Abstract
Introduction
Ivacaftor, a cystic fibrosis transmembrane regulator (CFTR) potentiator is currently approved for use in individuals with class III gating mutations and the R117H mutation, a non-gating mutation with residual functioning CFTR. Nevertheless, ivacaftor may also be effective in individuals who have CF mutations giving rise to a residual functioning protein. However, aside from case reports involving a single patient, little data exist on the use of ivacaftor in such individuals.
Methods
A real life pragmatic report wherein seven adults with mutations resulting in a CFTR with residual function were prescribed ivacaftor. Four individuals with similar mutations acted as comparison. We assessed lung function, body mass index, sweat chloride; the number of acute respiratory exacerbations and health related quality of life.
Results
Patients with residual functioning CFTR showed significant improvement or stabilization in all parameters up to 3 years following the start of ivacaftor. Those with similar mutations and who did not receive ivacaftor worsened.
Conclusion
We report the use of ivacaftor in seven adults with various Class IV and V non-gating CFTR mutation with residual functioning protein and we demonstrate improvement in several clinical parameters.
Keywords: Cystic fibrosis, Ivacaftor, CFTR, Residual function
1. Introduction
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene resulting in impaired transport of chloride ion across cell membranes. CF mutations are generally grouped into six classes based on whether the defect impacts production, trafficking, function or stability of the CFTR protein. Ivacaftor, a CFTR potentiator [1] (Vertex, Boston, MA) was initially approved for CF patients with class III mutations, also known as gating mutations. In a landmark study, the administration of ivacaftor to people with CF and at least one copy of G551D, the most common gating mutation, produced improvement in FEV1 and body mass index (BMI) as well as a decrease in sweat chloride [2]. Further studies on other non-G551D gating mutations extended the use of Ivacaftor to individuals with other class III mutations [3].
The use of ivacaftor was further extended when a trial examined its efficacy in subjects with R117H, a class IV mutation with partial channel activity. This study demonstrated a 5% increase in FEV1 in those over 18 years of age, who presumably have more advanced disease. Improvements were also noted in sweat chloride, BMI and in the Cystic Fibrosis Questionnaire Revised (CFQ-R) [4]. Based on these results, ivacaftor was then approved for use in people with CF and the R117H mutation [5], but not in those with other CFTR mutations. Prior studies of ivacaftor, albeit in vitro, demonstrated considerable improvements in chloride transport in non-gating CFTR mutations suggesting that ivacaftor could benefit CF individuals with mutations other than those involving strictly gating defects [6]. Indeed, in a patient with the P67L mutation, the benefits of ivacaftor were demonstrated [7]. To our knowledge, aside from single case reports, there have been no publications on the use of ivacaftor in CF patients who have mutation with residual functioning protein (classes IV and V). We report the use of ivacaftor in seven CF adults with various class IV and V mutations.
2. Methods
All patients who were cared for at our adult CF center and who had at least one CFTR mutation resulting in a protein with residual function were asked to participate. The determining factor for inclusion in a treatment or non-treatment group was whether the patients' health plan would approve payment for ivacaftor. Since this drug is not currently approved for people with CF and mutations with residual functioning protein, apart from the R117H mutation [5]; its cost of approximately US$300,000/year renders it prohibitive for patients to purchase on their own, or for the CF center to supply it to them. In seven patients, the health insurance carriers agreed to provide the drug. In four others, ivacaftor could not be obtained, and these individuals served as non-treatment comparisons. None of the patients included in this report had CF related diabetes mellitus or CF related liver disease and all had sinus disease. Sweat chloride was measured in five patients who received ivacaftor in our CFF-accredited institutional laboratory using the pilocarpine iontophoresis method [8]. The mean of two measurements performed at least one week apart was used. All data were collected at visits when patients were in their usual state of health, taking their usual maintenance medications and without an acute CF exacerbation. We defined an acute exacerbation as decreased exercise tolerance with increased cough; increased sputum; absence from school or work; decreased appetite and increased adventitial sounds on lung examination [10], [11]. Additionally, an acute exacerbation required the addition of new oral or intravenous antibiotics to the patient's regimen. The study was approved by the Northwell health system institutional review board.
