Abstract
Common bile duct (CBD) stones are generally associated with greater elevations of alkaline phosphatase and gamma-glutamyl transpeptidase levels than aspartate aminotransferase and alanine aminotransferase levels. However, some patients with CBD stones show markedly increased aminotransferase levels, sometimes leading to the misdiagnosis of liver disease. Therefore, the aim of this study was to investigate the clinicopathologic features of patients with CBD stones and high aminotransferase levels.
This prospective cohort study included 882 patients diagnosed with CBD stones using endoscopic retrograde cholangiopancreatography (ERCP). Among these patients, 38 (4.3%) exhibited aminotransferase levels above 400 IU/L without cholangitis (gallstone hepatitis [GSH] group), and 116 (13.2%) exhibited normal aminotransferase levels (control group). We compared groups in terms of clinical features, laboratory test results, radiologic images, and ERCP findings such as CBD diameter, CBD stone diameter and number, and periampullary diverticulum. Liver biopsy was performed for patients in the GSH group.
GSH patients were younger and more likely to have gallbladder stones than control patients, implying a higher incidence of gallbladder stone migration. Also, GSH patients experienced more severe, short-lasting abdominal pain. ERCP showed narrower CBDs in GSH patients than in control patients. Histological analysis of liver tissue from GSH patients showed no abnormalities except for mild inflammation.
Compared with control patients, GSH patients were younger and showed more severe, short-lasting abdominal pain, which could be due to a sudden increase of CBD pressure resulting from the migration of gallstones through narrower CBDs. These clinical features could be helpful not only for the differential diagnosis of liver disease but also for investigating the underlying mechanisms of liver damage in obstructive jaundice. Moreover, we propose a new definition of “gallstone hepatitis” based on the specific clinicopathologic characteristics observed in our patients.
Keywords: aminotransferase, choledocholithiasis, gallstone hepatitis
1. Introduction
Precise interpretation of abnormalities in the results of liver function tests is necessary for the diagnostic work-up for hepatobiliary disease. In general, abnormalities in liver function can be classified into 2 patterns: a hepatocellular pattern, in which aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are elevated higher than alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (γ-GT) levels,[1] and a cholestatic pattern, in which ALP and γ-GT levels are elevated higher than AST and ALT levels.[2,3] Patients who have obstructive jaundice due to a common bile duct (CBD) stone generally exhibit the cholestatic pattern, with relatively low aminotransferase levels.[4,5] Although some patients with a CBD stone in combination with cholangitis show marked elevation of aminotransferase levels, other CBD stone patients exhibit elevated aminotransferase levels without cholangitis,[6,7] which sometimes leads to the misdiagnosis of liver diseases such as viral hepatitis, drug-induced hepatitis, or ischemic liver injury. Therefore, clinical investigation of the characteristics of patients with a CBD stone and markedly elevated aminotransferase levels without the presence of cholangitis could aid in rapid diagnosis and treatment. However, few studies of this patient population have been conducted, and these studies are limited by their retrospective nature and the fact that factors such as cholangitis, which can affect aminotransferase levels, were not excluded.[8–11] The aim of this prospective study was to characterize the clinicopathologic features of patients with a CBD stone and markedly elevated serum aminotransferase levels without the presence of cholangitis.
