Abstract
As a widely-applied alternative therapy, acupuncture is gaining popularity in Western society. One challenge that remains, however, is incorporating it into mainstream medicine. One solution is to combine acupuncture with other conventional, mainstream treatments. In this study, we investigated the combination effect of acupuncture and the antidepressant fluoxetine, as well as its underlying mechanism using resting state functional connectivity (rsFC) in patients with major depressive disorders.
Forty-six female depressed patients were randomized into a verum acupuncture plus fluoxetine or a sham acupuncture plus fluoxetine group for eight weeks. Resting-state fMRI data was collected before the first and last treatments. Results showed that compared with those in the sham acupuncture treatment, verum acupuncture treatment patients showed 1) greater clinical improvement as indicated by Montgomery–Åsberg Depression Rating Scale (MADRS) and Self-Rating Depression Scale (SDS) scores; 2) increased rsFC between the left amygdala and subgenual anterior cingulate cortex (sgACC)/preguenual anterior cingulate cortex (pgACC); 3) increased rsFC between the right amygdala and left parahippocampus (Para)/putamen (Pu). The strength of the amygdala-sgACC/pgACC rsFC was positively associated with corresponding clinical improvement (as indicated by a negative correlation with MADRS and SDS scores). Our findings demonstrate the additive effect of acupuncture to antidepressant treatment and suggest that this effect may be achieved through the limbic system, especially the amygdala and the ACC.
Keywords: Depression, Acupuncture, Resting-state functional connectivity, Amygdala, Limbic system, Fluoxetine, Combination effect
Highlights
-
•
We investigated amygdala rsFC change before and after real and sham acupuncture plus fluxoetine treatment.
-
•
Real acupuncture plus fluoxetine treatment significantly increased rsFC between the left amygdala and ACC.
-
•
Real acupuncture plus fluoxetine treatment significantly increased rsFC between the right amygdala and right parahippocampus.
-
•
The rsFC increase is significantly associated with HAMD score change.
1. Introduction
Major depressive disorder (MDD) is a common disorder that affects a large proportion of the population by significantly impairing their occupational, social, and academic functioning (Lehtinen and Joukamaa, 1994, Johnson et al., 1992). Current treatments of antidepressant medications may be provided as an initial primary treatment for MDD, but they are far from satisfactory (Rush et al., 2003, Sackeim, 2001) due to undesirable side effects and a delay in the onset of therapeutic action (Arroll et al., 2005, Zhu et al., 2013).
In recent years, acupuncture has become a promising and effective alternative treatment for depression (Jorm et al., 2002, Kessler et al., 2001; Im. Quah-Smith et al., 2013; Im. Quah-Smith et al., 2012). Studies suggest that acupuncture may work by inducing the release of norepinephrine, serotonin, and dopamine in the central nervous system (Ulett and Han, 1998, Siedentopf et al., 2005), all of which play a role in the pathophysiology of MDD (Belmaker & Agam, 2008).
As a popular alternative and complementary modality, acupuncture is gaining popularity in Western society. One challenge that remains, however, is incorporating it into mainstream medicine. One solution is to combine acupuncture with other conventional, mainstream treatments. Using the treatment of depression as an example, accumulating evidence has indicated that acupuncture combined with antidepressant medications is more effective than antidepressants alone, safe, well-tolerated, and has an early onset of action (Zhang et al., 2009, Zhu et al., 2013) However, the lack of understanding regarding the underlying mechanisms of the combinative treatment of acupuncture and antidepressant medications has significantly slowed the attempt to incorporate this treatment into mainstream medicine.
In recent years, brain imaging techniques have been applied to investigate the physiopathology of depression and revealed that MDD is associated with structural and functional abnormalities in brain circuits involved in emotional processing, self-representation, reward, and external stimulus (stress, distress) interactions (Davidson et al., 2002, Hasler and Northoff, 2011;Damoiseaux and Greicius, 2009, Lu et al., 2012, Mwangi et al., 2012; D. A. Pizzagalli, 2011, Silbersweig, 2013). Many brain regions including the amygdala, hippocampus, insula, ventral striatum, ventral anterior cingulate gyrus, and prefrontal cortex are involved in the neural pathology and development of MDD (Lawrence et al., 2004, Phillips et al., 2015, Phillips et al., 2003a, Phillips et al., 2003b) and subthreshold depression (Hwang et al., 2016). Among these brain regions, one of the most well-studied regions is the amygdala.
As part of the limbic system, the amygdala plays a vital role in emotional processing, fear learning, and motivation behaviors (Adolphs et al., 1994, Cardinal et al., 2002, Mears and Pollard, 2016, Phelps et al., 2004, Phelps and LeDoux, 2005). Studies showed that compared with healthy controls, MDD patients showed abnormal elevated activity in the amygdala when presented with negative stimuli (Phillips et al., 2015, Sheline et al., 2010, Siegle et al., 2002, Siegle et al., 2007). In a more recent meta-analysis, Ma (Ma, 2015) found that antidepressant medication in patients with mood disorders affects the amygdala by decreasing its activity during negative emotions and increasing its activity during positive emotions.
