ABSTRACT
Coordination of the specific functions of α5β1 and αvβ3 integrins is crucial for the precise regulation of cell adhesion, spreading and migration, yet the contribution of differential integrin-specific crosstalk to these processes remains unclear. To determine the specific functions of αvβ3 and α5β1 integrins, we used nanoarrays of gold particles presenting immobilized, integrin-selective peptidomimetic ligands. Integrin binding to the peptidomimetics is highly selective, and cells can spread on both ligands. However, spreading is faster and the projected cell area is greater on α5β1 ligand; both depend on ligand spacing. Quantitative analysis of adhesion plaques shows that focal adhesion size is increased in cells adhering to αvβ3 ligand at 30 and 60 nm spacings. Analysis of αvβ3 and α5β1 integrin clusters indicates that fibrillar adhesions are more prominent in cells adhering to α5β1 ligand, while clusters are mostly localized at the cell margins in cells adhering to αvβ3 ligand. αvβ3 integrin clusters are more pronounced on αvβ3 ligand, though they can also be detected in cells adhering to α5β1 ligand. Furthermore, α5β1 integrin clusters are present in cells adhering to α5β1 ligand, and often colocalize with αvβ3 clusters. Taken together, these findings indicate that the activation of αvβ3 integrin by ligand binding is dispensable for initial adhesion and spreading, but essential to formation of stable focal adhesions.
KEYWORDS: block copolymer micellar nanolithography, focal adhesion, integrin crosstalk, integrins, peptidomimetic, receptor clustering, surface nanopatterning
Introduction
Cell-extracellular matrix (ECM) interactions, mediated by integrins, are crucial for cell adhesion, migration, proliferation and differentiation. Upon binding to ECM proteins, the lateral clustering of integrins and the recruitment of intracellular adhesome proteins to the attachment site leads to the formation of focal adhesions (FAs) and the assembly of actin stress fibers.1 Various integrin types participate in the formation and maturation of FAs. Notably, FA formation involves several integrins (e.g., α5β1 and αvβ3), each of which is known to perform a different function.2-4
The selective enrichment of α5β1 integrins in fibrillar adhesions, which arise from mature FAs following the centripetal translocation of the receptors, is important for fibronectin fibrillogenesis, whereas αvβ3 integrins remain in FAs, exerting stabilizing functions.2,3,5-7 Accordingly, it was proposed that this localization pattern and segregation could be due to the specific mechanotransduction functions and signaling pathways associated with α5β1 and αvβ3 integrins. Thus, identification of the integrin-specific triggers for specific biochemical and biomechanical changes in FAs remain a challenge, necessitating novel methods that differentially control integrin activation and localization.
The functional diversity of α5β1 and αvβ3 integrins has been shown through their regulation of cell adhesion forces. The catch bond established by β1 integrin with fibronectin might adjust adhesion strength according to the underlying mechanical tension, while αvβ3 integrin binding enables the structural reinforcement of the integrin-actin linkage.4,8 Downstream signaling requires the cooperation of both integrin types, since expression of both αv- and α5β1 integrins is necessary to induce myosin II activation in rigidity sensing and migration signaling.9-11 An increase in cell traction forces is due to local activation of β1 but not β3 integrins; however, enhancing the expression of αvβ3 integrin can compensate for the loss of α5β1 integrin in force transmission.12-14
Specific activation of α5β1 and αvβ3 integrins in adhesion-mediated responses has thus far been investigated either by culturing cells on specific ECM proteins, by altering their expression profile, or by using RGD-based antagonists.3,5,9,15 Recently, material surfaces for in vitro studies have been coated with highly selective compounds that bind and specifically activate α5β1 or αvβ3 integrins.13,16-18 Ligand immobilization and receptor activation are prerequisites for αvβ3 integrin clustering and β1 integrin activation within FAs.19,20
To control the clustering of integrins we have developed surface patterning strategies that enable the presentation of integrin ligands at high spatial resolution.21,22 (Given that spacing below 60 nm promotes and stabilizes FA formation, we recently determined that RGD ligand spacing modulates β3 integrin activation and force transmission.23 Here, we combine tunable ligand spacing by surface patterning with the immobilization of α5β1 or αvβ3 integrin selective ligands,16 to show that α5β1 integrin clustering enhances cell spreading, and is dependent on ligand spacing: only at spacings below 60 nm, mature FAs are formed. Furthermore, αvβ3 integrin clustering is essential to this process.
Results
Cell adhesion to α5β1 integrin selective ligands leads to faster spreading, and an increase in projected cell area
We first monitored human osteosarcoma U2OS cells spreading on nanopatterned surfaces with gold nanoparticles spaced 30, 60, or 90 nm apart, and functionalized with either α5β1 or αvβ3 integrin selective ligands. Cell spreading kinetics during the first 60 min of adhesion is shown in Fig. 1 (see also Supplementary Movies 1-6, and Fig. S1). The smaller spacing led to a marked increase in cell spreading velocity and projected cell area, compared to cell spreading on substrates with larger spacings, regardless of the type of ligand immobilized on the surfaces. At distances of 30 nm and 60 nm, the projected cell area was greater, and its progression faster, when cells bound to the surface via α5β1 integrins (Fig. 1A and B and Fig. S1). Such differences were not observed on the substrate with 90 nm particle spacing (Fig. 1A). Moreover, the maximal area of cells adhering to α5β1 integrin ligands at 30 nm spacing was significantly greater than that displayed by cells adhering to αvβ3 integrin ligands at that spacing (Fig. 1B). As the interparticle spacing increased, the maximal cell area of cells adhering to either ligand became comparable.
Figure 1.
