consider the population aged below 35 years, a prevalence in the specific population of 0.9% is observed for both sexes. In contrast from the 1990s to the principal methods of infection of the epidemic, a significant alteration in composition by age of the infected population is evident: 70+ year old HCV+ subjects increased from 28.1% in 1995 to 45% in 2014. The median age in 1995 was 61 years, which increased to 68 years in 2014. Therefore, the distribution of HCV+ subjects by age group highlights a large imbalance toward the older groups: only 6.4% of positive subjects are aged between 18 and 34 years, with a prevalence of 0.9%; 48.3% of positive subjects are aged between 35 and 69 years, with a prevalence of 2.6%; and 45.3% of positive subjects are aged over 70 years, with a specific prevalence of 7.5%.
Therefore, the peak of the epidemic with the greater number of serious consequences has been overcome.[5] By 2014, the cases of cirrhosis and its complications were less than approximately 56% compared with the case studies in 1995. The dynamics of the phenomenon generated the assumption that the peak of the epidemic was attained prior to 2005 and prior to the estimate obtained by Deuffic-Burban, which estimated that the peak of the epidemic in Italy would occur in 2008.
The evident predominance of adult and elderly subjects, with an old or very old infection, inevitably entails a significant number of HCV RNA+ subjects in the advanced stages of the illness. According to our estimates, approximately 400,000 subjects have cirrhosis, decompensated cirrhosis, and hepatocarcinoma, with an average age of 69.5 years and a median age of 70 years.
The distribution by age and Metavir score F4 and F4s (F4s also includes the subjects who are affected by hepatocarcinoma) is shown in Fig. 4.
Figure 4.
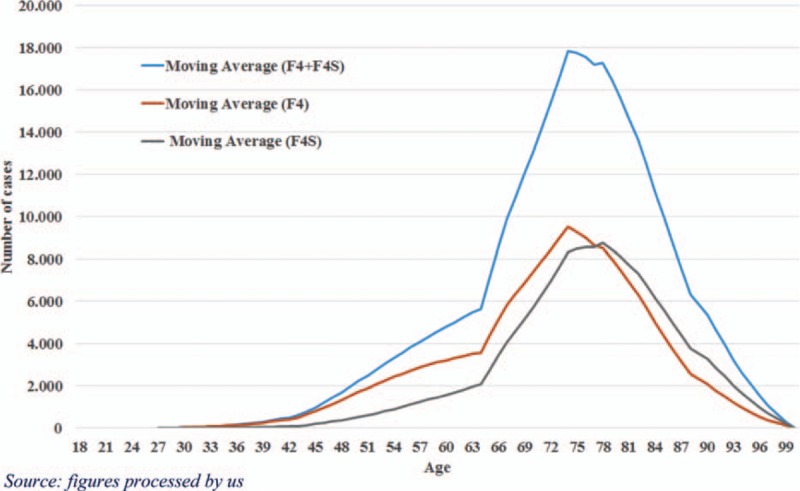
Metavir score F4 and F4s distribution age of patients (2014).
A total of 52.9% (95% CrI: 48.6%–56.7%) of the subjects belong to genotype 1a1b; 27.7% (95% CrI: 23.9%–31.5%) of the subjects belong to genotype 2a2b; 11.8% (95% CrI: 9.0%–14.9%) of the subjects belong to genotype 3, and 7.7% (95% CrI: 1.4%–14.0%) of the subjects belong to genotype 4 (refer to Table 5).
Table 5.
Distribution by age group, genotype, and Metavir score∗.