2.1. Statistical analysis
The program SPSS version 21 (IBM, Armonk, NY) was used for analysis. Normally distributed results were expressed as mean ± sd and comparison was made using the t-test. Non-normally distributed data were expressed as median (IQR) using the rank sum test to determine significance. We used the Department of Psychology at the University of Miami Excel program to calculate CFQ-R scores [9].
3. Results
All patients who received ivacaftor had been on the drug for at least three years. Those who did not receive ivacaftor were also followed for three years. We attempted to collect data one month following administration of ivacaftor and every 3 months thereafter. Improvements in FEV1%predicted, BMI and CFQ-R and sweat chloride were noted as early as one month following the administration of ivacaftor. Subsequently, we observed no further improvement in any parameter. Because of this, and for the sake of clarity, we show data at baseline and at 3 years.
At three years following the administration of ivacaftor, %predicted FEV1 increased from a baseline value of 50 (27, 56) to 60 (33, 55), p < 0.05. In the 4 individuals who did not receive ivacaftor, the %predicted FEV1 was 61 ± 15 at year 1 and 54 ± 14 at year 3. Individuals who received ivacaftor had an increase in BMI from 19.5 ± 2 at baseline to 22.3 ± 3 at three years (p < 0.05), while the group in which the drug could not be obtained, the BMI at baseline was 22 ± 3 and 21 ± 3 at year three. Individuals who received ivacaftor, demonstrated an increase in CFQ-R from 50 ± 5 at baseline to 95 ± 5 (p < 0.05). In those not taking the drug, we found no change (48 ± 6 to 50 ± 4). The number of acute respiratory exacerbations decreased from an average of 4.4 ± 2 exacerbations annually to 2 ± 2 (p < 0.5) in the ivacaftor group. In those who could not receive ivacaftor, the number of exacerbations was not statistically different 4.6 ± 2 at baseline to 5.5 ± 3 at three years.
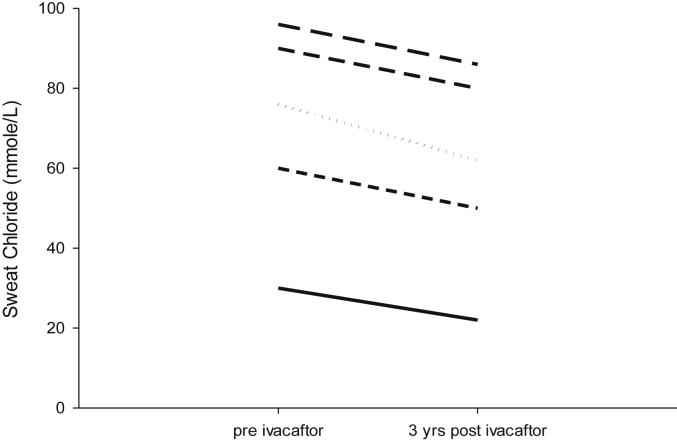
Table 1 demonstrates the characteristics of all patient. Fig. 1 demonstrates results on 5 patients who underwent sweat chloride measurements prior to and at 3 years following ivacaftor administration. While the sweat chloride decreased in each patient, Due to the small number of patients and the large variance in baseline sweat chloride, we could not demonstrate statistical significance. Finally, we examined sputum microbiology and found no change. The bacteria present prior to administering ivacaftor were present 3 years later.
Table 1.