Seed-based resting-state functional connectivity (rsFC) is a method of functional brain imaging that can assess the temporal dependency of brain regions (seeds) during rest (Biswal et al., 1995). rsFC allows for the study of the function of one or several brain regions in relation to its functional network and how the network contributes to brain procedures (Hwang et al., 2015; Jessica S. Damoiseaux & Greicius, 2009). It has also widely been used to reveal the underlying mechanisms of different diseases (Sun et al., 2012; J. Wang et al., 2013a, Wang et al., 2013b, Wang et al., 2013c, Zheng et al., 2014), including depression (L. Wang et al., 2013a, Wang et al., 2013b, Wang et al., 2013c; L. Wang et al., 2015). Previous studies suggested that acupuncture can significantly modulate rsFC in healthy subjects (Bai et al., 2009, Dhond et al., 2008, Hui et al., 2009; P. Liu et al., 2009, Qin et al., 2008, Zhong et al., 2012) and patient populations, such as patients with knee osteoarthritis (X. Chen et al., 2015, Egorova et al., 2015), migraine (Li et al., 2016a, Li et al., 2016b, Li et al., 2016a, Li et al., 2016b, Zhao et al., 2014) and Alzheimer's disease (Z. Wang et al., 2014). Few studies (Yi et al., 2012), however, have analyzed rsFC changes resulting from repeated acupuncture treatments for MDD.
In this study, we investigated rsFC changes in the left and right amygdala before and after verum acupuncture plus fluoxetine and sham acupuncture plus fluoxetine in patients with MDD. Considering that brain activity dysfunction may vary between male and female subjects (Altemus et al., 2014, Bangasser and Valentino, 2014), we only recruited female patients to increase the homogeneity of this study. We hypothesized that in contrast to sham acupuncture treatment, verum acupuncture treatment can modulate amygdala-related rsFC. In addition, rsFC changes may be associated with changes in the depressive symptoms of patients.
2. Materials and methods
2.1. Participants
The study protocol was approved by the Institutional Review Board of the 2nd Affiliated Hospital of Guangzhou University of Chinese Medicine. The study was enrolled online on the Chinese Clinical Trial Registry (ChiCTR) (www.chictr.org.cn, ChiCTR-TRC-14,005,228). All patients were provided written informed consent before participation in the study. Patients with MDD were recruited for this study through community postings, and all eligible participants were required to meet the specific inclusion/exclusion criteria.
2.1.1. Inclusion criteria
1) Women who met the criteria of depression in ICD-10; 2) standard score of Self-Rating Depression Scale (SDS) ≥ 53 or total score of Montgomery-Asberg depression rating scale (MADRS) ≥ 14; 3) aged 30–60 years old and able to provide voluntary informed consent; 4) right-handed; 5) primary school education or higher; and 6) normal cognitive functioning, with no aphasia or intellectual disabilities.
2.1.2. Exclusion criteria
1) Refused to sign informed content; 2) pregnant or lactating women; 3) women with metallic implants; 4) used antipsychotics or antidepressants within a month before the study; 5) consumption of alcohol or illicit substances; 6) patients with severe damage of liver or kidney function, or with neurological deficits, rheumatologic disorders, cardiac disease, diabetes, malignant tumors or any other significant systemic disorders that might affect the results; 7) presence of severe psychoses (schizophrenia, mania, paranoid psychosis or depression with suicidal intent) or dementia; 8) presence of any somatic diseases (such as cerebral infarction, cerebral hemorrhage, Parkinson's or cerebral tumor) or any other significant systemic disorders that might affect the results.
2.2. Intervention
All participants received fluoxetine (20 mg po qd) once per day plus verum or sham acupuncture treatment based on randomization.
2.3. Verum and sham acupuncture administration
In this study, we applied abdominal acupuncture, an acupuncture modality (Zhiyun, 2001) that has been proven to be effective for depression (Cheng and Tang, 2007, Lyons et al., 2012; X. Y. Wang et al., 2010, Wu et al., 2012). The advantage of abdominal acupuncture is that it only produces mild sensations, possibly making it more acceptable to patients.
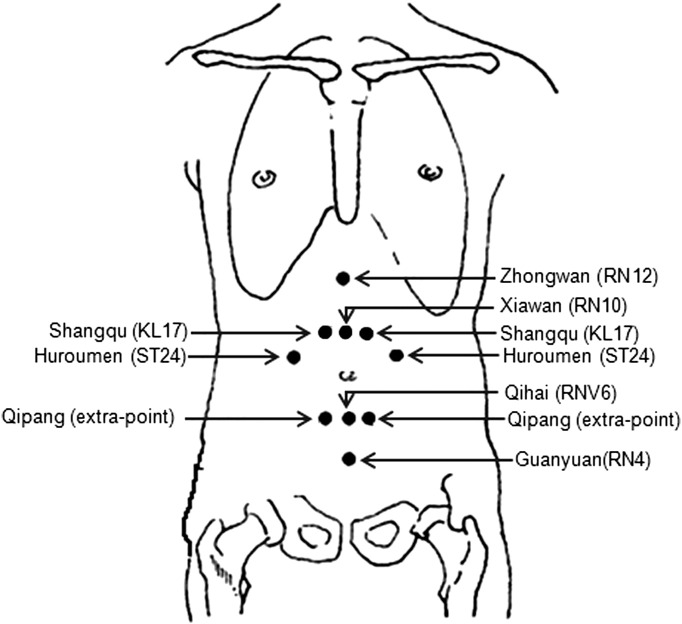
Abdominal acupuncture is based on the Chinese Traditional Medicine theory that CV 8 (umbilicus) plays a crucial role in propelling and regulating the flow of Qi (Zhiyun, 2001). Abdominal acupuncture uses only acupoints in the abdomen because these acupoints are close to important meridians and can easily communicate with five zang and six fu organs through channels like Conceptional Vessel, Governor Vessel, Thoroughfare Meridian and Belt Meridian. Based on a previous study from our group (X. Y. Wang et al., 2008), the acupoints applied in this study were: Zhongwan (RN12), Xiawan (RN10), Qihai (RN6), Guanyuan (RN4), Shangqu (KL17), Huaroumen (ST24), and Qipang (extra-point) (Fig. 1). This prescription could harmonize zang-fu five viscera, tonify qi, and replenish blood to relieve depression. An acupuncturist with > 3 years of experience performed all acupuncture treatments.
Fig. 1.
Locations of acupoints applied in this study.