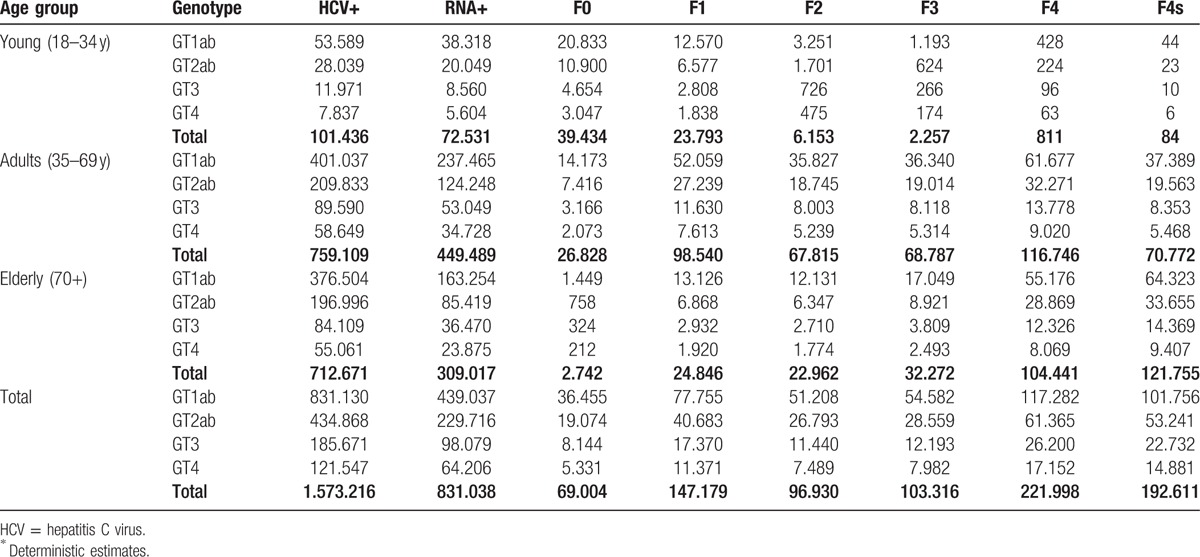
In the case of formulated emersion, the NHS should be aware and engaged in the treatment of 385,553 (95% CrI: 275,351–520,819) subjects, which accounts for 47% of the HCV RNA+ subjects. The assumed cases of emersion are strictly related to the gravity of the illness and inevitably entail an even greater percentage of elderly subjects. Among the emerged subjects, the 70+-year-olds account for 47.32% (95% CrI: 38.56%–54.71%) compared with only 2.78% (95% CrI: 1.60%–4.53%) of subjects aged below 34 years.
4. Discussion
In methodological terms, the estimated prevalence in Italy poses a number of challenges, which primarily derive from changes in the etiology of the infection over time.
Therefore, the model that we have adopted employs the meta-prevalences that are estimated based on epidemiological studies of Italian populations that are applied by cohorts with the risk of uniform infection. We incorporated the estimates using a Markovian model and a correction based on the supermortality caused by the large percentage of subjects that became infected a long time ago.
Regarding HCV infection, Italy is undoubtedly a peculiar case among Western countries. The Italian endemic load is unquestionably higher than the endemic load in other countries. However, the struggle against the transmission of the epidemic with the greatest impact and the introduction of increasingly effective specific treatments has curbed the spread of HCV since the 1990s. The model confirms a significant reduction in prevalence over the last 20 years, which is estimated at 2.6%, and the predominance of the percentage of elderly subjects in advanced stages of the disease: cirrhosis, decompensated cirrhosis, and hepatocarcinoma, of which 70+-year-olds account for 55% of the total number of these subjects.
The main peculiarity of the infection in Italy, therefore, is the vast difference in the epidemiological structure of the cases, with a predominance in Italy, of cases in the preserological period compared with recent cases, which are primarily related to drug abuse.
Our studies have confirmed that the prevalence of HCV among youth and adults in Italy is comparable to the prevalence of other developed countries: for west European countries, Gower et al[1] has estimated a prevalence of 0.9% (95% confidence interval: 0.7%–1.5%). Therefore, the difference can be interpreted as the result of the high prevalence of elderly and very elderly subjects. However, the model highlights that the most serious effects of the disease (cirrhosis, decompensated cirrhosis, and hepatocarcinoma) have peaked, and a further gradual and significant reduction over the coming years is projected due to new treatments that ensure very high percentages of SVR.
The change of the infection's etiology over time has also determined the considerable diversity in prevalence across the country, with a higher prevalence in southern Italy by 1.8% compared with central–northern Italy (3.8% vs 2.0%). With regard to conditions of seropositivity, the higher prevalence decreases to 0.8% for subjects with a viral load (1.9% vs 1.1%).
Although they generate the hope of eradicating this disease, at least locally, the introduction of new and highly effective antiretroviral treatments on the market poses a problem of sustainability due to the high cost of these treatments.
These financial constraints are probably directing Italian health authorities[34] toward priority treatment of more serious cases—cases that have already emerged or will emerge in the forthcoming years as a result of occasional specimens or the evolution of the disease.
This type of strategy inevitably entails the treatment of elderly or very elderly subjects, which does not bode well for the rapid eradication of the disease.
The assumptions that are adopted with respect to emergence have produced an estimated percentage of 47% of HCV RNA+ subjects, who are unknown to the health services.
