ABSTRACT
Cancer-secreted exosomes influence tumor microenvironment and support cancer growth and metastasis. MiR-210 is frequently up-regulated in colorectal cancer tissues and correlates with metastatic disease. We investigated whether exosomes are actively released by HCT-8 colon cancer cells, the role of exosomal miR-210 in the cross-talk between primary cancer cells and neighboring metastatic cells and its contribution in regulating epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET).
After 7 d of culture, a subpopulation of viable HCT-8 cells detached the monolayer and started to grow in suspension, suggesting anoikis resistance and a metastatic potential. The expression of key proteins of EMT revealed that these cells were E-cadherin negative and vimentin positive further confirming their metastatic phenotype and the acquisition of anoikis resistance. Metastatic cells, in the presence of adherently growing HCT-8, continued to grow in suspension whereas only if seeded in cell-free wells, were able to adhere again and to form E-cadherin positive and vimentin negative new colonies, suggesting the occurrence of MET.
The chemosensitivity to 5 fluorouracil and to FOLFOX-like treatment of metastatic cells was significantly diminished compared to adherent HCT-8 cells. Of note, adherent new colonies undergoing MET, were insensitive to both chemotherapeutic strategies. Electron microscopy analysis demonstrated that adherently growing HCT-8, actually secreted exosomes and that exosomes in turn were taken up by metastatic cells. When exosomes secreted by adherently growing HCT-8 were administered to metastatic cells, MET was significantly inhibited. miR-210 was significantly upregulated in exosomes compared to its intracellular levels in adherently growing HCT-8 cells and correlated to anoikis resistance and EMT markers.
Exosomes containing miR-210 might be considered as EMT promoting signals that preserve the local cancer-growth permissive milieu and also guide metastatic cells to free, new sites of dissemination.
KEYWORDS: Colon cancer, chemoresistance, EMT, exosomes, metastasis, miR-210, MET
Introduction
A continuous cross-talk between cancer cells and local/distant environments is required for effective tumor growth and systemic dissemination.1 Intercellular communication can occur through cell-cell direct contact mediated by gap junctions as well as by extracellular vesicles.2 Exosomes, are 30–100 nm phospholipid bilayer-enclosed vesicles surrounding a small cytosol which are increasingly recognized as key autocrine and paracrine mediators of cell-cell communication through the transport of bioactive molecules such as mRNAs, proteins and microRNAs (miRNAs).3 Exosomes from breast cancer cells transfer miRNAs to normal cells, stimulate them to become cancerous4 and promote cell invasion.5 It has been suggested that the formation of the “pre-metastatic niches” depends on tumor-derived exosomes but the contribution of miRNAs has not been addressed.6 The elegant study of Lieberman and her group also clearly demonstrated that the metastatic capability can be transferred between metastatic and non-metastatic cancer cells via exosomes containing miR-200.7
Epithelial–mesenchymal transition (EMT), characterized by the loss of epithelial traits and the acquisition of a mesenchymal phenotype is involved in cancer progression and metastasis.8 If EMT is critical to the initial transformation from benign to invasive carcinoma, its reverse process, the mesenchymal-to-epithelial transition (MET), is central for the latter stages of the metastatic process.8 Several miRNAs have been reported to control multiple aspects of EMT and MET and to support tumor progression and metastasis.9,10
MiR-210 is frequently up-regulated in colorectal cancer tissues and its expression correlates with aggressive disease and metastasis.11 A recent study demonstrated that the circulating levels of miR-210 are significantly elevated in colorectal cancer patients and correlate to the occurrence of distant metastasis.12 Although these studies suggest that mir-210 is associated to colon cancer metastasis they did not show whether colon cancer cells indeed secrete exosomes containing miR-210 and their relevance in the paracrine communication with nearby disseminated cancer cells.
On these basis, by using an in vitro model of spontaneous metastasis of colon cancer cells, we aimed at investigating whether exosomes are actively released by cancer cells to modify the local tumor environment, the role of exosomal miR-210 in the cross-talk between primary cancer cells and metastasis and its contribution in regulating EMT and MET.
