Abstract
To analyze whether Physical activity (PA) reduces mortality risk at thirteen years' follow-up in a population-based cohort of Spanish older adults. The NEDICES (Neurological Disorders in Central Spain) is a prospective population-based survey of older adults (age ≥ 65 years) that comprised 5278 participants at baseline. A modified version of the Rosow-Breslau questionnaire was applied to categorize the PA (sedentary, light, moderate and high) and dates of death were collected from the Official Spanish Death Registry. Cox regression models adjusted for different covariates (age, sex, marital status, smoking, previous stroke, Parkinson disease, incident dementia, body mass index, comorbidity indexes and functional assessment) were used to evaluate the hazard of death at thirteen years' interval according to different levels of PA. 1710 deaths (52.9% men vs. 47.1% women) were identified among 3633 individuals at thirteen years' follow-up. Hazard ratios (HRs) of the light, moderate, and high PA groups (vs. sedentary group) were 0.64 (95% confidence interval (CI) [0.56, 0.72], p < 0.001), 0.61 (95% CI [0.53, 0.70], p < 0.001) and 0.48 (95% CI [0.41, 0.55], p < 0.001), respectively. Significant dose effects were observed between light versus the sedentary group and intense versus the moderate group. PA prevents long-term mortality in older Spanish adults, with the highest intensity levels being those related to the lowest risk of mortality. These findings indicate that health policies for old age care should include PA as one of the main targets.
Keywords: Aging, Health, Physical activity, Mortality, Population-based study
Highlights
-
•
Physical activity is associated with a lower risk of mortality in older adults.
-
•
This protective effect seemed slightly higher for women.
-
•
Most studies didn't show a dose-effect response.
-
•
Promotion of physical activity should be a priority for health agencies.
1. Introduction
Physical activity (PA) is defined as “any bodily movement produced by skeletal muscles that requires energy expenditure, including activities undertaken while working, playing, carrying out household chores, travelling, and engaging in recreational pursuits” (“WHO | Global Recommendations on Physical activity for Health”). Considering that physical inactivity is linked to the development of major chronic diseases such as cardiovascular disease, diabetes, or certain types of cancer (“WHO | The world health report, 2002), different patterns of PA (type, intensity, frequency and duration) are recommended throughout the week for older adults (“WHO | Global Recommendations on Physical activity for Health”).
In this context, the association between physical activity and premature mortality has been discussed since the 1950s. Morris and Heady (1953) released the first report describing a significant inverse relation between occupational physical activity and cardiovascular mortality (Morris and Heady, 1953). From then on, different studies have explored this association, with recent meta-analyses which reflect a lower risk for all mortality causes in people who carry out PA (Nocon et al., 2008, Löllgen et al., 2009, Loef and Walach, 2012, Hupin et al., 2015, Samitz et al., 2011). However, this association has not been consistent across different populations (Hayasaka et al., 2009, Brown et al., 2012, Ottenbacher et al., 2012, Shortreed et al., 2013), and the presence of dose effect (a linear relationship between increase of PA intensity and reduction of mortality) is controversial (Löllgen et al., 2009). Such discrepancies could be explained by the effect of population characteristics, differences in periods of follow-up (short- vs. long-term) and profile of PA analysed (e.g., type, intensity, frequency and duration). Finally, evidence has been mainly derived from studies involving middle-aged subjects, whereas research based on aged population are scarcer (Ueshima et al., 2010, Paganini-Hill et al., 2011).
The aim of this study is to analyze whether PA is a protective factor against mortality at a thirteen-year follow-up in a population-based sample of Spanish older people. Moreover, we tested whether the intensity of PA (light, moderate, or high) was associated with a dose-effect response. This research has implications in term of seeking strategies to prevent early mortality in older adults.
2. Methods
2.1. Participants
Data for this study were collected from the Neurological Disorders in Central Spain (NEDICES) cohort, a population-based survey of older people's (age 65 years and older) main age-related conditions, including Parkinson's disease, essential tremor, stroke and dementia. This research is part of a main study which analysed the association between the risk of incident dementia and PA (Llamas-Velasco et al., 2015).