Before acupuncture administration, each participant was asked to lie in a supine position and to wear a mask over her eyes. Then, the participant's abdomen was exposed and the skin was disinfected at the acupuncture points. Afterwards, an acupuncture specialist inserted fine needles (0.22 mm × 40 mm) through short plastic tubes or sheaths. The needles were inserted intramuscularly to a depth of 15–20 mm and were left in situ for 20 min. The patient's abdomen was covered with a basket underneath a sheet during the treatment period. Patients received abdominal acupuncture once a day for the first three days and subsequently once every three days for the remainder of the 8-week trial. We chose this paradigm so that the effect of acupuncture could be accumulated quickly, enhancing the confidence and compliance of patients at the beginning of the study.
For sham acupuncture, the acupoints were the same as in the real acupuncture group. Before sham abdominal acupuncture administration, each patient was asked to lie in a supine position and put a mask over her eyes. Then, the subject's abdomen was exposed and the acupoint areas were disinfected. Later, short plastic needle sheaths not containing any needles were tapped against the skin of the patient’s acupoints, but no needles were inserted into the skin through the sheaths. Then, as with the verum treatment, the patient’s abdomen was covered with a basket underneath a sheet during the ‘treatment’ period. The time and frequency of the sham abdominal acupuncture treatments were exactly the same as in the verum acupuncture group.
2.4. Clinical outcomes
MADRS and SDS were used to assess the clinical outcomes. Both were evaluated at time points before the first treatment and after the final treatment.
2.5. MRI data acquisition
The fMRI brain imaging acquisition was conducted on a 1.5 Tesla Siemens Avanto MR scanner. T2*-weighted functional images encompassing the whole brain were acquired with the gradient-echo EPI sequence (echo time: 30 ms, repetition time: 2000 ms, data matrix: 64 × 64, field of view: 240 mm, flip angle: 90°, slice thickness: 4 mm, interslice gap: 0.88 mm, 31 slices paralleled to the AC-PC line, and 180 time points acquired in 6 min). Two 6-minute resting-state fMRI scans were applied while the subjects were required to keep their eyes closed to relax the mind but not to fall asleep and not to think of anything in particular. High-resolution brain structural images were also acquired with a T1-weighted three-dimensional multi-echo magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (repetition time: 1900 ms, echo time: 2.3 ms, data matrix: 256 × 256, field of view: 256 mm × 256 mm, slice thickness 1 mm, flip angle: 15°, and 176 sagittal slices covering the whole brain).
2.6. Statistical analysis
2.6.1. Clinical data analysis
Statistical analysis was performed using SPSS 19.0 Software (SPSS Inc., Chicago, IL, USA). Two sample t-test was used to compare baseline measurements between groups, and analysis of covariance (ANCOVA) was applied to compare MADRS and SDS scores before and after treatments between groups. Age was included in the model to adjust for its effects.
2.6.2. Resting-state fMRI data analysis
Functional data were preprocessed using SPM8 (Statistical Parametric Mapping. Welcome Department of Cognitive Neurology, London, UK; implemented by MATLAB R2012b, Math Works, Inc., Natick, MA, USA). During the preprocessing, images were realigned, segmented, and co-registered to each subject's high-resolution T1 scan, which was used in normalizing to the standard Montreal Neurological Institute (MNI) template. Images were also smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel, filtered with a frequency window of 0.008–0.09 Hz. Subsequently, we segmented the brain into gray matter, white matter, and cerebrospinal fluid (CSF) for the removal of temporal confounding factors (white matter and CSF) (Whitfield-Gabrieli & Nieto-Castanon, 2012). Finally, data were then submitted to motion correction using the artifact detection toolbox (http://www.nitrc.org/projects/artifact_detect/). Time points in subjects' images were marked as outliers if the global signal exceeded three standard deviations from the mean or if scan-to-scan motion exceeded 0.5 mm deviation (Redcay et al., 2013).
Resting-state functional connectivity analysis was conducted using the CONN toolbox v15.g (Whitfield-Gabrieli & Nieto-Castanon, 2012) (http://www.nitrc.org/projects/conn). Bilateral amygdala seeds were generated from the AAL, (Rolls et al., 2002) using WFU-Pick Atlas software (Maldjian et al., 2003) (Fig. 2a). Functional connectivity measures were computed between a seed region of interest (ROI) and every other voxel in the brain. In the calculations, we first extracted the residual BOLD time course from a given seed and then estimated its first-level correlation maps by computing Pearson’s correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were transformed into Fisher’s ‘Z’-scores, which increases normality and allows for improved second-level General Linear Model analyses.
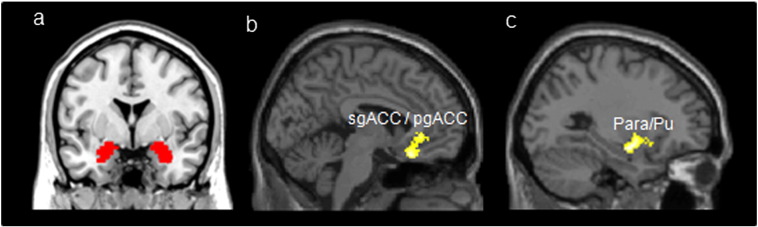
Fig. 2.
Amygdala seed locations and brain regions with significant changes (post minus pre) of rsFC with the amygdala modulated by the verum acupuncture plus fluoxetine compared with sham acupuncture plus fluoxetine treatment. Abbreviations: sgACC, subgenual anterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; Para, parahippocampus; Pu, Putamen.
Amygdala seed-to-voxel functional connectivity was estimated for each subject. Between-group analyses were performed to compare the rsFC change (post-treatment minus pre-treatment) between the two groups. In the calculations, two sample t-tests were adopted and a threshold of a voxel-wise p < 0.005 (uncorrected) and cluster-level p < 0.05 family-wise error correction was applied.
