ABSTRACT
Transcranial direct current stimulation (tDCS) has been reported to be effective for alleviation of neuropsychiatric and neurological conditions as well as enhancement of memory and cognition. Despite the positive effects of tDCS in humans, its mechanism of action remains poorly understood. Recently, we reported that astrocytes, a major glial cell type in the brain, show an increase in intracellular Ca2+ levels during tDCS in the cerebral cortex of the awake mouse. This tDCS-induced elevation in astrocytic Ca2+ has subsequently been demonstrated to be important for cortical plasticity. In this commentary article, we discuss possible interpretations and implications of our findings from the viewpoint of neuron-glia interactions.
keywords: astrocytes, G protein-coupled receptors, neuromodulation, noradrenaline, norepinephrine, transcranial direct current stimulation (tDCS)
Introduction
Transcranial direct current stimulation (tDCS) is the application of constant, weak-intensity electrical current to the brain through the skull for a prolonged duration. For humans, typical parameters are a current intensity ranging from 1–2 mA and a duration ranging from 10–30 minutes.1 tDCS has been shown to have positive effects on brain function, including memory enhancements, accelerated motor function rehabilitation, alleviation of depressive symptoms, and slower progression of neurodegeneration in Alzheimer disease patients (for reviews, see refs. 2, 3). tDCS can be performed with simple electronics powered by commercially available batteries. Remarkably, while tDCS is practiced in neuropsychiatry and most probably by many curious minds, the mechanism by which these positive effects emerge is not well understood.
Simulation studies and animal experiments have been performed to unveil the underlying mechanisms of tDCS. For example, multiple simulation studies suggest subthreshold depolarization of neurons around the anodal site and hyperpolarization around the cathodal site,4-6 confirming the early intracellular recording study that showed neuronal depolarization in rodents.7 Learning enhancement has also been confirmed using tDCS in rodents,8 which allows for molecular manipulations and in vitro acute brain slice experiments and thus provides a foundation to investigate tDCS mechanisms. As a result, there is a general consensus that tDCS-induced synaptic plasticity is expressed in a manner dependent on the N-methyl-D-aspartate (NMDA) receptor.9,10 Furthermore, an influential study demonstrated the involvement of the brain-derived neurotrophic factor (BDNF) and activation of the tropomyosin receptor kinase B (trkB), a receptor for BDNF.11 The involvement of BDNF has been further supported by a recent study reporting that tDCS induces epigenetic changes in the BDNF gene locus that lasts for over several days.12
While in vitro acute slice experiments are powerful in assessing synaptic physiology, they are often compromised by truncation of long-range connections, incubation temperature, and inflammatory reactions caused by the preparation. In our recent study, we performed in vivo transcranial calcium (Ca2+) imaging in mice during tDCS and found that astrocytes, a major glia cell type, play a role in tDCS-induced cerebral plasticity. Encouragingly, a mouse behavioral experiment indicated an initial sign of alleviation of depression-like behavior after tDCS.13 Here, we extend our discussion of this original publication to consider how astrocytic activation fits the existing models of tDCS.
Cortex-wide Ca2+ imaging during tDCS
We made transgenic mice that have high G-CaMP7 (an improved version of the well-known Ca2+ indicator protein G-CaMP14) expression in astrocytes and a large population of excitatory neurons in the cortex and hippocampus (G7NG817 mouse13). Notably, the G-CaMP7 expression of the G7NG817 mouse is sufficient to allow cortex-wide transcranial functional imaging with a standard fluorescence stereo microscope. For example, visualization of single barrel columns and cortex-wide slow oscillations (0.5 to 2 Hz, also known as UP/DOWN states) is feasible in urethane-anesthetized mice. In these cases, the G-CaMP7 signals are predominantly of neuronal origin as they are usually shorter than one second, most likely reflecting action potential-driven Ca2+ influx since GPCR-triggered Ca2+ oscillations are of longer duration. Conversely, cytosolic Ca2+ signals of astrocytes are generally larger in magnitude and longer in duration by at least several-folds because they are triggered by G protein-coupled receptors (GPCRs) that signal to inositol trisphosphate (IP3) receptors, leading to the release of Ca2+ from internal stores. Volume-transmitted neuromodulators such as noradrenaline (norepinephrine) and acetylcholine are known to activate astrocytic15-18 and neuronal GPCRs. On the intracellular side, the IP3 receptor type 2 (IP3R2) is the main IP3 receptor in astrocytes, and the ablation of IP3R2 results in the diminishment of large and long-lasting cytosolic Ca2+ elevations in astrocytes.15,19-22 Using G7NG817 mice, we described large and long-lasting cytosolic Ca2+ elevations in astrocytes within several seconds after the onset of tDCS (0.1 mA, for 10 minutes). Importantly, cortex-wide tDCS-induced Ca2+ elevations were not observed in mice deficient of IP3R2 (IP3R2−/−;G7NG817+/−),13 suggesting that tDCS-induced Ca2+ elevations are of astrocytic origin.