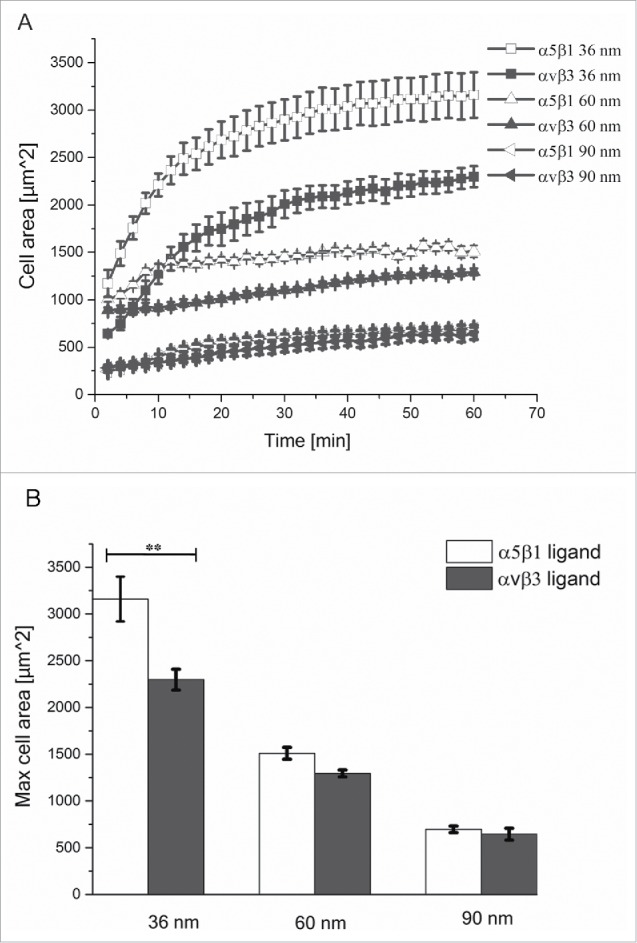
Cell spreading kinetics on nanopatterned surfaces functionalized with integrin selective ligands. (A) Progression of projected cell area during spreading on nanopatterned surfaces with interparticle distances of 30, 60, or 90 nm, and functionalized with α5β1 (white) and αvβ3 (black) integrin selective ligands. (B) Maximum projected cell area on the different surfaces. Error bars indicate SEM of 3 independent repeats.
Cells adhering to the selective αvβ3 integrin ligands form larger focal adhesions
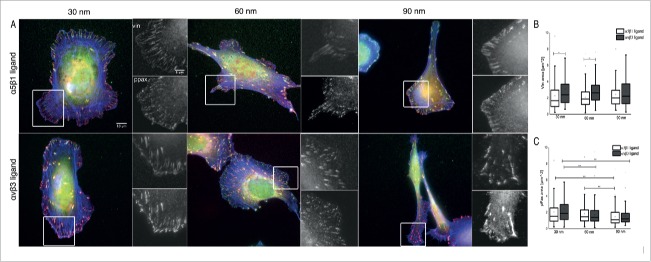
To determine the effects of integrin type and integrin lateral spacing on focal adhesion size and composition, cells were immunostained for vinculin, phospho-paxillin (PY118), and actin after 4 hr of adhesion to the surfaces (Fig. 2). Notably, cells formed peripheral FAs when adhering to αvβ3 integrin ligands, and fibrillar structures when adhering to the α5β1 integrin ligand. Vinculin clusters were larger in cells adhering to the αvβ3 integrin ligand at all spacings, compared to clusters formed on the α5β1 integrin ligand (Fig. 2A, and Fig. 2B, box plot). Significant differences in vinculin cluster size are observed only in cells adhering to the αvβ3 integrin ligand at 30 and 60 nm spacings (Fig. 2A, small inserts left and middle), whereas at the 90 nm spacing, only a small increase in cluster size was seen, compared to cells adhering to the α5β1 integrin ligand (Fig. 2A small inserts right).
Figure 2.
Focal adhesions in cells adhering to nanopatterned surfaces functionalized with integrin α5β1 and αvβ3 integrin selective ligands. (A) Indirect immunofluorescence staining of vinculin (green), phosphorylated paxillin (red), and actin (blue) in U2OS cells. Insets are a magnification of separate stainings for vinculin and phosphorylated paxillin, in the cell region delineated by the white box. Cells adhering for 4 hr to α5β1 (first row) and αvβ3 integrin selective ligands (second row) at spacings of 30 nm (left), 60 nm (middle), and 90 nm (right) were imaged by wide-field microscopy. (B) Analysis of vinculin cluster size; and (C) Analysis of phosphorylated paxillin (PY118) cluster size in U2OS cells. Box plots indicate cluster area values between 25% and 75%, and whiskers between 10% and 90% of the data range. The line in the box plot indicates the median value. p* < 0.001.
The phosphorylation of paxillin promotes the assembly of focal adhesions, while non-phosphorylated paxillin is commonly associated with fibrillar adhesions.24 In FAs, paxillin phosphorylation of cells adhering to the 2 different integrin ligands shows a similar trend (Fig. 2A and Fig. 2C, box plot). For each integrin ligand type, however, the spacing between gold nanoparticles affects paxillin phosphorylation. In cells adhering to the αvβ3 integrin ligand, a significant difference in paxillin tyrosine phosphorylation is observed between cells adhering to substrates with all 3 interparticle spacings, while for the α5β1 integrin ligand, such a difference is observed only when comparing the 60 nm and 90 nm spacings.
It is noteworthy that activities promoting differential spreading and focal adhesion organization, both induced by αvβ3 and α5β1 integrin ligands on nanopatterns, can readily be obtained with the generic adhesive proteins vitronectin and fibronectin, respectively. This is illustrated in Fig. S2, showing spreading and FA formation patterns similar to those described above, based on labeling of the cells for diverse plaque proteins. In this experiment, cells adhering to fibronectin and vitronectin coatings were further treated with inhibitory antibodies that block αvβ3 and α5β1 adhesion, respectively. It was further shown that when both integrins were allowed to interact with the matrix, the “α5β1 phenotype” was dominant.
