Abstract
Background. There are several mechanisms by which global climate change may impact malaria transmission. We sought to assess how the increased frequency of extreme precipitation events associated with global climate change will influence malaria transmission in highland areas of East Africa.
Methods. We used a differences-in-differences, quasi-experimental design to examine spatial variability in the incidence rate of laboratory-confirmed malaria cases and malaria-related hospitalizations between villages (1) at high versus low elevations, (2) with versus without rivers, and (3) upstream versus downstream before and after severe flooding that occurred in Kasese District, Western Region, Uganda, in May 2013.
Results. During the study period, 7596 diagnostic tests were performed, and 1285 patients were admitted with a diagnosis of malaria. We observed that extreme flooding resulted in an increase of approximately 30% in the risk of an individual having a positive result of a malaria diagnostic test in the postflood period in villages bordering a flood-affected river, compared with villages farther from a river, with a larger relative impact on upstream versus downstream villages (adjusted rate ratio, 1.91 vs 1.33).
Conclusions. Extreme precipitation such as the flooding described here may pose significant challenges to malaria control programs and will demand timely responses to mitigate deleterious impacts on human health.
Keywords: Malaria, climate change, epidemiology, Uganda, disasters, flooding
(See the editorial commentary by Caminade, Mclntyre, and Jones on pages 1300–1.)
The effect of global climate change on the incidence of vector-borne diseases, including malaria, is of both great interest and debate [1, 2]. Many analyses have suggested that rising global temperatures may result in a resurgence of malaria [3–5]. One region that has received particular attention is the East African highlands. A number of studies have attributed a resurgence of malaria in this region, especially at higher altitudes, to warming surface temperatures and changing rainfall patterns [5–8]. Others, however, are more skeptical of long-term impacts on climate [9, 10].
In addition to rising global temperatures, the manifold effects of climate change also entail an increased frequency of weather extremes, such as droughts and floods [11, 12]. Approximately 50% of global disasters between 2003 and 2012 were attributable to extreme precipitation and flooding [13]. Populations in developing countries are thought to be particularly at risk of adverse health consequences from floods, given unregulated land use in flood-prone areas, limited public-health infrastructure, and inadequate emergency response capability [14].
Over the past 100 years, East Africa has become wetter on average by around 10%–20% [15]. The frequency of major flood events in this region has also been increasing [16]. Uganda is not only expected to see a relatively large increase in the mean annual temperature, but the country may also experience increases in extreme precipitation [8, 15, 17]. Like warming temperatures, weather extremes—particularly heavy precipitation and flooding—have been associated with increasing malaria transmission [18]. Changing precipitation patterns can have both short-term and long-term effects on vector habitats and disease transmission [4, 19]. However, there is little empirical evidence outside of field reports and mathematical models about the relationship between individual flood events and malaria transmission.
On 1 May and again on 5 May 2013, heavy rains exacerbated existing glacial snowmelt [20, 21] and submerged 9 subcounties of Kasese District, Western Region, Uganda. Using this event as a natural experiment, we sought to estimate the temporal and spatial changes in malaria epidemiology associated with flooding in this rural, malaria-endemic area of western Uganda. The overall objective of the study was to estimate the excess burden of malaria attributable to flooding and, more specifically, to identify the geographic areas most vulnerable to postflood epidemic malaria, using the method of differences in differences.
METHODS
Study Site
The Bugoye Level III Health Center (BHC), located in Kasese District (0° 18′ N, 30° 5′ E), functions as the primary referral center for the Bugoye subcounty, serving a rural population of approximately 50 000 residents. Clinical officers, nurses, midwives, and laboratory technicians from the Ugandan Ministry of Health staff the health center, and care is available at no cost to patients. Malaria rapid diagnostic tests (mRDTs) were first introduced at the BHC in 2011 [22].
The climate in Bugoye permits year-round malaria transmission. There are semiannual transmission peaks that occur December–February and May–July, following the end of the traditional rainy seasons [23]. The geography of the Bugoye subcounty is also highly varied. Narrow river valleys and steep hillsides with elevations up to 2000 m define the westernmost villages of the subcounty along the boundary of the Rwenzori National Park. In contrast, the villages located to the east and southeast are composed of relatively level terrain at elevations nearly 1000 m lower than those to the west. Three large rivers—the Mubuku to the north, the Nyabyagi to the south, and the Sabo, which bisects the middle of the subcounty—flow down the hillsides from west to east.
The flooding of May 2013, considered the worst since April 1966, swept away houses, cattle, and infrastructure, including multiple bridges, the municipal wastewater treatment facility, and the district hospital. More than 1000 houses were destroyed, and nearly 10 000 residents were displaced [24]. Heavy rains caused overflowing of the Mubuku and Sabo rivers in the Bugoye subcounty, one of 9 affected subcounties in the region.