tDCS-assisted in vivo sensory plasticity
One of the core questions in neuron-glia interactions is functional roles of astrocytic Ca2+ signaling in neuronal information processing (for reviews, see refs. 23-26). One of the hypotheses is that astrocytic Ca2+ signaling is involved in synaptic plasticity, owing to the morphological characteristics of astrocytes that surround synapses.
We demonstrated that tDCS enhances sensory-evoked potentials and neuronal Ca2+ responses in the adult cerebral cortex. Interestingly, the Ca2+ response of neuropil was enhanced in superficial layers (layer 2/3) of the cortex, but not in layer 4 where sensory thalamic input arrives. This observation could be related to the phenomenon that layer 4 plasticity disappears as the animal matures, especially after the critical period.27,28 Similar to a human study,9 the tDCS-assisted plasticity in our study is NMDAR-dependent as the plasticity was blocked by AP-5. Remarkably, IP3R2 knockout mice did not develop sensory response enhancement by tDCS, suggesting a crucial role of astrocytic Ca2+ elevation.
What is the pathway that connects astrocytic Ca2+ elevation to brain plasticity? There are at least several candidates, which may operate in parallel. First, Ca2+-dependent gliotransmission—secretion of bioactive molecules from astrocytes—can occur to influence synapses. A wide variety of gliotransmitters have been suggested in literature, including adenosine triphosphate (ATP), glutamate, D-serine, and tumor necrosis factor α (TNFα).29 Considering the NMDAR dependency, the NMDAR co-agonist D-serine is a sound candidate, as previously demonstrated in in vitro hippocampal plasticity30,31 and in vivo sensory plasticity in the cortex.15 However, other gliotransmitters such as ATP and TNFα are also implied in brain plasticity and hence remain as candidate gliotransmitters. Second, astrocytic Ca2+ elevation has been shown to decrease the extracellular ionic balance of potassium (K+), leading to an increase in synaptic efficacy.32 Third, astrocytic Ca2+ may be linked to morphological plasticity and integrity of tri-partite synapses.33 Fourth, a change in the extracellular volume and interstitial fluid exchange through astrocytes (i.e. the glymphatic system)34,35 may influence the metaplasticity of synapses. Finally, tDCS on humans has been reported to alter cerebral blood flow in a polarity dependent manner,36 while astrocytic involvement in functional hyperemia is controversial to date.37,38
Literature suggests that cortical potentiation is preferentially expressed at the anodal site over the cathodal site. A simpleminded explanation is depicted in Fig. 1. In short, astrocytic Ca2+ elevation occurs in wide areas of the cortex, including the contralateral side. This astrocytic Ca2+ elevation could act as a permissive factor that allows plasticity at active synapses, such that synapses close to the anode are depolarized and those close to the cathodes are hyperpolarized due to the electric field. Peri-anodal synapses are more susceptible to LTP-type synaptic plasticity, because NMDARs are in part gated by post-synaptic membrane depolarization (Fig. 1).
Figure 1.
