ABSTRACT
Medulloblastoma is an aggressive primitive neuroectodermal tumor of the cerebellum that is rare in adults. Medulloblastomas fall into 4 prognostically significant molecular subgroups that are best defined by experimental gene expression profiles: the WNT pathway, sonic hedgehog (SHH) pathway, and subgroups 3 and 4 (non-SHH/WNT). Medulloblastoma of adults belong primarily to the SHH category. Vismodegib, an SHH-pathway inhibitor FDA-approved in 2012 for treatment of basal cell carcinoma, has been used successfully in the setting of chemorefractory medulloblastoma, but not as a first-line therapy. In this report, we describe a sustained response of an unresectable multifocal form of adult medulloblastoma to vismodegib. Molecular analysis in this case revealed mutations in TP53 and a cytogenetic abnormality, i17q, that is prevalent and most often associated with subgroup 4 rather than the SHH-activated form of medulloblastoma. Our findings indicate that vismodegib may also block alternate, non-canonical forms of downstream SHH pathway activation. These findings provide strong impetus for further investigation of vismodegib in clinical trials in the first-line setting for pediatric and adult forms of medulloblastoma.
KEYWORDS: Medulloblastoma, sonic hedgehog, targeted therapy, vismodegib
Introduction
Medulloblastoma is an aggressive primitive neuroectodermal tumor of the cerebellum with a propensity for distant metastasis and a high recurrence rate.1,2 It is the most common primary intracranial malignancy in the pediatric population, but it is quite rare in adults, with an approximate incidence of 0.5 cases per 1,000,000 per year.2,3 Due to its rarity in adults, treatment modalities have been adapted from pediatric protocols, and there have been no randomized trials to establish standard-of-care treatment of adult medulloblastoma.
There is rising interest in the use of molecular targeted therapy, and very specifically SHH inhibitors, to treat medulloblastomas. One such inhibitor, vismodegib (GDC-0449), acts by inhibiting mutated smoothened (SMO) receptors, and thus overcoming regulation by PTCH1 (Fig. 1). To date, several phase I studies using vismodegib have reported temporary response in patients with chemorefractory medulloblastoma.4,5 In these reports, response has been limited to several months. In a recent phase II study of 31 adults with recurrent medulloblastoma receiving vismodegib, 3 patients with SHH-subgroup type displayed progression-defined response (defined as radiologic response lasting 8 weeks).6 Here, we present the case of an exceptional responder, a patient with an unresectable/multifocal form of adult medulloblastoma that has responded completely to SHH inhibition with vismodegib in the first-line setting, with sustained response lasting nearly 3 years to date.
Figure 1.
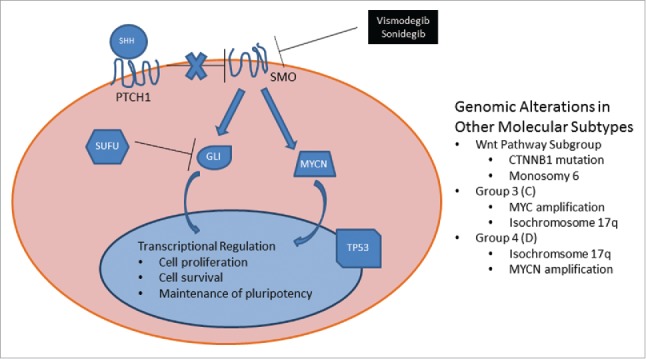
Schematic of molecular subgroups of medulloblastoma, including the SHH and alternate molecular pathways. Signaling in the SHH subgroup of medulloblastoma and recurring genomic abnormalities observed in other subtypes. Sonic hedgehog (SHH) ligand binds PTCH1 receptor, relieving this receptor's baseline inhibition of SMO. Active SMO prevents SUFU inhibition of GLI and leads to GLI nuclear translocation and transcriptional activation as well as transcription-independent upregulation of MYCN with subsequent downstream transcriptional regulation. The FDA-approved molecular inhibitors vismodegib and sonidegib block SHH-mediated downstream activation by direct inhibition of SMO receptor. Common genomic aberrations in the SHH subtype include: 1) germline or somatic mutations of PTCH1, SMO, or SUFU; 2) amplification of 2q (GLI2) or 2p (MYCN); or deletion of 9q (PTCH1), 10q (SUFU), or 17p (TP53). Note that i17q functionally leads to deletion of 17p. Other genomic alterations recurrently observed in the other 3 subtypes of medulloblastoma are listed in the right-hand text box. The molecular subtypes are best categorized by gene expression profiling, and many of the genomic alterations are not specific for an individual molecular subtype.