Study Design
We used a quasi-experimental design and differences-in-differences regression analysis to examine malaria diagnostic testing results and malaria-related hospitalizations before and after severe flooding in Kasese District. Results of malaria diagnostic tests, defined as either microscopy or mRDT, were obtained from health center laboratory registries. Information on patients admitted with a diagnosis of malaria was collected from inpatient medical records. For each record, we abstracted the following information: age, sex, village of residence, and diagnostic test result.
To estimate associations between geographic factors and malaria risk, we completed village-level geographic information system (GIS) mapping of the subcounty and surrounding environs, composing an area of approximately 55 km2. These data were entered into ArcGIS (Redlands, California) to create a reference map, from which we calculated the mean elevation and area of each village. We divided the data into quartiles based on mean elevation (Figure 1) and defined villages by the presence or absence of a flood-affected river within or along the village boundaries. In a secondary analysis based on observed geographical trends, we subdivided the villages with flood-affected rivers into 2 categories: “upstream” (defined as <6 km from the river source) and “downstream” (>6 km).
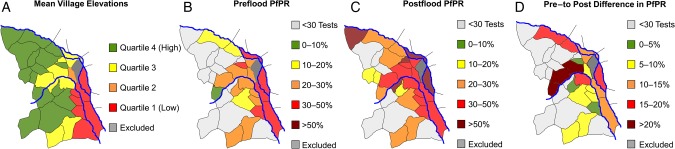
Figure 1.
A, Mean village elevations divided in quartiles, highlighting the generally higher terrain to the west of the subcounty and the lower terrain to the southeast between the Mubuku and Sabo rivers. B, Plasmodium falciparum test positivity rate (PfPR) in each village before the flood, with the highest rates in villages at lower elevation and along the major rivers. C, PfPR in each village after the flood, with relatively high positivity rates in the majority of villages. D, Absolute difference in the PfPR from the preflood to postflood periods. The PfPR for villages with <30 tests performed in the respective period is not shown.
Of the 32 villages in the subcounty, we included 31 villages in the analyses. We excluded 1 village, where international relief organizations established temporary camps with healthcare services for individuals displaced by flooding. We included 2 villages outside of the Bugoye subcounty but for which BHC is the closest medical center. Village populations were taken from the 2014 national census, from which we estimated a 3% annual population growth rate [25]. Villages that are traditionally subdivided for administrative purposes, such as Muramba 1 and Muramba 2, were considered 1 village for the analysis.
Statistical Analysis
To assess the impact of flooding on malaria transmission, we selected 2 primary outcomes of interest: (1) the P. falciparum test positivity rate (PfPR), defined as the percentage of malaria diagnostic tests positive for P. falciparum malaria per 100 tests conducted [26], and (2) the incidence rate of severe malaria, as measured by the number of malaria-related inpatient admissions per village per month, using 2014 census data to provide village-level population offsets. Our primary explanatory variable of interest was calendar time, which we divided into a 12-month “preflood” period (May 2012 to April 2013) and a 12-month “postflood” period (June 2013 to May 2014).
We first graphically depicted trends in diagnostic test results and inpatient malaria admissions rates over calendar time to examine patterns of malaria outcomes in relation to the flooding. We then compared malaria incidence before and after the flood by fitting generalized linear models and estimated the incidence rate ratio (IRR) of hospitalization before and after the flood, using generalized negative binomial regression models. We accounted for clustering within villages, using a clustered sandwich estimator. We added potentially confounding covariates in multivariable models including age, sex, the presence of an affected river in the village, mean village elevation, and rainy versus dry season. We included in multivariable regression models all variables that were significant in univariable models with a prespecified P value of <.25 [27]. A resulting P value of <.05 in final models was considered statistically significant.
We then used the method of difference in differences [28] to examine the preflood and postflood differences in malaria incidence according to the presence or absence of a flood-affected river within or along the village boundaries, the proximity to the river source (upstream vs downstream), and the mean village elevation. Differences-in-differences models have been increasingly used in public health to study the effects of external events or policies at different times by treating the event as a quasi-experiment [29, 30]. Whereas conventional observational studies are valid only if there are no underlying secular trends in the outcomes of interest, the difference-in-differences study design addresses this problem by using a comparison group that is experiencing the same trends but is not exposed to the event or policy. This method allows the investigator to subtract out background changes.