αvβ3 integrin clusters are present in cells adhering to the α5β1 integrin selective ligand
We next utilized indirect immunofluorescence staining and expression of fluorescence proteins to study the localization patterns of α5 and αvβ3 integrins in cells adhering to nanopatterned surfaces presenting α5β1 and αvβ3 integrin selective ligands (Fig. 3, and Supplementary Movies 7 and 8). Representative cells adhering to α5β1 or αvβ3 integrin ligands are shown in Fig. 3A for the 30 nm spacing, and in Fig. 3B for the 60 nm spacing. Cells adhering to the 90 nm nanopatterns are not shown, since the integrin clusters were hardly detectable, and the small focal adhesion clusters that did form were essentially identical for both ligands (Fig. 2).
Figure 3.
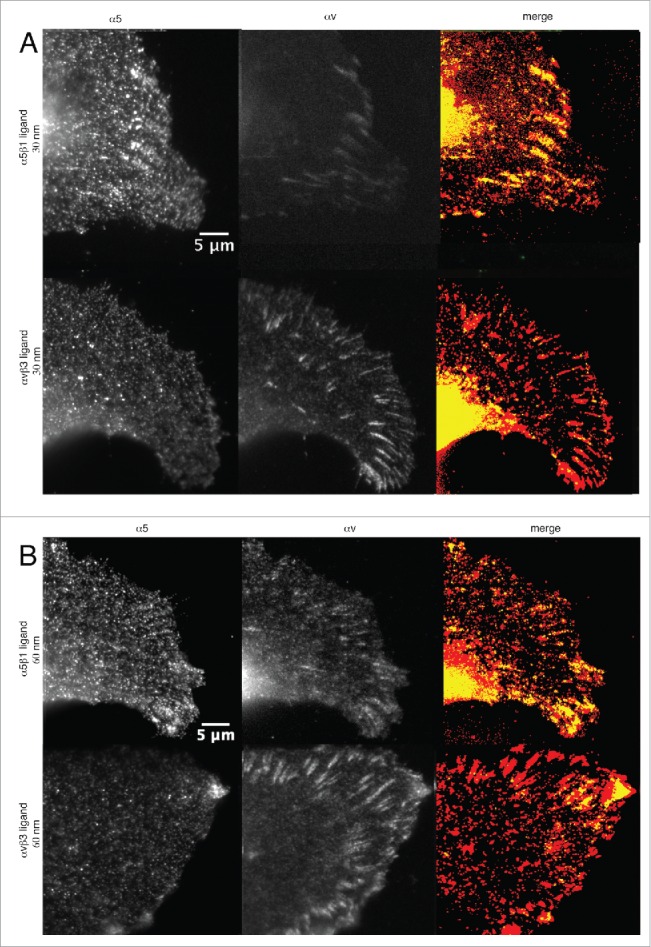
α5 and αvβ3 clusters in U2OS cells adhering to nanopatterned surfaces functionalized with α5β1 and αvβ3 integrin selective ligands. (A) Cells adhering to surfaces with 30 nm interparticle spacing; and (B) Cells adhering to surfaces with 60 nm particle spacing. Upper row: Cells adhering to α5β1 integrin selective ligands. Lower row: Cells adhering to αvβ3 integrin selective ligands. Left: Staining for α5 clusters. Middle: Staining for αvβ3 clusters. Right: Lookup table displaying the colocalization of α5 and αvβ3 integrin clusters (pixel with positive signals for both integrins are shown in yellow).
Interestingly, αvβ3 integrin clusters were observed in all cells adhering to the 2 integrin selective ligands, regardless of particle spacing (Fig. 3A and B, lower middle panel), similar to cells adhering to fibronectin-coated surfaces (Fig. S3A, upper row). At the 30 nm spacing, αvβ3 and α5 integrin clusters assembled and colocalized at the periphery of cells adhering to the α5β1 integrin selective ligand. When adhering to a 60 nm-spaced nanopatterned surface, αvβ3 integrin clusters were still present, though smaller in size, compared to those on the 30 nm-spaced surface. In cells adhering to the αvβ3 integrin selective ligand, only αvβ3 integrin clusters were present, since α5 staining appears very diffuse, and very few fibrillar adhesions were observed (Fig. 3A and B, lower left panel). The assembly of αvβ3 integrin clusters was comparable to that observed in cells adhering to vitronectin-coated surfaces (Fig. S3A, lower row).
Inhibition of αvβ3 integrins does not impair adhesion and spreading, but hinders focal adhesion assembly
Integrins that are not bound to the immobilized ligands can still be recruited to focal adhesions via their cytoplasmic components, as was previously shown, using chimeric integrins, in which different integrin transmembrane and cytoplasmic domains were fused to an irrelevant extracellular domain.25 Given that integrin can be recruited to FAs via their cytoplasmic domains, we further investigated the specific contribution of αvβ3 integrin recruitment for adhesion and FA assembly, by means of integrin function blocking. Here, prior to being seeded on the surfaces, cells in suspension were incubated with the soluble form of the peptidomimetic ligands, which selectively bind to α5β1 or αvβ3 integrins, but lack the thiol group that binds to the gold nanopatterns.26 Thus, the receptors are still physically present on the cell membrane, yet the selective ligand binds to them, and inhibits their function.
