Abstract
The aim of the study was to identify factors associated with undiagnosed human immunodeficiency virus (HIV) infection among men who have sex with men (MSM) and male-to-female transgender women in Lima, Peru.
We analyzed characteristics of 378 MSM and transgender women recruited from 2 sexually transmitted infection (STI) clinics in Lima, Peru. Descriptive analyses compared: (A) HIV-uninfected, (B) previously undiagnosed HIV-infected, and (C) previously diagnosed HIV-infected participants. Multivariable logistic regression models identified: (1) correlates of previously undiagnosed HIV-infection among participants thought to be HIV-uninfected (B vs A); and (2) correlates of previously undiagnosed HIV-infection among HIV-infected participants (B vs C). Subanalysis identified correlates of frequent HIV testing among participants thought to be HIV-uninfected.
Among participants, 31.0% were HIV-infected; of those, 35.0% were previously undiagnosed. Among participants thought to be HIV-uninfected (model 1), recent condomless receptive anal intercourse and last HIV test being over 1-year ago (compared to within the last 6-months) were associated with increased odds of being previously undiagnosed HIV-infected (adjusted odds ratio [aOR] = 2.43, 95% confidence interval [95%CI] = 1.10–5.36; aOR = 2.87, 95%CI = 1.10–7.53, respectively). Among HIV-infected participants (model 2), recent condomless receptive anal intercourse was again associated with previously undiagnosed HIV-infection (aOR = 2.54, 95%CI = 1.04–6.23). Achieving post-secondary education and prior syphilis infection were associated with lower odds of having previously undiagnosed HIV-infection (aOR = 0.35, 95%CI = 0.15–0.81; aOR = 0.32, 95%CI = 0.14–0.75, respectively).
Reporting semiannual testing was associated with higher educational attainment, identifying as a transgender woman, or reporting a history of syphilis (aOR = 1.94, 95%CI = 1.11–3.37; aOR = 2.40, 95%CI = 1.23–4.70; aOR = 2.76, 95%CI = 1.62–4.71, respectively). Lower odds of semiannual testing were associated with recent condomless insertive anal intercourse or reporting a moderate or high self-perceived risk of acquiring HIV (aOR = 0.56, 95%CI = 0.33–0.96; aOR = 0.32, 95%CI = 0.18–0.59 and aOR = 0.43, 95%CI = 0.21–0.86, respectively).
In our study, undiagnosed HIV-infection was associated with recent condomless receptive anal intercourse, infrequent HIV testing, lower education, and absence of prior syphilis diagnosis. Infrequent HIV testing was associated with lower education, not identifying as transgender, recent condomless insertive anal intercourse, absence of prior syphilis diagnosis, and higher self-perceived risk of HIV. Further efforts to decrease HIV transmission and increase HIV-serostatus awareness should be directed towards effectively promoting condom use and frequent HIV testing, integrated with STI management.
Keywords: Lima, men who have sex with men, Peru, risk factors, transgender women, undiagnosed HIV infection
1. Introduction
In Peru, the HIV epidemic primarily affects men who have sex with men (MSM) and male-to-female transgender women.[1] Recent data suggest that 12.4% of Peruvian MSM are infected with human immunodeficiency virus (HIV), with estimates as high as 22.3% in the city of Lima.[1] Further, the HIV prevalence is estimated as high as 30.0% among transgender women.[2] Due to HIV infections being concentrated among MSM and transgender women, it is crucial to strengthen HIV prevention, management, and care among these key population.
Frequent HIV testing with immediate linkage to care and access to treatment for those previously undiagnosed are pillars of the “treatment as prevention” and “test and treat” models.[3–5] MSM and transgender women who are unaware of their HIV-infection are not only delaying their own access to antiretroviral therapy (ART), but are also more likely to unknowingly transmit HIV to uninfected sex partners.[6–8]
In the United States, the Centers for Disease Control and Prevention (CDC) and United States Preventive Services Task Force (USPSTF) have recommended at least annual HIV testing of MSM and quarterly testing as guided by clinical discretion.[9,10] Recent reports show that about 59% to 67% of MSM in the United States get tested at least annually with 37% to 44% testing even more frequently.[11,12] In contrast, the United Nations General Assembly Special Session (UNGASS) Declaration of Commitment in 2005 made recommendations for semiannual testing and counseling for MSM.[1] The Peruvian National Ministry of Health (MoH) program's guideline on periodic medical check-ups recommends quarterly visits for MSM and transgender women, with opt-in HIV testing and counseling, but few MSM and transgender women test more than once yearly.[1,13,14] A recent study showed only 6.2% of MSM and transgender women tested semi-annually in Lima.[13] Low testing frequency results in a higher proportion of HIV infected individuals who are unaware of their HIV-infection status. Previous research has demonstrated that low testing frequency is correlated with low perceived HIV risk, limited access to testing services, fear of test results, unawareness of the availability of effective treatment, and perceived stigma associated with HIV and HIV testing.[1,13,15]
Surveillance studies of MSM and transgender women in Lima have suggested between 62.5% to 90.0% of HIV-infected individuals are unaware of their HIV status.[6,16,17] In contrast, studies occurring at STI clinics, where HIV testing is often sought after or recommended, have found rates of HIV-serostatus unawareness <30%.[18,19]
Our study aimed to further characterize the socio-demographic factors, sexual risk behaviors, alcohol and drug use, syphilis history, and HIV testing history that were associated with previously undiagnosed HIV-infection among high-risk MSM and transgender women in Lima, Peru.