3. Results
Of all forty-six subjects (22 in the verum group, 24 in the sham group) who participated the study, 36 subjects (18 in the verum group, 18 in the sham group) were scanned at week 0 and week 8. Six subjects from the sham acupuncture group dropped out (2 due to discomfort from treatment (headache and diarrhea), 1 due to a scheduling conflict, and 3 due to having had only one scan), and four subjects from the verum acupuncture group dropped out (3 due to having had only one fMRI scan and 1 due to a scheduling conflict). Thus, all of the clinical and imaging analyses were based on these 36 subjects.
No significant differences were found between the two groups in age (t(34) = 0.22, p = 0.83) and MADRS (F(1,33) = 0.01, p = 0.97) and SDS (F(1,33) = 0.01, p = 0.94) scores at baseline (Table 1). ANCOVA analysis revealed significant differences in both MADRS and SDS scores between the verum acupuncture and sham acupuncture groups. The verum acupuncture group showed significantly greater clinical improvement (post minus pre-treatment, MADRS, F(1,33) = 10.86, p < 0.01; SDS, F (1,33) = 8.21, p < 0.01) compared to sham acupuncture (Table 1).
Table 1.
Demographic and Clinical Characteristics of participants in this study. Abbreviations: MADRS, Montgomery-Asberg depression rating scale; SDS, Self-rating depression scale.
| Characteristic | Conditions | Acupuncture mean (SD) | Sham mean (SD) |
|---|---|---|---|
| No. of participants who completed the study | 18 | 18 | |
| Age (years old) | 44.5 (10.47) | 43.78 (9.10) | |
| MADRS | Pre-treatment | 22.94 (7.36) | 22.83 (9.17) |
| Post-treatment | 5.44 (5.37) | 14.06 (4.39) | |
| SDS | Pre-treatment | 47.833 (6.46) | 47.44 (9.23) |
| Post-treatment | 26.83 (6.46) | 34.94 (5.40) |
3.1. Resting state functional connectivity results
Baseline analysis showed that there was no significant rsFC difference between the two groups at baseline. After 8 weeks of treatment, participants in the verum acupuncture group showed increased rsFC at the left amygdala-subgenual anterior cingulate cortex (sgACC)/pregeunal antieror cingulated cortex (pgACC) and right amygdala-paraphippocampus (Para)/putman (Pu) compared to baseline (Table 2). The sham acupuncture plus fluoxetine group showed decreased rsFC between the right amygdala-left Para/Pu after eight weeks of treatment (Table 2).
Table 2.
Regions showed significantly increased resting-state functional connectivity between the amygdala and other brain regions after acupuncture treatment and sham treatment, controlling for age as a covariate (voxel-wise, p < 0.005, uncorrected; cluster –wise, p < 0.05, FWE corrected). Abbreviations: AUC, acupuncture; sgACC, subgenual anterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; Para, parahippocampus; Pu, putamen.
| Group | Condition | Seed | Brain region | Cluster size mm3 |
Peak coordinate (MNI space) |
Peak Z-value | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| ACU plus fluoxetine | Post-treatment > pre-treatment | Left amygdala | Left sgACC/pgACC | 278 | − 4 | 24 | − 20 | 3.78 |
| Right amygdala | Left Para/Pu | 325 | − 28 | − 2 | − 12 | 3.86 | ||
| Sham plus fluoxetine | Post-treatment < pre-treatment | Right amygdala | Left Para/Pu | 359 | − 28 | − 2 | − 12 | 3.53 |
| ACU plus fluoxetine > Sham plus fluoxetine | Post-treatment > pre-treatment | Left amygdala | Left sgACC/pgACC | 310 | − 14 | 28 | − 22 | 3.64 |
| Right amygdala | Left Para/Pu | 352 | − 28 | − 2 | − 12 | 3.74 | ||
We also compared the increased rsFC (post-treatment minus pre-treatment) between the two groups. The results showed that the verum group showed significantly increased rsFC between the left amygdala and left sgACC/pgACC (Table 2, Fig. 2b) compared to the sham acupuncture group. The right amygdala showed significantly increased rsFC with the left Para/Pu (Table 2, Fig. 2c) in the verum acupuncture group compared to the sham acupuncture group.
To explore the association between rsFC and each of the corresponding clinical outcomes, we extracted the Fisher ‘Z’ value (a sphere of 3 mm radius around the peak voxel) of the left sgACC/pgACC and the Pu/Para, and then applied a multiple regression analysis controlling for the age effect across all participants. The results showed that the increase of rsFC between the left amygdala and sgACC/pgACC was significantly negatively associated with MADRS (p = 0.02, FDR corrected) and SDS (p = 0.01, FDR corrected) scores across all subjects.
4. Discussion
In this study, we investigated amygdala-related rsFC changes before and after eight weeks of acupuncture plus fluoxetine treatment as compared with sham acupuncture plus fluoxetine in female depressive disorder patients. Our results revealed a significant remission of depressive symptoms in the verum acupuncture plus fluoxetine group compared with the control group. In addition, we also found that verum acupuncture plus fluoxetine treatment can significantly increase the rsFC of the amygdala with brain regions associated with emotion and affect modulation. The rsFC changes in the amygdala were also significantly associated with a reduction in symptom severity as indicated by MADRS and SDS scores, implying that acupuncture plus fluoxetine treatment may achieve treatment effects by modulating the rsFC of the amygdala.