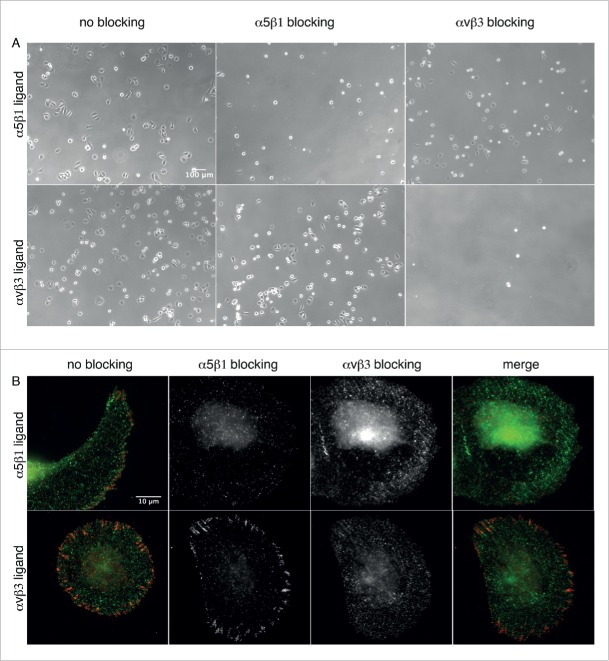
Blocking of α5β1 integrins in U2OS cells plated on the α5β1 integrin selective ligand inhibited adhesion, whereas blocking of αvβ3 integrins did not affect the adhesion of U2OS cells on these substrates (Fig. 4A, upper row). Accordingly, cells adhering to the αvβ3 integrin selective ligand could still attach and spread when α5β1 integrins were blocked, whereas no cell adhesion was observed when αvβ3 integrins were blocked (Fig. 4A, lower row). Thus, the ligands mediated by either integrin type proved to be specific for adhesion.
Figure 4.
α5β1 and αvβ3 integrin blocking. (A) Phase contrast micrographs of U2OS cells incubated with α5β1 and αvβ3 integrin selective ligands, and seeded on nanopatterned surfaces functionalized with these ligands. Upper row: Cells adhering to α5β1integrin selective ligands. Lower row: Cells adhering to αvβ3 integrin selective ligands. Left: No integrin blocking. Middle: α5β1 integrin blocking. Right: αvβ3 integrin blocking. (B) Indirect immunofluorescence staining of α5 (green) and αvβ3 clusters (red) in U2OS cells pre-incubated with the integrin selective ligands. Cells were seen to adhere to nanopatterned surfaces functionalized with α5β1 (upper row) and αvβ3 integrin selective ligands (lower row).
We next performed staining for α5β1 and αvβ3 integrins in U2OS cells, in which α5β1 and αvβ3 integrins were pre-blocked with selective ligands (Fig. 4B). Interestingly, αvβ3 integrin blocking did not impair cell adhesion and spreading, but both α5β1 and αvβ3 integrin clusters were not observed in U2OS cells adhering to the α5β1 integrin selective ligand (Fig. 4B, upper row). Blocking of α5β1 integrins in cells adhering to αvβ3 integrin selective ligands does not impair neither adhesion and spreading, nor assembly of αvβ3 integrin clusters (Fig. 4B, lower row). Similarly, in cells adhering to vitronectin we blocked α5β1 integrin to prevent integrin binding to secreted fibronectin (Fig. S3B, middle row). Thus, in this condition cell adhesion is mediated only by αvβ3 integrin, while α5β1 integrin clustering is inhibited. We observed that adhesion and spreading were less efficient than in cells plated on fibronectin (Fig. S3B, upper row) since cells appear smaller and less cells could attach to the substrate, but αvβ3 integrin clusters were present at the cell periphery. When plated on a mixture of fibronectin and vitronectin, spreading and formation of both α5β1 and αvβ3 integrin clusters was restored (Fig. S3B, lower row).
These results suggest that the adhesion of cells to specific ECM proteins influences cell spreading and integrin clustering, where α5β1 integrin is important for the initial spreading and αvβ3 integrin supports the stabilization of FAs.
Discussion
In this study, we aimed to elucidate the differential effects of matrix adhesion via α5β1 and αvβ3 integrins on cell spreading and FA assembly. To that end, we applied surface nanopatterning consisting of 8 nm-sized gold nanoparticles with interparticle spacings of 30, 60 or 90 nm, and functionalized with α5β1 and αvβ3 integrin selective ligands.16,21 On such surfaces, it is possible to control the binding sites of single integrins to the surface, and to modulate the lateral clustering of the receptors during cell adhesion.