2. Methods
2.1. Study population
The study sample included MSM and transgender women aged 18-years or older who were recruited for a 2-year cohort study from 2 sexually transmitted infection (STI) clinics in Lima, Peru. Participants were eligible for participation in the study if they were considered to be at high-risk for HIV and STIs by reporting specific risky sexual behaviors and/or had a clinical history of syphilis, genital ulcerative disease, and/or HIV-infection. Detailed inclusion criteria for the study are described elsewhere.[20] Written informed consent was obtained from subjects after explanation of the study. This study was approved by the Ethics Committee at Universidad Peruana Cayetano Heredia institutional review board (IRB no. 61522).
2.2. Instrument
Participant baseline characteristics were collected using a 139-question survey. Questions addressed social/environmental characteristics of participants, gender identification, sexual role (insertive anal sex, receptive anal sex, or both insertive and receptive anal sex), history of syphilis, knowledge of HIV, presumed HIV-infection status, frequency of HIV testing, date of last sexual encounter, number and type of sex partners in the past 3-months, and alcohol and drug use. The 10-item Alcohol Use Disorder Identification Test (AUDIT) was used to further characterize alcohol use.[21] A score of 8 or above to define hazardous or harmful alcohol use was the cutoff used in our survey since this is the score at which the World Health Organization recommends intervening clinically.[21] Participants were considered to have a recurrent sex partner if they reported that among their sex partners one or more were stable sex partners with whom they had engaged in sex multiple times over the past 3-months.
2.3. Laboratory analysis
At enrollment, participants were tested for HIV-infection using a rapid HIV antibody test (Determine, Alere Inc., Waltham, MA, USA) and fourth generation enzyme immunoassay (EIA) (GenscreenTM ULTRA HIV Ag-Ab, Bio-Rad, France). Those with a reactive EIA and/or positive rapid HIV antibody test had a confirmatory western blot (WB) (NEW LAV BLOT I, Bio-Rad, France) that is required for entry into care in Peru.
2.4. Classification of subjects
Participants were classified into 3 categories according to their self-reported HIV status and the results of HIV testing performed at the enrollment visit: (A) “HIV-uninfected” was defined as having a negative rapid test and nonreactive EIA; (B) “previously undiagnosed HIV-infected” was defined as having a positive rapid test and/or reactive EIA with confirmatory WB test as well as reporting that their last HIV test was negative or that they had never been tested; and (C) “previously diagnosed HIV-infected” was defined as having a positive rapid test and/or reactive EIA along with confirmatory WB test as well as reporting that their last HIV test was positive.
2.5. Statistical analysis
Participants who recorded responses to all variables of interest were included in the final analysis. Descriptive statistics were used to summarize the study population. Socio-demographic, health, and behavioral characteristics of study participants were stratified by HIV-infection and awareness status. Continuous variables were described by inter-quartile ranges (IQR) and medians. For each variable of interest, 2 bivariate analyses were performed (1) previously undiagnosed HIV-infected compared to HIV-uninfected MSM and transgender women (B vs A), and (2) previously undiagnosed HIV-infected compared to previously diagnosed HIV-infected MSM and transgender women (B vs C). Subanalyses were performed on HIV-uninfected and previously undiagnosed HIV-infected participants to identify variables associated with reporting an HIV testing interval of every 6 months or less. Pairwise chi-squared tests with Bonferroni correction were used to analyze the bivariate analyses. Corresponding to the bivariate analyses, multivariable analyses were conducted including variables found to have a P < 0.10 in their respective bivariate analysis. Crude and adjusted odds ratios (aOR) with corresponding 95% confidence intervals (95%CI) were reported for the multivariable models. Statistical significance in the multivariable analyses was set at P < 0.05 and variables that were not found to be significant were sequentially removed from the analysis until all variables had a P < 0.05. Statistical analysis was conducted using STATA v13 (College Station, TX).
3. Results
A total of 401 MSM and transgender women were enrolled in the study, of whom 378 (94.3%) had complete data on all variables of interest and were included in this analysis. Table 1 shows the characteristics of MSM and transgender women who were HIV-uninfected, previously undiagnosed HIV-infected, and previously diagnosed HIV-infected. The mean age of all participants was 31.6 years old. Overall 31.0% (117/378) of our study participants were HIV-infected. Among those who were HIV-infected, 35.0% (41/117) were previously undiagnosed HIV-infected at study enrollment.
Table 1.
Characteristics of men who have sex with men and transgender women by HIV-infection and awareness status in Lima, Peru.