Briefly, the NEDICES study was carried out in three well-defined geographic areas of central Spain, to obtain a representative cohort of older people with different socioeconomic backgrounds. Thus, participants were selected from population censuses of three communities: Las Margaritas, a working-class neighbourhood in Getafe (Greater Madrid); Lista, a professional-class neighbourhood in the Salamanca district (Central Madrid); and 38 villages from the agricultural region of the Arévalo country (125 km northwest of Madrid). A signed informed consent was obtained from all participants at the time of enrolment. Ethical standards committees on human research at the University Hospitals “12 de Octubre” (Madrid) and “La Princesa” (Madrid) approved the protocol of the study as complying with the Declaration of Helsinki. A detailed account of the study population and methods were previously reported (Bermejo et al., 2001, Morales et al., 2004).
It is a longitudinal study with two surveys: baseline wave (1994–1995) and incidence wave (1997–98) among the same population. At the time of their baseline assessment, 5278 population based older people (57.6% women with a mean age of 74.31 ± 6.97; 53.1% without a certificate of primary school) were interviewed using a 500-item screening questionnaire that assessed demographic data, medical conditions, current medication and lifestyle (e.g., consumption of alcohol, smoking habits, physical activity, self-reported health). A short form of the questionnaire was mailed to participants who were unavailable for face-to-face or telephone screening.
2.2. Measures and testing procedure
2.2.1. Assessment of daily physical activity
The PA of individuals was collected at baseline (1994–1995) using an adapted modified version (four items) of the Rosow-Breslau physical function measure (Rosow and Breslau, 1966). The measure assesses usual tasks performed by community-dwelling older adults (e.g., walking half a mile, walking up and down two flights of stairs, performing heavy housework) and its test-retest reliability (r = 0.81) and has been assessed in the Established Populations for Epidemiologic Studies of the Elderly (Smith et al., 2010). Therefore, the measure seems sufficiently stable for longitudinal analyses, and concurrent validity was previously established (Reuben et al., 1990). This measure has also been related to functional disability and mortality in older populations (Brock et al., 1994, Thomas and Lichtenstein, 1986).
In this survey, trained interviewers asked the participants “How many hours do you dedicate daily to.....” (a) sedentary lifestyle (i.e., only minimal house chores or short walks at home); (b) light physical activity (i.e., regular house chores, walks independently at home); (c) moderate physical activity (i.e., regular house chores, walks up to one kilometre per day); (d) high activity (i.e., performs heavy housework, walks more than one kilometre or practices any sport regularly). Therefore, PA was classified into four groups (sedentary, light, moderate and high PA). These groups were formed with the aim of categorizing PA under a dose-effect hypothesis (Löllgen et al., 2009, Lee and Skerrett, 2001).
2.2.2. Indicators of health
Self-rated health was assessed with one question (“In general terms, how would you describe your health: very good, good, fair, poor, or very poor?” rated with 1 [very good] to 5 [very poor] points). Meanwhile, the Charlson comorbidity index was calculated based on Romano's adaptation (Romano et al., 1993). The following diseases were included: myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic lung disease, connective tissue disease, ulcer, chronic liver disease, diabetes, hemiplegia, moderate or severe kidney disease, diabetes with end organ damage, tumour, leukaemia, lymphoma, moderate or severe liver disease, malignant tumour, metastasis and/or AIDS.
2.2.3. Mortality data source
Follow-up data on death were collected until December 31, 2007. The date of “all causes” of death was obtained from the Official Spanish Death Registry (INE). In Spain, all deceased individuals receive a death certificate, completed by a doctor, at the time of death. The certificate is then sent to the local police authority in the municipality where the person had been living, and the information is recorded in the National Register.