Previously, we demonstrated that a ≤ 60 nm separation of gold nanoparticles functionalized with cyclic RGD ligands favors cell adhesion and focal adhesion assembly, as a sufficient number of ligands are available for interactions with the receptors.21,27 Furthermore, we determined that at a spacing of ≥70 nm, cells exhibited increased motility and focal adhesion instability, resulting in excessive retraction events.22 More recently, by combining surface nanopatterning with molecular tension probes, we could show that the lateral clustering of integrins affects integrin tension at single bonds with RGD ligands, a process tightly coupled to actomyosin-driven tension.23 However, this critical nanoscale spacing between cyclic RGD peptides left unclear the question whether the α5β1 and/or αvβ3 integrins that recognize these ligands, would cooperate and contribute in a manner similar to the formation of focal adhesions upon modulation of integrin clustering. Toward this aim, α5β1 and αvβ3 integrin selective peptidomimetics have been developed and linked to gold and titanium substrates to test the specific adhesion of α5β1 or αvβ3-expressing fibroblasts to the prepared surfaces.18,28 The use of such selective ligands for cell adhesion studies is a powerful means to determine the different functions of these integrin types, without perturbing the expression of the cell receptors themselves.3
The effects on cell adhesion dynamics and focal adhesion formation, seen following selective α5β1 or αvβ3 integrin clustering, reveal major differences between the 2 integrins. Although delayed and reduced spreading on surfaces with 90 nm particle spacings is still observed for both ligands, spreading kinetics are enhanced in cells adhering to the α5β1 integrin selective ligand at spacings of 30 and 60 nm, whereas spreading of cells adhering to the αvβ3 integrin selective ligand remains slower (Fig. 1 and Fig. S1). As shown in the kymograph analysis, the increased spreading in cells that bind to the α5β1 integrin selective ligand is due to increased lamellipodial protrusions. Thus, at the cellular level, adhesion dynamics to the selective ligands reflect that previously observed for cell adhesion to fibronectin and vitronectin coatings.6
The possibility that increased spreading mediated by α5β1 integrin binding dominates over αvβ3 integrins, rather than blocking of spreading being dominant, is validated by observations that spreading of cells plated on mixed fibronectin and vitronectin coatings resembles that occurring on fibronectin (Baruch Zimerman, unpublished findings; and Fig. S3). Charo IF et al.29 suggested that the increased spreading on fibronectin is due to the cooperative effect of α5β1 and αvβ3 integrins in promoting recognition and binding to fibronectin. The kinetics of spreading and formation of protrusions at the cell edges regulate cell traction forces,30 which are dependent on β1 integrin activation, as determined by means of knockout cells, blocking approaches, and selective binding of this integrin type to specific ligands.12,13 The reinforcing behavior of β1 integrin bonds appears to be necessary for spreading, since reduced surface expression of α5β1 integrins negatively impacts spreading and stress fiber formation, but increases cortical actin assembly.31
Here, we observed that blocking α5β1 integrins in cells adhering to the αvβ3 integrin selective ligand, and blocking αvβ3 integrins in cells adhering to the α5β1 integrin selective ligand, do not impair cell adhesion and spreading. Rather, both α5β1 and αvβ3 integrin clusters are negatively affected only when αvβ3 integrin is blocked; i.e., that α5β1 selective adhesion to α5β1 ligands is not sufficient for focal adhesion formation when αvβ3 is blocked, and cannot be part of the adhesion cluster (Fig. 4, as compared with Fig. 3A). Also, αvβ3 alone cannot form clusters on the α5β1 ligand, when α5β1 is blocked (Fig. 4B).
A feedback mechanism involving integrin clustering and traction force generation may underlie mechanotransduction. Recently, Balcioglu HE et al.14 reported that αvβ3 integrin-mediated adhesion enables FA assembly even at low matrix stiffness, while traction force magnitude remains unvaried. It could be speculated that activated αvβ3 integrins might be important in traction force modulation, which is intimately tied to receptor lateral spacing and FA assembly. In fact, αvβ3 integrin cluster size appears to be dependent on such spacing, since clusters forming on surfaces with 30 nm particle spacing, are larger than those seen with 60 nm-spaced particles (Fig. 3). Moreover, our observations that vinculin cluster size is modulated by particle spacing on both integrin ligands, and that the clusters are larger in cells adhering to the αvβ3 integrin selective ligand, indicate that FAs are more stable and turnover might be reduced. The larger vinculin clusters in cells adhering to αvβ3 integrin implies also that higher forces might be present in these cells and contribute also to the stability of FAs, since vinculin is in the force-transducing layer and regulates force transmission within FAs (Fig. 2).23,32 On the contrary, FA signaling appears to be independent of the type of integrin bound to the surface, as indicated by staining for phosphorylated paxillin clusters (Fig. 2).
The fine-tuning of FA assembly might due to the following factors: (i) lateral spacing between single integrins and their clustering; (ii) the type of integrin recruited; and (iii) spatial distribution of different integrin types in FAs. The driving force behind αvβ3 integrin lateral association requires a certain density of activated proteins to maintain both the cluster, and FA stability.19 Rossier O. et al.7 reported that within FAs, β1 and β3 exhibit distinct dynamics at the nanoscale, due to differences in the relative amounts of each integrin type: the β3 immobile fractions are most abundant in FAs due to bond formation with talin and F-actin, whereas β1 integrins are less enriched, and exhibit rearward movements. The coupling of αvβ3 integrins with F-actin, and the observation of ventral F-actin waves, suggest a dependence on the extracellular environment, necessitating cycles of engagement and disengagement of integrin bonds to the extracellular matrix.33 Thus, the presence of αvβ3 integrin clusters in FAs of cells adhering to the α5β1 integrin selective ligand could be explained, considering that αvβ3 integrin clusters are physically necessary to stabilize FAs and to link to actin fibers, even when these integrins are not bound to the ligand on the surface (Fig. 3). It should be also noted that on the nanopatterned substrates cells cannot assemble extracellular matrix because of the presence of the polyethylene glycol layer between the nanoparticles, which prevents any protein binding and adsorption. Thus, the fibrillar structures formed in cells adhering to the α5β1 ligand should not be considered as functional fibrillar adhesion arising from the translocation of α5β1 integrins upon assembly of extracellular matrix fibers.2,5
Here, we demonstrated that the clustering behavior and selective binding of α5β1 and αvβ3 integrins regulate cell adhesion and FA assembly. We achieved spatial control of integrin clustering type by using a tool for surface nanopatterning of integrin ligands, which enables precise control of receptor localization. However, the mechanism underlying the spatio-temporal regulation of bound and non-bound integrins in FAs still remains unclear. The current understanding of FA molecular composition is limited, though recent studies using high-resolution microscopy have begun to elucidate the dynamics of FAs at the nanoscale, and its impact on signaling. Combining our nanopatterning tools with high-resolution microscopy approaches will enable us to elucidate the specific dynamics of α5β1 and αvβ3 integrins localized in a FA, by means of controlled ligand/receptor spatial organization. Thus, it would be possible to determine not only the spatial organization of single bound and non-bound integrins, but also how different integrin types signal each other, in response to chemical and physical properties of the extracellular matrix. Future studies should also clarify the effects of integrin mobility and spatial organization in clusters on cell signaling responses.