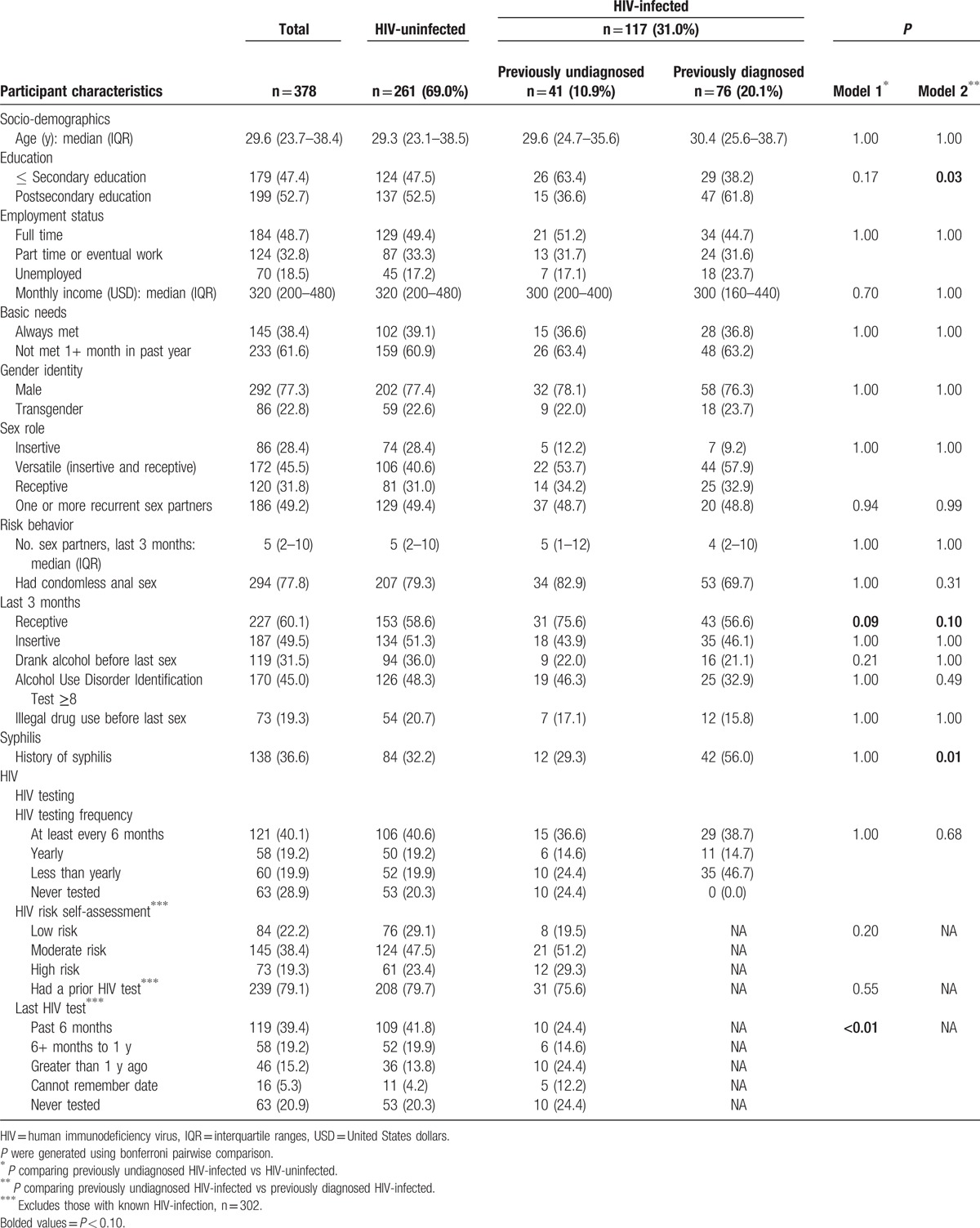
In the bivariate analysis comparing study participants who were previously undiagnosed HIV-infected and HIV-uninfected (B vs A), those with previously undiagnosed HIV-infection were more likely to have engaged in condomless receptive anal intercourse in the past 3-months (75.6%) than participants who were HIV-uninfected (58.6%) (P = 0.09). Also, HIV-uninfected participants were more likely to have had an HIV test within the past 6 months (41.8%) than previously undiagnosed HIV-infected participants (24.4%) (P < 0.01). Among participants who were HIV-infected (B vs C), those who had attained a lower overall education level were more likely to be previously undiagnosed HIV-infected (63.4%) than previously diagnosed HIV-infected (38.2%) (P = 0.03). Condomless receptive anal intercourse in the past 3-months was reported more frequently by previously undiagnosed HIV-infected participants (75.6%) than those previously diagnosed HIV-infected (56.6%) (P = 0.10). Participants reporting a history of syphilis were more likely to be previously diagnosed HIV-infected (56.0%) than previously undiagnosed HIV-infected (29.3%) (P = 0.01).
Multivariable analysis among participants thought to be HIV-uninfected (B vs A) showed some differences between previously undiagnosed HIV-infected and HIV-uninfected MSM and transgender women (Table 2A). Higher odds of having a previously undiagnosed HIV-infection rather than being HIV-uninfected were associated with: recent condomless anal intercourse (aOR = 2.43, 95%CI = 1.10–5.36) or having had a previous HIV test over 1-year ago rather than having been tested within the past 6-months (aOR = 2.87, 95%CI = 1.10–7.53).
Table 2.
(A) Multivariable logistic regression analysis of factors associated with having previously undiagnosed HIV-infection compared to being HIV-uninfected among men who have sex with men and transgender women in Lima, Peru.