2. Methods
2.1. Patients
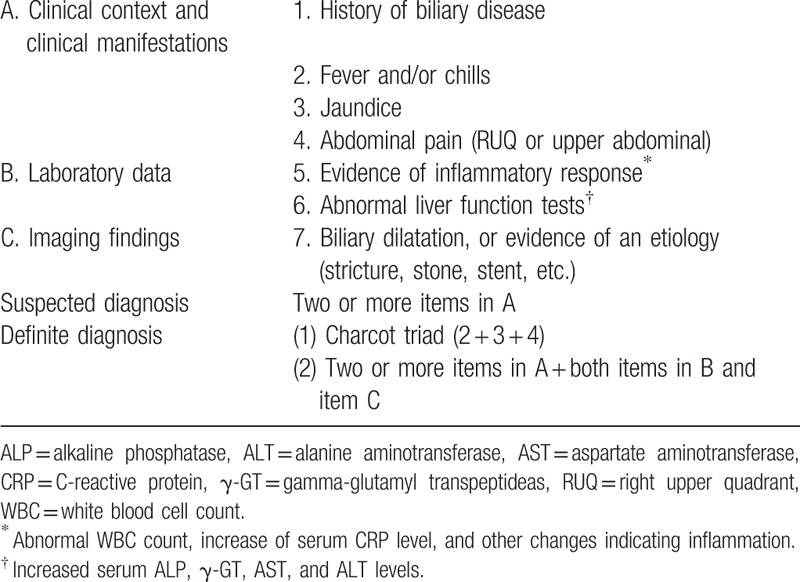
Between June 2006 and June 2015, patients confirmed to have a CBD stone by endoscopic retrograde cholangiopancreatography (ERCP) were enrolled in this study, which was conducted at Gangnam Severance Hospital in Seoul, Korea. Patients with underlying hepatobiliary disease such as viral hepatitis (i.e., hepatitis A, B, or C virus infection), autoimmune hepatitis, hepatolithiasis, and liver cirrhosis, which can affect aminotransferase levels, were excluded from analysis. Patients who had cholangitis upon hospital admission, used hepatotoxic agents or herbs or were alcoholics, previously had endoscopic sphincterotomy, or who showed moderate elevations (38–399 IU/L) of aminotransferase levels were also excluded from analysis (Fig. 1). According to the 2007 Tokyo guidelines (Table 1), cholangitis was diagnosed if there were signs of inflammation in laboratory test results or radiological images. The remaining patients were divided into 2 groups; the gallstone hepatitis (GSH) group consisted of patients with aminotransferase levels >400 IU/L upon hospital admission, and the control group consisted of patients with normal AST (≤37 IU/L) and ALT (≤46 IU/L) levels. In order to clarify the characteristics of GSH, we defined the control group as patients with normal aminotransferase who were judged to have minimal liver damage. This study was approved by the Institutional Review Board at Gangnam Severance Hospital (approval number: 4-2012-0165) and registered at ClinicalTrials.gov (identifier: NCT02647593).
Figure 1.
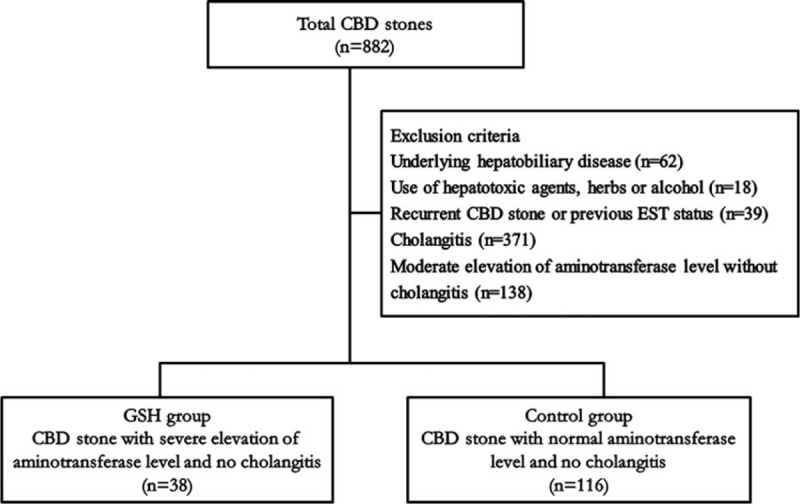
Patient selection process. CBD = common bile duct, EST = endoscopic sphincterotomy, GSH = gallstone hepatitis.
Table 1.
Diagnostic criteria for acute cholangitis.
2.2. Data acquisition and procedures
Demographic and clinical variables, including gender, age, symptoms (i.e., abdominal pain, duration, severity, and location), radiologic findings (i.e., gallbladder distension, gallbladder stones, and intrahepatic duct dilatation), ERCP findings (i.e., CBD diameter, diameter and number of CBD stones, and periampullary diverticulum), and laboratory findings (i.e., white blood cell count, levels of C-reactive protein, AST, ALT, total bilirubin, ALP, γ-GT, amylase, and lipase) were evaluated for patients in the GSH and control groups. Abdominal pain severity was measured by a numeric rating scale: no pain (0), mild pain (1–3), moderate pain (4–6), or severe pain (7–10). Laboratory tests were performed upon hospital arrival, after ERCP, and before leaving the hospital.
Three endoscopists (DKL, SJL, and SIJ) who had performed ERCP over 1000 times carried out the procedure using an endoscopy system (TJF 240, Olympus, Tokyo, Japan).
Choleystectomy was performed for patients in both groups who had gallbladder stones. Liver biopsy was performed for patients in the GSH group who underwent elective cholecystectomy. Liver tissue (3 × 6–7 mm) was obtained using endoscopic scissors while cholecystectomy was being performed in the operating room. Hemorrhage sites were controlled using electrocautery.