Materials and methods
Preparation and functionalization of nanopatterned surfaces
Nanopatterned substrates were prepared by block copolymer micelle nanolithography, as previously described.34,35 Briefly, glass coverslips were either dip-coated or spin-coated with a monolayer of polystyrene-block-poly[2-vinylpyridine(HAuCl4)] (Sigma Aldrich 520918 HauCl4) diblock copolymer micelles in o-xylene. To obtain the interparticle distances of 30, 60 and 90 nm, polystyrene (288)-block-poly (2-vinylpyridine)(119), polystyrene (1056)-block-poly (2-vinylpyridine)(671) and polystyrene (1824)-block-poly (2-vinylpyridine)(523) (Polymer Source Inc. P4554-S2VP) were used. Following plasma treatment, the gold ions on the surfaces were reduced to gold and the polymer micelles were removed, resulting in quasi-hexagonal patterns of gold nanoparticles. To prevent cell adhesion and protein deposition between these nanoparticles, substrates were passivated with polyethylene glycol (PEG) (2000)-triethoxysilane.36 The nanoparticles were then functionalized with the integrin selective peptidomimetic ligands, which bind either α5β1 or αvβ3 integrins, at a concentration of 25 µM in MilliQ water for 4 hr at room temperature.16 The unbound ligands were removed by gentle shaking, and the samples were thoroughly rinsed with MilliQ water overnight. The surfaces were further washed with sterile PBS, prior to cell experiments. To characterize the patterned surfaces, the samples were imaged with scanning electron microscopy (LEO 1530 Gemini, Carl Zeiss). The interparticle distances and the order of the hexagonal patterns were determined by analyzing the micrographs with a custom-made Image J plug-in, written by Dr. Philippe Girard (University of Heidelberg).
Cell cultures
Human osteosarcoma U2OS cells (ATCC, HTB-96) were cultured in DMEM supplemented with 10% FBS, 1% L-glutamine and 1% penicillin streptomycin (all from Gibco Laboratories, 10938-025, 10500-064, 25030-024, 15140-122) at 37°C and 5% CO2. Prior to the experiments, cells were serum starved overnight. During the experiments, cells were gently detached with Accutase (Gibco, A11105-01) and seeded at a density of 600 cells/mm2 in DMEM supplemented with 1% FBS. For transfection, cells were seeded at a density of 1 × 105 cells/well in a 6-well plate, until they reached 80% confluency. Cells were transfected using Lipofectamin 2000 (Invitrogen 11668027) and 2 µg αv-mApple plasmid (Addgene 54866) in Opti-MEM (Gibco 31985-062) for 32 hr. Cells were then detached with Trypsin (Gibco, 25300-054) and plated on the nanopatterned surfaces.
Cell adhesion and spreading analysis
U2OS cells were seeded on the substrates, and allowed to adhere for 10 min. Time-lapse microscopy was then carried out at 37°C and 5% CO2. Phase contrast images of 5 random fields from each sample were acquired every 10 min over a period of 8 hr, using a DeltaVision RT system (Applied Precision, Inc.) on an Olympus IX inverted microscope equipped with a a 20x/0.50 Ph1 UPlanFl. Cell imaging was carried out with a cooled CCD camera (Photometrics); images were acquired with the Resolve 3D program. ImageJ software, version 1.48v (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997-2009) was used to: (i) measure cell area; (ii) generate kymographs (using the Multiple Kymograph Plug-in from J. Rietdorf, FMI Basel, and A. Seitz, EMBL Heidelberg); and (iii) adjust image brightness and contrast levels for presentation. Data (cell n = 15 for the 30 nm particle spacing; n = 22 for the 60 nm spacing; and n = 15 for the 90 nm spacing) were plotted in OriginLab 9.1; standard deviations and standard errors of the mean were calculated with the same software.
Immunocytochemical staining and fluorescence microscopy
For immunocytochemical staining, cells were plated for 4 hr on the nanopatterned surfaces. Cells were then washed with warm PBS, and fixed in 3.7% paraformaldehyde in PBS for 20 min. Post-fixation, cells were permeabilized with 0.1% TritonX-100 in PBS for 5 min, blocked with 1% bovine serum albumin (BSA) in PBS, and incubated for 1 hr with the following antibodies: mouse anti-human αvβ3 integrin (Millipore, MAB1976), rat anti-human α5-integrin (MABII, kindly provided by K. Yamada), mouse anti- human vinculin (Sigma, V9131), and rabbit anti-human zyxin (Synaptic Systems, 307011). Actin stress fibers were labeled with phalloidin-TRITC (Sigma, P1951). To visualize labeled membrane proteins, fixed cells were treated with secondary antibodies for 45 min (Invitrogen A-21238, A-11006, A-11001, A-11078). After washing with PBS, coverslips were mounted in Elvanol (Mowiol 4-88, Karl Roth & Co GmbH, 0713.1). Immunofluorescent images were obtained with the Delta Vision Spectris System, as described above. Cells were examined with a 60x/1.4 UPlanApo oil immersion objective (Olympus).
Focal adhesion size was measured by using ImageJ 1.48v; the results of the measurements were displayed in a box plot plotted with OriginLab 9.1. The statistical significance of variation in focal adhesion size for the different groups was determined by applying the Mann-Whitney U test in GraphPad Prism, version 6.0.
Integrin blocking experiments
For integrin blocking experiments, cells were resuspended in DMEM containing 1% FBS and 1% BSA. Afterwards, 100 µl of the cell suspension (1 × 106 cells/ml) was incubated with 10 µl of thiol-free ligands (25 µM) for 30 min at 4°C. The cells were then plated on substrates functionalized with α5β1- or αvβ3 integrin selective ligands, and fixed after 4 hr. Prior to fixation, samples were rinsed twice with PBS to remove unattached cells. Fixed cells were stained, as described above in the previous paragraph on immunocytochemical staining.