Clinical case report
A 51-year-old male with history of basal cell carcinoma presented with a 3-month history of unremitting headaches, dizziness, expressive aphasia, agraphia, difficulty with balance, and tinnitus. Neurologic evaluation revealed dysmetria of the left upper extremity; no nystagmus was noted. Brain magnetic resonance imaging (MRI) revealed a heterogeneous abnormality in the cerebellum, presenting as a poorly defined mass involving both cerebellar hemispheres and the vermis (Fig. 2A & C). There was no mass effect on the fourth ventricle or evidence of hydrocephalus.
Figure 2.
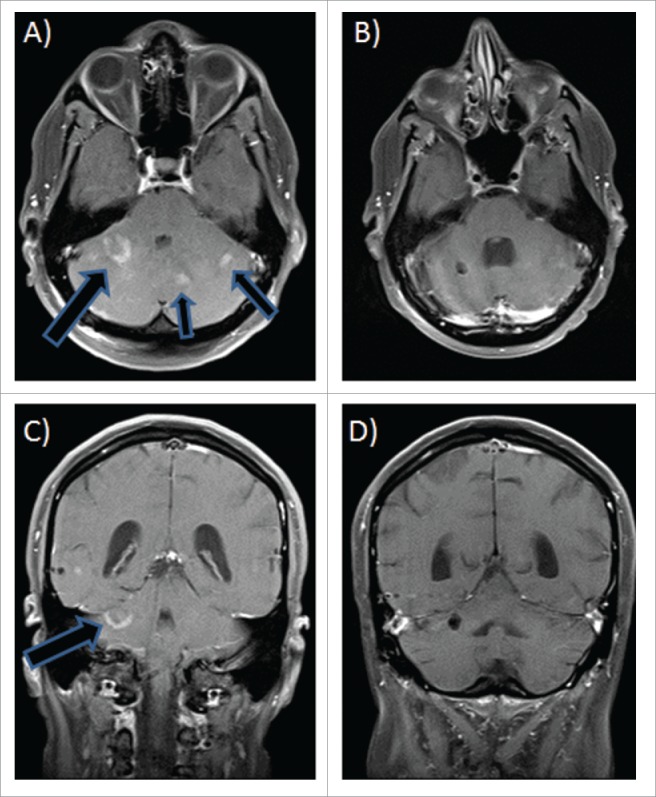
MRI images of unresectable multifocal medulloblastoma at time of diagnosis (A and C) and after 4 months of vismodegib therapy (B and D). Images are shown in axial sections with contrast (top) and coronal sections (bottom).
Biopsy of the mass showed a small round blue cell tumor infiltrating the surrounding cerebellar parenchyma (Fig. 3A–F). No desmoplastic features were present, but the small biopsy precluded definitive assessment. Immunohistochemical stains were strongly positive for synaptophysin, with weak glial fibrillary acidic protein (GFAP) and patchy chromogranin positivity. Nuclear staining for β-catenin was absent. CD3, CD20, LCA, cytokeratin, and TTF-1 were negative. Ki-67 showed a high proliferation index (30–50% of tumor cells). A diagnosis of medulloblastoma was made based on morphology, immunophenotype, and anatomic location.
Figure 3.