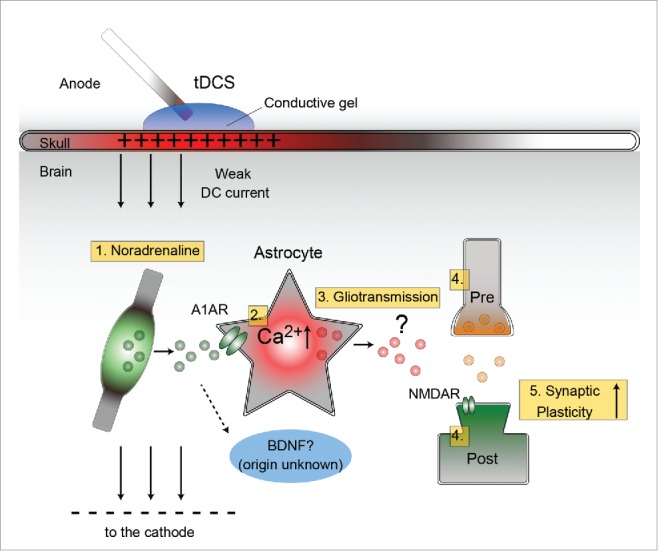
Schematic diagram for tDCS-induced Ca2+ elevation in the mouse cortex (modified from ref. 13). 1. Direct current activates noradrenergic fibers or boutons to release noradrenaline (NA). 2. Volume-transmitted NA induces astrocytic Ca2+ elevation thorough the α-1 adrenergic receptor (A1AR). BDNF signaling pathways have also been reported to be activated by tDCS. The causal link between NA and BDNF is yet to be shown. 3. Gliotransmitters (glutamate, ATP, D-serine, etc.) are possibly released from astrocytes. 4. Direct current also induces subthreshold depolarization of neuronal processes at the peri-anodal side. 5. NMDAR-dependent long-term synaptic plasticity is promoted for the active synapses.
Noradrenergic activation and alleviation of depressive behavior
In our experiments, tDCS-induced Ca2+ elevation is blocked by noradrenergic neuron ablation or α-1 adrenergic receptor (A1AR) blockade, suggesting that tDCS results in a noradrenaline release in the cortex. Supposing that noradrenaline release is a direct effect of tDCS, the question arises as to why noradrenaline, but not other neurotransmitters, appears to be responsible to trigger astrocytic Ca2+ elevation. We speculate that other transmitters like glutamate, gamma aminobutyric acid (GABA), and acetylcholine (ACh) are secreted as well. However, glutamate and GABA are subject to rapid uptake by respective transporters. ACh is a volume-transmitted neuromodulator; however, its enzymatic breakdown is quite fast due to the abundance of acetylcholinesterase. The efficient elimination of these neurotransmitters from the extracellular space could be the reason why their astrocytic receptors are not activated. More detailed information on cellular morphology and distributions of enzymes, transporters, and receptors will allow simulation studies to predict the dwell times of various neurotransmitters in the extracellular space.
The A1AR is a Gq-type GPCR abundantly expressed astrocytes. For instance, a cell type specific transcriptomic database suggests predominant expression of A1ARs over muscarinic ACh receptors.39 Also, in vivo Ca2+ imaging studies have shown that A1AR is the principal receptor for spontaneous and startle-stimulus-driven Ca2+ elevations in astrocytes.17,18 A recent study showed that tDCS induces epigenetic changes, including the BDNF locus in the mouse brain.12 Considering another recent report that noradrenergic β receptor signaling promotes NMDAR-dependent long-term potentiation through epigenetic changes in neurons,40 tDCS-induced noradrenergic signaling conceivably contributes to neural plasticity and epigenetic modifications, in consort with the astrocyte-mediated signaling.
Noradrenaline and serotonin enhancers (e.g. by inhibiting reuptake) are commonly used to treat depression. In addition to cortical plasticity, we have shown that the same tDCS application (0.1 mA, for 10 minutes) can alleviate a mouse model of depression. This effect lasted for at least several days despite single dose application. Similar to tDCS-assisted cortical plasticity experiments, the antidepressant tDCS effect was dependent on A1ARs and IP3R2s. These results propose the possibility that certain aspects of depression are treatable through noradrenaline signaling. Further, in line with work that showed positive effects of glial ATP transmission,41 our data support the view that astrocytes are considered to be a potential therapeutic target for depression. Given that astrocytes cover the vasculature by their endfoot processes, astrocytes could have easier access to drugs administered through the bloodstream. Future drug discovery studies may find astrocytic GPCR activation effective in treating mood disorders.