Consistent with previous pilot studies (Manber et al., 2010; J. I. Quah-Smith et al., 2005, Roschke et al., 2000; W. D. Wang et al., 2013a, Wang et al., 2013b, Wang et al., 2013c; X. Y. Wang et al., 2008), we observed that acupuncture stimulation plus fluoxetine could effectively reduce the symptoms of depression. Interestingly, a paired t-test comparing pre and post-treatment clinical outcomes also indicates that the symptoms in the sham acupuncture plus fluoxetine group significantly decreased after treatment (MADRS: p < 0.001; SDS: p < 0.001). We speculate that this may be attributed to the treatment effect of fluoxetine. The additional effect of verum acupuncture demonstrated that acupuncture can be combined with pharmacological treatment to achieve a greater therapeutic effect in MDD patients.
Antidepressants including fluoxetine have been widely used as a basic treatment for MDD (Gibbons et al., 2012, Kong et al., 2006). However, antidepressant treatments may produce undesirable side effects and a delay in the onset of therapeutic action (Arroll et al., 2005, Zhu et al., 2013). In this study, we found that combining acupuncture with fluoxetine can produce greater improvement in MDD patients, which is consistent with findings from previous studies investigating the combinative effects of acupuncture and other treatments (Duan et al., 2008; Y. Liu et al., 2008, Roschke et al., 2000, Zhang et al., 2009, Zhu et al., 2013).
We found that after 8 weeks of treatment, the verum acupuncture plus fluoxetine treatment induced increased rsFC between the left amygdala and sgACC/pgACC and between the right amygdala and left putamen/parahippocampus. The sham acupuncture plus fluoxetine treatment induced decreased rsFC between the right amygdala and left Pu/Para. A direct comparison between the two groups showed that verum acupuncture plus fluoxetine induced increased rsFC between the left amygdala and sgACC/pgACC and between the right amygdala and Pu/Para compared with the sham group.
The sgACC, located underneath the genu of the corpus callosum, and the pgACC, anterior to the genu of the corpus callosum, are both affective subdivisions of the anterior cingulate cortex (Felger et al., 2015, Phillips et al., 2008; D. A. Pizzagalli, 2010). Specifically, the sgACC is implicated in automatic behavioral control, and the pgACC is involved in automatic attentional control based in part on their functional interaction with limbic structures such as the amygdala (DeRubeis et al., 2008, Drevets et al., 2008, Etkin et al., 2006; D. A. Pizzagalli, 2010, Salvadore et al., 2010). Compared with healthy controls, MDD patients show elevated activity in the amygdala and ACC areas in response to negative stimuli (Felger et al., 2015, Salvadore et al., 2009), and they also show abnormal reduced functional connectivity between the amygdala and ACC (Anand et al., 2009, Felger et al., 2015, Lui et al., 2011, Matthews et al., 2008, Veer et al., 2010). In addition, the region also plays an important role in the self-regulation of pain such as placebo analgesia (Bingel et al., 2006, Eippert et al., 2009, Kong et al., 2006, Kong et al., 2013, Petrovic et al., 2002) and acupuncture treatment of chronic pain (X. Chen et al., 2014; X. Chen et al., 2014, Egorova et al., 2015, Li et al., 2016a, Li et al., 2016b).
Both the sgACC and pgACC are considered as biomarkers to antidepressant response (Felger et al., 2015, Phillips et al., 2015; D. Pizzagalli et al., 2001; D. A. Pizzagalli, 2011) or the target of deep brain stimulation treatments (Felger et al., 2015, Hamani et al., 2010, Salvadore et al., 2010) in MDD. For instance, previous studies found that antidepressant treatments can increase the connectivity of the amygdala-sgACC during the perception of sad faces (C. H. Chen et al., 2008) and threatening stimuli (Pezawas et al., 2005), and the connectivity of the pgACC-amygdala can be used to predict antidepressant response to ketamine in a working memory task context (Salvadore et al., 2010).
We also found increased rsFC between the right amygdala and a cluster of brain regions including the left parahippocampus and putamen in the verum acupuncture group. The parahippocampus is associated with memory encoding and retrieval, and it is also linked to emotional processing (Fu et al., 2007, Gosselin et al., 2006, Smith et al., 2004, Van den Stock et al., 2012). The connectivity of the parahippocampus with the amygdala has been proposed to mediate contextual processing and emotion, thus facilitating emotion understanding and expectations of the environment (Aminoff et al., 2013, LaBar and Cabeza, 2006). The altered rsFC between the amygdala and parahippocampus is a powerful discrimination between MDD and healthy controls (Zeng et al., 2012). Chen et al. (C. H. Chen et al., 2008) found that after eight weeks of antidepressant treatment with fluoxetine hydrochloride, the coupling of the amygdala with the parahippocampus was normalized to the levels of healthy controls.
The putamen is implicated in reward and motivation (Felger et al., 2015, Fu et al., 2007, Robbins and Everitt, 1996). Previous fMRI studies have found that individuals suffering from depression have lower activation in the putamen during the perception of happy faces (Critchley et al., 2000, Lawrence et al., 2004, Phan et al., 2002). A similar study (Hamilton & Gotlib, 2008) in adults with MDD using the bilateral amygdala as seeds found that patients showed greater amygdala-putamen connectivity compared with healthy controls during successful encoding of negative emotional memories. With antidepressant treatment, Chen et al. (C. H. Chen et al., 2008) observed increased coupling of the amygdala and putamen in depressed subjects, which is consistent with our results.
We also found that that acupuncture treatment produces different effects on the left and right amygdala rsFC, and the changes of left amygdala rsFC showed a negative association with depression symptoms. A recent fMRI study (C. H. Chen et al., 2008) showed that antidepressant treatments enhanced the connection between the left amygdala and right lateral prefrontal cortex in response to unmasked negative faces in depressed patients. Mccabe et al. (McCabe & Mishor, 2011) found reduced rsFC between the right amygdala and frontal cortex in healthy subjects when they were administrated with the antidepressants citalopram and reboxetine for seven days. We did not find amygdala and prefrontal cortex connectivity changes in our study. We speculate this is mainly due to using a different treatment modality, method, and patient population.