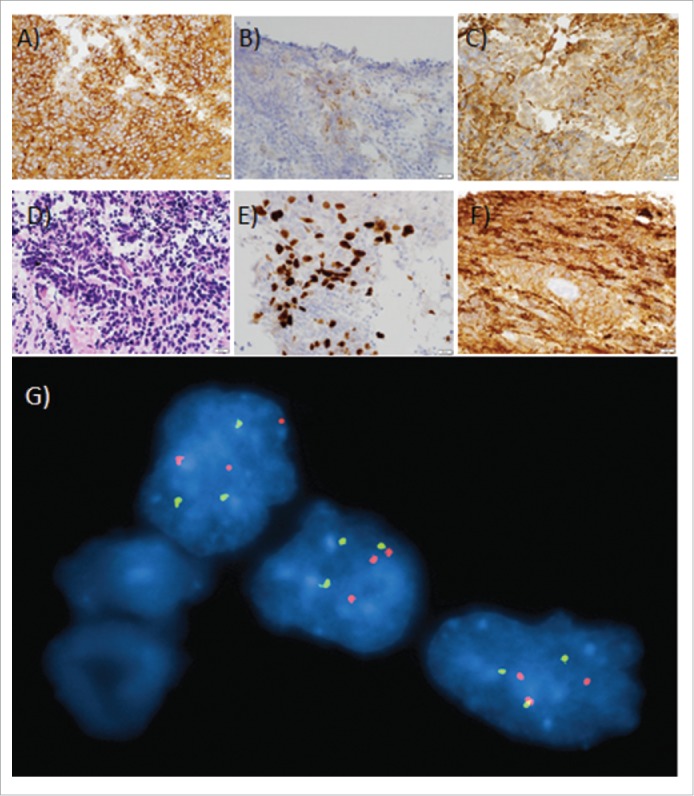
Histopathology staining of biopsied tumor from this patient. Scale bars = 20 µm (lower right-hand corner of each image). (A) 40x original magnification of β-catenin immunoperoxidase stain: Diffuse moderately strong cytoplasmic immunostaining of tumor cells for β-catenin. No nuclear staining is present. (B) 40x original magnification of chromogranin immunoperoxidase stain: Patchy moderate granular cytoplasmic staining of neoplastic cells for chromogranin. (C) 40x original magnification of GFAP immunoperoxidase stain: Patchy weak to moderately strong cytoplasmic staining of neoplastic cells for GFAP. (D) 40x original magnification of hematoxylin and eosin; Primitive appearing cells with high nuclear to cytoplasmic ratios, focally arranged in a vaguely nested pattern, infiltrate the cerebellar molecular layer. (E) 40x original magnification of KI-67 immunoperoxidase stain: Strong nuclear staining of neoplastic cells for KI-67 (30 – 50% of tumor cells) indicating a high proliferative fraction. (F) 40x original magnification of Synaptophysin immunoperoxidase stain: Diffuse moderately strong cytoplasmic staining of neoplastic cells, with darker staining of the background infiltrated neuropil. (G) FISH performed on a touch imprint slide shows 3 signals each for 17p11.2 (orange) and 17q21 (green). Of the interphase cells examined, 80.5% had 3 signals each for the 17p11.2 and 17q21 probes. This signal pattern is consistent with either trisomy 17 or with an isodicentric chromosome composed of 2 copies of a chromosome 17 joined in mirror image in their short arms, distal to the 17p11.2 probe.
The amount of tissue was insufficient to initiate cultures for G-banding, but fluorescence in situ hybridization (FISH) on limited touch preps demonstrated 3 copies of RAI1 (17p11.2) and RARA (17q21) in 161 of 200 cells, which likely represented an isodicentric chromosome with a breakpoint in proximal 17p and deletion of distal 17p (Fig. 3G). Isochromosome 17q (i17q) is one of the most frequent recurring abnormalities in medulloblastoma, occurring in approximately 40–50% of cases, predominantly within the Group 4/D subtype in adults.7 It has been reported to be a negative prognostic factor;8,9 in childhood medulloblastoma, it has been commonly associated with mutations in TP53 and desmoplastic histology10, which was consistent with what we found in our case despite its occurrence in an adult. A predominant difference is that studies in the childhood form associated 17p loss with the SHH subgroup.10
The patient's clinical workup found no evidence of systemic disease at the time of diagnosis, including cytologic evaluation of cerebrospinal fluid. Initial therapy included craniospinal irradiation (1980 cGy to the brain in 11 fractions; 3600 cGy to the spine in 20 fractions). One week into this course, the patient developed headaches and lethargy. Cranial computed tomography scanning demonstrated ventriculomegaly requiring endoscopic third ventriculostomy and placement of an external ventriculostomy drain. Further complications included intractable nausea and vomiting, malnutrition, anorexia, radiation-induced pancytopenia, and lower extremity deep venous thrombosis requiring anticoagulation.