Adult neurogenesis, cognition and depression
Enhancements in adult neurogenesis has been implied to be a potential mechanism for long-term tDCS effects such as cognitive enhancement and antidepressant effects (for reviews, see refs. 42-44). Recently, Braun et al. showed that multi-session tDCS facilitated rehabilitation after focal cerebral ischemia and promoted adult neurogenesis in a polarity-independent manner.45 The duration of positive effects for depressive behavior in our experiment may be related to adult neurogenesis. Among neurotrophic factors for neurogenesis, BDNF has been shown to be related to tDCS,11,12 as mentioned earlier. BDNF is also well-recognized to have antidepressant effects.46 Interestingly, in dissociated hippocampal neuron culture, application of noradrenaline was reported to increase BDNF47 Although the origin of tDCS-induced BDNF is yet to be identified, one possibility is the induction of BDNF through activation of adrenergic receptors. Other possible sources of BDNF include microglia48 and circulation.49 In addition to neurogenesis, NMDAR-dependent synapse formation of adult-born neurons in the dentate gyrus has been shown to be related to astrocytic Ca2+ signaling and vesicular release of D-serine.50 Neurogenesis and the subsequent spine-genesis can be a viable candidate mechanism that explains the long-term effects of tDCS and should be studied in the future by molecular genetics that can manipulate neurogenesis (e.g., ref. 51).
Scaling to human tDCS
While we demonstrate that tDCS application resulted in a cortex-wide elevation of Ca2+ in mice, Ca2+ elevation was the largest at the anodal site and attenuated with distance. This hints at the possibility that the Ca2+ elevation is a function of electric field, which has been simulated in recent studies.52 One potential mechanism for the noradrenaline release is by action potentials reaching at noradrenergic axon boutons. If the applied electric field is strong enough to elicit action potentials in noradrenergic nerve fibers, (which was not monitored in ref. 13), the Ca2+ response should be more evenly distributed because of the diffuse pattern of noradrenergic innervation. Moreover, other kinds of axons, including the abundant glutamatergic axons, should also produce action potentials; in which case, it would have a profound effect on a mouse's behavioral state. As a matter of fact, no obvious changes were observed in the frequency of Ca2+ events in G-CaMP7 positive, presumably glutamatergic, cortical neurons imaged in G7NG817 mice. No obvious behavioral changes were observed during or after tDCS in mice, either. Conversely, we did not find compelling evidence that contradict terminal secretion of neuromodulators by tDCS, although the exact mechanism is yet to be identified. Assuming electric field-dependent neuromodulator secretion, whether tDCS induces cortex-wide Ca2+ elevation occurs in the human brain would depend on the spread of the electric field. Given Ca2+ imaging on human brain is not yet possible, we will need to rely on modeling studies to assess the spatial spread of Ca2+ elevation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs. Derek L. Buhl, and Youichi Iwai for comments on earlier versions of the manuscript.
Funding
This work was supported by the RIKEN Brain Science Institute, KAKENHI grants (26117520, 16K13116, 16H01888), and HFSP (RGP0036/2014).