2.3. Statistical analysis
Statistical analyses were performed using Student t tests and chi-square tests. Multiple logistic regression analysis was used to determine independent predictors of GSH. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
3.1. Demographic and clinical characteristics of the GSH and control groups
A total of 882 patients had CBD stones confirmed by ERCP during the study period. Of these patients, 62 with underlying hepatobiliary disease, 18 who used hepatotoxic agents or herbs or were alcoholics, 39 who had endoscopic sphincterotomy or recurrent CBD stones, 471 who had cholangitis upon hospital admission, and 138 who showed moderate elevations of aminotransferase levels were excluded, leaving 38 patients (4.3%) in the GSH group and 116 patients (13.2%) in the control group (Fig. 1). Ten patients in the GSH group were misdiagnosed as having liver disease, resulting in the delay of diagnosis and treatment such as ERCP. Also, 69 of the 882 patients (7.8%) were diagnosed with gallstone pancreatitis, and 3 patients (0.3%) were diagnosed with both GSH and gallstone pancreatitis.
Patients in the GSH group were younger on average than patients in the control group. Also, the incidence of abdominal pain among patients in the GSH group was significantly higher than that among patients in the control group. Compared to control patients, GSH patients had more moderate to severe abdominal pain, classified as a score of 5 or higher, which lasted for relatively short periods less than 48 hours. There was no significant difference in the site of abdominal pain