Brain MRI following completion of radiation therapy showed a marked decrease in the size and enhancement of the tumor, with no evidence of spinal metastasis. However, as the patient had continued neurologic compromise and poor performance status, he was not considered to be a candidate for platinum-based chemotherapy. In light of the preponderance of Hedgehog pathway activation in adult medulloblastoma, the Hedgehog inhibitor vismodegib was initiated on the basis of compassionate use. Within four months, there was no evidence of abnormal enhancing lesions at previous sites of known medulloblastoma (Fig. 2B & D). The patient remained on vismodegib for 22 months without evidence of disease recurrence before he elected to stop treatment. He remains disease-free as of 7 months after cessation of therapy. Given his remarkable response, clinical testing of his diagnostic biopsy for somatic mutations in the SHH pathway was considered, but insufficient tissue remained in the archived block.
Retrospective analysis of residual diagnostic cytologic preparations
Although clinical analyses were hampered by the limited quantity of specimen, a residual cytologic touch preparation from the patient's initial diagnosis was available for additional research testing after appropriate patient consent. DNA extracted from this specimen was sequenced on a targeted Next Generation Sequencing (NGS) assay for 21 genes commonly mutated in solid and hematologic malignancies, but no sequence mutations were identified by this panel including within TP53.
A qPCR assay was used to assess copy number alterations (CNAs) commonly observed in medulloblastoma. This testing confirmed the deletion of one copy of TP53 inferred by the clinical FISH results, but showed no CNAs in SUFU (10q), PTCH1 (9q), or GLI2 (2q), which are known drivers of the SHH molecular subtype (Fig. 4A). Furthermore, no clear CNAs were observed in MYC (8q), MYCN (2p), or OTX2 (14q), which also show enrichment in particular molecular subtypes (Fig. 4B).
Figure 4.
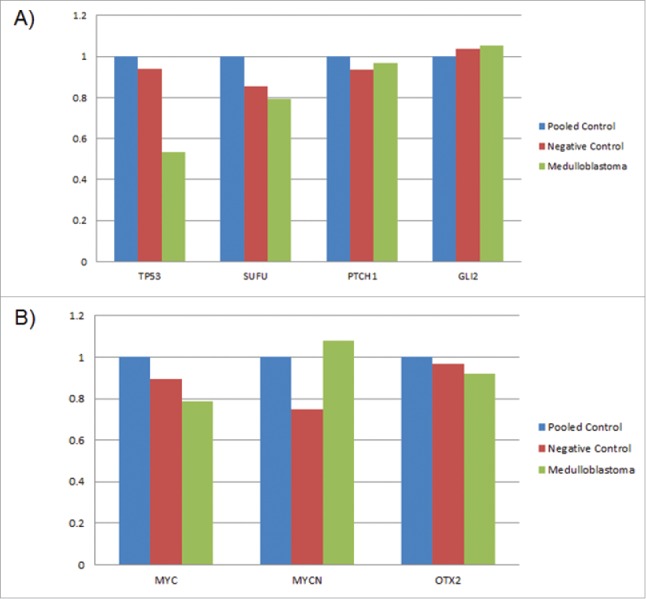
Copy Number Alteration Analysis. Normalized copy number (CN) ratios for genes commonly deleted or amplified in medulloblastoma. A negative control and the tumor sample were compared by qPCR to a pooled (10 individual) control as a copy number benchmark. The pooled control is normalized to a CN ratio = 1; experimental ratios that drop below 0.7 or rise above 1.3 are considered to be deleted or amplified, respectively. The 0.53 ratio for TP53 in the medulloblastoma correlates with FISH results indicating deletion of distal 17p. No other genes passed cut-off criteria for copy number alteration.
Finally, somatic activating mutations within SMO have been reported to occur in several hotspots within exons 4, 6, and 910. Targeted Sanger sequencing was performed on the residual specimen, but no mutations were identified, including the most commonly identified alteration at p.L412.
Clinical medical genetics evaluation
Due to his personal history of basal cell carcinoma, the patient was evaluated for family history, clinical evidence, and genetic testing for Gorlin syndrome/ Nevoid Basal Cell Carcinoma Syndrome (NBCCS). This is an autosomal dominant syndrome characterized by basal cell carcinomas and jaw keratocysts associated with a spectrum of facial and skeletal abnormalities. The incidence may be as high as 1 in 20,000 births,11 and sequence mutations or deletions of PTCH1 are identified in up to 85–90% of cases.12 The clinical assessment is summarized in Table 1, and germline sequencing with deletion/duplication analysis of PTCH1 performed at a commercial reference laboratory was reported negative. In light of these findings, a clinical diagnosis of Gorlin syndrome was deemed unlikely but not definitively excluded.