Materials and methods
Cell line and metastatic cells
HCT-8 cells (gift from Dr. Coronnello, University of Florence) were maintained in DMEM (Invitrogen, Life technologies, Carlsbad, USA) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/mL penicillin-streptomycin, 1% l-glutamine (200 mmol/L), 4.5 g/L glucose and grown in 5% CO2. After 48 hours from seeding, several cells able to survive in suspension, spontaneously appeared in the culture medium. These cells were interpreted as potentially metastatic and their number was assessed at different time points from seeding (48 h, 72 h, 96 h; 7 d, 10 d, 15 d). Cell viability was measured by trypan blue (Invitrogen).
Immunocytochemistry
To characterize the phenotype of cells growing in suspension, the expression of E-cadherin and vimentin was assessed by immunocytochemistry. Cells were prepared as follows: a) HCT-8 cells seeded on histological slides, b) potentially metastatic cells placed on histological slides by 500xg centrifugation for 5 minutes, c) potentially metastatic cells seeded in HCT-8 free-wells and collected as soon as they formed adherent visible colonies. Samples were fixed in 4% formaldehyde pH 7.4 for 10 min and pre-incubated in 0.5% triton and 1.5% bovine serum albumin (BSA) (Sigma Aldrich, Milan, Italy) for 15 min at room temperature. Immunostaining was performed by incubation for 24 h at 4°C with mouse monoclonal anti E-cadherin at final dilution of 1:50 (Millipore, Billerica, MA, USA) or goat-polyclonal anti-Vimentin at final dilution 1:40 (Sigma Aldrich) followed by Alexa Fluor 488 Goat anti mouse (1:333) or Alexa Fluor 568 Donkey anti Goat (1:333) (Invitrogen).
Image acquisition and analysis
Microscopic analysis was performed with a fluorescence microscope (Labophot-2, Nikon, Tokyo, Japan) connected to a CCD camera. Ten photomicrographs (∼100 cells/microscopic field) were randomly taken for each sample and fluorescence was measured using ImageJ 1.33 image analysis software (http://rsb.info.nih.gov/ij). Results were expressed in arbitrary units.
Exosomes isolation
Exosomes were obtained from HCT-8 culture media at day 7 post seeding by using the Exosome Precipitation Solution (Macherey-Nagel, Düren, Germany) following the manufacturer's instructions.
Transmission electron microscopy
HCT-8 (106 cells) were fixed in 4% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 8 h at 4°C. Cells were then embedded in epoxy resin and routinely processed. In order to evaluate the interaction between metastatic cells and exosomes, 105 metastatic cells were incubated for 30 min at 37°C with 10% exosomes suspension obtained as described above. At the end of the incubation, cells were fixed and embedded for transmission electron microscopy. Samples were cut in sections about 70 nm thick and examined with a Jeol 1010 transmission electron microscope (Jeol, Tokyo, Japan) at 80 kV.
RNA extraction from exosomes and from adherent HCT-8 cells
Total RNA from exosomes was extracted using the NucleoSpin miRNAs Plasma kit (Macherey-Nagel, Düren, Germany). Total RNA from adherent HCT-8 cells was isolated using the miRNeasy Mini Kit (Qiagen Duesseldorf, Germany). Reverse transcription of RNA was performed using the miScript Reverse Transcription Kit according to manufacturer's instructions (Qiagen). In the case of exosomal miRNA, cDNAs were preamplified with MiScript Pre-AMP (Qiagen).
mir-210 expression in exosomes and in adherent HCT-8 cells
Quantitative real-time RT-PCR was performed in 7900HT Fast Real-Time PCR System (Applied Biosystems, California, USA) using miScript SYBR Green PCR Kit and miScript Primer Assay according to manufacturer's instructions (Qiagen). Cel-miR-39 was used for the normalization of both exosomal and intracellular miRNAs levels according to Turchinovich et al.13 After amplification, melting curve analysis was performed to ensure the specificity of the products. The expression levels of miR-210 were calculated as 2ˆ−ΔΔCT.
