Abstract
Objective:
To study the effects of natalizumab treatment on subgroups of circulating peripheral blood B cell populations.
Methods:
We studied the proportions and absolute numbers of CD19+CD20+, CD10+, and CD5+ B cell populations, and determined very late activation antigen-4 and chemokine receptor CXCR3, CCR5, and CCR6 expression on B cells in the peripheral blood of 14 natalizumab-treated patients with relapsing-remitting multiple sclerosis. Five blood samples per patient were obtained longitudinally before and during the first year of treatment. Blood samples were analyzed by 6-color flow cytometry.
Results:
Proportions of B cells and CD10+ pre–B cells were significantly increased, and very late activation antigen-4 expression on the B cell surface was significantly decreased already after 1 week of natalizumab treatment. Natalizumab-induced sustained increase in the proportion and absolute number of CXCR3-expressing B cells was statistically significant after 1 month of treatment. There were no changes in the proportions of CCR5- or CCR6-expressing B cells.
Conclusions:
The rapid and persistent increase in circulating CXCR3-expressing B cells in response to natalizumab treatment possibly reflects the relevance of this chemokine receptor in controlling migration of B cells into the CNS in humans in vivo.
Trafficking of leukocytes into the inflamed CNS is a multistep process involving both adhesion molecules, such as integrin very late activation antigen 4 (VLA-4), and chemokine receptors.1 This migration is prevented by an anti-VLA-4 antibody, natalizumab, which efficiently reduces relapses in relapsing-remitting multiple sclerosis. By rendering the activated leukocytes trapped in circulation, natalizumab treatment allows the detection and study of leukocyte subgroups potentially important for multiple sclerosis (MS) pathogenesis. B cells are increasingly recognized as active players in the pathogenesis of MS. They are found within the active lesions in MS brain,2 but it is not well known how natalizumab affects B cell trafficking into the CNS. However, VLA-4 is known to be expressed on B cells,3 and selective elimination of VLA-4 from B cells reduced leukocyte recruitment and susceptibility to CNS autoimmunity in an animal model of MS, thus providing mechanistic proof for the role of VLA-4 in B cell trafficking into the CNS.4 The CXCR3 chemokine receptor has also been hypothesized to be involved in regulating the accumulation of B cells into the CNS.5 The aim of this prospective study was to evaluate longitudinally the effects of natalizumab treatment on peripheral blood B cell populations and chemokine receptor expression, with special reference to CNS homing determinants expressed by these cells.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethical committee of the Hospital District of Southwestern Finland. All patients gave written informed consent.
Patients.
Fourteen patients with relapsing-remitting MS (10 women) initiating natalizumab treatment (Tysabri, 300 mg IV every 4 weeks; Biogen Idec, Cambridge, MA) at the neurology outpatient clinic of the Division of Clinical Neurosciences at the Turku University Hospital, Turku, Finland (December 2008 to December 2009), were prospectively recruited into the study. Characteristics included the following: mean age 37.2 (range 21–46) years; disease duration from MS diagnosis 1.9 (0–3.75) years; Expanded Disability Status Scale score 3.7 (1–7.5); number of relapses 4.1 (1–10, total) and 1.9 (0–5, year before); and disease-modifying therapy year before, β-interferon (n = 6) and glatiramer acetate (n = 1). The sample size was determined by the number of patients initiating natalizumab treatment and willing to participate during the recruitment period of 1 year. Heparinized blood samples (10 mL) were obtained shortly before the initiation of treatment and at 1 week and 1, 3, and 12 months of treatment. A total of 64 prospective blood samples were collected. Two patients discontinued the treatment (after 1 and 8 months [infusion reaction and fear of progressive multifocal leukoencephalopathy, respectively]), and 3 patients did not show up at 1 week. One patient experienced a relapse 10 days after initiation of natalizumab treatment and received a course of methylprednisolone pulse therapy.
Flow cytometry.
Immunofluorescence staining was performed using whole blood (ethylenediaminetetraacetic acid–anticoagulated peripheral blood, 100 μL) with the following antibodies: CD19-PE-Cy7, CD5-APC, CD10-APC, CD19-APC, CD20-APC-Cy7, CD45-APC-Cy7, CD45-PerCP-Cy5.5, CD4-APC, CD3-PE-Cy7 (BD Biosciences, Franklin Lakes, NJ); CD49d, CD183-PE, CD196-PE, CD195-FITC, CD3-PE-Cy7, CD3-PerCP-Cy5.5 (BD Pharmingen, San Diego, CA). Samples were processed according to EuroFlow Standard Operating Procedure for sample preparation and staining (euroflow.org) and analyzed using flow cytometry (FACSCanto; BD, San Jose, CA). Rationale for the choice of target molecules was the following: CD19 and CD20 were included as B cell determinants. CD10 was included as a pre–B cell marker. CD183/CXCR3 and CD49d/VLA-4 evaluation on B cells was the hypothesis-driven main objective of the study. CD45, CD3, and CD4 were used for gating purposes. Other chemokine receptors and cell surface molecules not immediately associated with B cell CNS homing (i.e., CD196/CCR6, CD195/CCR5, CD5) were included as controls. From each tube, 100,000 events were collected, and data were analyzed using FACSDiva version 6.1.3 (BD). On the same day, the blood lymphocyte counts were obtained from differential white blood cell count analyzed at 0, 1, 3, and 12 months using Sysmex XE2100 (Sysmex, Kobe, Japan) automated hematology analyzer according to standard clinical laboratory procedures, and absolute counts for B cell subpopulations were calculated from lymphocyte counts with assistance of flow cytometry results.