Table 1.
Evaluation of major and minor criteria for Gorlin syndrome/Nevoid Basal Cell Carcinoma Syndrome (NBCCS).
| Major Criteria | Patient Findings |
|---|---|
| Lamellar calcification of the falx cerebri | Negative |
| Jaw keratocyst | Unlikely based on dental x-rays, but not definitively excluded by a current orthopantogram |
| 2 or more palmar/plantar pits | Negative |
| Multiple basal cell carcinomas (> 5) or diagnosis before age 30 | Negative; patient diagnosed with one BCC at age of 48 |
| First degree relative with Gorlin |
Negative; but known family medical history was limited |
|
Minor Criteria |
Patient Findings |
| Childhood medulloblastoma | Negative; patient diagnosed at age of 51 |
| Lympho-mesenteric or pleural cysts | Negative |
| Macrocephaly (OFC >97th centile) | Positive |
| Cleft lip/palate | Negative |
| Rib or vertebrae anomalies | Negative |
| Polydactyly | Negative |
| Cardiac fibroma | Negative |
| Cataracts or ocular developmental abnormalities | Negative |
Methods
Consent and clinical testing
Informed consent was obtained directly from the patient for chart review and all research testing performed, with concurrent waiver from the Institutional Review Board. Germline testing for Gorlin syndrome (PTCH1) was sent to Ambry Genetics (Aliso Viejo, CA).
Fluorescence in situ hybridization (FISH)
Due to the very small size of the submitted tissue, insufficient tissue remained after the preparation of touch imprints to initiate cultures for a G-banded chromosome analysis. FISH was prepared on a touch imprint slide with probes to 17p11.2 (SpectrumOrange) and 17q21 (SpectrumGreen) (Abbott Molecular, Des Plaines, IL, USA).
Targeted next generation sequencing
A sequencing library was prepared using a custom designed microfluidics-based (Fluidigm Corporation, South San Francisco, CA) PCR amplicon target enrichment for the following genes: TP53, BRAF, KRAS, NRAS, HRAS, EGFR, PIK3CA, MET, IDH1, IDH2, KIT, PDGFRA, ERBB2, WT1, FLT3, CALR, JAK2, MPL, NPM1,GATA1, and MYD88. Sequencing was performed on an Illumina MiSeq (San Diego, CA) with 300 base pair paired end reads and analyzed using a custom somatic variant calling pipeline.13
Copy number analysis
A qPCR method to confirm CNV calls has been described recently.14 Briefly, primers are designed for targets of interest and GAPDH is used for normalization. A pooled, sex-matched control (of peripheral blood from 10 healthy individuals) is compared to the analytic sample using a delta-delta method normalizing target Ct to GAPDH Ct. To verify that individual results are not spurious, a negative control consisting of a single sex-match healthy individual is also compared to the pooled control. The pooled control is normalized to a CNA ratio = 1; experimental ratios that drop below 0.7 or rise above 1.3 are considered to be deleted or amplified, respectively.
Sanger sequencing
Primers flanking exons 4, 6, and 9 of SMO were designed and appended with M13 sequencing tags. Cycling parameters followed a touchdown PCR approach.15 Amplification products were separated on an ethidium bromide stained 1% agarose gel and purified with the QIAquick Gel Extractin Kit (Qiagen). Cycle sequencing products were generated using BigDye Terminator chemistry (Applied Biosystems) and analyzed on a 3130xl Genetic Analyzer (Applied Biosystems).