Colonies formation
Metastatic cells were isolated from HCT-8 culture media by 500xg centrifugation for 5 min. 4 × 103 cells were seeded in 24 well plates and maintained for 72 h in DMEM or in DMEM containing 5% or 10% of exosomes obtained as described above. Adherent new colonies were then counted.
Chemotherapy sensitivity
The chemosensitivity of the 3 cell clones was assessed. Adherent HCT-8 cells, metastatic cells and adherent new colonies were treated for 72 h with 5-fluorouracil (5FU) 10−6 M 14 and with a FOLFOX-like treatment (5FU 10−6 M + Oxaliplatin 2 × 10−7 M) (Sigma Aldrich, Milan, Italy). Cells viability was assessed by MTS assay (Promega Corporation, WI). The optical density of the chromogenic product was measured at 490 nm.
Statistical analysis
Data were expressed as means ± SEM of 3 independent experiments. Statistical analysis was performed by one-way analysis of variance, followed by the Student–Newman–Keuls multiple comparison post hoc test or by Mann-Whitney test. Calculations were done using a GraphPad Prism 4.0 (GraphPad software, San Diego, CA).
Results
A subpopulation of viable HCT-8 cells spontaneously goes in suspension
After 48h post-seeding, a number of HCT-8 cells spontaneously began to disassociate, migrated away from the cell monolayer and remained fully viable in the culture media. The number of these cells significantly increased from the seventh to the fifteenth day post-seeding (Fig. 1).
Figure 1.
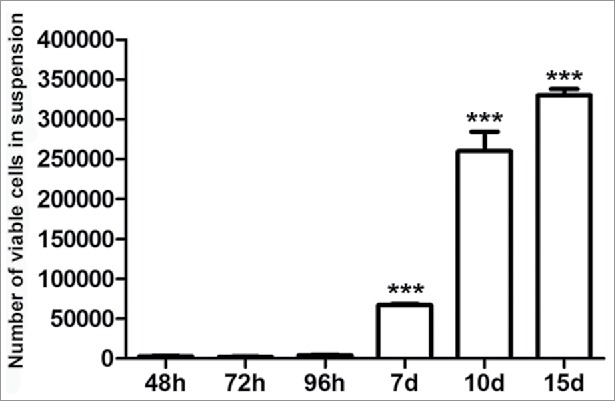
At different time points post seeding, a subpopulation of viable HCT-8 cells spontaneously goes in suspension. Data are expressed as means ± SEM of 3 independent experiments. ***p < 0.001 vs 48h.
The key epithelial-mesenchymal transition (EMT) markers Vimentin and E-cadherin, are differentially expressed in adherent HCT-8 and in those in suspension
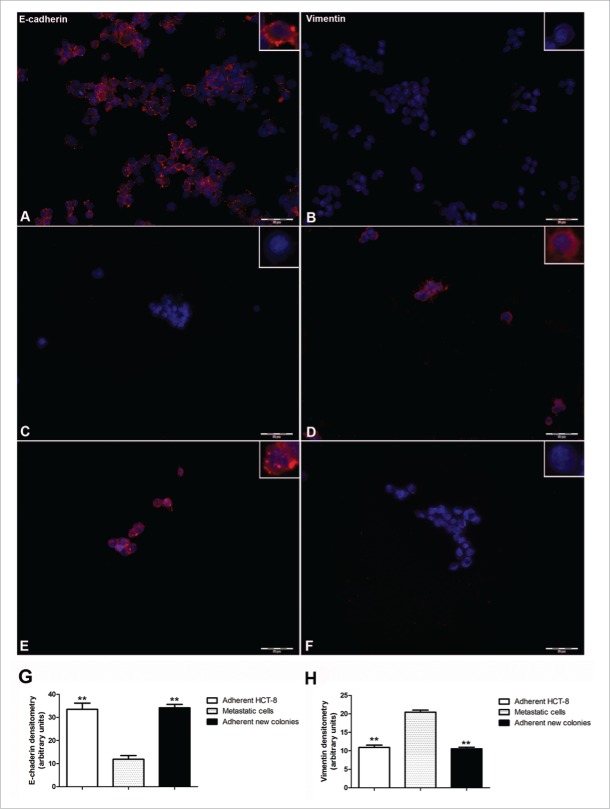
At day 7 from seeding, immunofluorescent staining for E-cadherin and Vimentin considerably differed when we compared to adherently growing HCT-8 cells with those in suspension. Suspended viable HCT-8 cells were E-cadherin negative (Fig. 2, panel C) and vimentin positive (Fig. 2, panel D) whereas the adherent originating HCT-8 cells, were E-cadherin positive (Fig. 2, Panel A) and vimentin negative (Fig. 2, panel B). Densitometric analysis further supported these results; in fact, suspended HCT-8 cells (namely metastatic cells) displayed significantly lower levels of E-cadherin and significantly higher levels of vimentin compared to the adherent originating HCT-8 cells (p < 0.01) (Fig. 2, panel G and panel H). Interestingly, these