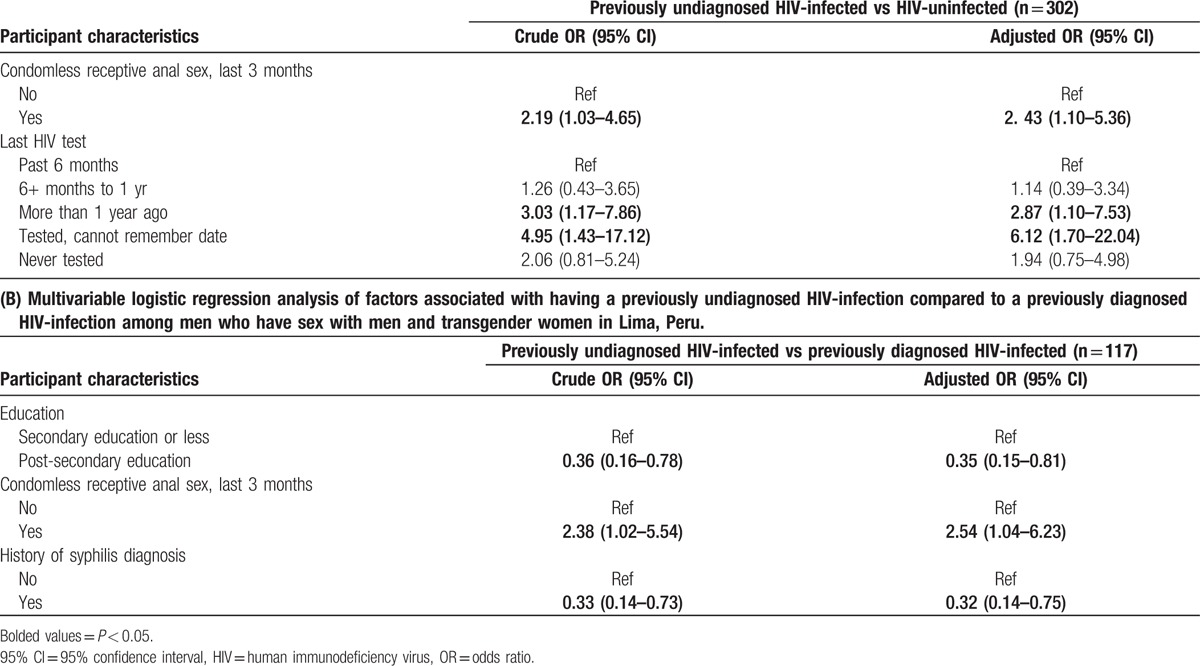
Multivariable analysis among HIV-infected participants (B vs C) also showed some differences between those with a previously undiagnosed HIV-infection and those with a previously diagnosed HIV-infection (Table 2B). Among this group, having engaged in condomless receptive anal intercourse in the last 3-months was associated with 2.54 times higher odds of having a previously undiagnosed HIV-infection (95%CI = 1.04–6.23). Lower odds of having a previously undiagnosed HIV-infection rather than previously diagnosed HIV-infection were associated with: achieving some post-secondary education (aOR = 0.35, 95%CI = 0.15–0.81) or having had a history of a syphilis infection (aOR = 0.32, 95%CI = 0.14–0.75).
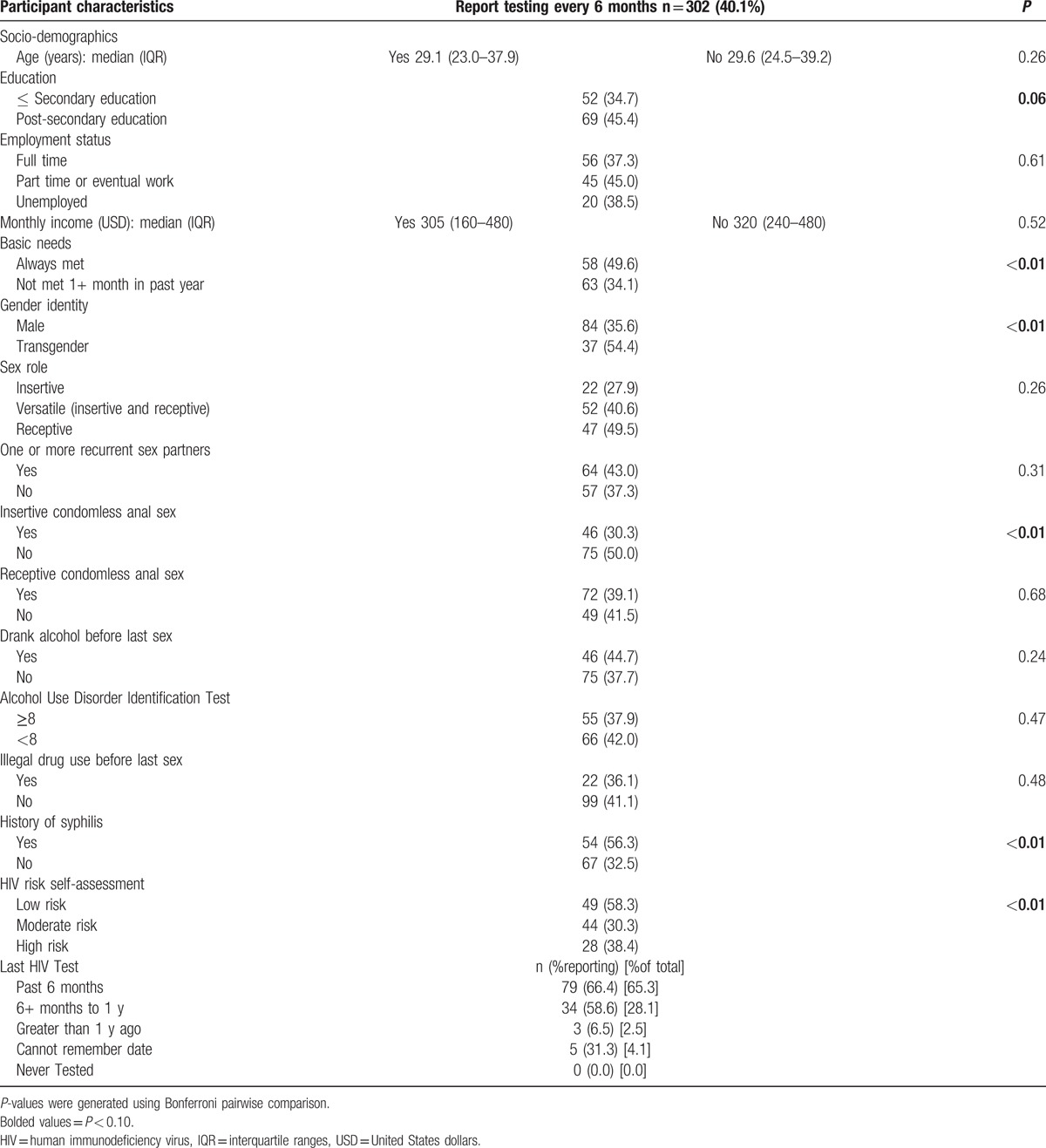
The subanalysis of MSM and transgender women who were HIV-uninfected and previously undiagnosed HIV-infected reporting an HIV testing interval of 6 months or less also revealed many correlates (Table 3). In the bivariate analysis, more frequent testing was associated with higher overall education level (45.4%) than lower overall education level (34.7%) (P = 0.06) and always having income to support their basic needs (49.6%) than not being able to afford their basic needs at least 1 month in the past year (34.1%) (P < 0.01). Frequent testing was also associated with identifying as a transgender woman (54.4%) than not as transgender (35.6%) (P < 0.01). Participating in condomless insertive anal sex within the past 3 months was associated with less frequent testing (30.3%) than not participating in condomless insertive anal sex (50.0%) (P < 0.01). Reporting a history of syphilis infection was associated with more frequent testing (56.3%) than not having had a prior syphilis infection (32.5%) (P < 0.01). Finally, reporting a low self-perceived risk of acquiring HIV was associated more frequent testing (58.3%) than reporting moderate (30.3%) or high (38.4%) self-perceived risk of acquiring HIV (P < 0.01).
Table 3.
Characteristics of HIV-uninfected and previously undiagnosed HIV-infected MSM who reported testing at least every 6 months.
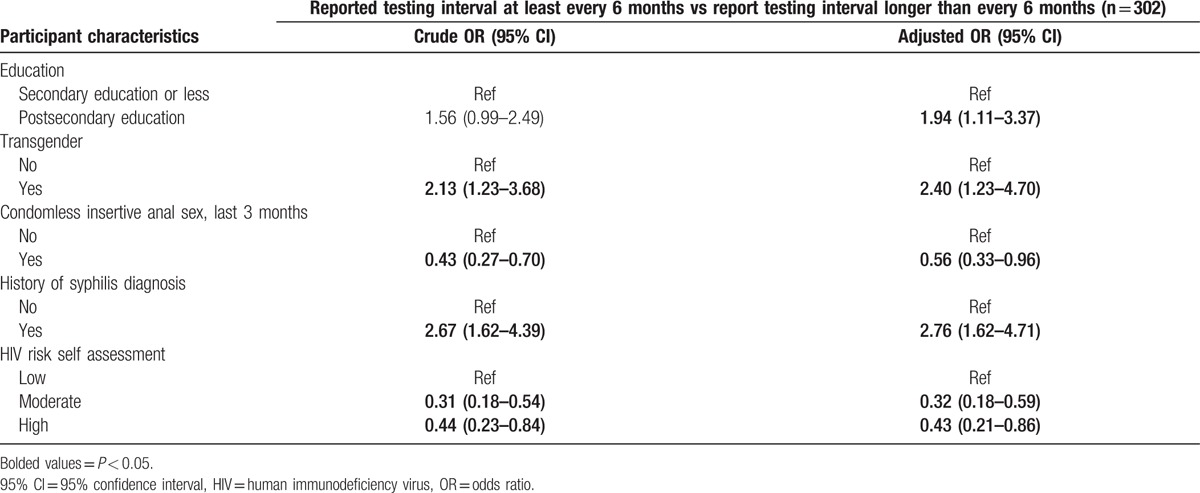
Multivariable analysis of this subanalysis (Table 4) found higher odds of reporting frequent testing among those with higher overall education attainment (aOR 1.94, 95%CI = 1.11–3.37), identifying as transgender women (aOR = 2.40, 95%CI = 1.23–4.70), or having a history of syphilis infection (aOR = 2.76, 95%CI = 1.62–4.71). Lower odds of reporting frequent testing were associated with recent condomless insertive anal sex (aOR = 0.56, 95%CI = 0.33–0.96) or having a moderate or high self-perceived risk of acquiring HIV compared to having a low self-perceived risk of acquiring HIV (aOR = 0.32, 95%CI = 0.18–0.59; aOR = 0.43, 95%CI = 0.21–0.86, respectively).