Therefore, we hope that proactive strategies and policies may be assessed to enable the emergence of unknown cases, especially among the younger members of the population at risk.
5. Conclusion
The proposed model elaborates estimates of the endemic load of HCV in Italy by adopting a meta-analysis of the studies of Italian populations and explicitly considering the changes in the etiology of the disease in the different cohorts (by year of birth) of the population and the impact of effective treatments since the 1990s.
A significant limitation of the proposed model is its lack of consideration of the endemic load of HCV in the immigrant population, which is undoubtedly increasing and originates in countries in which the disease is endemic.
Another limitation is the adoption of emergence estimates based on clinical experience, of which the impact has been assessed by a Probability Sensitivity Analysis.
The proposed model aims to support policymakers in the determination of rational action plans by providing estimates of the emerged and nonemerged infected population by age, gender, gravity, and genotype, as well as by geographical area. In the future, the model may contribute to simulations of the costs and outcomes of the different action strategies that are adopted by the health authorities.
Supplementary Material
Footnotes
Abbreviations: CrI = credible interval, HCV = hepatitis C virus, NHS = National Health Service, PegIFN = pegylated interferon, RBV = ribavirin, SVR = sustained viral response.
Author distribution: All authors conceived and designed the study. RLM, FS, and DdA—performed data extraction and drafted the manuscript. RLM—biostatistician—provided methodological guidance of this manuscript as well as reviewed the statistics. All authors had access to the data and a role in writing the manuscript. All authors were accountable for all aspects of the work and ensured that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding/support: The study received an unconditioned grant from BMS. None of the funding sponsors were involved in the study design, data collection, management, analysis, and interpretation of the data; and preparation, review, and or approval of this manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61:S45–S57. [DOI] [PubMed] [Google Scholar]
- 2.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 2008; 48:148–162. [DOI] [PubMed] [Google Scholar]
- 3.Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, et al. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis 1995; 171:1607–1610. [DOI] [PubMed] [Google Scholar]
- 4.Raptopoulou M, Touloumi G, Tzourmakliotis D, et al. Significant epidemiological changes in chronic hepatitis C infection: results of the nationwide HEPNET-GREECE cohort study. Hippokratia 2011; 15:26–31.PMID: 21607032. [PMC free article] [PubMed] [Google Scholar]
- 5.Deuffic-Burban S, Deltenre P, Buti M, et al. Predicted effects of treatment for HCV infection vary among European countries. Gastroenterology 2012; 143:974–985.e14. [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. Hepatitis B and C in the EU neighborhood: prevalence, burden of disease and screening policies. Stockholm, September 2010 ISBN 978-92-9193-213-9. doi 10.2900/30933. [Google Scholar]
- 7.Mariano A, Tomba G, Tosti ME, Spada E, Mele A. Future burden of hepatitis C virus infection: the case of Italy. In: 41st Annual Meeting of the European Association for the Study of the Liver (EASL). Vienna, Austria, April 26–30 2006. [Google Scholar]
- 8.daCosta DiBonaventura M, Yuan Y, Wagner JS, et al. The burden of viral hepatitis C in Europe: a propensity analysis of patient outcomes. Eur J Gastroenterol Hepatol 2012; 24:869–877. [DOI] [PubMed] [Google Scholar]
- 9.Stroffolini T, Menchinelli M, Taliani G, et al. High prevalence of hepatitis C virus infection in a small central Italian town: lack of evidence of parenteral exposure. Ital J Gastroenterol 1995; 27:235–238. [PubMed] [Google Scholar]
- 10.Raffaele A, Valenti M, Iovenitti M, et al. High prevalence of HCV infection among the general population in a rural area of central Italy. Eur J Epidemiol 2001; 17:41–46. [DOI] [PubMed] [Google Scholar]
- 11.Sagnelli E, Stroffolini T, Mele A, et al. The importance of HCV on the burden of chronic liver disease in Italy: a multicenter prevalence study of 9997 cases. J Med Virol 2005; 75:522–527. [DOI] [PubMed] [Google Scholar]
- 12.Stroffolini T, D’Egidio PF, Aceti A, et al. DAVIS Drug Addicted, HCV Prevalence in Italy an Epidemiological, Observational, Cross-Sectional, Multicenter Study Participating Centers. Hepatitis C virus infection among drug addicts in Italy. J Med Virol 2012; 84:1608–1612. [DOI] [PubMed] [Google Scholar]