References
- [1].Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, et al.. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2015; 127:1031-48; PMID:26652115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, et al.. Transcranial direct current stimulation: State of the art 2008. Brain Stimul 2008; 1:206-23; PMID:20633386; http://dx.doi.org/ 10.1016/j.brs.2008.06.004 [DOI] [PubMed] [Google Scholar]
- [3].Kuo M-F, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 2014; 85 Pt 3:948-60; http://dx.doi.org/ 10.1016/j.neuroimage.2013.05.117 [DOI] [PubMed] [Google Scholar]
- [4].Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2009; 2:215-228.e3; PMID:20161507; http://dx.doi.org/ 10.1016/j.brs.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 1965; 28:166-85; PMID:14244793 [DOI] [PubMed] [Google Scholar]
- [6].Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro J Physiol Blackwell Science Ltd; 2004; 557:175-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol 1964; 172:369-82; PMID:14199369; http://dx.doi.org/ 10.1113/jphysiol.1964.sp007425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009; 106:1590-5; PMID:19164589; http://dx.doi.org/ 10.1073/pnas.0805413106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002; 125:2238-47; PMID:12244081; http://dx.doi.org/ 10.1093/brain/awf238 [DOI] [PubMed] [Google Scholar]
- [10].Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol Blackwell Publishing Ltd; 2003; 553:293-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010; 66:198-204; PMID:20434997; http://dx.doi.org/ 10.1016/j.neuron.2010.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Podda MV, Cocco S, Mastrodonato A, Fusco S, Leone L, Barbati SA, Colussi C, Ripoli C, Grassi C. Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci Rep 2016; 6:22180; http://dx.doi.org/ 10.1038/srep22180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, Mikoshiba K, Itohara S, Nakai J, Iwai Y, et al.. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 2016; 7:11100; http://dx.doi.org/ 10.1038/ncomms11100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ohkura M, Sasaki T, Sadakari J, Gengyo-Ando K, Kagawa-Nagamura Y, Kobayashi C, Ikegaya Y, Nakai J. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS One 2012; 7:e51286; PMID:23240011; http://dx.doi.org/ 10.1371/journal.pone.0051286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 2011; 31:18155-65; PMID:22159127; http://dx.doi.org/ 10.1523/JNEUROSCI.5289-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 2008; 18:2789-95; PMID:18372288; http://dx.doi.org/ 10.1093/cercor/bhn040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014; 82:1263-70; PMID:24945771; http://dx.doi.org/ 10.1016/j.neuron.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ding F, O'Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. α1-Adrenergic receptors mediate coordinated Ca(2+) signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 2013; 54:387-94; PMID:24138901; http://dx.doi.org/ 10.1016/j.ceca.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Petravicz J, Fiacco TA, McCarthy KD. Loss of Ins(1,4,5)P3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 2008; 28:4967-73; PMID:18463250; http://dx.doi.org/ 10.1523/JNEUROSCI.5572-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca(2+) detection and modulation of synaptic release by astrocytes. Nat Neurosci 2011; 14:1276-84; PMID:21909085; http://dx.doi.org/ 10.1038/nn.2929 [DOI] [PubMed] [Google Scholar]
- [21].Kanemaru K, Sekiya H, Xu M, Satoh K, Kitajima N, Yoshida K, Okubo Y, Sasaki T, Moritoh S, Hasuwa H, et al.. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep 2014; 8:311-8; PMID:24981861; http://dx.doi.org/ 10.1016/j.celrep.2014.05.056 [DOI] [PubMed] [Google Scholar]
- [22].Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci 2015; 18:708-17; PMID:25894291; http://dx.doi.org/ 10.1038/nn.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron 2008; 59:932-46; PMID:18817732; http://dx.doi.org/ 10.1016/j.neuron.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Araque A, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 2014; 81:728-39; PMID:24559669; http://dx.doi.org/ 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity Nat Rev Neurosci 2014; 15:327-35 [DOI] [PubMed] [Google Scholar]
- [26].Hirase H, Iwai Y, Takata N, Shinohara Y, Mishima T. Volume transmission signalling via astrocytes. Phil Trans R Soc B 2014; 369:20130604; PMID:25225097; http://dx.doi.org/ 10.1098/rstb.2013.0604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol 1992; 67:197-202; PMID:1552319 [DOI] [PubMed] [Google Scholar]
- [28].Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci 1992; 12:1826-38; PMID:1578273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 2005; 6:626-40; PMID:16025096; http://dx.doi.org/ 10.1038/nrn1722 [DOI] [PubMed] [Google Scholar]