2.3. Statistical analysis
Statistical analyses were performed with the SAS software, version 9.3 (SAS Institute Inc, 2011). Baseline characteristics of the groups were compared using analysis of variance (ANOVA) tests for numerical variables and χ2 tests for categorical variables. Mortality (for all causes) rates per 1000 person-years were calculated based on the baseline cohort, and Cox's proportional-hazards models (95% confidence interval) were used to test the association between PA and the risk of mortality at 13 years. The effect of different covariates (age, sex, marital status, smoking, previous stroke, Parkinson disease, incident dementia, body mass index [BMI], functional assessment measured by adapted Spanish version of Pfeffer's Functional Activities Questionnaire (FAQ; Olazaran et al., 2005), were controlled in two independent Cox regression models: the first one included self-reported health, and the second one included the Charlson index. Age was introduced as a time-dependent covariate to adjust for the effect of aging across the study period. Finally, Kaplan-Meier method was used to calculate mean survival times, and log-rank tests were performed to assess the significance of the difference between survival curves based on PA levels (sedentary, light, moderate and high).
To test the dose-effect hypothesis, the number of hours was weighted, multiplying the sedentary category by 1, the light PA category by 1.2, the moderate PA category by 1.4, and the high PA category by 1.8. Next, different cut-off points were calculated based on the quartile distribution to classify the subjects as follows: ≤ 15.6 h (sedentary group), ≤ 17.6 h (light PA group), ≤ 19.4 h (moderate PA group), and > 19.4 h (high PA group). The presence of a dose effect was tested using the incremental coding procedure.
3. Results
Of the 5278 participants screened for neurological disorders at baseline, 306 prevalent dementia cases (5.8%) were excluded from further analyses. Therefore, 4972 participants were classified as non-demented at baseline. Of them, 3633 participants from the final cohort had information about PA. No significant differences were found between this final subsample and participants without PA assessment (N = 1339) in terms of sex (p = 0.08), but the latter individuals were slightly older (74.4 ± 7.06 vs. 73.6 ± 6.45, p < 0.05) and had fewer years of schooling (3.9 ± 4.34 vs. 7.0 ± 5.15, p < 0.05) than the selected subsample. The flow chart of this survey is shown in Fig. 1.
Fig. 1.
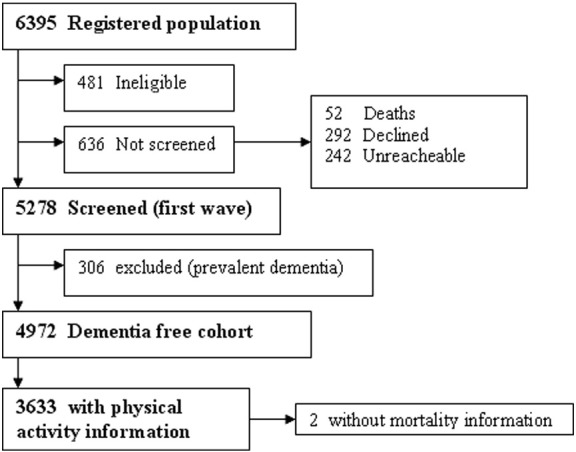
Flow chart of the study.
Table 1 compares the baseline characteristics of the groups according to the level of PA. 975 individuals were classified as leading a “sedentary lifestyle,” 1022 as “light PA,” 771 as “moderate PA,” and 865 as “high PA”. As shown, the sedentary group was significantly older, and the percentage of men was higher in comparison with PA groups. The sedentary group also showed lower educational level, higher proportion of smokers and history of prior stroke than individuals from the PA groups. Functional status was within the normal range in all groups, but the sedentary group obtained a worse performance. Finally, the sedentary group had worse health indicators (Charlson index and self-rated health) than the PA groups.
Table 1.
Characteristics of participants stratified by the intensity of physical activity.