- 13.Brandolini M, Novati S, De Silvestri A, et al. Prevalence and epidemiological correlates and treatment outcome of HCV infection in an Italian prison setting. BMC Public Health 2013; 13:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loviselli A, Oppo A, Velluzzi F, et al. Independent expression of serological markers of thyroid autoimmunity and hepatitis virus C infection in the general population: results of a community-based study in north-western Sardinia. J Endocrinol Invest 1999; 22:660–665. [DOI] [PubMed] [Google Scholar]
- 15.Kondili LA, Chionne P, Costantino A, et al. Infection rate and spontaneous seroreversion of anti-hepatitis C virus during the natural course of hepatitis C virus infection in the general population. Gut 2002; 50:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendino GM, Mariano A, Surace P, et al. Prevalence and etiology of altered liver tests: a population-based survey in a Mediterranean town. Hepatology 2005; 41:1151–1159. [DOI] [PubMed] [Google Scholar]
- 17.Osella AR, Misciagna G, Leone A, et al. Epidemiology of hepatitis C virus infection in an area of Southern Italy. J Hepatol 1997; 27:30–35. [DOI] [PubMed] [Google Scholar]
- 18.Mazzeo C, Azzaroli F, Giovanelli S, et al. Ten year incidence of HCV infection in northern Italy and frequency of spontaneous viral clearance. Gut 2003; 52:1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellentani S, Pozzato G, Saccoccio G, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut 1999; 44:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guadagnino V, Stroffolini T, Rapicetta M, et al. Prevalence, risk factors and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in Southern Italy. Hepatology 1997; 26:1006–1011. [DOI] [PubMed] [Google Scholar]
- 21.Maio G, d’Argenio P, Stroffolini T, et al. Hepatitis C virus infection and alanine transaminase levels in the general population: a survey in a southern Italian town. J Hepatol 2000; 33:116–120. [DOI] [PubMed] [Google Scholar]
- 22.Maggi G, Armitano S, Brambilla L, et al. Hepatitis C infection in an Italian population not selected for risk factors. Liver 1999; 19:427–431. [DOI] [PubMed] [Google Scholar]
- 23.Di Stefano R, Stroffolini T, Ferraro D, et al. Endemic hepatitis C virus infection in a Sicilian town: further evidence for iatrogenic transmission. J Med Virol 2002; 67:339–344. [DOI] [PubMed] [Google Scholar]
- 24.Cozzolongo R, Osella AR, Elba S, et al. Epidemiology of HCV infection in the general population: a survey in a southern Italian town. Am J Gastroenterol 2009; 104:2740–2746. [DOI] [PubMed] [Google Scholar]
- 25.Guadagnino V, Stroffolini T, Caroleo B, et al. Hepatitis C virus infection in an endemic area of Southern Italy 14 years later: evidence for a vanishing infection. Dig Liver Dis 2013; 45:403–407. [DOI] [PubMed] [Google Scholar]
- 26.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67:974–978. [DOI] [PubMed] [Google Scholar]
- 27.Remis RS. Modelling the Incidence and Prevalence of Hepatitis C Infection and its Sequelae in Canada, 2007. Ottawa: Public Health Agency of Canada; 2007. [Google Scholar]
- 28.Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013; 57:2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. Antiviral Drugs Advisory Committee Meeting. PC April 27–28, 2011, Silver Spring, MD 2011. [Google Scholar]
- 30.Marascio N, Matera G, Quirino A, et al. Eleven-year distribution pattern of hepatitis C virus in southern Italy. J Pathog 2012; 2012:631095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marascio N, Liberto MC, Barreca1ì GS, et al. Update on epidemiology of HCV in Italy: focus on the Calabria Region. BMC Infect Dis 2014; 14 suppl 5:S2.http://www.biomedcentral.com/1471-2334/14/S5/S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Ambrosio R, Aghemo A, De Francesco R, et al. The association of IL28B genotype with the histological features of chronic hepatitis C is HCV genotype dependent. Int J Mol Sci 2014; 15:7213–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cenci M, Massi M, Alderisio M, et al. Prevalence of hepatitis C virus (HCV) genotypes and increase of type 4 in central Italy: an update and report of a new method of HCV genotyping. Anticancer Res 2007; 27:1219–1222. [PubMed] [Google Scholar]
- 34.AIFA. 2015. (https://www.agenziafarmaco.gov.it/piattaformaAlgoritmi/index.php/survey/index). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