Finally, we calculated the topographic convergence index (TCI) [31], which estimates drainage patterns based on a mathematical measurement of upslope drainage and the slope of the terrain for our geographic area of interest. High values indicate areas with large upslope drainage area and relatively low slope, leading to higher potential for water to slow or stagnate. As the slope increases or the upslope drainage area decreases, the potential for water to pool in these regions drops, indicated by a drop in the TCI. All data were analyzed with Stata 12.1 (College Station, TX).
Ethics Statement
Ethical approval of the study was provided by the institutional review boards of Partners Healthcare and the Mbarara University of Science and Technology. Informed consent was not required by the ethical review committees because of the programmatic nature of original data collection and retrospective review of data.
RESULTS
Diagnostic Test Positivity Rate
A total of 10 134 individuals underwent diagnostic testing for malaria by mRDT and/or microscopy during the study period. Village of residence information was not available for 630 test results (6.2%). Missing data were more common in the postflood than the preflood period (6.7% vs 5.2%; P = .004). We excluded data from 1858 individuals (18.3%) who presented for testing from villages outside of the defined study area.
Complete clinical and demographic information was available for 7596 individuals who presented from villages in the defined study area during the observation period (Table 1). The median age of those undergoing testing was 13 years, with approximately 2 in 3 being female. The majority of those who had a malaria diagnostic test performed (6757 [89.0%]) presented from villages containing a flood-affected river.
Table 1.
Characteristics of Patients Undergoing Diagnostic Testing for Malaria During Each Study Period
| Variable | Preflood Period | Postflood Period | P Value |
|---|---|---|---|
| Patients tested | 2311 (30.4) | 5285 (69.6) | … |
| Age, y | |||
| Median (IQR) | 14 (2.5–25) | 13 (4–27) | .068 |
| >15 | 1098 (47.5) | 2443 (46.3) | |
| 5–15 | 453 (19.6) | 1403 (26.6) | <.001 |
| <5 | 760 (32.9) | 1436 (27.2) | |
| Male sex | 879 (38.1) | 1968 (37.3) | .66 |
| Rainy seasona | 1148 (49.7) | 2696 (51.0) | .28 |
| Village of residence | |||
| Contains flood-affected river | 2060 (89.1) | 4697 (88.9) | .75 |
| Elevation quartile | |||
| 4 (highest) | 359 (14.9) | 792 (15.0) | |
| 3 | 780 (33.8) | 1775 (33.6) | |
| 2 | 464 (20.1) | 1004 (19.0) | .64 |
| 1 (lowest) | 722 (31.2) | 1713 (32.4) | |
| Diagnostic testing | |||
| RDT | |||
| Performed | 1850 (80.1) | 4881 (92.4) | <.001 |
| Positive result | 421 (22.8) | 1894 (38.8) | <.001 |
| Microscopy | |||
| Performed | 475 (19.9) | 586 (7.6) | <.001 |
| Positive result | 179 (37.8) | 313 (53.4) | <.001 |
Data are no. (%) of patients, unless otherwise indicated.
Abbreviations: IQR, interquartile range; RDT, rapid diagnostic test.
a Defined as March–May and September–November.
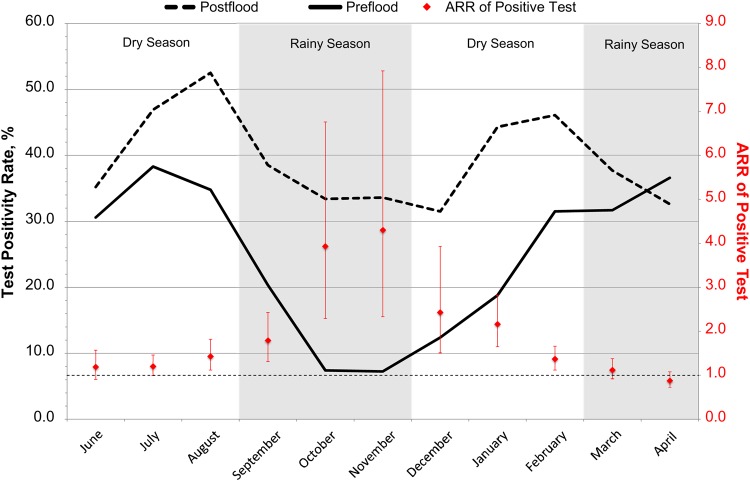
More than twice as many patients underwent malaria testing in the postflood period as compared to the preflood period (5285 vs 2311). The test positivity rate increased from 25.8% to 39.4% from the preflood to the postflood period (unadjusted relative risk [RR], 1.53; 95% confidence interval [CI], 1.42–1.65; P < .001; Supplementary Table 1). This relationship remained statistically significant after adjustment for confounding variables, such that 47% more tests were positive in the postflood period than the preflood period (adjusted RR [ARR], 1.47; 95% CI, 1.36–1.58; P < .001). When analyzed by month, the increased percentage of tests with positive results was most pronounced in November, typically a low transmission period, when the percentage of tests with positive results increased from approximately 7.3% to 33.6% from the preflood to the postflood period (ARR, 4.30; 95% CI, 2.33–7.92; P < .001; Figure 2).