between groups. Examinations such as abdomen ultrasonography or abdominal computer tomography prior to ERCP showed that the incidence of gallbladder stones was higher in GSH patients than in control patients. ERCP showed that GSH patients had narrower CBDs than control patients. Laboratory tests revealed significant differences between groups in AST, ALT, total bilirubin, ALP, and γ-GT levels, whereas white blood cell count and levels of amylase, lipase, and C-reactive protein were similar between groups (Table 2). The removal of CBD stones under ERCP was associated with improved liver function test results at discharge (Fig. 2).
Table 2.
Comparisons of the clinical findings related to GSH.
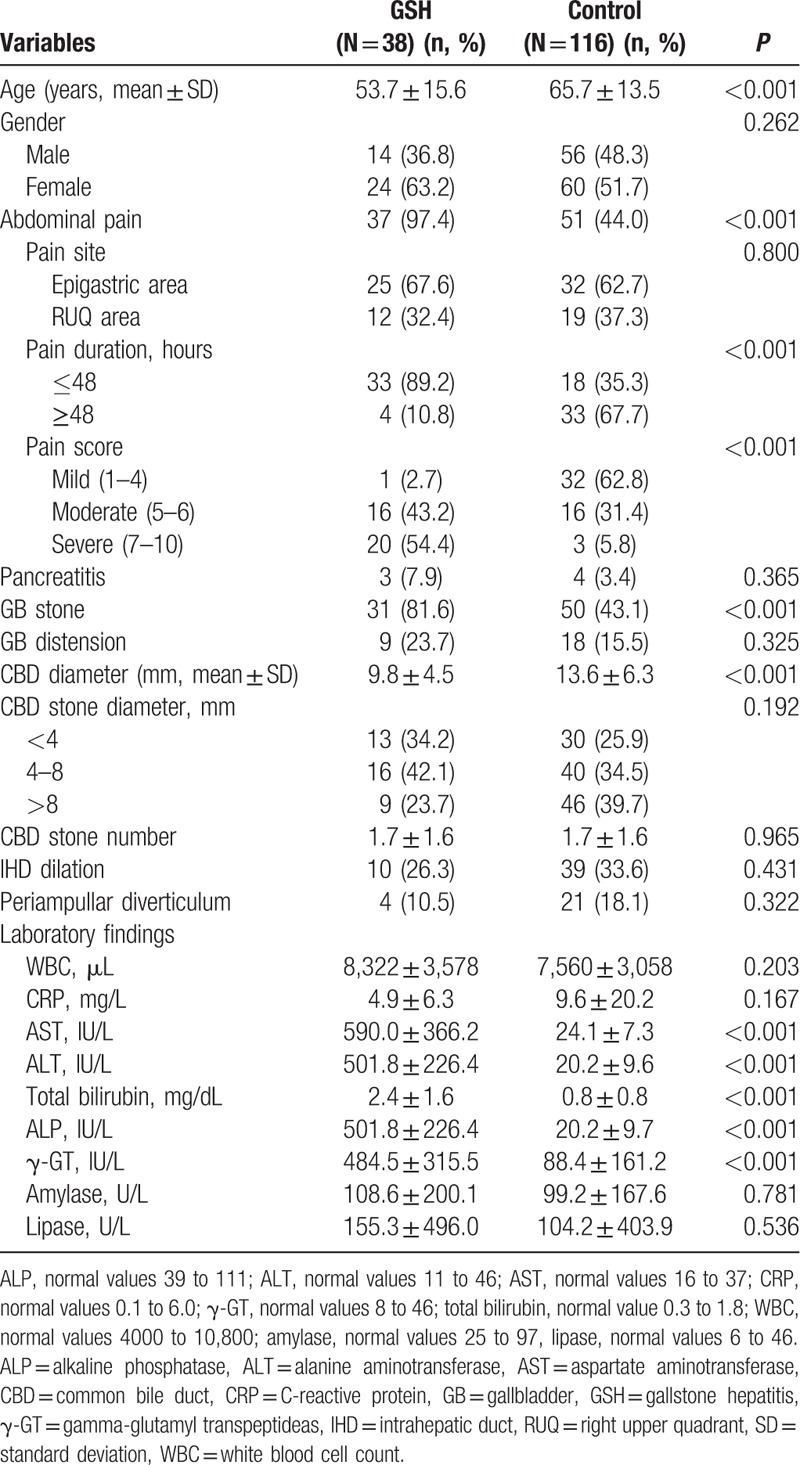
Figure 2.
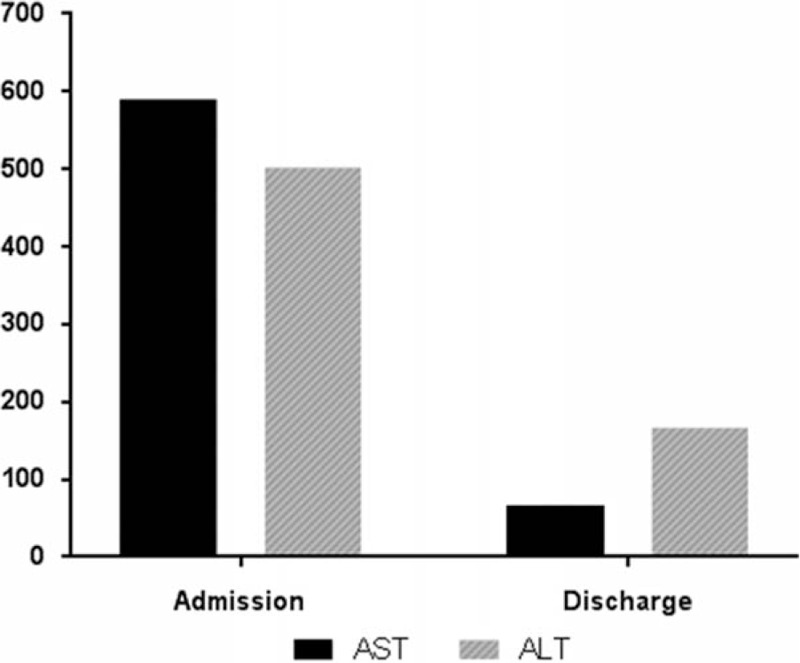
AST and ALT levels at hospital admission and discharge for CBD patients in the gallstone hepatitis group. The removal of CBDs was associated with improved liver function test results at discharge. ALT = alanine transaminase, AST = aspartate aminotransferase, CBD = common bile duct.
3.2. Liver pathology
For 5 patients in the GSH group, liver biopsy was performed during cholecystectomy an average of 11.2 days after hospital admission. No patients showed any specific abnormalities except minimal inflammatory changes (Fig. 3A and B).
Figure 3.
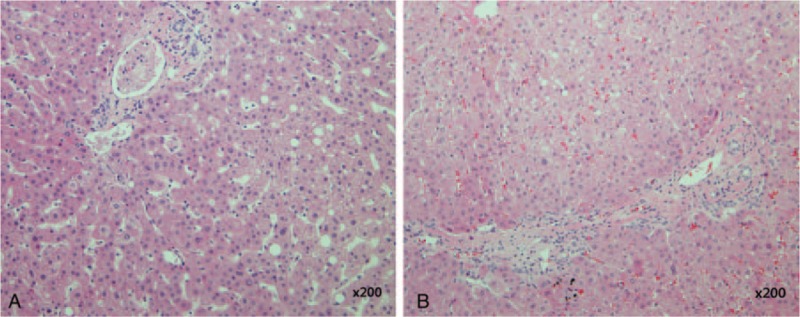
Representative photomicrographs of liver tissue in gallstone hepatitis group. (A) Very mild sinusoidal lymphocytosis (H&E stain, 200× magnification). (B) Very mild portal inflammation (H&E stain, 200× magnification).