metastatic cells, only if seeded in cell-free wells, formed new, adherent, E-cadherin positive and vimentin negative colonies (Fig. 2, panel E and panel F), suggesting the occurrence of MET. Densitometric analysis confirmed that the adherent new colonies expressed significantly lower levels of vimentin and higher E-cadherin levels compared to metastatic cells (p < 0.01) (Fig. 2, panel G and panel H).
Figure 2.
Immunocytochemical evaluation of the expression of E-cadherin (panel A) and vimentin (panel B) in adherently growing HCT-8. Expression of E-cadherin (panel C) and vimentin (panel D) in metastatic cells growing in suspension. Expression of E-cadherin (panel E) and vimentin (panel F) in adherent new colonies obtained by seeding metastatic cells in cell-free wells (20x magnification; Barr 100 µm objective). Panel G: Densitometric analysis of E-cadherin in adherently growing HCT-8, in metastatic cells growing in suspension and in adherent new colonies obtained by seeding metastatic cells in cell-free wells. Panel H: Densitometric analysis of vimentin in adherently growing HCT-8, in metastatic cells growing in suspension and in adherent new colonies obtained by seeding metastatic cells in cell-free wells. **p < 0.01 vs metastatic cells growing in suspension.
HCT-8 secrete exosomes which in turn interact with neighboring metastatic cells
In the cytoplasm of HCT-8 cells, transmission electron microscopy (TEM) highlighted several intracellular multi-vesicle bodies (MVBs) carrying bi-lipidic membrane-bound exosome-like vesicles (Fig. 3, panel A). Metastatic cells did not show any MVBs in their cytoplasm but several round bodies compatible with exosome-like vesicles, were identified closely to their membrane. In some cases these round bodies appeared fused with the metastatic cells membrane (Fig. 3, panel B and C).
Figure 3.
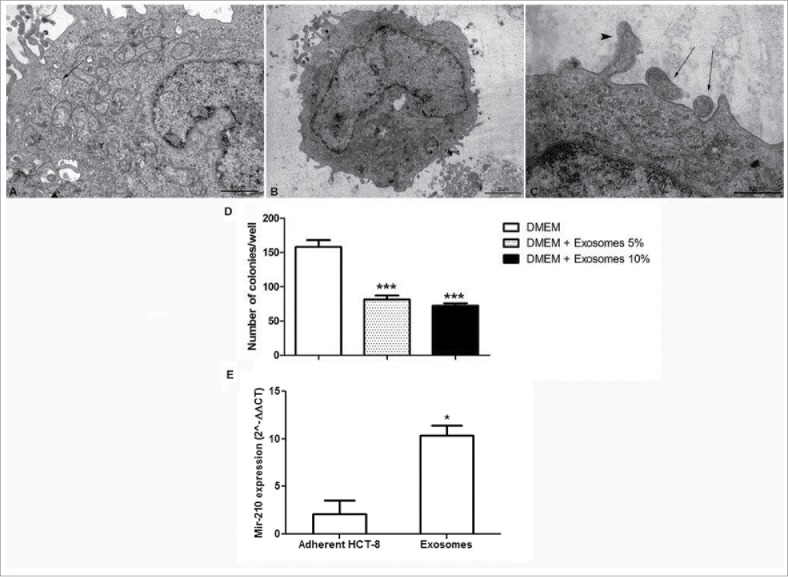
Morphological analysis of exosomes. (Panel A) Transmission electron microscopy of HCT-8 cells showing micro vesicles enclosing bilipidic layer-bound exosomes (black arrow) (20,000X, scale bar 1 µm). (Panel B) Metastatic cell cultured with 10% exosome suspension (10,000X, scale bar 2 µm). (Panel C) Higher magnification of panel B. Two microvescicles are located closed to cell membrane (black arrows), one appears fused with cell membrane (arrow head); (50,000X, scale bar 500 nm). (Panel D) Number of adherent new colonies formed by metastatic cells in the presence and absence of exosomes ***p < 0.001 vs DMEM. (Panel E) miR-210 expression in adherently growing HCT-8 and in exosomes, analyzed using real-time RT-PCR. *p < 0.05.