Figure 2.
Change in test positivity by month before and after flooding. Abbreviation: ARR, adjusted risk ratio.
We identified significant interaction effects between geographic characteristics and malaria test positivity (Table 2), which we subsequently explored in the differences-in-difference analysis.
Table 2.
Interactions Between Village Characteristics and Study Periods and the Risk of a Positive Malaria Test Result
| Characteristic | Preflood Period |
Postflood Period |
Interaction | ||
|---|---|---|---|---|---|
| RR | P Value | RR | P Value | P Value | |
| Contains river | 1.01 | .92 | 1.50 | <.001 | .003 |
| Location from river | |||||
| Downstream | Reference | Reference | <.001 | ||
| Upstream | 0.86 | <.001 | 1.02 | .41 | |
| Elevation quartile | |||||
| 1 (lowest) | Reference | Reference | |||
| 2 | 0.76 | .005 | 0.70 | <.001 | .44 |
| 3 | 0.73 | <.001 | 0.96 | .24 | .004 |
| 4 (highest) | 0.60 | <.001 | 0.59 | <.001 | .90 |
Abbreviation: RR, relative risk.
Presence of a Flood-Affected River
In the preflood period, the test positivity rate was 25.8% in villages that bordered a flood-affected river and 25.5% in those that did not. In the postflood period, test positivity rates were statistically similar in those villages farther from a river (PfPR, 27.0%; P = .66) but were significantly higher in villages bordering the flood-affected rivers (PfPR, 40.9%; P < .001). Using a regression model stratified by the presence or absence of a river and adjusted for confounding variables, we see that there was little relative change from the preflood to post flood period in villages farther from a river (ARR, 1.03; 95% CI, .81–1.33; P = .78) but a significantly higher risk of a positive test in those villages bordering a flood-affected river (ARR, 1.53; 95% CI, 1.41–1.66 P < .001). Overall, there was an increase of 30% in the risk of an individual having a positive malaria diagnostic test result in the postflood period in villages bordering a flood-affected river, compared with villages farther from a river (ARR, 1.30; 95% 1.16–1.46; P < .001).
Upstream Versus Downstream
Among those villages bordering a flood-affected river, in the preflood period, the test positivity rate was 29.1% in the downstream villages and 21.3% in the upstream villages. In the postflood period, however, the test positivity rate was 40.4% (P < .001) in the downstream villages and 41.6% (P < .001) in the upstream villages. Again, using a regression model stratified by the relative location of the village along the river and adjusted for confounding variables, we found that there was larger relative change from the preflood to the postflood period in upstream villages (ARR, 1.91; 95% CI, 1.67–2.20; P < .001), compared with downstream villages (ARR, 1.33; 95% CI, 1.20–1.46; P < .001). In the postflood period, there was no difference in the risk of an individual having a positive malaria diagnostic test result in the upstream villages, compared with the downstream villages (ARR, 0.98; 95% 0.95–1.01; P = .24), whereas in the preflood period, upstream villages were relatively protected.
Elevation
The effect of elevation was largely consistent between the preflood and postflood periods, with higher elevations experiencing a lower risk of malaria transmission during both periods (Table 2).
Individual Villages
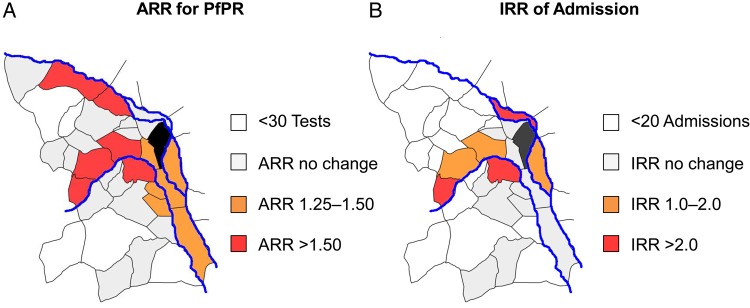
In addition to the overall increase in the test positivity rate from the preflood to the postflood period at the subcounty level, significant increases were identified in 8 individual villages (Figure 3A). A flood-affected river was present in all 8 villages with significant changes in the relative malaria risk. The greatest magnitude of effect was observed in the upstream villages. When the results of changes in the risk of a positive test result at the village level were overlaid on the convergence index (Figure 4), we see that the villages with the largest relative risk for an increase in the test positivity rate were upstream villages, which generally have well-organized, branching drainage networks. In contrast, the downstream villages, which were less impacted in the postflood period, generally had disorganized drainage networks consistent with the more level terrain.