| Characteristic | Sedentary (N = 975) |
Light PA (N = 1022) |
Moderate PA (N = 771) |
High PA (N = 865) |
P |
|---|---|---|---|---|---|
| Age (years) | 75.1 ± 7.01 | 73.4 ± 6.32 | 72.9 ± 6.13 | 72.7 ± 5.90 | < 0.00011 |
| Sex (% female) | 479 (49.1%) | 587 (57.4%) | 426 (55.3%) | 550 (63.6%) | < 0.001 |
| Education (years) | 6.34 ± 5.02 | 7.33 ± 4.92 | 7.29 ± 5.18 | 7.05 ± 5.44 | < 0.001 |
| Marital status | 583 (60.0%) | 621 (60.8%) | 490 (63.6%) | 503 (58.2%) | 0.159 |
| Current drinking | 309 (31.8%) | 349 (34.3%) | 274 (35.6%) | 321 (37.1%) | 0.103 |
| Current smoking | 134 (13.8%) | 128 (12.5%) | 96 (12.5%) | 82 (9.5%) | < 0.05 |
| BMIa | 27.24 ± 5.47 | 27.74 ± 5.06 | 27.47 ± 4.86 | 27.33 ± 6.71 | < 0.05 |
| FAQb | 3.01 ± 5.33 | 1.71 ± 4.00 | 1.46 ± 3.52 | 1.19 ± 3.17 | < 0.0001 |
| Parkinson Disease | 20 (2.1%) | 17 (1.7%) | 10 (1.3%) | 8 (0.9%) | 0.231 |
| Previous stroke | 65 (6.7%) | 45 (4.4%) | 21 (2.7%) | 22 (2.5%) | < 0.001 |
| Charlson-Romano index | 0.80 ± 0.76 | 0.70 ± 0.77 | 0.65 ± 0.66 | 0.67 ± 0.67 | < 0.001 |
| Self-related healthc | 125 (13.0%) | 96 (9.5%) | 80 (10.4%) | 110 (12.8%) | 0.001 |
| Incident dementia | 67 (8.4%) | 31 (3.5%) | 20 (2.9%) | 16 (2.0%) | 0.0001 |
Note: Data are given as mean ± standard deviation or frequencies (%).
Body mass index.
Functional Activities Questionnaire of Pfeffer. A high scores means worse functional capacity.
Poor or very poor (%).
The cohort was followed from baseline for an interval of 10 ± 3.9 years. 1710 deaths (905 men [52.9%] and 805 women [37.1%]) were registered at follow-up (p < 0.001). Mortality rates, per 1000 person-years, according to age, social factors and lifestyle in men and women are shown in Table 2.
Table 2.
Mortality rates, per 1000 person-years, according to age, social factors and lifestyle.
| p-y |
Deaths |
Rate |
p |
Rate |
||||
|---|---|---|---|---|---|---|---|---|
| Men |
p |
Women |
p |
|||||
| Total | 36,580 | 1710 | 46.7 | 69.93 | 0.0001 | 37.05 | 0.0001 | |
| Age | ||||||||
| < 70 years | 13,859 | 322 | 23.23 | 36.51 | 13.58 | |||
| 70–85 years | 21,088 | 1165 | 55.24 | 71.47 | 44.39 | |||
| > 85 years | 1632 | 223 | 136.62 | 0.0001 | 154.57 | 0.000 | 127.01 | 0.0001 |
| Education | ||||||||
| No formal education | 19,349 | 919 | 47.50 | 60.80 | 39.43 | |||
| Primary school or higher | 17,231 | 791 | 45.90 | 0.43 | 61.05 | 0,93 | 34.10 | < 0.05 |
| Marital status | ||||||||
| Married | 14,146 | 722 | 51.04 | 68.93 | 46.68 | |||
| Single | 22,425 | 986 | 43.97 | 0.001 | 59.02 | < 0.05 | 26.39 | 0.0001 |
| Current drinking | ||||||||
| No | 23,866 | 1129 | 47.30 | 68.18 | 39.15 | |||
| Yes | 12,656 | 576 | 45.51 | 0.45 | 54.91 | 0.0001 | 28.78 | 0.001 |
| Current smoking | ||||||||
| No | 32,360 | 1471 | 45.46 | 60.34 | 37.27 | |||
| Yes | 4170 | 238 | 57.06 | 0.0001 | 63.30 | 0,52 | 32.23 | 0,46 |
| Physical activity | ||||||||
| Sedentary | 8641 | 585 | 67.70 | 81.68 | 55.20 | |||
| Light | 10,439 | 463 | 44.35 | 58.37 | 35.12 | |||
| Moderate | 8066 | 343 | 42.52 | 55.52 | 33.04 | |||
| High | 9433 | 319 | 33.81 | 0.0001 | 43.70 | 0.0001 | 28.68 | 0.0001 |
p-y = person-years.