Table 4.
Multivariable logistic regression analysis of factors associated with reporting a testing frequency of at least every 6 months among HIV-uninfected and previously undiagnosed HIV-infected men who have sex with men and transgender women in Lima, Peru.
4. Discussion
We conducted a cross-sectional analysis of high-risk MSM and transgender women seeking STI services in Lima, Peru. Among those who were HIV-infected about one-third were previously undiagnosed at study enrollment. Correlates of previously undiagnosed HIV-infection were recent condomless receptive anal intercourse, infrequent HIV testing, lower education, and absence of prior syphilis infection. Many participants reported a testing frequency of at least every 6 months (40.1%) as recommended by the UNGASS Declaration of Commitment.[1] Correlates of reporting an HIV testing frequency of at least every 6 months were associated with higher educational attainment, identifying as transgender, not reporting recent condomless insertive anal intercourse, having a prior syphilis diagnosis, and reporting a lower overall self-perceived risk of acquiring HIV.
Consistent with current literature on HIV prevalence and undiagnosed HIV-infection, our study revealed social, behavioral, and health factors associated with participants being unaware of their HIV serostatus.[6,8,15,22–25] Among HIV-infected MSM and transgender women in our study, 35.0% reported they had never been previously diagnosed. That proportion, lower than figures from surveillance studies (62.5–90%), is similar to figures reported in other studies in STI clinics (28.8–30.0%).[6,16–19] However, it is important to recognize that due to the inclusion criteria of our cohort study favoring MSM and transgender women at higher risk of HIV and syphilis, the estimation of undiagnosed HIV-infection may not be representative.
Our finding that at least semiannual testing was associated with higher HIV-awareness rates support the importance of strategies to increase frequent HIV testing. Even though up to quarterly testing is currently recommended by the Peruvian National HIV/STI Program, previous reports suggest few MSM and transgender women in Peru actually test that frequently.[15] Others have suggested that decreasing stigmatization and improving access to HIV testing services are critical to increase testing.[14,15,26] In the USA, where MSM are more aware of their HIV serostatus than in Peru, testing is more frequent and frequent testing has been shown to be cost-effective.[24,27] Additionally, consistent with the literature, our study found higher educational attainment was associated with participants knowing their HIV-infection status.[11,28] This suggests that strategies that encourage and facilitate frequent HIV testing among MSM and transgender women, particularly those with lower educational attainment, could be beneficial.[28]
A history of syphilis infection was also more common among previously diagnosed HIV-infected participants than previously undiagnosed HIV-infected participants. Our finding is consistent with current literature of factors associated with HIV-awareness.[8] Syphilis infection may drive people to seek care earlier by causing a visible lesion requiring treatment, and STI care-seeking increases the likelihood of concomitant HIV testing.[29,30] Untreated syphilis infection has also been shown to increase susceptibility to acquiring HIV and may have been a major risk factor for infection among these participants.[30] Other studies have shown an association between recent syphilis infection and HIV serostatus awareness. Those findings reinforce the need for integrated HIV testing services in STI treatment programs.[31]
HIV-uninfected and previously diagnosed HIV-infected participants reported less receptive condomless anal intercourse within the last 3 months than previously undiagnosed HIV-infected participants. In principle, this finding indicates more recent risk behavior and increased probability of recent infection. Additionally, HIV awareness has been well documented to reduce risky sexual behaviors.[6,23,32–34] In 1 meta-analysis in the USA, authors demonstrated that HIV serostatus awareness reduced condomless anal intercourse among MSM by as much as 68%.[33] Similarly, studies of HIV serostatus awareness in Colombia showed that being diagnosed HIV-infected decreased condomless anal intercourse and risky behaviors such as drug or alcohol use prior to intercourse.[23] Therefore, raising serostatus awareness may reduce sexual risk behavior and subsequently reduce HIV transmission.[23,32–34]
Infrequent HIV testing among those thought to be HIV-uninfected was associated with lower educational attainment, not identifying as transgender, recent condomless insertive anal intercourse, absence of prior syphilis diagnosis, and higher self-perceived risk of acquiring HIV. Similar to our finding that lower educational attainment is associated with previously undiagnosed HIV-infection, it was also associated with infrequent testing, reinforcing the need for directed interventions to increase HIV testing among this group.[11,28] Consistent with prior literature, individuals with prior syphilis history or identifying as transgender reported more frequent testing suggesting recognition of being at higher risk of acquiring HIV among these groups.[13] Within our study, recent condomless insertive anal intercourse or higher self-perceived risk of acquiring HIV were correlated with lower overall testing frequency. This suggests, as previously demonstrated, that those at higher risk of acquiring HIV need more targeted interventions within and outside of STI testing centers to increase the number of frequent testers and subsequently decrease HIV transmission.[3–6,11,15] Both opt-out models at STI clinics and social media innovations that normalize and promote frequent testing could prove beneficial in reaching these higher risk groups.[35,36]
Considering that almost 50% of our study population reported having a recurrent sex partner within the last 3 months, additional interventions to support couples notification and services could have positive impact on HIV testing and uptake of prevention behaviors.[37] Interventions to reach couples with prevention interventions and HIV treatment are important, especially in the context of high rates of HIV-infection and potential HIV sero-discordance.[38] MSM and transgender women at high HIV risk in discordant relationships can benefit from pre-exposure antiretroviral prophylaxis.[39,40] Similarly, incentivized community programs allow an opportunity to create social engagement among MSM and transgender women supporting earlier and more frequent HIV testing.[12]
4.1. Limitations
Our study population was restricted to MSM and transgender women at higher risk for HIV and syphilis in Lima. As such, our findings may not be generalizable to the entire population of MSM and transgender women in Peru. We also did not analyze what individuals perceived as barriers to HIV testing or why they may not be aware of their HIV serostatus. Further work should be done to characterize what MSM and transgender women perceive as barriers to frequent testing. Since the answers to our survey were self-reported, our study may be subject to recall bias and social desirability bias, including underreporting of HIV-infection (i.e., some individuals who were aware of their HIV-infection may have preferred to report that they were uninfected, which may result in their misclassification as “previously undiagnosed HIV-infected”). We also recognize that our testing algorithm is unable to distinguish acute HIV infections from chronic HIV infections. Further work should be done to find correlates of acute HIV infection in order to create strategies that effectively prevent transmission.