Supplementary Material
Abbreviations
- ECM
Extracellular matrix
- FAs
focal adhesions
- PEG
polyethylene glycol
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mrs. Barbara Morgenstern for her invaluable editorial help with the manuscript, and Dr. Janet Askari (University of Manchester, UK) for helpful discussions. J.P.S. is the Weston Visiting Professor at the Weizmann Institute of Science, and is a member of the Heidelberg Cluster of Excellence CellNetworks. B.G is the Erwin Neter Professor of Cell and Tumor Biology. A.C.A. and J.P.S. are members of the collaborative research cluster SFB1129 from the German Research Foundation.
Funding
Financial support was provided by the European Research Council under the European Union's Seventh Framework Program (FP/2007-2013)/ERC Grant Agreement no. 294852, and the BMBF/MPG network MaxSynBio.
References
- [1].Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 2009; 10:21-33; PMID:19197329; http://dx.doi.org/ 10.1038/nrm2593 [DOI] [PubMed] [Google Scholar]
- [2].Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz B-Z, Lin S, Lin DC, Bershadsky AD, Kam Z, et al.. Dynamics and segregation of cell–matrix adhesions in cultured fibroblasts. Nat Cell Biol 2000; 2:191-6; PMID:10783236; http://dx.doi.org/ 10.1038/35008607 [DOI] [PubMed] [Google Scholar]
- [3].Danen EHJ, Sonneveld P, Brakebusch C, Fässler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 2002; 159:1071-86; PMID:12486108; http://dx.doi.org/ 10.1083/jcb.200205014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roca-Cusachs P, Gauthier N, del Rio A, Sheetz MP. Clustering of alpha5beta1 integrins determines adhesion strength whereas alphavbeta3 and talin enable mechanotransduction. Proc Natl Acad Sci USA 2009; 106:16245-50; PMID:19805288; http://dx.doi.org/ 10.1073/pnas.0902818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pankov R, Cukierman E, Katz B-Z, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha5beta1 integrins promotes early fibronectin fibrillogenesis. J Cell Biol 2000; 148:1075-90; PMID:10704455; http://dx.doi.org/ 10.1083/jcb.148.5.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 2011; 3:1-21; PMID:21441590; http://dx.doi.org/ 10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rossier O, Octeau V, Sibarita J-B, Leduc C, Tessier B, Nair D, Gatterdam V, Destaing O, Albigès-Rizo C, Tampé R, et al.. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat Cell Biol 2012; 14:1057-67; PMID:23023225; http://dx.doi.org/ 10.1038/ncb2588 [DOI] [PubMed] [Google Scholar]
- [8].Friedland JC, Lee MH, Boettinger D. Mechanically activated integrinswitch controls α5β1 function. Science 2009; 323:642-4; PMID:19179533; http://dx.doi.org/ 10.1126/science.1168441 [DOI] [PubMed] [Google Scholar]
- [9].Schiller HB, Hermann M-R, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk K-E, Théry M, et al.. beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 2013; 15:625-36; PMID:23708002; http://dx.doi.org/ 10.1038/ncb2747 [DOI] [PubMed] [Google Scholar]
- [10].Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals-distinct functions of α5β1and αvβ3 integrins in regulating cell behaviour. IUBMB Life 2009; 61:731-8; PMID:19514020; http://dx.doi.org/ 10.1002/iub.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wolfenson H, Bershadsky A, Henis YI, Geiger B. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci 2011; 124:1425-32; PMID:21486952; http://dx.doi.org/ 10.1242/jcs.077388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lin GL, Cohen DM, Desai RA, Breckenridge MT, Gao L, Humphries MJ, Chen CS. Activation of beta 1 but not beta 3 integrin increases cell traction forces. FEBS Lett 2013; 587:763-9; PMID:23395612; http://dx.doi.org/ 10.1016/j.febslet.2013.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rahmouni S, Lindner A, Rechenmacher F, Neubauer S, Sobahi TRA, Kessler H, Cavalcanti-Adam EA, Spatz JP. Hydrogel micropillars with integrin selective peptidomimetic functionalized nanopatterned tops: a new tool for the measurement of cell traction forces transmitted through αvβ3- or α5β1-integrins. Adv Mater 2013; 25:5869-74; PMID:23913640; http://dx.doi.org/ 10.1002/adma.201301338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balcioglu HE, van Hoorn H, Donato DM, Danen EHJ, Erik . Integrin expression profile modulates orientation and dynamics of force transmission at cell matrix adhesions. J Cell Sci 2015; 128:1316-26; PMID:25663698; http://dx.doi.org/ 10.1242/jcs.156950 [DOI] [PubMed] [Google Scholar]
- [15].Mould AP, Craig SE, Byron SK, Humphries MJ, Jowitt TA. Disruption of integrin–fibronectin complexes by allosteric but not ligand-mimetic inhibitors. Biochem J 2014; 464:301-13; PMID:25333419; http://dx.doi.org/ 10.1042/BJ20141047 [DOI] [PubMed] [Google Scholar]
- [16].Rechenmacher F, Neubauer S, Polleux J, Mas-Moruno C, De Simone M, Cavalcanti-Adam EA, Spatz JP, Fässler R, Kessler H. Functionalizing αvβ3- or α5β1-selective integrin antagonists for surface coating: A method to discriminate integrin subtypes in vitro. Angew Chem Int Ed 2012; 52:1572-5; PMID:23345131; http://dx.doi.org/ 10.1002/anie.201206370 [DOI] [PubMed] [Google Scholar]