Rate = mortality per 1000 person-years.
The unadjusted Cox regression model showed that the PA groups (light, moderate and high) have a lower risk of mortality at 13 years compared with the sedentary lifestyle group (Table 3). Even when the Cox model was adjusted by controlling for significant covariates of the univariate analyses (age, sex, marital status, smoking, previous stroke, Parkinson disease, incident dementia, body mass index, functional assessment, self-rated health and Charlson-Romano comorbidity index), any level of PA versus sedentary lifestyle remained as a protective factor against mortality. These findings were also consistent when the sample was stratified by sex and taking into account different health indicators (Charlson index and self-rated health) in parallel regression models (see Table 3), except for the moderate PA strata in Model 2, which showed a trend toward significance (p = 0.07). Incremental coding procedure revealed the existence of a significant dose effect (not linear) with a lower risk of mortality in the light PA group compared to the sedentary group (p < 0.0001), and in the intense PA group versus the moderate PA group (p < 0.0001). No dose effect was found between moderate and level groups.
Table 3.
Cox regression models: risk of mortality and physical activity level.
| PA groups | HRs | 95% HR CI | p | Men |
Women |
||
|---|---|---|---|---|---|---|---|
| HRs | p | HRs | p | ||||
| Model without adjustment for covariates | |||||||
| Light | 0.64 | 0.56–0.72 | < 0.0001 | 0.70 | < 0.0001 | 0.62 | < 0.0001 |
| Moderate | 0.61 | 0.53–0.70 | < 0.0001 | 0.66 | < 0.0001 | 0.58 | < 0.0001 |
| High | 0.48 | 0.41–0.55 | < 0.0001 | 0.51 | < 0.0001 | 0.50 | < 0.0001 |
| Model 1 (adjusted bya & self reported health) | |||||||
| Light | 0.76 | 0.65–0.89 | < 0.0001 | 0.75 | 0.007 | 0.75 | 0.016 |
| Moderate | 0.84 | 0.71–0.98 | 0.030 | 0.86 | 0.161 | 0.75 | 0.023 |
| High | 0.64 | 0.54–0.75 | < 0.0001 | 0.58 | < 0.0001 | 0.66 | < 0.0001 |
| Model 2 (adjusted bya & Charlson Index) | |||||||
| Light | 0.79 | 0.68–0.92 | 0.003 | 0.82 | 0.056 | 0.76 | 0.019 |
| Moderate | 0.86 | 0.73–1.01 | 0.071 | 0.92 | 0.444 | 0.73 | 0.015 |
| High | 0.67 | 0.56–0.79 | < 0.0001 | 0.64 | < 0.0001 | 0.65 | < 0.0001 |
The sedentary group was taken as reference (n = 790).
HR: Hazard Ratio; CI: Interval confidence.
Age, sex, marital status, smoking, previous stroke, Parkinson disease, incident dementia, body mass index, functional assessment.
We also derived survival probability at 13 years' follow-up with the Kaplan-Meier method. Fig. 2 shows the survival probability for the different levels of PA, showing a less favourable survival rate in the sedentary group.
Fig. 2.
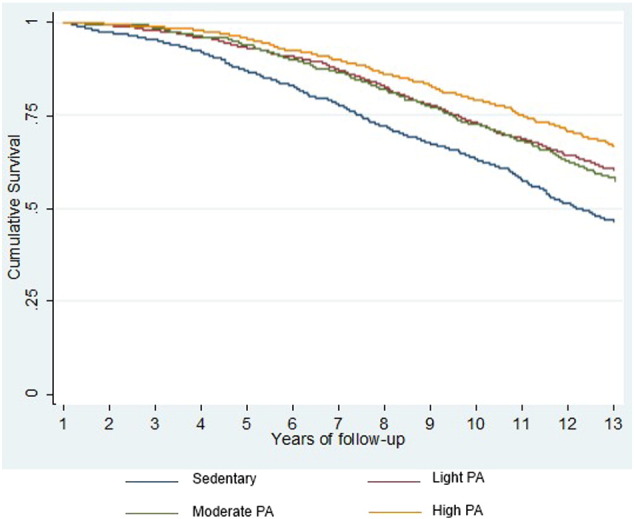
Kaplan-Meier curves comparing survival according to level of physical activity.