- [30].Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc Natl Acad Sci U S A 2003; 100:15194-9; PMID:14638938; http://dx.doi.org/ 10.1073/pnas.2431073100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010; 463:232-6; PMID:20075918; http://dx.doi.org/ 10.1038/nature08673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 2012; 5:ra26; PMID:22472648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tanaka M, Shih P-YY, Gomi H, Yoshida T, Nakai J, Ando R, Furuichi T, Mikoshiba K, Semyanov A, Itohara S. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol Brain 2013; 6:6; PMID:23356992; http://dx.doi.org/ 10.1186/1756-6606-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al.. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4:147ra111; PMID:22896675; http://dx.doi.org/ 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al.. Sleep drives metabolite clearance from the adult brain. Science 2013; 342:373-7; PMID:24136970; http://dx.doi.org/ 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wachter D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Kutschenko A, Rohde V, Liebetanz D. Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp Neurol 2011; 227:322-7; PMID:21147105; http://dx.doi.org/ 10.1016/j.expneurol.2010.12.005 [DOI] [PubMed] [Google Scholar]
- [37].Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 2016; 19:182-9; http://dx.doi.org/ 10.1038/nn.4201 [DOI] [PubMed] [Google Scholar]
- [38].Nuriya M, Hirase H. Involvement of astrocytes in neurovascular communication. Prog Brain Res 2016; 225:41-62; PMID:27130410; http://dx.doi.org/ 10.1016/bs.pbr.2016.02.001 [DOI] [PubMed] [Google Scholar]
- [39].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al.. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34:11929-47; PMID:25186741; http://dx.doi.org/ 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maity S, Jarome TJ, Blair J, Lubin FD, Nguyen PV. Noradrenaline goes nuclear: epigenetic modifications during long-lasting synaptic potentiation triggered by activation of β-adrenergic receptors. J Physiol 2016; 594:863-81; PMID:26574176; http://dx.doi.org/ 10.1113/JP271432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, et al.. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 2013; 19:773-7; PMID:23644515; http://dx.doi.org/ 10.1038/nm.3162 [DOI] [PubMed] [Google Scholar]
- [42].Bennabi D, Pedron S, Haffen E, Monnin J, Peterschmitt Y, Van Waes V. Transcranial direct current stimulation for memory enhancement: from clinical research to animal models. Front Syst Neurosci 2014; 8:159; PMID:25237299; http://dx.doi.org/ 10.3389/fnsys.2014.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pelletier SJ, Cicchetti F. Cellular and molecular mechanisms of action of transcranial direct current stimulation: Evidence from in vitro and in vivo models. Int J Neuropsychopharmacol 2015; 18:pyu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ebajemito JK, Furlan L, Nissen C, Sterr A. Application of transcranial direct current stimulation in neurorehabilitation: The modulatory effect of sleep. Front Neurol 2016; 7:54; PMID:27092103; http://dx.doi.org/ 10.3389/fneur.2016.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Braun R, Klein R, Walter HL, Ohren M, Freudenmacher L, Getachew K, Ladwig A, Luelling J, Neumaier B, Endepols H, et al.. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp Neurol 2016; 279:127-36 [DOI] [PubMed] [Google Scholar]
- [46].Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol 2010; 70:289-97; http://dx.doi.org/ 10.1002/dneu.20758 [DOI] [PubMed] [Google Scholar]
- [47].Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal 2007; 19:114-28; PMID:16876982; http://dx.doi.org/ 10.1016/j.cellsig.2006.05.028 [DOI] [PubMed] [Google Scholar]
- [48].Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci 1996; 16:2508-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Radka SF, Hoist PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res 1996; 709:122-30; PMID:8869564; http://dx.doi.org/ 10.1016/0006-8993(95)01321-0 [DOI] [PubMed] [Google Scholar]
- [50].Sultan S, Li L, Moss J, Petrelli F, Cassé F, Gebara E, Lopatar J, Pfrienger FW, Bezzi P, Bischofberger J, et al.. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 2015; 88:957-72; http://dx.doi.org/ 10.1016/j.neuron.2015.10.037 [DOI] [PubMed] [Google Scholar]
- [51].Niibori Y, Yu T-S, Epp JR, Akers KG, Josselyn SA, Frankland PW. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun 2012; 3:1253; http://dx.doi.org/ 10.1038/ncomms2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rahman A, Lafon B, Bikson M. Multilevel computational models for predicting the cellular effects of noninvasive brain stimulation. Prog Brain Res 2015; 222:25-40; PMID:26541375; http://dx.doi.org/ 10.1016/bs.pbr.2015.09.003 [DOI] [PubMed] [Google Scholar]