- [17].Guasch J, Conings B, Neubauer S, Rechenmacher F, Ende K, Rolli CG, Kappel C, Schaufler V, Micoulet A, Kessler H, et al.. Segregation versus colocalization: orthogonally functionalized binary micropatterned substrates regulate the molecular distribution in focal adhesions. Adv Mater 2015; 27:3737-47; PMID:25981929; http://dx.doi.org/ 10.1002/adma.201500900 [DOI] [PubMed] [Google Scholar]
- [18].Fraioli R, Rechenmacher F, Neubauer S, Manero JM, Gil J, Kessler H, Mas-Moruno C. Mimicking bone extracellular matrix: integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf B 2015; 128:191-200; PMID:25637448; http://dx.doi.org/ 10.1016/j.colsurfb.2014.12.057 [DOI] [PubMed] [Google Scholar]
- [19].Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol 2005; 171:383-92; PMID:16247034; http://dx.doi.org/ 10.1083/jcb.200503017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Askari JA, Tynan CJ, Webb SED, Martin-Fernandez ML, Ballestrem C, Humphries MJ. Focal adhesions are sites of integrin extension. J Cell Biol 2010; 188:891-903; PMID:20231384; http://dx.doi.org/ 10.1083/jcb.200907174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arnold M, Cavalcanti-Adam EA, Glass R, Blümmel J, Eck W, Kantlehner M, Kessler H, Spatz JP. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 2004; 5:383-8; PMID:15067875; http://dx.doi.org/ 10.1002/cphc.200301014 [DOI] [PubMed] [Google Scholar]
- [22].Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz JP. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J 2007; 92:2964-74; PMID:17277192; http://dx.doi.org/ 10.1529/biophysj.106.089730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Y, Medda R, Liu Z, Galior K, Yehl K, Spatz JP, Cavalcanti-Adam EA, Salaita K. Nanoparticle tension probes patterned at the nanoscale: Impact of integrin clustering on force transmission. Nano Lett 2014; 14:5539-46; PMID:25238229; http://dx.doi.org/ 10.1021/nl501912g [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci 2006; 120:137-48; PMID:17164291; http://dx.doi.org/ 10.1242/jcs.03314 [DOI] [PubMed] [Google Scholar]
- [25].LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol 1992; 117:437-47; PMID:1373145; http://dx.doi.org/ 10.1083/jcb.117.2.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Neubauer S, Rechenmacher F, Beer AJ, Curnis F, Pohle K, D'Alessandria C, Wester H-J, Reuning U, Corti A, Schwaiger M, et al.. Selective imaging of the angiogenic relevant integrins α5β1 and αvβ3. Angew Chem Int Ed 2013; 52:11656-9; PMID:24115324; http://dx.doi.org/ 10.1002/anie.201306376 [DOI] [PubMed] [Google Scholar]
- [27].de Beer AGF, Cavalcanti-Adam EA, Majer G, Lopez-García M, Kessler H, Spatz JP. Force-induced destabilization of focal adhesions at defined integrin spacings on nanostructured surfaces. Phys Rev E 2010; 81:051914-7; PMID:20866268; http://dx.doi.org/ 10.1103/PhysRevE.81.051914 [DOI] [PubMed] [Google Scholar]
- [28].Rechenmacher F, Neubauer S, Mas-Moruno C, Dorfner PM, Polleux J, Guasch J, Conings B, Boyen H-G, Bochen A, Sobahi TR, et al.. A molecular toolkit for the functionalization of titanium-based biomaterials that selectively control integrin-mediated cell adhesion. Chem Eur J 2013; 19:9218-23; PMID:23744802; http://dx.doi.org/ 10.1002/chem.201301478 [DOI] [PubMed] [Google Scholar]
- [29].Charo IF, Nannizzi L, Smith JW, Cheresh DA. The vitronectin receptor alphavbeta3 binds fibronectin and acts in concert with alpha5beta1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol 1990; 111:2795-800; PMID:1703545; http://dx.doi.org/ 10.1083/jcb.111.6.2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dobereiner HG, Dubin-Thaler BJ, Giannone G, Sheetz MP. Force sensing and generation in cell phases: analyses of complex functions. J Appl Physiol 2005; 98:1542-6; PMID:15772064; http://dx.doi.org/ 10.1152/japplphysiol.01181.2004 [DOI] [PubMed] [Google Scholar]
- [31].Davey G, Buzzai M, Assoian RK. Reduced expression of alpha5beta1 integrin prevents spreading-dependent cell proliferation. J Cell Sci 1999; 112:4663-72; PMID:10574714 [DOI] [PubMed] [Google Scholar]
- [32].Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature 2010; 468:580-4; PMID:21107430; http://dx.doi.org/ 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Case LB, Waterman CM. Adhesive F-actin waves: A novel integrin-mediated adhesion complex coupled to ventral actin polymerization. PLoS One 2011; 6:e26631-13; PMID:22069459; http://dx.doi.org/ 10.1371/journal.pone.0026631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Spatz JP, Mössmer S, Hartmann C, Möller M, Herzog T, Krieger M, Boyen H-G, Ziemann P, Kabius B. Ordered deposition of inorganic clusters from micellar block copolymer films. Langmuir 2000; 16:407-15; http://dx.doi.org/ 10.1021/la990070n [DOI] [Google Scholar]
- [35].Glass R, Möller M, Spatz JP. Block copolymer micelle nanolithography. Nanotechnology 2003; 14:1153-60; http://dx.doi.org/ 10.1088/0957-4484/14/10/314 [DOI] [Google Scholar]
- [36].Blümmel J, Perschmann N, Aydin D, Drinjakovic J, Surrey T, López-García M, Kessler H, Spatz JP. Protein repellent properties of covalently attached PEG coatings on nanostructured SiO2-based interfaces. Biomaterials 2007; 28:4739-47; PMID:17697710; http://dx.doi.org/ 10.1016/j.biomaterials.2007.07.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.