4. Discussion
The main finding of this prospective population-based study is that, compared to sedentary lifestyle, PA is associated with a lower risk of mortality for all causes in older adults after controlling for the effect of several covariates. These results confirm previous epidemiological evidence about the protective role of PA in the mortality rates (Brown et al., 2012, Ueshima et al., 2010, Paganini-Hill et al., 2011, Woodcock et al., 2011, Wu et al., 2015, Ramalho et al., 2015), which is basically explained by the preventive effect of PA in neurological disorders and other chronic conditions (“WHO | The world health report, 2002”; Llamas-Velasco et al., 2015).
Interestingly, regression models showed that this protective effect seemed slightly higher for women than for men in almost all strata analyses, which is consistent with the results of Hupin et al. (Hupin et al., 2015), who showed a 32% reduction in mortality risk in women compared to 14% in men (Hupin et al., 2015). Likewise, Samitz et al. (Samitz et al., 2011) also showed significant risk ratios (0.58 women vs. 0.72 men) for all domains of PA (occupational PA, exercise and sports). However, other studies and the National Health and Nutrition Examination Survey (NHANES), which used an accelerometer to measure PA, did not find this moderator effect in older populations (Woodcock et al., 2011, Wu et al., 2015, Long et al., 2015). A possible explanation is that men and women may also overestimate or underestimate, respectively, their PA level (Löllgen et al., 2009, Wu et al., 2015). Hormone levels, estrogenic metabolism and body fat distribution also have been proposed, but the effect of these factors may be reduced in older samples (Hupin et al., 2015).
Significant dose effects were identified comparing light versus sedentary PA groups and high versus moderate PA groups, which is also consistent with the results obtained by Hupin et al. (Hupin et al., 2015). However, there is a non-linear relationship between the risk of mortality and PA, with larger benefits associated with slight changes at the lower levels of PA compared to the same increment at high or moderate PA levels, as shown previously in other studies (Löllgen et al., 2009, Woodcock et al., 2011). In other words, we assume that PA prevents mortality at any level, but its effect is not proportional at each level. In this sense, Samitz et al. found a larger reduction in mortality when PA time is increased per week but only in several domains of PA (exercise and sports) (Samitz et al., 2011).
Several physiological mechanisms have been proposed to explain the effect of PA as a preventive factor against mortality. For instance, PA reduces the incidence of cardiovascular risk factors (Type 2 diabetes mellitus, hypertension, etc.), improves cardiorespiratory fitness, modulates the stress cascade, changes body composition or weight and prevents falls, osteoporotic fractures and disability (Wu et al., 2015, Nelson et al., 2007, Murtagh et al., 2015).
This study has several limitations. Information about PA was collected by a self-report questionnaire that was more weighted toward functionality at home and walking abilities, whereas other studies have used a daytime actigraphy to measure PA objectively (Fishman et al., 2016). In addition, non-exercise PA in daily life was not measured, which may play an important role in the benefits of total activity. The amount of PA was only recorded at baseline, making it impossible to report changes in exercise patterns over time. As other longitudinal studies, is difficult to know and is a possible bias, if people more healthy are hence physically more active, or people physically more active are hence more healthy. It should also be noted that the group without PA information was older and less educated than the selected sample, which limits generalization. Finally, the effect of PA on mortality was analysed using a single long-term follow up interval.
In this research, several strengths should be highlighted. All participants were selected from a prospective population-based study. In this regard, a broad spectrum of older Spaniards (socio-economically diverse) was analysed. Complete death information of almost the entire cohort (i.e., except for 2 individuals) was available. Finally, substantial confounders related to mortality (e.g., self-rated health and education) were controlled in the analyses (DeSalvo et al., 2006, Brehaut et al., 2004).