3.3. Multivariate analysis
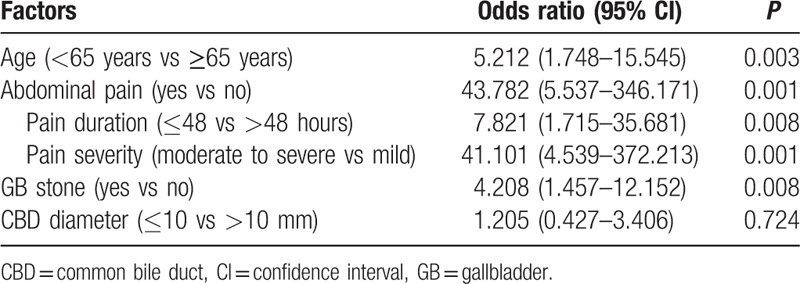
Multivariate logistic regression analysis showed that the incidence, duration, and intensity of abdominal pain, the presence of gallbladder stones, and age were independent risk factors for GSH (Table 3).
Table 3.
Predictive factor of gallstone hepatitis in multivariate logistic regression analysis.
4. Discussion
We investigated the clinicopathologic features of patients with a CBD stone and high aminotransferase levels without the presence of cholangitis. Among 882 patients with CBD stones, 38 (4.3%) were diagnosed with GSH, and these patients showed distinct features such as severe and short-lasting abdominal pain, relatively young age, the presence of gallbladder stones, and narrow CBDs as measured by ERCP. In patients with CBD stones and aminotransferase levels ≤400 IU/L who did not have cholangitis, the AST level was 149.5 ± 72.3 IU/L and the ALT level was 183 ± 91.3 IU/L. Age (59.8 ± 17.3 years), CBD diameter (14.5 ± 7.8 mm), and GB stone frequency (48.3%) showed no significant differences compared with the control group. The difference in abdominal pain frequency (60.8%) was significant compared with the control group, but not the GSH group. No difference was detected in pain duration or severity compared with the control group. Therefore, none of the clinicopathologic features evaluated were significantly associated with the moderately increased aminotransferase levels.
A previous study of patients with obstructive jaundice showed that 98% and 93% of patients had AST and ALT levels lower than 400 IU/L, respectively, and less than 7% of patients had AST or ALT levels higher than 400 IU/L.[4,12] We found that 16.5% of patients diagnosed with a CBD stone had aminotransferase levels higher than 400 IU/L, and 4.3% of CBD stone patients had high serum levels of aminotransferases without the presence of cholangitis (GSH group). Some patients had high levels of aminotransferases but did not meet inclusion criteria for the GSH group (12.2%). Although a GSH component may exist in these patients, we excluded them from analysis to investigate the exact clinical characteristics of GSH.
Levels of ALP and γ-GT, which are considered cholestatic liver enzymes, generally increase under conditions in which biliary obstruction occurs. [2,3] Anciaux et al demonstrated that serum ALP and γ-GT were elevated within the 1st 3 days in 100% of patients with biliary obstruction, whereas aminotransferase levels were elevated in up to 88% of patients, with a mean of 102 to 150 IU/L for AST and ALT.[13] Although a more dramatic elevation in aminotransferase levels has been considered to be indicator of choledocholithiasis, it is still unclear whether it indicates acute hepatitis in clinical practice.[9,14] Nathwani et al showed that among 478 patients with CBD stones, 2.3% had normal aminotransferase levels, and 27.8%, 18.4%, and 9.6% of patients had levels higher than 250, 500, and 1000 IU/L, respectively.[9] However, this report did not provide detailed information such as presenting symptoms (e.g., cholangitis-associated symptoms), ERCP findings, or the intensity and pattern of abdominal pain. Also, previous studies did not rule out cholangitis, which can affect aminotransferase levels.[8–11,15] In the present study, however, we strictly excluded several factors that can affect aminotransferase levels, including cholangitis.