Figure 3.
Map demonstrating the relationships between river distribution and changes in malaria risk in the preflood versus postflood periods. A dose-response effect of flood and distance to major rivers can be seen, whereby the regions closest to rivers and at the highest altitudes had the greatest relative increase in positive malaria test results and malaria-related admissions following the flood. Villages without direct access to rivers and at lower elevations were generally less affected. Abbreviations: ARR, adjusted risk ratio; PfPR, Plasmodium falciparum test positivity rate.
Figure 4.
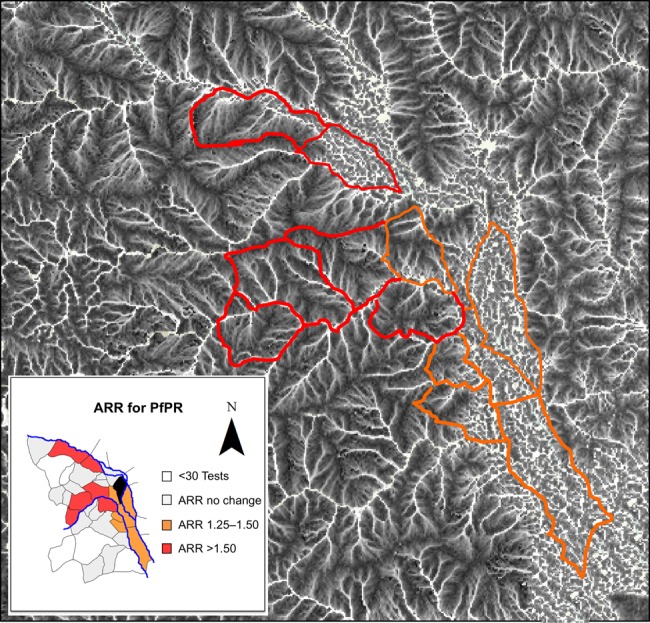
Overlay of the adjusted relative risk of a positive malaria test result on the topographic convergence index (TCI). Areas in white represent wet areas, where precipitation is expected to converge. The image demonstrates the well-organized, branching drainage networks seen in the westernmost or upstream villages, compared with the flat lowlands in the east, where runoff can be expected to accumulate. Abbreviations: ARR, adjusted risk ratio; IRR, incident risk ratio; PfPR, Plasmodium falciparum test positivity rate.
Malaria Admissions
Over the study period, 1744 patients were admitted to the inpatient ward with a diagnosis of malaria. Only 25 patients (1.4%) did not have village of residence information included in the clinical record. We excluded 229 individuals (13.1%) who presented for testing from villages outside of the defined study area and 200 patients (11.5%) who presented from the village where the temporary camps were established.
Relevant demographic and clinical data was available for >98% of included individuals. The only significant difference in the demographic characteristics of admitted patients was a greater proportion of patients aged 5–15 years old in the postflood period, with a lower proportion of those <5 years of age (Supplementary Table 3).
The number of patients admitted with a diagnosis of malaria increased by >50% in the postflood period (781 vs 504; P < .001). Additionally, malaria-related admissions as a proportion of all medical admissions increased significantly (54.1% vs 45.4%; P < .001). After adjustment for the presence of a river and mean village elevation, the incidence rate ratio (AIRR) of admissions for malaria remained significantly higher in the postflood periods, compared with the preflood period (AIRR, 1.40; 95% CI, .1.16–1.69; P < .001; Supplementary Table 2). Similar to the trends seen with PfPR, we found that all 5 villages that experienced increasing incidence of malaria admissions after the flood were in close proximity to a flood-affected river (Figure 3B). We found trends in the interaction effects between flooding, the presence of a river, the relative location along the river (upstream vs downstream), or village elevation on the rates of malaria admission, but these associations did not reach the threshold of statistical significance.
DISCUSSION
Our results demonstrate a significant and sustained increase in malaria transmission following catastrophic flooding in a rural, highland area of western Uganda. We observed an increase of approximately 30% in the risk of an individual having a positive malaria diagnostic test in the postflood period in villages bordering a flood-affected river compared with villages farther from a river. Among villages bordering a flood-affected river, we saw a larger relative impact on upstream versus downstream villages (ARR, 1.91 vs 1.33). We also observed a 40% increase in the incidence rate of malaria admission in the postflood period, using the conventional preflood versus postflood analysis, but did not observe similar changes using the differences-in-differences model, a finding that may reflect the smaller absolute number of admissions from affected areas. Overall, these results suggest that the increasingly frequent extreme precipitation events associated with global climate change have great potential to adversely affect malaria-related health in highland areas of East Africa.