Study population characteristics.
| Patient | Sex | Age at diagnosis | Age baseline | Mutation | PS/PI | Micro-biology | Baseline FEV1% | Baseline sweat Cl | |
|---|---|---|---|---|---|---|---|---|---|
| Ivacaftor | #1 | F | 18 | 30 | R347P/L1065P | PS | Ps | 80 | 97 |
| #2 | F | 16 | 28 | R347P/L1065P | PS | Ps, MRSA | 74 | 95 | |
| #3 | F | 30 | 48 | 2789 + 5G/R1066C | PS | Ac | 34 | 65 | |
| #4 | M | 12 | 28 | S912X/D579G | PI | Ps, MSSA | 35 | 66 | |
| #5 | F | 28 | 43 | del F508/R352Q | PS | Sm | 34 | 80 | |
| #6 | M | 51 | 72 | G542X/D1152H | PS | Ps | 40 | 22 | |
| #7 | M | 51 | 56 | W1282X/D1152H | PS | Ps, MSSA | 65 | 33 | |
| No therapy | #8 | F | 65 | 72 | del F508/D1152H | PS | MRSA | 69 | 83 |
| #9 | F | birth | 34 | del F508/R352Q | PS | Sm | 64 | 75 | |
| #10 | F | 28 | 56 | del F508/R334W | PS | Ps | 56 | 66 | |
| #11 | F | 8 | 22 | 3849 + 10KBC > T/3849 + 10KBC > T | PS | Ps, MRSA | 55 | 88 |
Ps- Pseudomonas species (none were multi-drug resistant); MRSA- Methicillin-resistant Staphylococcus Aureus; MSSA- Methicillin-sensitive Staphylococcus Aureus; Ac- Achromobacter xylosoxidans; Sm- Stenotrophomonas. PS/PI = pancreatic sufficient/insufficient.
Fig. 1.
Sweat chloride was measured in 5 patients at baseline (pre-ivacaftor) and at 3 years on the drug.
4. Discussion
We demonstrate that the administration of ivacaftor to individuals with CF mutations that produce a CFTR protein with some function resulted in improvement in the %predicted FEV1, BMI, CFQ-R and a decrease in the number of acute exacerbations. We also show a non-significant decrease in the sweat chloride in 5 patients on ivacaftor. To our knowledge, this study includes the largest number of CF patients with mutations resulting in a partially functioning protein, and spans a wide range of mutations, ages and disease severity. Furthermore, patients were followed for up to three years on ivacaftor. The non-treatment group was also followed for three years and included CF patients with similar age, mutations and disease burden. In this group, the disease worsened. We failed to detect changes in sputum microbiology in our group of patients who took ivacaftor. While we performed sputum microbiology, more sensitive techniques such as culture-independent methods for characterizing bacteria, together with a molecular phylogenetic approach, might reveal a different picture.
Interestingly, the Food and Drug Administration (FDA) did not grant approval to Vertex for its supplemental New Drug Application (sNDA) to use ivacaftor in CF patients with residual function mutations [12]. Vertex's sNDA application was based on preclinical data and results from a Phase 2a trial. This study included 24 patients with 23 mutations. The study consisted of two, two-week treatment cycles of ivacaftor followed by two treatment cycles of placebo, or vice-versa. While ivacaftor increased %predicted FEV1 compared to placebo, the FDA determined that it cannot approve the drug based on these findings alone. Our results support and supplement these data. The patients in this report had some of the twenty-three mutations included in the phase 2a trial: 2789+5G- > A (patient 3 who stabilized with ivacaftor), 3849 + 10kbC- > T (patient 11 who deteriorated without ivacaftor), D579G (patient 4 who improved with ivacaftor) and D1152H (patients 6 and 7 both of whom improved with ivacaftor and patient 8 who could not receive the drug and worsened).
Our report is not a controlled trial. It was neither blinded, nor was it randomized. Consequently, this report is susceptible to bias even though we demonstrate statistically significant improvements in several important parameters. We were unable to randomize our patients to treatment or non-treatment groups. Indeed, the patient's assignment depended on whether the insurance carrier approved payment tor this medication or not. Nevertheless, this is a real life, pragmatic report and provides strong evidence for the potential benefits of ivacaftor in these CF individuals. Additionally, we report the largest group of CF patients (seven) with residual function mutations to receive ivacaftor who were followed for three years.