In patients with biliary obstruction, the mechanism underlying elevated serum aminotransferase levels remains unknown. In an animal model, normal CBD hydrostatic pressure is 10 to 15 cm H2O (7.4–11 mm Hg), and nonsphincteric biliary obstruction increases pressure to 25 to 40 cm H2O (18–30 mm Hg).[16,17] In humans, CBD pressure is increased in biliary obstruction along with an increase in the frequency of retrograde propagation of the phasic wave, which contributes to a rapid increase in biliary hydrostatic pressure.[18,19] These changes also lead to increased hepatocellular permeability, aminotransferase production, and hepatocyte toxicity caused by bile acid, which in turn results in increased aminotransferase levels.[20,21] When the bile duct is occluded, hepatocytes undergo inflammatory damage. Previous studies show that rats with bile duct ligation show not only increased phagocytosis of Kupffer cells but also increased production of several cytokines and platelet-activating factor.[22–24] Additionally, activated neutrophils increase in number and accumulate in the liver following bile duct ligation, thus aggravating liver damage.[25–28]
Previous studies indicate that liver test results rapidly improve after the removal of a CBD stone within 3 to 14 days in patients with choledocholithiasis and marked elevation of aminotransferase levels.[9,11] The present study also shows that liver function test results dramatically improved within a relatively short time period after the removal of a CBD stone under ERCP. Also, histological examination of liver biopsy tissue revealed only mild inflammation in GSH patients. Given that the liver is an organ capable of rapid recovery, our observations suggest that liver recovery may occur more quickly if the removal of CBD stones is timely executed.
Because the diameter of the bile duct is dilated in aged individuals, compared with younger patients, bile duct pressure is less elevated in aged patients with acute biliary obstruction which may result in lower serum aminotransferase level.[29–32] Also, elderly patients are less susceptible to pain than younger patients, resulting in delayed hospital visit.[29,33,34] Furthermore, the presence of gallbladder stones is thought to be a factor contributing to the rapid elevation of CBD pressure through gallstone migration. Primary choledocholithiasis progresses slowly from stone formation to symptom expression to anatomical changes caused by the stone, such as stone-induced bile duct dilation. On the other hand, migrated secondary gallstones can occlude distal CBDs without such changes, leading to rapid increases in bile duct pressure. Retrograde pressure due to increased bile duct pressure could possibly induce liver parenchymal destruction with bilo-lymphatic and bilo-venous reflux. Although the gallbladder could serve as a reservoir for the sudden rise of intraductal pressure,[35] migrated stones and edema of the cystic duct could disturb the role of the gallbladder in buffering pressure. Thus, younger patients may exhibit narrower CBDs and abrupt increases in intrabile duct pressure, which could contribute to dramatically elevated aminotransferase levels and severe abdominal pain. Clinically, the drastic rise of aminotransferase levels in patients with CBD stones can be misdiagnosed as liver disease, which often results in the delay of diagnosis and treatment. Given that abdominal pain is not the main symptom of liver disease, and its intensity is low, this study provides important evidence that severe abdominal pain is a clue for differential diagnosis. Also, despite the elevated pressure caused by ductal obstruction, we found that the incidence of GSH (4.3%) was lower than that of gallstone pancreatitis (7.8%), which could be due to anatomical differences in pancreatic and bile ducts and buffering capability.
The term “gallstone hepatitis” was suggested in 1991 by Isogai et al,[11] who defined GSH as consisting of severe abdominal pain, marked elevated serum transaminase levels (≥300 IU/L), and gallstones, usually discovered in the dilated biliary tract, including the by ultrasonography. At that point in time, examinations using ERCP were not common, and CBD stones tended to be managed conservatively in cases that were not emergencies. Therefore, Isogai et al[10] considered GSH as risk factor for symptomatic bile duct stone or cholangitis. In contrast to these previous studies,[10,11] we excluded patients with cholangitis, which can increase aminotransferase levels, and we compared GSH patients with control patients who had normal aminotransferase levels. Therefore, we suggest a new definition of “gallstone hepatitis” based on the clinicopathologic features observed in our study.
Our study has several limitations. First, if liver biopsy is performed at the time point when GSH is diagnosed, when aminotransferase levels have reached their peak, the characteristics of GSH could be confirmed more accurately. However, this was not done in the present study due to ethical consideration. Therefore, our biopsy results are representative of the recovery stage after acute liver injury, and they indicate complete livery recovery after biliary decompression through endoscopic management. Second, we did not perform multiple deep biopsies but rather a single biopsy in a superficial and peripheral area of the liver, which cannot reveal histological changes of the entire liver. Third, a small number of patients in the GSH group underwent liver biopsy; thus, the biopsy results might not be confirmative. Finally, because magnetic resonance cholangiopancreatography and endoscopic ultrasound are not covered by insurance in our country, they were not used as initial diagnostic tools. To our knowledge, however, our study is the 1st to describe the clinicopathologic characteristics of patients with a CBD stone and marked aminotransferase elevation without cholangitis. Furthermore, our study has the strengths of being a long-term, prospective, single-center study.