Our study identified a number of geographical factors of postflood malaria that may inform emergency response and malaria control programs. First, the observed impacts of the flood were most pronounced in villages along flood-affected rivers. This finding is consistent with previous work showing that most productive breeding sites are usually located close to rivers and streams [32, 33] and that households in close proximity to breeding sites generally exhibit higher adult mosquito densities [34].
Second, we found the greatest relative increase in malaria test positivity in upstream areas. When we overlaid the postflood malaria risk map on TCI results (Figure 4), we found that the upstream villages were generally those with organized, branching drainage systems. Under normal conditions, these areas are well drained, and accessible surface water is largely limited to fast-moving streams that are unsuitable breeding habitats [35, 36]. However, during periods of extreme, prolonged precipitation, well-drained areas may become saturated to the extent that stagnant pools are formed, creating ideal habitats for the Anopheles vector [32, 35]. Despite the greater relative impact on upstream villages, the absolute effect of the flood was most pronounced in lower downstream villages. In such areas, stagnant pools are a permanent feature of the terrain, which favors stable breeding habitats and malaria transmission even in the absence of flooding.
Third, we observed a 3-month lag between the flooding and the peak of the postflood malaria epidemic. This delay may be the result of heavy rainfall flushing out existing breeding sites. Lyndsay et al hypothesized that such a flushing effect could explain the lower postflood malaria prevalence in northeastern Tanzania seen after the heavy precipitation of the 1997–1998 El Nino southern oscillation [37]. However, as the floodwaters recede, the vectors can reestablish productive habitats in new areas typically with a delay of 6–8 weeks, although this may vary by geographic and climactic conditions [38]. In Mozambique, for example, malaria incidence peaked approximately 1 month after flooding, while in northeastern Kenya and southwest Uganda, peak incidence was seen 2–3 months after flooding, which is consistent with our findings [39–41]. In our study, the impact of flooding on malaria transmission was greatest during what is typically the low transmission season, with a >4-fold increased risk of a positive malaria test in the postflood period, compared with the preflood period (Figure 2). We hypothesize that this “unseasonal epidemic” was due to surface waters from the flood that accumulated and persisted, allowing mosquito larvae to complete the full life cycle at times and in areas not normally hospitable to breeding [42].
Our study has a number of important policy and programmatic implications. First, the initial delay between the flood and the postflood epidemic may provide an important opportunity for targeted interventions to mitigate the postflood epidemic [43]. Additionally, our results show that control efforts must be sustained, as the effects of the flood on malaria transmission may continue for up to 1 year after the initial event. Finally, our study demonstrates the importance of understanding local microenvironments. Most of the villages we studied averaged <2 km2 in size, yet we observed stark contrasts in both the preflood and postflood epidemiology of malaria, even between neighboring villages.
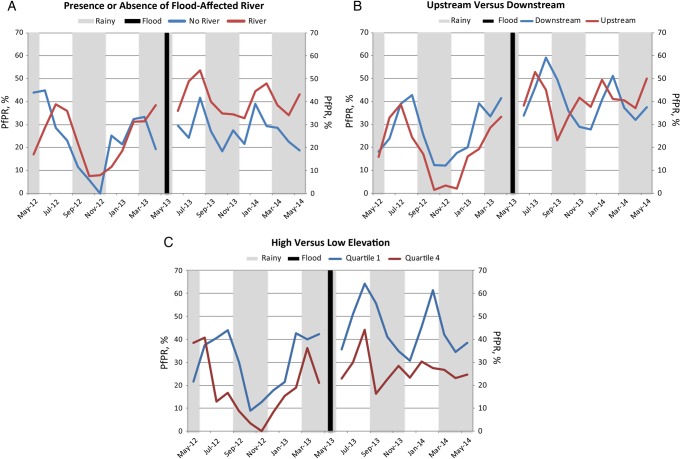
Our study has a number of methodological limitations. While more robust than conventional preevent versus postevent analyses, the difference-in-differences study design relies on the assumption that preexisting trends in malaria transmission would have been the same in the postflood period in the absence of severe flooding. Differential trends in the exposed and unexposed villages in the period leading up to the flooding would have biased our results. We assessed these trends prior to the “shock” of the flood by examining the parallel trends and presenting graphical displays of the raw data that clearly demonstrate this assumption was met (Figure 5).
Figure 5.
Monthly trends in diagnostic test positivity rate in the preflood and postflood periods between villages with and those without a flood-affected river (A), villages upstream and those downstream from the river source (B), and at high (quartile 4) and low (quartile 1) elevations (C). Abbreviation: PfPR, Plasmodium falciparum test positivity rate.