Discussion
Here, we present a case of unresected adult medulloblastoma that responded completely to SHH inhibition with vismodegib in the first-line setting after craniospinal irradiation. We identified a cytogenetic abnormality (i17q) that is not specific, but is more frequently associated with group 4/D medulloblastoma than with the SHH subtype. Furthermore, this abnormality has been reported as an independent poor prognostic factor in previous studies. Nonetheless, based on the preponderance of SHH subtype in adults and the relative lack of other options, vismodegib given on a compassionate use basis resulted in a phenomenal response in this patient. In context of our patient's extraordinary response to vismodegib, related pathways markers SMO, SUFU, and downstream transcription factor GLI2 were all unaffected in this patient's tumor. We did identify genomic deletion of one copy of TP53 via FISH and copy number analysis, suggesting that this tumor was activated through mechanisms mostly associated with group 4 medulloblastomas that carry intermediate prognosis and have a higher rate of metastasis than the SHH subgroup.16 Our findings thus suggest that vismodegib can have significant effects through an alternate non-canonical SHH-activation pathway. TP53 mutations are associated with pediatric forms of medulloblastoma and are considered rare in adults.10,17 Loss of 17p (as seen here) is enriched in TP53 mutant cases, but has been most reported with childhood cases.10 Our evaluation was not exhaustive in comparison to gene expression profiling tools used to define categories of medulloblastoma in clinical research settings, and the limited amount of tissue available for testing highlights the difficulty in identifying sensitive and specific genomic biomarkers that accurately identify cases that may benefit from SHH inhibition.
Historically, the prognosis of medulloblastoma has been clinically assessed based on factors such as the histopathologic subtype (e.g., desmoplastic vs large cell anaplastic) and the presence of residual disease after resection. The recent identification of molecular drivers involved in medulloblastoma tumorigenesis, including Wnt and SHH, has enabled the identification of additional prognostic markers.18 SHH tumors are associated with desmoplastic histology in the vast majority of cases, in both infant (89%) and adult tumors (nearly all), but only 25% of cases in children.19 As many as 84% of adult medulloblastoma tumors may show evidence of activation of the SHH pathway.20 When considering both pediatric and adult cases, most studies report a prevalence of approximately 28%.19 Notably, more than half of medulloblastoma cases are driven by the SHH pathway in the infant population as well as adults.19 Interestingly, WNT pathway activation is less frequent in adult medulloblastomas.20 Further studies have substantiated these differences in pathway activation as compared to pediatric cases. Evidence to date suggests that SHH pathway activation in adults does not correlate with a favorable prognosis.20 In a meta-analysis of 550 cases, Kool et al. reported an approximately 70% overall survival rate of adults with SHH medulloblastomas by 5 years, but with significant subsequent rise in mortality, evidenced by a 30% overall survival rate by 150 months (12.5 years).19
Agents targeting the sonic hedgehog (SHH) pathway have been investigated in recurrent adult and pediatric medulloblastomas because a significant subgroup of this disease is driven by this molecular pathway.6,16,19 In the case we describe in this report, our patient had a complete and sustained radiologic response over 2 y on vismodegib in the first-line setting after standard craniospinal irradiation. However, this exceptional response occurred in a patient whose tumor did not harbor alterations in SMO or in other canonical SHH pathway-associated drivers; this indicates that vismodegib may inhibit non-canonical alterations that activate the SHH pathway downstream (Fig. 1). In light of these findings, further exploration of vismodegib efficacy in other molecular subgroups of medulloblastoma is warranted. In addition, in the burgeoning era of molecularly targeted therapy, vismodegib merits strong consideration and investigation as an appropriate form of first-line treatment, replacing cytotoxic chemotherapies that have traditionally been used to treat adult forms of the disease based on data from trials performed in the pediatric population.
Supplementary Material
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We would like to thank the patient for providing written consent to report the results of his case. We thank Michael Franklin, M.S. for editing this manuscript.