In conclusion, patients in GSH group tended to be young and experience moderate to severe abdominal pain lasting for relatively short periods of time. Also, ERCP and radiologic imaging showed narrow CBDs and gallbladder stone migration in GSH patients. Taken together, we assume that marked elevation of aminotransferase levels is induced under conditions in which intrabile duct pressure dramatically surges. Also, these clinical features can be helpful not only for differential diagnosis with liver disease but also for investigating the underlying mechanisms of liver damage in obstructive jaundice. Therefore, we propose defining “gallstone hepatitis” as the including: severe abdominal pain, marked elevated of serum aminotransferase levels (≥400 IU/L), the confirmation of a CBD stone through various imaging techniques (e.g., abdomen ultrasonography, abdominal computed tomography, and ERCP), the absence of cholangitis, the exclusion of diseases or risk factors that increase aminotransferase levels, and the rapid recovery of liver injury after gallstone removal.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CBD = common bile duct, ERCP = endoscopic retrograde cholangiopancreatography, γ-GT = gamma-glutamyl transpeptidase, GSH = gallstone hepatitis.
CWH and SIJ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Skrede S, Blomhoff JP, Gjone E. Biochemical features of acute and chronic hepatitis. Ann Clin Res 1976; 8:182–199. [PubMed] [Google Scholar]
- 2.Scharschmidt BF, Goldberg HI, Schmid R. Current concepts in diagnosis. Approach to the patient with cholestatic jaundice. N Engl J Med 1983; 308:1515–1519. [DOI] [PubMed] [Google Scholar]
- 3.Stain SC, Marsri LS, Froes ET, et al. Laparoscopic cholecystectomy: laboratory predictors of choledocholithiasis. Am Surg 1994; 60:767–771. [PubMed] [Google Scholar]
- 4.Ellis G, Goldberg DM, Spooner RJ, et al. Serum enzyme tests in diseases of the liver and biliary tree. Am J Clin Pathol 1978; 70:248–258. [DOI] [PubMed] [Google Scholar]
- 5.Clermont RJ, Chalmers TC. The transaminase tests in liver disease. Medicine (Baltimore) 1967; 46:197–207. [DOI] [PubMed] [Google Scholar]
- 6.Kim SW, Shin HC, Kim IY. Transient arterial enhancement of the hepatic parenchyma in patients with acute cholangitis. J Comput Assist Tomogr 2009; 33:398–404. [DOI] [PubMed] [Google Scholar]
- 7.Balthazar EJ, Birnbaum BA, Naidich M. Acute cholangitis: CT evaluation. J Comput Assist Tomogr 1993; 17:283–289. [DOI] [PubMed] [Google Scholar]
- 8.Jeon WJ, Han JH, Seo JC, et al. Clinical features of patients with choledocholithiasis showing high levels of aminotransferases. Korean J Gastroenterol 2006; 47:213–217. [PubMed] [Google Scholar]
- 9.Nathwani RA, Kumar SR, Reynolds TB, et al. Marked elevation in serum transaminases: an atypical presentation of choledocholithiasis. Am J Gastroenterol 2005; 100:295–298. [DOI] [PubMed] [Google Scholar]
- 10.Isogai M, Hachisuka K, Yamaguchi A, et al. Biochemical prediction of acute cholangitis and symptomatic bile duct stones by gallstone hepatitis. HPB Surg 1995; 8:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isogai M, Hachisuka K, Yamaguchi A, et al. Etiology and pathogenesis of marked elevation of serum transaminase in patients with acute gallstone disease. HPB Surg 1991; 4:95–105.discussion 106–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrine SK. Schiff's diseases of the liver. Gastroenterology 1999; 116:1501–1502. [DOI] [PubMed] [Google Scholar]
- 13.Anciaux ML, Pelletier G, Attali P, et al. Prospective study of clinical and biochemical features of symptomatic choledocholithiasis. Dig Dis Sci 1986; 31:449–453. [DOI] [PubMed] [Google Scholar]
- 14.Abbruzzese A, Jeffery RL. Marked elevations of serum glutamic oxalacetic transaminase and lactic dehydrogenase activity in chronic extraheptic biliary disease. Am J Dig Dis 1969; 14:332–338. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox CM, Kim H, Trevino J, et al. Prevalence of normal liver tests in patients with choledocholithiasis undergoing endoscopic retrograde cholangiopancreatography. Digestion 2014; 89:232–238. [DOI] [PubMed] [Google Scholar]