Statistical analyses.
Statistical analyses were performed separately for each antibody using SAS System (SAS Institute, Cary, NC) for Windows 9.4. If residuals were normally distributed, time comparisons were done using mixed-model repeated-measures analysis of variance with heterogeneous compound symmetry covariance structure and followed by Dunnett adjustment for pairwise comparisons. Otherwise, comparisons were made using signed-rank or paired t test with Bonferroni adjustment multiple comparisons. Samples taken during natalizumab treatment were compared to the baseline sample. Corrected 2-sided p values <0.05 were considered statistically significant.
RESULTS
Natalizumab treatment led to increased absolute numbers of circulating B cells, and B cell subpopulations (table, figure). The increase in circulating B cells was seen already after 1 month of treatment, and it persisted throughout the study period. Proportionately, the increase was greatest among CD10+CD19+CD20+ pre–B cells (median 8.3-fold) and CXCR3+CD19+ B cells (4.7-fold). Natalizumab-induced persistent increase in the proportion of CD19+CD20+ B cells of all peripheral blood lymphocytes was observed already after 1 week of treatment (p = 0.004 at 1 week, p = 0.0005 at 1 month, p = 0.002 at 3 months, p = 0.008 at 12 months; table, figure). Natalizumab treatment increased the proportion of CD10+CD19+CD20+ pre–B cells during the first 3 months (p = 0.02, p = 0.01, p = 0.004, and p = 0.2, respectively; table, figure). The proportion of VLA-4+CD19+ cells decreased already after 1 week of natalizumab treatment (p < 0.0001 at all time points; table, figure). There was no change in the proportion of CD5+CD19+ native transitional B cells (table, figure). The proportion of CXCR3+CD19+ cells was increased after 1 month (p = 0.014 at 1 and 3 months, p = 0.0058 at 12 months; table, figure). Natalizumab-treatment did not alter the proportion of CCR6+CD19+ or CCR5+CD19+ cells (table, figure).
Table.
Natalizumab-induced increases in cell counts and alterations in proportions of circulating B cells in patients with multiple sclerosis
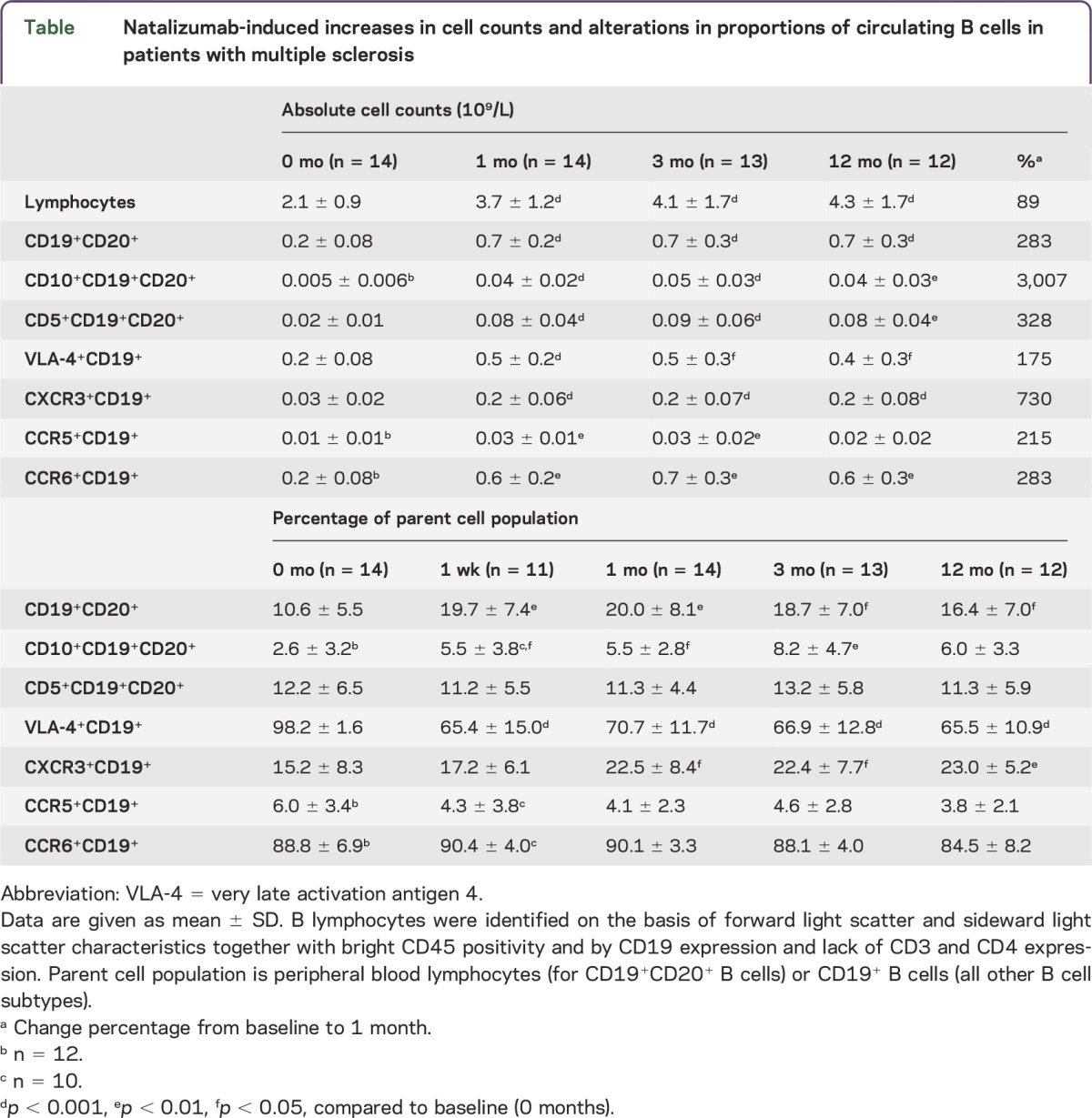
Figure. Effect of natalizumab treatment on circulating B cell subpopulations and chemokine receptor expression by circulating B cells.
(A–H) The absolute number of total lymphocytes and all investigated B cell populations and surface molecules, except CCR5 at 12 months, increases significantly and persistently in patients with multiple sclerosis during natalizumab treatment. (I) The proportion of CD19+CD20+ B cells of all peripheral blood lymphocytes increases in patients with multiple sclerosis already after 1 week of natalizumab treatment. (J) The proportion of CD10+CD19+CD20+ pre–B cells increases already after 1 week of natalizumab treatment. (K) The proportion of CD5+CD19+CD20+ B cells is not altered during natalizumab treatment. (L) The proportion of VLA-4+CD19+ cells decreases already after 1 week of natalizumab treatment. (M) The proportion of CCR5+CD19+ cells is not altered during natalizumab treatment (N) The proportion of CCR6+CD19+ cells is not altered during natalizumab treatment. (O) The proportion of CXCR3+CD19+ cells increased after 1 month of natalizumab treatment. (P) The proportion of CXCR3+CD19+ cells increased in 11 of 13 patients from baseline to 3 months during natalizumab treatment. Median, lower and upper quartiles, and range (A–H) or mean ± standard error (I–O) is shown except (P), where lines represent intraindividual changes.