5. Conclusions
We report improvement in CF disease in seven individuals who were followed for three years on ivacaftor compared to their baseline (pre-initiation of ivacaftor). These data provide evidence supporting the possibility of beneficial effects of CFTR potentiators in people with CFTR mutations producing residual functioning protein. While there appears to be a need for a randomized double blinded trial using ivacaftor, the low number of CF patients with these relevant genotypes makes such an undertaking challenging. Nevertheless, our data support alternative trial designs for these difficult to study CF subpopulations.
Conflict of interest
None of the authors have any relationship of any manner with Vertex.
References
- 1.Van Goor F., Hadida S., Grootenhuis P.D., Burton B., Cao D., Neuberger T. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U. S. A. 2009;106(44):18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramsey B., Davies J., McElvaney N.G., Tullis E., Bell S., Dřevínek P., Griese M., McKone E., Wainwright C., Konstan M., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S., Dong Q., Rodriguez S., Yen K., Ordoñez C., Elborn J., for the VX08-770-102 Study Group CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Boeck K., Munck A., Walker S., Faro A., Hiatt P., Gilmartin G., Higgins M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Moss R.B., Flume P.A., Elborn J.S., Cooke J., Rowe S.M., McColley S.A., Rubenstein R.C., Higgins M., VX11-770-110 (KONDUCT) Study Group Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir. Med. 2015;3(7):524–533. doi: 10.1016/S2213-2600(15)00201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration Approves KALYDECO® (ivacaftor) for Use in People with Cystic Fibrosis Ages 6 and Older Who Have the R117H Mutation. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=889027. (Accessed 18 January 2016).
- 6.Van Goor F., Yu H., Burton B., Hoffman B.J. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J. Cyst. Fibros. 2014;13(1):29–36. doi: 10.1016/j.jcf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Yousef S., Solomon G., Brody A., Rowe S., Colin A. Improved clinical and radiographic outcomes after treatment with ivacaftor in a young adult with cystic fibrosis with the P67L CFTR mutation. Chest. 2015;147(3):e79–e82. doi: 10.1378/chest.14-1198. [DOI] [PubMed] [Google Scholar]
- 8.Collie J.T., Massie R.J., Jones O.A., LeGrys V.A., Greaves R.F. Sixty-five years since the New York heat wave: advances in sweat testing for cystic fibrosis. Pediatr. Pulmonol. 2014;49(2):106–117. doi: 10.1002/ppul.22945. [DOI] [PubMed] [Google Scholar]
- 9.Scoring the CFQ-R. The Cystic Fibrosis Questionnaire-Revised. A Health-Related Quality of Life Measure. http://www.psy.miami.edu/cfq_QLab/scoring.html. (Accessed 18 January 2016).
- 10.Abbott J.A., Holt A.A., Hart A.M., Morton L., MacDougall B.M., Pogson B.G., Milne B., Rodgers H.C., Conway S.P. What defines a pulmonary exacerbation? The perceptions of adults with cystic fibrosis. J. Cyst. Fibros. 2009;8:356–359. doi: 10.1016/j.jcf.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Stenbit A.E., Flume P.A. Pulmonary exacerbations in cystic fibrosis. Curr. Opin. Pulm. Med. 2011;17(6):442–447. doi: 10.1097/MCP.0b013e32834b8c04. [DOI] [PubMed] [Google Scholar]
- 12.Vertex Receives Complete Response Letter from U.S. FDA for Use of KALYDECO® (ivacaftor) in People with Cystic Fibrosis Ages 2 and Older with One of 23 Residual Function Mutations. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=953584. (Accessed 14 February 2016).