5. Conclusions
In conclusion, with improving serostatus awareness being central to HIV prevention and treatment efforts, our study adds understanding of undiagnosed HIV-infection among MSM and transgender women. Undiagnosed HIV-infections in these populations were associated with lower educational attainment and engaging in condomless receptive anal intercourse in the last 3-months. HIV-positive serostatus awareness was associated with having had an HIV test within the past 6-months and having had a previous syphilis infection. Infrequent testing was associated with lower educational attainment, not identifying as transgender, recent condomless insertive anal intercourse, absence of prior syphilis infection or having a higher self-perceived HIV risk. Our findings demonstrate there is continued need to prioritize strategies to promote condom use among MSM and transgender women and facilitate that a higher proportion of that population tests frequently, with a focus on those at risk for infrequent testing. Our findings also reinforce the importance of offering HIV testing to all individuals when they present for diagnosis or treatment of other STIs.
Footnotes
Abbreviations: 95%CI = 95% confidence interval, aOR = adjusted odds ratio, AUDIT = Alcohol Use Disorder Identification Test, CDC = Centers for Disease Control and Prevention, EIA = enzyme immunoassay, HIV = human immunodeficiency virus, IQR = inter-quartile ranges, MoH = Ministry of Health, MSM = men who have sex with men, STI = sexually transmitted infection, UNGASS = United Nations General Assembly Special Session, USPSTF = United States Preventive Services Task Force, WB = Western blot.
Meetings at which parts of data were presented: None.
Funding: This study was funded through National Institutes of Health/National Institute Allergy and Infectious Diseases #1R01AI099727–01. Author Jeremy Chow is also funded through the National Institutes of Health/National Institute of Mental Health #5T32MH080634-09.
The authors have no conflicts of interest to disclose.
References
- 1.Beyrer C, Wirtz AL, Walker D, et al. The Global HIV Epidemics among Men Who Have Sex with Men. Washington, D.C: The World Bank; 2011. [Google Scholar]
- 2.Silva-santisteban A, Raymond HF, Salazar X, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav 2012; 16:872–881. [DOI] [PubMed] [Google Scholar]
- 3.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 4.Beyrer C, Sullivan PS, Sanchez J, et al. A call to action for comprehensive HIV services for men who have sex with men. Lancet 2012; 380:424–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cambiano V, Rodger AJ, Phillips AN. ’Test-and-treat’: the end of the HIV epidemic? Curr Opin Infect Dis 2011; 24:19–26. [DOI] [PubMed] [Google Scholar]
- 6.Vagenas P, Ludford KT, Gonzales P, et al. Being unaware of being HIV-infected is associated with alcohol use disorders and high-risk sexual behaviors among men who have sex with men in Peru. AIDS Behav 2014; 18:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metsch LR, Pereyra M, Messinger S, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis 2008; 47:577–584. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer L, Furegato M, Foschia JP, et al. Undiagnosed HIV infection in a population of MSM from six European cities: results from the Sialon project. Eur J Public Health 2015; 25:494–500. [DOI] [PubMed] [Google Scholar]
- 9.Branson BM, Handsfield HH, Lampe MA, et al. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17. [PubMed] [Google Scholar]
- 10.Moyer VA. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2013; 159:51–60. [DOI] [PubMed] [Google Scholar]
- 11.Velter A, Barin F, Bouyssou A, et al. HIV prevalence and sexual risk behaviors associated with awareness of HIV status among men who have sex with men in Paris, France. AIDS Behav 2013; 17:1266–1278. [DOI] [PubMed] [Google Scholar]
- 12.Down I, Ellard J, Triffitt K, et al. Factors associated with recent previous HIV testing among a sample of recently HIV-diagnosed gay men in Australia: a cross-sectional study. Sex Transm Infect 2015; 91:189–193. [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Deiss RG, Segura ER, et al. A cross-sectional study of low HIV testing frequency and high-risk behaviour among men who have sex with men and transgender women in Lima, Peru. BMC Public Health 2015; 15:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salud Md. Directiva sanitaria para la atención médica periódica a las/os trabajadoras/es sexuales y HSH. In: Salud Md, editor. Lima, Peru: Ministerio de Salud; 2009. [Google Scholar]