*p < 0.05, **p < 0.01, ***p < 0.001 compared to baseline (0 months). m = month; VLA-4 = very late activation antigen 4; w = week.
DISCUSSION
B cells express high levels of the adhesion-mediating integrin molecule VLA-4.3 Hence, blocking VLA-4 using natalizumab most likely both enhances the release of B cells from lymphoid tissues and bone marrow, and prevents B cell entry from blood into tissue. This results with increased numbers of circulating B cells as shown in this study and by others.6,7 Our result of the apparent partial decrease in B cell VLA-4 expression following natalizumab treatment is in line with previous results.8 In addition to adhesion molecules, chemokine receptors and chemokines contribute to recruitment of leukocytes into sites of inflammation.1 Not much is known, however, about the specific chemokine receptors mediating entry of B cells into the CNS under inflammatory conditions. We show here for the first time that the proportion of circulating B cells expressing the chemokine receptor CXCR3 is significantly and persistently increased during natalizumab treatment. CXCR3 is expressed in a subset of circulating B cells and, of note, CXCR3 expression on B cells in the CSF is higher in active MS patients than in controls.5,9 It is thus possible that the increase in the proportion of CXCR3-expressing circulating B cells following natalizumab treatment is related to the reduced migration ability of these cells into the inflamed CNS. Moreover, the observed reduction in the level of CXCR3-binding chemokines within the CNS following natalizumab treatment10 might hamper the trafficking of CXCR3+ B cells into CNS, which further contributes to the increased proportion of these cells in the blood. Natalizumab treatment did not alter the proportions of CCR5+ or CCR6+ B cells, highlighting the fact that natalizumab has divergent effects on various B cell populations expressing various homing determinants. In addition, natalizumab seems to influence differently the proportions of CXCR3-expressing circulating B and T cells, as CXCR3+CD8+ T cells were shown to decrease in circulation during natalizumab treatment.11
Our study demonstrates the early (demonstrable already after 1 month) and persistent (up to 1 year) increase in absolute numbers of circulating CD19+CD20+ B cells and CD10+CD19+CD20+ pre–B cells during natalizumab treatment, which probably is caused by the rapid release of these cells from lymphoid tissues and bone marrow due to VLA-4 blockage.6,7 This phenomenon is so pronounced that it leads to an increase in absolute numbers of any B cell population studied (table). However, when proportions of various B cell subpopulations are calculated, the only increase is observed in CXCR3-expressing B cells.