The knowledge of PA as a protective factor of mortality in late-life periods is of special interest for preventive strategies. Mace et al. showed that 65% of adults aged 65 or older did not meet the guidelines for regular PA (at least 150 min of PA per week) (Mace et al., 2016). At this point, physical inactivity has mortality implications (Wen et al., 2011), so the promotion of PA should be a priority for health agencies (Heath et al., 2012). To sum up, PA in older adults (aged 65 and older) seems to be a protective factor against mortality for all causes. Specific dose effects were identified in several groups, but there is no significant reduction of risk at intermediate levels of PA. Future studies should focus on how various types, intensities and frequencies of PA could influence the risk of early mortality.
References
- Bermejo F., Gabriel R., Vega S., Morales J.M., Rocca W.A., Anderson D.W. Neurological disorders in Central Spain (NEDICES) study group: problems and issues with door-to-door, two-phase surveys: an illustration from Central Spain. Neuroepidemiology. 2001;20:225–231. doi: 10.1159/000054794. [DOI] [PubMed] [Google Scholar]
- Brehaut J.C., Raina P., Lindsay J. Does cognitive status modify the relationship between education and mortality? Evidence from the Canadian study of health and aging. Int. Psychogeriatr. 2004;16:75–91. doi: 10.1017/s1041610204000080. [DOI] [PubMed] [Google Scholar]
- Brock D.B., Lemke J.H., Branch L.G., Evans D.A., Berkman L.F. Mortality and physical functioning in epidemiologic studies of three older populations. J. Aging Soc. Policy. 1994;6:21–37. doi: 10.1300/j031v06n03_04. [DOI] [PubMed] [Google Scholar]
- Brown W.J., McLaughlin D., Leung J. Physical activity and all-cause mortality in older women and men. Br. J. Sports Med. 2012;46:664–668. doi: 10.1136/bjsports-2011-090529. [DOI] [PubMed] [Google Scholar]
- DeSalvo K.B., Bloser N., Reynolds K., He J., Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. J. Gen. Intern. Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman E.I., Steeves J.A., Zipunnikov V. Association between objectively measured physical activity and mortality in NHANES. Med. Sci. Sports Exerc. 2016 Feb;5 doi: 10.1249/MSS.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S., Shibata Y., Ishikawa S. Jichi medical school cohort study group: physical activity and all-cause mortality in Japan: the Jichi medical school (JMS) cohort study. J. Epidemiol. 2009;19:24–27. doi: 10.2188/jea.JE20080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath G.W., Parra D.C., Sarmiento O.L. Lancet physical activity series working group: evidence-based intervention in physical activity: lessons from around the world. Lancet. 2012;380:272–281. doi: 10.1016/S0140-6736(12)60816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupin D., Roche F., Gremeaux V. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥ 60 years: a systematic review and meta-analysis. Br. J. Sports Med. 2015;49:1262–1267. doi: 10.1136/bjsports-2014-094306. [DOI] [PubMed] [Google Scholar]
- Lee I.M., Skerrett P.J. Physical activity and all-cause mortality: what is the dose-response relation? Med. Sci. Sports Exerc. 2001;33:459–471. doi: 10.1097/00005768-200106001-00016. [DOI] [PubMed] [Google Scholar]
- Llamas-Velasco S., Contador I., Villarejo-Galende A., Lora-Pablos D., Bermejo-Pareja F. Physical activity as protective factor against dementia: a prospective population-based study (NEDICES) J. Int. Neuropsychol. Soc. 2015;21:861–867. doi: 10.1017/S1355617715000831. [DOI] [PubMed] [Google Scholar]
- Loef M., Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev. Med. 2012;55:163–170. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Löllgen H., Böckenhoff A., Knapp G. Physical activity and all-cause mortality: an updated meta-analysis with different intensity categories. Int. J. Sports Med. 2009;30:213–224. doi: 10.1055/s-0028-1128150. [DOI] [PubMed] [Google Scholar]
- Long G., Watkinson C., Brage S. Mortality benefits of population-wide adherence to national physical activity guidelines: a prospective cohort study. Eur. J. Epidemiol. 2015;30:71–79. doi: 10.1007/s10654-014-9965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace C.J., Kerse N., Maddison R. Descriptive epidemiology of physical activity levels and patterns in new Zealanders in advanced age. J. Aging Phys. Act. 2016;24:61–71. doi: 10.1123/japa.2014-0230. [DOI] [PubMed] [Google Scholar]