There are several limitations in our study. First, we used the total anatomical structure of the amygdala as seeds, while amygdala subregional structures, including the centromedial (stress), superficial (socio-affective), and basolateral (learning and memory) amygdala are involved in different emotion processing functions (Alarcon et al., 2015, Etkin et al., 2004). Further focus on the rsFC of amygdala subregions is still needed. Second, we only recruited female depressed patients to increase the homogeneity of the study, and thus, our external validity may only be applied to interpret the effects of acupuncture on female patients. More study is needed to validate these findings in male patients. Finally, we only focus on the added effect of acupuncture treatment to fixed doses of fluoxetine in this study; there is no placebo fluoxetine, which prevents us from exploring the effect and mechanism of fluoxetine as well as the interaction between fluoxetine and acupuncture.
In conclusion, we found that acupuncture plus fluoxetine treatment significantly modulates the rsFC between the amygdala and sgACC/pgACC in patients with MDD compared to sham acupuncture plus fluoxetine. This rsFC change is associated with clinical improvement. We believe this modulation effect may at least partly represent the underlying mechanism of acupuncture treatment of MDD when it is combined with fluoxetine.
Conflict of interest statement
All authors declare no conflict interests.
Acknowledgment
The study was funded by a 2013 project of the South Korean Health Ministry (CIMI-13-01-32). Jian Kong is supported by R01AT006364, R01AT008563, R21AT008707, and P01 AT006663 from NIH/NCCIH.
Contributor Information
Bo Liu, Email: lbgdhtcm@163.com.
Jian Kong, Email: kongj@nmr.mgh.harvard.edu.
References
- Adolphs R., Tranel D., Damasio H., Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Alarcon G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. NeuroImage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M., Sarvaiya N., Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014;35(3):320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171(3):189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroll B., Macgillivray S., Ogston S., Reid I., Sullivan F., Williams B. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann. Fam. Med. 2005;3(5):449–456. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Qin W., Tian J., Dai J., Yang W. Detection of dynamic brain networks modulated by acupuncture using a graph theory model. Prog. Nat. Sci. 2009;19(7):827–835. [Google Scholar]
- Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker R.H., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bingel U., Lorenz J., Schoell E., Weiller C., Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1–2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Suckling J., Ooi C., Fu C.H., Williams S.C., Walsh N.D. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33(8):1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- Chen X., Spaeth R.B., Freeman S.G., Scarborough D.M., Hashmi J.A., Wey H.Y. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol. Pain. 2015;11:67. doi: 10.1186/s12990-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Spaeth R.B., Retzepi K., Ott D., Kong J. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci. Rep. 2014;4:6482. doi: 10.1038/srep06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Tang Q.S. Abdominal acupuncture in treating liver-qi stagnation and spleen deficiency in the elderly with post-stroke depression: A randomized and controlled observation. J. Clin. Rehabil. Tissue Eng. Res. 2007:39. [Google Scholar]
- Critchley H., Daly E., Phillips M., Brammer M., Bullmore E., Williams S. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum. Brain Mapp. 2000;9(2):93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Pizzagalli D., Nitschke J.B., Putnam K. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- DeRubeis R.J., Siegle G.J., Hollon S.D. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat. Rev. Neurosci. 2008;9(10):788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond R.P., Yeh C., Park K., Kettner N., Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D.M., Tu Y., Chen L.P. Assessment of effectiveness of electroacupuncture and fluoxetine for treatment of depression with physical symptoms. Zhongguo Zhen Jiu. 2008;28(3):167–170. [PubMed] [Google Scholar]
- Egorova N., Gollub R.L., Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. NeuroImage Clin. 2015;9:430–435. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Bingel U., Schoell E.D., Yacubian J., Klinger R., Lorenz J. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A., Klemenhagen K.C., Dudman J.T., Rogan M.T., Hen R., Kandel E.R. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(6):1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.H., Williams S.C., Brammer M.J., Suckling J., Kim J., Cleare A.J. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am. J. Psychiatry. 2007;164(4):599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Gibbons R.D., Hur K., Brown C.H., Davis J.M., Mann J.J. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch. Gen. Psychiatry. 2012;69(6):572–579. doi: 10.1001/archgenpsychiatry.2011.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N., Samson S., Adolphs R., Noulhiane M., Roy M., Hasboun D. Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain. 2006;129(Pt 10):2585–2592. doi: 10.1093/brain/awl240. [DOI] [PubMed] [Google Scholar]
- Hamani C., Mayberg H., Stone S., Laxton A., Haber S., Lozano A.M. The subcallosal cingulate gyrus in the context of major depression. Biol. Psychiatry. 2010;69(4):301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Gotlib I.H. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol. Psychiatry. 2008;63(12):1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G., Northoff G. Discovering imaging endophenotypes for major depression. Mol. Psychiatry. 2011;16(6):604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- Hui K.K., Marina O., Claunch J.D., Nixon E.E., Fang J., Liu J. Acupuncture mobilizes the brain's default mode and its anti-correlated network in healthy subjects. Brain Res. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.W., Egorova N., Yang X.Q., Zhang W.Y., Chen J., Yang X.Y. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.W., Xin S.C., Ou Y.M., Zhang W.Y., Liang Y.L., Chen J. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. J. Psychiatr. Res. 2016;76:111–120. doi: 10.1016/j.jpsychires.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Greicius M.D. 2009. Greater Than the Sum of Its Parts: A Review of Studies Combining Structural Connectivity and Resting-State Functional Connectivity (Vol. 213): Brain Structure and Function. [DOI] [PubMed] [Google Scholar]