It has been recognized that VLA-4 has an important role in mediating T lymphocyte entry into the CNS, but adhesion molecules controlling the B cell entry have been much less understood. Our study suggests that some of the treatment effect of natalizumab might be attributable to prevented entry of disease-relevant B cells into the CNS. The beneficial effect on CNS inflammation of B cell VLA-4 expression elimination in mice supports this hypothesis.4 However, the mechanistic demonstration of the role of CXCR3 in B cell entry into the inflamed CNS remains to be seen.
ACKNOWLEDGMENT
The authors thank Ms. Emma Smith for valuable assistance with data management, and Drs. Marja-Terttu Pelliniemi and Anri Tienhaara for fruitful discussions and advice for designing the study protocol. Tommi Kauko and Jaakko Matomäki are acknowledged for help with the statistical evaluation.
GLOSSARY
- MS
multiple sclerosis
- VLA-4
very late activation antigen 4
AUTHOR CONTRIBUTIONS
Maija Saraste: analysis of the data, drafting and revising the manuscript for intellectual content. Tarja-Leena Penttilä: design of the antibody panels for flow cytometry analysis, analysis of the data. Laura Airas: design and conceptualization of the study, critical revision of manuscript for intellectual content.
STUDY FUNDING
This investigator-initiated study was supported by a grant from Biogen Idec. Maija Saraste is financially supported by the University of Turku Doctoral Programme of Clinical Investigation, the Maud Kuistila Memory Foundation, the Finnish MS Foundation, and the Orion Research Foundation. The sponsor had no influence on the analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper.
DISCLOSURE
M. Saraste received travel funding from the Turku University Foundation, received research support from the University of Turku Doctoral Programme of Clinical Investigation, the Maud Kuistila Memory Foundation, The Finnish MS Foundation, The Orion Research Foundation. T.-L. Penttilä reports no disclosures. L. Airas served on the scientific advisory board for Teva, Roche, Biogen, Genzyme, Novartis, received travel funding and/or speaker honoraria from Teva, Genzyme, Biogen Idec, Sanofi-Aventis, Merck, Novartis, received research support from Merck, Novartis, Biogen Idec. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev 2012;248:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michel L, Touil H, Pikor NB, Gommerman JL, Prat A, Bar-Or A. B cells in the multiple sclerosis central nervous system: trafficking and contribution to CNS-compartmentalized inflammation. Front Immunol 2015;6:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niino M, Bodner C, Simard ML, et al. . Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol 2006;59:748–754. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann-Horn K, Sagan SA, Bernard CC, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Ann Neurol 2015;77:902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sørensen TL, Roed H, Sellebjerg F. Chemokine receptor expression on B cells and effect of interferon-beta in multiple sclerosis. J Neuroimmunol 2002;122:125–131. [DOI] [PubMed] [Google Scholar]
- 6.Krumbholz M, Meinl I, Kümpfel T, Hohlfeld R, Meinl E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008;71:1350–1354. [DOI] [PubMed] [Google Scholar]
- 7.Mattoscio M, Nicholas R, Sormani MP, et al. . Hematopoietic mobilization: potential biomarker of response to natalizumab in multiple sclerosis. Neurology 2015;84:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putzki N, Baranwal MK, Tettenborn B, Limmroth V, Kreuzfelder E. Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur Neurol 2010;63:311–317. [DOI] [PubMed] [Google Scholar]
- 9.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood 2000;95:627–632. [PubMed] [Google Scholar]
- 10.Mellergård J, Edström M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler 2010;16:208–217. [DOI] [PubMed] [Google Scholar]
- 11.Kivisäkk P, Healy BC, Viglietta V, et al. . Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology 2009;72:1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]