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, this research was also supported by the Litman Family Fund for Cancer Research; the Randy Shaver Cancer Research and Community Fund; Minnesota Masonic Charities; and the Masonic Cancer Center and Department of Medicine, Division of Hematology, Oncology and Transplantation at the University of Minnesota (E.L.).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114:97-109; PMID:17618441; http://dx.doi.org/ 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordana MT, Schiffer P, Lanotte M, Girardi P, Chio A. Epidemiology of adult medulloblastoma. Int J Cancer 1999; 80:689-92; PMID:10048968; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19990301)80:5%3c689::AID-IJC10%3e3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 3.Carrie C, Lasset C, Alapetite C, Haie-Meder C, Hoffstetter S, Demaille MC, Kerr C, Wagner JP, Lagrange JL, Maire JP, et al.. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer 1994; 74:2352-60; PMID:7922986; http://dx.doi.org/ 10.1002/1097-0142(19941015)74:8%3c2352::AID-CNCR2820740821%3e3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Stewart CF, Ellison DW, Kaste S, Kun LE, Packer RJ, Goldman S, Chintagumpala M, Wallace D, Takebe N, et al.. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin Cancer Res 2013; 19:6305-12; PMID:24077351; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et al.. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 2009; 361:1173-8; PMID:19726761; http://dx.doi.org/ 10.1056/NEJMoa0902903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, et al.. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: Results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol 2015; 33:2646-54; PMID:26169613; http://dx.doi.org/ 10.1200/JCO.2014.60.1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, Benner A, von Deimling A, Scheurlen W, Perry A, et al.. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol 2011; 29:2717-23; PMID:21632505; http://dx.doi.org/ 10.1200/JCO.2011.34.9373 [DOI] [PubMed] [Google Scholar]
- 8.Pan E, Pellarin M, Holmes E, Smirnov I, Misra A, Eberhart CG, Burger PC, Biegel JA, Feuerstein BG. Isochromosome 17q is a negative prognostic factor in poor-risk childhood medulloblastoma patients. Clin Cancer Res 2005; 11:4733-40; PMID:16000568; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-0465 [DOI] [PubMed] [Google Scholar]
- 9.Lamont JM, McManamy CS, Pearson AD, Clifford SC, Ellison DW. Combined histopathological and molecular cytogenetic stratification of medulloblastoma patients. Clin Cancer Res 2004; 10:5482-93; PMID:15328187; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0721 [DOI] [PubMed] [Google Scholar]
- 10.Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V, Piro RM, Esparza LA, Markant SL, Remke M, et al.. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell 2014; 25:393-405; PMID:24651015; http://dx.doi.org/ 10.1016/j.ccr.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, Lalloo F. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 2010; 152A:327-32; PMID:20082463; http://dx.doi.org/ 10.1002/ajmg.a.33139 [DOI] [PubMed] [Google Scholar]
- 12.Evans DG FP. Nevoid Basal Cell Carcinoma Syndrome. 2002 Jun 20 [Updated 2015 Oct 1] In: Pagon RA AM, Ardinger HH, et al., editors, ed. GeneReviews® [Internet] Seattle (WA: ): University of Washington, Seattle, 1993-2015. [PubMed] [Google Scholar]
- 13.Yang R, Nelson AC, Henzler C, Thyagarajan B, Silverstein KA. ScanIndel: a hybrid framework for indel detection via gapped alignment, split reads and de novo assembly. Genome Med 2015; 7:127; PMID:26643039; http://dx.doi.org/ 10.1186/s13073-015-0251-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onsongo G, Baughn LB, Bower M, Henzler C, Schomaker M, Silverstein KAT, Thyagarajan B. CNV-RF: A random forest based copy number variation detection method using next generation sequencing. J Mol Diagnostics 2016; In Press. [DOI] [PubMed] [Google Scholar]
- 15.Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 1991; 19:4008; PMID:1861999; http://dx.doi.org/ 10.1093/nar/19.14.4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al.. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012; 123:465-72; PMID:22134537; http://dx.doi.org/ 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlin AM, Hollegaard MV, Wibom C, Andersson U, Hougaard DM, Deltour I, Hjalmars U, Melin B. CCND2, CTNNB1, DDX3X, GLI2, SMARCA4, MYC, MYCN, PTCH1, TP53, and MLL2 gene variants and risk of childhood medulloblastoma. J Neurooncol 2015; 125:75-8; PMID:26290144; http://dx.doi.org/ 10.1007/s11060-015-1891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald TJ, Aguilera D, Castellino RC. The rationale for targeted therapies in medulloblastoma. Neuro Oncol 2014; 16:9-20; PMID:24305711; http://dx.doi.org/ 10.1093/neuonc/not147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, et al.. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 2012; 123:473-84; PMID:22358457; http://dx.doi.org/ 10.1007/s00401-012-0958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Halabi H, Nantel A, Klekner A, Guiot MC, Albrecht S, Hauser P, Garami M, Bognar L, Kavan P, Gerges N, et al.. Preponderance of sonic hedgehog pathway activation characterizes adult medulloblastoma. Acta Neuropathol 2011; 121:229-39; PMID:21107850; http://dx.doi.org/ 10.1007/s00401-010-0780-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