- 15.Blas MM, Alva IE, Cabello R, et al. Risk behaviors and reasons for not getting tested for HIV among men who have sex with men: an online survey in Peru. PLoS ONE 2011; 6:e27334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo R, Konda KA, Leon SR, et al. Human Immunodeficiency Virus (HIV) and Sexually Transmitted Infection (STI) incidence and associated risk factors among high-risk MSM and male-to-female transgender women in Lima, Peru. J Acquir Immune Defic Syndr 2015; 69:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JL, Konda KA, Silva-Santisteban A, et al. Sampling methodologies for epidemiologic surveillance of men who have sex with men and transgender women in Latin America: an empiric comparison of convenience sampling, time space sampling, and respondent driven sampling. AIDS Behav 2014; 18:2338–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Brumer AG, Konda KA, Salvatierra HJ, et al. Prevalence of HIV, STIs, and risk behaviors in a cross-sectional community- and clinic-based sample of men who have sex with men (MSM) in Lima, Peru. PLoS One 2013; 8:e59072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark JL, Konda KA, Segura ER, et al. Risk factors for the spread of HIV and other sexually transmitted infections among men who have sex with men infected with HIV in Lima, Peru. Sex Transm Infect 2008; 84:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deiss RG, Leon SR, Konda KA, et al. Characterizing the syphilis epidemic among men who have sex with men in Lima, Peru to identify new treatment and control strategies. BMC Infect Dis 2013; 13:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 22.Beyrer C, Baral SD, Van griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380:367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zea MC, Reisen CA, del Río-González AM, et al. HIV prevalence and awareness of positive serostatus among men who have sex with men and transgender women in Bogotá, Colombia. Am J Public Health 2015; 105:1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torian L, Chen M, Rhodes P, et al. HIV surveillance–United States, 1981–2008. Centers for Disease Control and Prevention Morb Mortal Wkly Rep 2011; 60:689–693. [PubMed] [Google Scholar]
- 25.Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol Rev 2004; 26:22–35. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez J, Lama JR, Kusunoki L, et al. HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. J Acquir Immune Defic Syndr 2007; 44:578–585. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson AB, Farnham PG, Sansom SL, et al. Cost-effectiveness of frequent HIV testing of high risk populations in the United States. J Acquir Immune Defic Syndr 2016; 71:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith A, Miles I, Le B, et al. Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men. MMWR Recomm Rep 2010; 59:1201–1207. [Google Scholar]
- 29.Van de laar MJ. HIV/AIDS and other STI in men who have sex with men--a continuous challenge for public health. Euro Surveill 2009. 14. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez J, Lama JR, Peinado J, et al. High HIV and ulcerative sexually transmitted infection incidence estimates among men who have sex with men in Peru: awaiting for an effective preventive intervention. J Acquir Immune Defic Syndr 2009; 51 suppl 1:S47–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J, Lo YR, Caceres CF, et al. WHO guidelines for HIV/STI prevention and care among MSM and transgender people: implications for policy and practice. Sex Transm Infect 2013; 89:536–538. [DOI] [PubMed] [Google Scholar]
- 32.Marks G, Millett GA, Bingham T, et al. Understanding differences in HIV sexual transmission among Latino and black men who have sex with men: The Brothers y Hermanos Study. AIDS Behav 2009; 13:682–690. [DOI] [PubMed] [Google Scholar]
- 33.Marks G, Crepaz N, Senterfitt JW, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr 2005; 39:446–453. [DOI] [PubMed] [Google Scholar]
- 34.Lauby JL, Millett GA, Lapollo AB, et al. Sexual risk behaviors of HIV-positive, HIV-negative, and serostatus-unknown Black men who have sex with men and women. Arch Sex Behav 2008; 37:708–719. [DOI] [PubMed] [Google Scholar]
- 35.Menacho LA, Galea JT, Young SD. Feasibility of recruiting peer educators to promote HIV testing using facebook among men who have sex with men in Peru. AIDS Behav 2015; 19 suppl 2:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baisley K, Doyle AM, Changalucha J, et al. Uptake of voluntary counselling and testing among young people participating in an HIV prevention trial: comparison of opt-out and opt-in strategies. PLoS One 2012; 7:e42108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crepaz N, Tungol-ashmon MV, Vosburgh HW, et al. Are couple-based interventions more effective than interventions delivered to individuals in promoting HIV protective behaviors? A meta-analysis. AIDS Care 2015; 27:1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagaraj S, Segura ER, Peinado J, et al. A cross-sectional study of knowledge of sex partner serostatus among high-risk Peruvian men who have sex with men and transgender women: implications for HIV prevention. BMC Public Health 2013; 13:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider K, Gray RT, Wilson DP. A cost-effectiveness analysis of HIV preexposure prophylaxis for men who have sex with men in Australia. Clin Infect Dis 2014; 58:1027–1034. [DOI] [PubMed] [Google Scholar]
- 40.Gomez GB, Borquez A, Caceres CF, et al. The potential impact of pre-exposure prophylaxis for HIV prevention among men who have sex with men and transwomen in Lima, Peru: a mathematical modelling study. PLoS Med 2012; 9:e1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]