Second, because we abstracted information from routinely collected clinical records, our study is subject to error in programmatic medical record data collection. In general, missing clinical or demographic data was rare, and we did not observe a differential rate of missing data by geographic location, although there was a small increase in the postflood period as compared to the preflood period (6.7% vs 5.2%; P = .004).
Finally, it is not possible to attribute this specific event to climate change or to predict whether similar events will occur in this or other flood-prone malaria-endemic areas. However, changing precipitation patterns over the last century and increasing frequency of extreme flooding events both in East Africa and other regions mandate increased attention to relationships between such events and human health [5, 8, 11, 16].
In summary, we have observed increased malaria transmission and morbidity following a major flood event in a highland area of western Uganda. These findings highlight that extreme weather conditions during periods of increased global weather emergencies have great potential to adversely affect human health in malaria-endemic and epidemic-prone areas. Events such as the flooding described here may pose significant challenges to malaria control and elimination programs, and will demand timely and sustained responses to prevent and mitigate deleterious impacts on human health.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Drs Alexander Tsai and Atheendar Venkataramani, along with John Lovette, for their editorial input on manuscript drafts; the Harvard Global Health Initiative and Thrasher Research Fund, for support to R. M. B.; and the National Institutes of Health, for support via grant K23MH099916 to M. J. S.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Patz JA, Epstein PR, Burke TA, Balbus JM. Global climate change and emerging infectious diseases. JAMA 1996; 275:217–23. [PubMed] [Google Scholar]
- 2.McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet 2006; 367:859–69. [DOI] [PubMed] [Google Scholar]
- 3.Tanser FC, Sharp B, le Sueur D. Potential effect of climate change on malaria transmission in Africa. Lancet 2003; 362:1792–8. [DOI] [PubMed] [Google Scholar]
- 4.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ 2000; 78:1136–47. [PMC free article] [PubMed] [Google Scholar]
- 5.Caminade C, Kovats S, Rocklov J et al. . Impact of climate change on global malaria distribution. Proc Natl Acad Sci U S A 2014; 111:3286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Ruiz Carrascal D, Pascual M. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 2014; 343:1154–8. [DOI] [PubMed] [Google Scholar]
- 7.Zhou G, Minakawa N, Githeko AK, Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci U S A 2004; 101:2375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leedale J, Tompkins AM, Caminade C, Jones AE, Nikulin G, Morse AP. Projecting malaria hazard from climate change in eastern Africa using large ensembles to estimate uncertainty. Geospat Health 2016; 11:393. [DOI] [PubMed] [Google Scholar]
- 9.Hay SI, Cox J, Rogers DJ et al. . Climate change and the resurgence of malaria in the East African highlands. Nature 2002; 415:905–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW, Hay SI. Climate change and the global malaria recession. Nature 2010; 465:342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith KR, Woodward A, Campbell-Lendrum D et al. . Human health: impacts, adaptation, and co-benefits. In: Climate change 2014: impacts, adaptation, and vulnerability. Part A: global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom, 2014:709–54. [Google Scholar]
- 12.Zhang X, Wan H, Zwiers FW, Hegerl GC, Min S-K. Attributing intensification of precipitation extremes to human influence. Geophys Res Lett 2013; 40:5252–7. [Google Scholar]
- 13.Guha-Sapir D, Below R, Ponserre S. Annual Disaster statistical review 2013: the numbers and trends. Brussels: Centre for Research on the Epidemiology of Disasters, 2014. [Google Scholar]
- 14.Haines A, Kovats RS, Campbell-Lendrum D, Corvalan C. Climate change and human health: impacts, vulnerability, and mitigation. Lancet 2006; 367:2101–9. [DOI] [PubMed] [Google Scholar]
- 15.Hepworth N, Goulden M. Climate change in uganda: understanding the implications and appraising the response. Edinburgh: LTS International, 2008. [Google Scholar]
- 16.Niang I, Ruppel OC, Abdrabo MA et al. . Africa. In: Barros VR, Field CB, Dokken DJ et al., eds. Africa. Climate change 2014: impacts, adaptation, and vulnerability. Part B: regional aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom and New York, NY: Cambridge University Press, 2014:1199–265. [Google Scholar]
- 17.Goulden M. Livelihood diversification, social capital and resilience to climate variability amongst natural resource dependent societies in Uganda [dissertation]. Norwich, United Kingdom: University of East Anglia, 2006. [Google Scholar]