- 16.Strasberg SM, Redinger RN, Dorn BC, et al. Effects of alteration of biliary pressure on bile composition – a method for study: primate biliary physiology. V. Gastroenterology 1971; 61:357–362. [PubMed] [Google Scholar]
- 17.Strasberg SM, Dorn BC, Small DM, et al. The effect of biliary tract pressure on bile flow, bile salt secretion, and bile salt synthesis in the primate. Surgery 1971; 70:140–146. [PubMed] [Google Scholar]
- 18.Csendes A, Sepulveda A, Burdiles P, et al. Common bile duct pressure in patients with common bile duct stones with or without acute suppurative cholangitis. Arch Surg 1988; 123:697–699. [DOI] [PubMed] [Google Scholar]
- 19.Toouli J, Geenen JE, Hogan WJ, et al. Sphincter of Oddi motor activity: a comparison between patients with common bile duct stones and controls. Gastroenterology 1982; 82:111–117. [PubMed] [Google Scholar]
- 20.Toyota N, Miyai K, Hardison WG. Effect of biliary pressure versus high bile acid flux on the permeability of hepatocellular tight junction. Lab Invest 1984; 50:536–542. [PubMed] [Google Scholar]
- 21.Watanabe N, Kojima S, Takashimizu S, et al. Initial site of bile regurgitation following extrahepatic biliary obstruction in living rats. J Gastroenterol Hepatol 2007; 22:1983–1992. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, Bethea NW, Baker GL, et al. Hepatic microcirculatory dysfunction during cholestatic liver injury in rats. Microcirculation 2003; 10:421–432. [DOI] [PubMed] [Google Scholar]
- 23.Bemelmans MH, Gouma DJ, Greve JW, et al. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology 1992; 15:1132–1136. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Chao W, Levine BA, et al. Role of platelet-activating factor in hepatic responses after bile duct ligation in rats. Am J Physiol 1992; 263 (5 Pt 1):G587–G592. [DOI] [PubMed] [Google Scholar]
- 25.Levy R, Schlaeffer F, Keynan A, et al. Increased neutrophil function induced by bile duct ligation in a rat model. Hepatology 1993; 17:908–914. [PubMed] [Google Scholar]
- 26.Parola M, Leonarduzzi G, Robino G, et al. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med 1996; 20:351–359. [DOI] [PubMed] [Google Scholar]
- 27.Koeppel TA, Trauner M, Baas JC, et al. Extrahepatic biliary obstruction impairs microvascular perfusion and increases leukocyte adhesion in rat liver. Hepatology 1997; 26:1085–1091. [DOI] [PubMed] [Google Scholar]
- 28.Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology 2000; 118:1157–1168. [DOI] [PubMed] [Google Scholar]
- 29.Hu KC, Wang HY, Chang WH, et al. Clinical presentations of patients from different age cohorts with biliary tract stone diseases. J Gastroenterol Hepatol 2014; 29:1614–1619. [DOI] [PubMed] [Google Scholar]
- 30.Barthet M, Spinoza S, Affriat C, et al. Influence of age and biliary lithiasis on the diameter of the common bile duct. Gastroenterol Clin Biol 1995; 19:156–160. [PubMed] [Google Scholar]
- 31.Perret RS, Sloop GD, Borne JA. Common bile duct measurements in an elderly population. J Ultrasound Med 2000; 19:727–730.quiz 731. [DOI] [PubMed] [Google Scholar]
- 32.Bachar GN, Cohen M, Belenky A, et al. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med 2003; 22:879–882.quiz 883–875. [DOI] [PubMed] [Google Scholar]
- 33.Pickering G, Jourdan D, Eschalier A, et al. Impact of age on pain perception and analgesic pharmacology. Presse Med 2001; 30:754–758. [PubMed] [Google Scholar]
- 34.Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med 2001; 17:433–456.v–vi. [DOI] [PubMed] [Google Scholar]
- 35.Sharara AI, Mansour NM, El-Hakam M, et al. Duration of pain is correlated with elevation in liver function tests in patients with symptomatic choledocholithiasis. Clin Gastroenterol Hepatol 2010; 8:1077–1082. [DOI] [PubMed] [Google Scholar]