- Johnson J., Weissman M.M., Klerman G.L. Service utilization and social morbidity associated with depressive symptoms in the community. Jama. 1992;267:1478–1483. [PubMed] [Google Scholar]
- Jorm A.F., Christensen H., Griffiths K.M., Rodgers B. Effectiveness of complementary and self-help treatments for depression. Med. J. Aust. 2002;176(Suppl):S84–S96. doi: 10.5694/j.1326-5377.2002.tb04508.x. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Soukup J., Davis R.B., Foster D.F., Wilkey S.A., Van Rompay M.I. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am. J. Psychiatry. 2001;158(2):289–294. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- Kong J., Gollub R.L., Rosman I.S., Webb J.M., Vangel M.G., Kirsch I. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J. Neurosci. 2006;26(2):381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Jensen K., Loiotile R., Cheetham A., Wey H.Y., Tan Y. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154(3):459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Cabeza R. Cognitive neuroscience of emotional memory. Nat. Rev. Neurosci. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lawrence N.S., Williams A.M., Surguladze S., Giampietro V., Brammer M.J., Andrew C. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol. Psychiatry. 2004;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lehtinen V., Joukamaa M. Epidemiology of depression: prevalence, risk factors and treatment situation. Acta. Psychiatr. Scand. Suppl. 1994;377:7–10. doi: 10.1111/j.1600-0447.1994.tb05794.x. [DOI] [PubMed] [Google Scholar]
- Li Z., Lan L., Zeng F., Makris N., Hwang J., Guo T. Cephalalgia; 2016. The Altered Right Frontoparietal Network Functional Connectivity in Migraine and the Modulation Effect of Treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu M., Lan L., Zeng F., Makris N., Liang Y. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci. Rep. 2016;6:20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Zhang Y., Zhou G., Yuan K., Qin W., Zhuo L. Partial correlation investigation on the default mode network involved in acupuncture: an fMRI study. Neurosci. Lett. 2009;462(3):183–187. doi: 10.1016/j.neulet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang Y.H., Jin M., Liu W.J. Study on clinical effect enhancement of acupuncture for depression with chronic pain treated with SSRI antidepressants. Zhongguo Zhen Jiu. 2008;33(8):689–691. [PubMed] [Google Scholar]
- Lu Q., Li H., Luo G., Wang Y., Tang H., Han L. Impaired prefrontal-amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci. Lett. 2012;523(2):125–130. doi: 10.1016/j.neulet.2012.06.058. [DOI] [PubMed] [Google Scholar]
- Lui S., Wu Q., Qiu L., Yang X., Kuang W., Chan R.C.K. Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry. 2011 doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- Lyons Z., van der Watt G., Shen Z., Janca A. Acupuncture and Chinese herbs as treatments for depression: an Australian pilot study. Complement. Ther. Clin. Pract. 2012;18(4):216–220. doi: 10.1016/j.ctcp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol. Psychiatry. 2015;20:311–319. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manber R., Schnyer R.N., Lyell D., Chambers A.S., Caughey A.B., Druzin M. Acupuncture for depression during pregnancy: a randomized controlled trial. Obstet. Gynecol. 2010;115(3):511–520. doi: 10.1097/AOG.0b013e3181cc0816. [DOI] [PubMed] [Google Scholar]
- Matthews S.C., Strigo I.A., Simmons A.N., Yang T.T., Paulus M.P. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J. Affect. Disord. 2008;111(1):13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- McCabe C., Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage. 2011;57(4):1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears D., Pollard H.B. Network science and the human brain: using graph theory to understand the brain and one of its hubs, the amygdala, in health and disease. J. Neurosci. Res. 2016 doi: 10.1002/jnr.23705. [DOI] [PubMed] [Google Scholar]
- Mwangi B., Ebmeier K.P., Matthews K., Steele J.D. Multi-centre diagnostic classification of individual structural neuroimaging scans from patients with major depressive disorder. Brain. 2012;135(Pt 5):1508–1521. doi: 10.1093/brain/aws084. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Kalso E., Petersson K.M., Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E.M., Verchinski B.A., Munoz K.E., Kolachana B.S. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., LeDoux J.E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Chase H.W., Sheline Y.I., Etkin A., Almeida J.R., Deckersbach T. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am. J. Psychiatry. 2015;172(2):124–138. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(9):829–833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D., Pascual-Marqui R.D., Nitschke J.B., Oakes T.R., Larson C.L., Abercrombie H.C. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am. J. Psychiatry. 2001;158(3):405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W., Tian J., Bai L., Pan X., Yang L., Chen P. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol. Pain. 2008;4:55. doi: 10.1186/1744-8069-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah-Smith I., Suo C., Williams M.A., Sachdev P.S. The antidepressant effect of laser acupuncture: a comparison of the resting Brain's default mode network in healthy and depressed subjects during functional magnetic resonance imaging. Med. Acupunct. 2013;25(2):124–133. doi: 10.1089/acu.2012.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah-Smith I., Wen W., Chen X., Williams M.A., Sachdev P.S. The brain effects of laser acupuncture in depressed individuals: an fMRI investigation. Med. Acupunct. 2012;24(3):161–171. [Google Scholar]
- Quah-Smith J.I., Tang W.M., Russell J. Laser acupuncture for mild to moderate depression in a primary care setting–a randomised controlled trial. Acupunct. Med. 2005;23(3):103–111. doi: 10.1136/aim.23.3.103. [DOI] [PubMed] [Google Scholar]