- 18.Watts N, Adger WN, Agnolucci P et al. . Health and climate change: policy responses to protect public health. Lancet 2015; 15:12. [DOI] [PubMed] [Google Scholar]
- 19.Pascual M, Cazelles B, Bouma MJ, Chaves LF, Koelle K. Shifting patterns: malaria dynamics and rainfall variability in an African highland. Proc R Soc B 2008; 275:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaggwa R, Hogan R, Hall B, eds. Enhancing the contribution of weather, climate and climate change to growth, employment and prosperity. Kampala, Uganda: UNDP/NEMA/UNEP Poverty Environment Initiative, 2009. [Google Scholar]
- 21.Taylor RG, Mileham L, Tindimugaya C, Mwebembezi L. Recent glacial recession and its impact on alpine riverflow in the Rwenzori Mountains of Uganda. J Afr Earth Sci 2009; 55:205–13. [Google Scholar]
- 22.Boyce RM, Muiru A, Reyes R et al. . Impact of rapid diagnostic tests for the diagnosis and treatment of malaria at a peripheral health facility in Western Uganda: an interrupted time series analysis. Malar J 2015; 14:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyce R, Reyes R, Ntaro M et al. . Association between HRP–2/pLDH rapid diagnostic test band positivity and malaria–related anemia at a peripheral health facility in Western Uganda. J Glob Health 2015; 5:020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Federation of the Red Cross and Crescent. DREF Preliminary Final Report. Uganda: Kasese Floods, 2014. [Google Scholar]
- 25.Uganda Bureau of Statistics. National population and housing census 2014: provisional results. Revised ed Kampala, Uganda: Uganda Bureau of Statistics, November 2014. [Google Scholar]
- 26.World Health Organization (WHO) Disease surveillance for malaria control: an operational manual. Geneva: WHO, 2012:6–19. [Google Scholar]
- 27.Hosmer D, Lemeshow S. Applied logistic regression. New York: Wiley, 2000. [Google Scholar]
- 28.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA 2014; 312:2401–2. [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee P, Venkataramani AS, Vijayan A, Wellen JR, Martin EG. THe effect of state policies on organ donation and transplantation in the United States. JAMA Intern Med 2015; 175:1323–9. [DOI] [PubMed] [Google Scholar]
- 30.McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med 2015; 372:1927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beven KJ, Kirkby MJ. A physically based variable contributing area model of basin hydrology. Hydrological Sciences Bulletin 1979; 24:43–69. [Google Scholar]
- 32.Zhou G, Munga S, Minakawa N, Githeko AK, Yan G. Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg 2007; 77:29–35. [PubMed] [Google Scholar]
- 33.Majambere S, Pinder M, Fillinger U et al. . Is mosquito larval source management appropriate for reducing malaria in areas of extensive flooding in The Gambia? A cross-over intervention trial. Am J Trop Med Hyg 2010; 82:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cano J, Descalzo MA, Moreno M et al. . Spatial variability in the density, distribution and vectorial capacity of anopheline species in a high transmission village (Equatorial Guinea). Malar J 2006; 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Githeko AK, Ototo EN, Guiyun Y. Progress towards understanding the ecology and epidemiology of malaria in the western Kenya highlands: opportunities and challenges for control under climate change risk. Acta Trop 2012; 121:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Githeko AK, Ayisi JM, Odada PK et al. . Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malar J 2006; 5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsay SW, Bodker R, Malima R, Msangeni HA, Kisinza W. Effect of 1997–98 El Nino on highland malaria in Tanzania. Lancet 2000; 355:989–90. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Flooding and communicable diseases fact sheet. Risk assessment and preventive measures. Geneva: World Health Organization, 2005. [Google Scholar]
- 39.Kondo H, Seo N, Yasuda T et al. . Post-flood--infectious diseases in Mozambique. Prehosp Disaster Med 2002; 17:126–33. [DOI] [PubMed] [Google Scholar]
- 40.Kilian AHA. Rainfall pattern, El Niño and malaria in Uganda. Trans R Soc Trop Med Hyg 1999; 93:22–3. [DOI] [PubMed] [Google Scholar]
- 41.Brown V, Abdir Issak M, Rossi M, Barboza P, Paugam A. Epidemic of malaria in north-eastern Kenya. Lancet 1998; 352:1356–7. [DOI] [PubMed] [Google Scholar]
- 42.Minakawa N, Munga S, Atieli F et al. . Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. Am J Trop Med Hyg 2005; 73:157–65. [PubMed] [Google Scholar]
- 43.Maes P, Harries AD, Van den Bergh R et al. . Can Timely Vector Control Interventions Triggered by Atypical Environmental Conditions Prevent Malaria Epidemics? A Case-Study from Wajir County, Kenya. PLoS One 2014; 9:e92386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.