- Morales J.M., FP B., Benito-León J. NEDICES study group: methods and demographic findings of the baseline survey of the NEDICES cohort. Public Health. 2004;118:426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Morris J.N., Heady J.A. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br. J. Ind. Med. 1953;10:245–254. doi: 10.1136/oem.10.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh E.M., Nichols L., Mohammed M.A., Holder R., Nevill A.M., Murphy M.H. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and metaanalysis of randomised control trials. Prev. Med. 2015;72:34–43. doi: 10.1016/j.ypmed.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Nelson M.E., Rejeski W.J., Blair S.N. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Nocon M., Hiemann T., Müller-Riemenschneider F., Thalau F., Roll S., Willich S.N. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- Olazaran J., Mouronte P., Bermejo F. Clinical validity of two scales of instrumental activities in Alzheimer's disease. Neurologia. 2005;20:395–401. [PubMed] [Google Scholar]
- Ottenbacher A.J., Snih S.A., Karmarkar A. Routine physical activity and mortality in Mexican Americans aged 75 and older. J. Am. Geriatr. Soc. 2012;60:1085–1091. doi: 10.1111/j.1532-5415.2012.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-Hill A., Kawas C.H., Corrada M.M. Activities and mortality in the elderly: the leisure world cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2011 May;66:559–567. doi: 10.1093/gerona/glq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho J.R., Mambrini J.V., César C.C. Physical activity and all-cause mortality among older Brazilian adults: 11-year follow-up of the Bambuí health and aging study. Clin. Interv. Aging. 2015 Apr;10:751–758. doi: 10.2147/CIA.S74569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben D.B., Siu A.L. An objective measure of physical function of elderly outpatients. The physical performance test. J. Am. Geriatr. Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- Romano P.S., Roos L.L., Jollis J.G. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J. Clin. Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- Rosow I., Breslau N. A Guttman health scale for the aged. J. Gerontol. 1966;21:556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- Samitz G., Egger M., Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose–response meta-analysis of cohort studies. Int. J. Epidemiol. 2011;40:1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2011. SAS® 9.3 DS2 Language Reference: Getting Started. [Google Scholar]
- Shortreed S.M., Peeters A., Forbes A.B. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham heart study. Heart. 2013;99:649–654. doi: 10.1136/heartjnl-2012-303461. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Blumenthal J.A., Hoffman B.M. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The world health report . Geneva, World Health Organization; 2002. Reducing Risks, Promoting Healthy Life. 2002. [Google Scholar]
- Thomas J.W., Lichtenstein R. Functional health measure for adjusting health maintenance organization capitation rates. Health Care Financ. Rev. 1986;7:85–95. [PMC free article] [PubMed] [Google Scholar]
- Ueshima K., Ishikawa-Takata K., Yorifuji T. Physical activity and mortality risk in the Japanese elderly a cohort study. Am. J. Prev. Med. 2010;38:410–418. doi: 10.1016/j.amepre.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Wen C.P., Wai J.P., Tsai M.K. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- Woodcock J., Franco O.H., Orsini N., Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 2011;40:121–138. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- World Health Organization Global Recommendations on Physical Activity for Health. 2010. http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/index.html Geneva. [PubMed]
- Wu C.Y., Hu H.Y., Chou Y.C., Huang N., Chou Y.J., Li C.P. The association of physical activity with all-cause, cardiovascular, and cancer mortalities among older adults. Prev. Med. 2015;72:23–29. doi: 10.1016/j.ypmed.2014.12.023. [DOI] [PubMed] [Google Scholar]