- Redcay E., Moran J.M., Mavros P.L., Tager-Flusberg H., Gabrieli J.D., Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front. Hum. Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Everitt B.J. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Joliot M., Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage. 2002;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- Roschke J., Wolf C., Muller M.J., Wagner P., Mann K., Grozinger M. The benefit from whole body acupuncture in major depression. J. Affect. Disord. 2000;57(1–3):73–81. doi: 10.1016/s0165-0327(99)00061-0. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M., Fava M. Depression, IV: STAR*D treatment trial for depression. Am. J. Psychiatry. 2003;160(2):237. doi: 10.1176/appi.ajp.160.2.237. [DOI] [PubMed] [Google Scholar]
- Sackeim H.A. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl. 16):10–17. [PubMed] [Google Scholar]
- Salvadore G., Cornwell B.R., Colon-Rosario V., Coppola R., Grillon C., Zarate C.A., Jr. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol. Psychiatry. 2009;65(4):289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G., Cornwell B.R., Sambataro F., Latov D., Colon-Rosario V., Carver F. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedentopf C.M., Koppelstaetter F., Haala I.A., Haid V., Rhomberg P., Ischebeck A., Buchberger W., Felber S., Schlager A., Golaszewski S.M. Laser acupuncture induced specific cerebral cortical and subcortical activations in humans. Lasers Med. Sci. 2005;20:68–73. doi: 10.1007/s10103-005-0340-3. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol. Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol. Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Silbersweig D. Default mode subnetworks, connectivity, depression and its treatment: toward brain-based biomarker development. Biol. Psychiatry. 2013;74(1):5–6. doi: 10.1016/j.biopsych.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Smith A.P., Henson R.N., Dolan R.J., Rugg M.D. fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage. 2004;22(2):868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Sun L., Cao Q., Long X., Sui M., Cao X., Zhu C. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naive boys with attention deficit hyperactivity disorder. Psychiatry Res. 2012;201(2):120–127. doi: 10.1016/j.pscychresns.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Ulett G.A., Han S., Han J.-S. Electroacupuncture: mechanisms and clinical application. Biol. Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Vandenbulcke M., Sinke C.B., de Gelder B. Affective scenes influence fear perception of individual body expressions. Hum. Brain Mapp. 2012;35(2):492–502. doi: 10.1002/hbm.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J., Ferrarini L., Milles J., Veltman D.J. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zuo X., Dai Z., Xia M., Zhao Z., Zhao X. Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol. Psychiatry. 2013;73(5):472–481. doi: 10.1016/j.biopsych.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Wang L., Dai Z., Peng H., Tan L., Ding Y., He Z. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum. Brain Mapp. 2013;35(4):1154–1166. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xia M., Li K., Zeng Y., Su Y., Dai W. The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum. Brain Mapp. 2015;36(2):768–778. doi: 10.1002/hbm.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.D., Lu X.Y., Ng S.M., Hong L., Zhao Y., Lin Y.N. Effects of electro-acupuncture on personality traits in depression: a randomized controlled study. Chin. J. Integr. Med. 2013;19(10):777–782. doi: 10.1007/s11655-013-1594-4. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Li X.Y., Deng A.J., Bo Z.Y. Comparative study on abdominal acupuncture and western medicine for treatment of menopause depressive disorder. Zhongguo zhen jiu = Chinese acupuncture & moxibustion. 2010;30(11):913–917. [PubMed] [Google Scholar]
- Wang X.Y., Yuan S.H., Yang H.Y., Sun Y.M., Cheng F.P., Zhang C.L. Abdominal acupuncture for insomnia in women: a randomized controlled clinical trial. Acupunct. Electrother. Res. 2008;33(1–2):33–41. doi: 10.3727/036012908803861203. [DOI] [PubMed] [Google Scholar]
- Wang Z., Liang P., Zhao Z., Han Y., Song H., Xu J. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer's disease. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wu J., Yeung A.S., Schnyer R., Wang Y., Mischoulon D. Acupuncture for depression: a review of clinical applications. Can. J. Psychiatr. 2012;57(7):397. doi: 10.1177/070674371205700702. [DOI] [PubMed] [Google Scholar]
- Yi Y., Xu F.-M., Xie P., Lv F.-j., Lin Y., Wu Y. Acupuncturing Taichong point for regulating the brain function of depression patients: restingstate fMRI study. Chin. J. Tradit. Chin. Med. Pharm. 2012;27(2):369–373. [Google Scholar]
- Zeng L.-L., Shen H., Liu L., Wang L., Li B., Fang P. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135(5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhang W.J., Yang X.B., Zhong B.L. Combination of acupuncture and fluoxetine for depression: a randomized, double-blind, sham-controlled trial. J. Altern. Complement. Med. 2009;15(8):837–844. doi: 10.1089/acm.2008.0607. [DOI] [PubMed] [Google Scholar]
- Zhao L., Liu J., Zhang F., Dong X., Peng Y., Qin W. Effects of long-term acupuncture treatment on resting-state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Wen J., Zhang L., Zhong J., Liang X., Ke W. Altered brain functional connectivity in hemodialysis patients with end-stage renal disease: a resting-state functional MR imaging study. Metab. Brain Dis. 2014;29(3):777–786. doi: 10.1007/s11011-014-9568-6. [DOI] [PubMed] [Google Scholar]
- Zhiyun B. On abdominal acupuncture therapy. Chin. Acupunct. Moxibustion. 2001;8:012. [Google Scholar]
- Zhong C., Bai L., Dai R., Xue T., Wang H., Feng Y. Modulatory effects of acupuncture on resting-state networks: a functional MRI study combining independent component analysis and multivariate Granger causality analysis. J. Magn. Reson. Imaging. 2012;35(3):572–581. doi: 10.1002/jmri.22887. [DOI] [PubMed] [Google Scholar]
- Zhu D., Gao Y., Chang J., Kong J. Placebo acupuncture devices: considerations for acupuncture research. Evid. Based Complement. Alternat. Med. 2013;2013:628907. doi: 10.1155/2013/628907. [DOI] [PMC free article] [PubMed] [Google Scholar]