The adhesion dynamic of metastatic cells is influenced by miR-210
To test directly if exosomes are indeed able to influence the adhesion of metastatic cells, 5 or 10 % of exosomes from HCT-8 was administered to metastatic cells. In the presence of exosomes, the number of adherent, new colonies expressing E-cadherin, was significantly decreased compared to control culture conditions, further supporting the role of exosomes also in the MET process (Fig. 3, panel D). Interestingly, we found that miR-210 was significantly upregulated in exosomes compared to adherently growing HCT-8 from which they arise (Fig. 3, panel E) and that these exosomes in turn were taken up by nearby disseminated cells (Fig. 3, panel B and C).
The three cell clones show different sensitivity to standard chemotherapy
As expected, adherent HCT-8 cells were sensitive to standard chemotherapy with a percentage of cell death of 30 % upon 5FU and of 50 % upon FOLFOX- like treatment. This sensitivity was significantly diminished in metastatic cells compared to adherent HCT-8 cells. Of note, adherent new colonies undergoing MET, were completely insensitive to both 5FU and FOLFOX- like treatment (Fig. 4).
Figure 4.
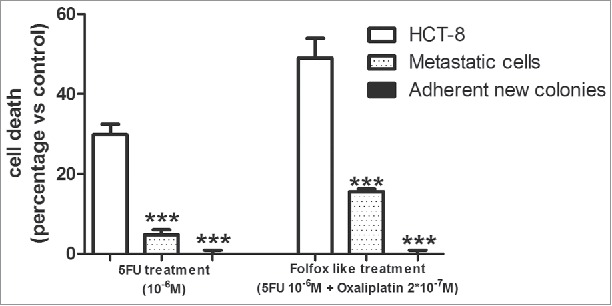
Chemotherapy sensitivity in the 3 different cell clones. Data are expressed as means ± SEM of 3 independent experiments. ***p < 0.001 vs adherent HCT-8 cells.
Discussion
The metastatic process remains one of the most unknown aspects of tumor biology and accounts for 90% of cancer mortality. The identification of the key molecular mechanisms regulating metastasis is therefore of paramount importance for the discovery of novel therapeutic approaches.15
Our results demonstrate that a sub-population of HCT-8 cells spontaneously disassociated, migrated away from the cell monolayer and remained viable in suspension. It might be argued that this phenomenon could be due to a physiological transition between adherent and suspended cells: if so, the number of cells in suspension would likely remains constant over time. Our findings instead demonstrated that this is not the case since the number of fully viable cells in suspension increased over time in culture medium, suggesting that HCT-8 actually and progressively underwent a phenotypic transition from an adhesive epithelial type to a subpopulation of cells with a metastatic-like phenotype. Interestingly, a similar behavior was reported by Tang et al. (2014) who discovered that HCT-8 cells can exhibit a dissociative, metastasis-like phenotype in vitro.16 Moreover, as barrier to metastases, cells normally undergo an apoptotic cell death called “anoikis” after they lose contact with their neighboring cells.17 In our model, a subpopulation of HCT-8 cells likely acquired resistance to anoikis and were thus able to survive after detachment from the “primary colonies.”
Increased invasive and metastatic potential has been associated to the epithelial-mesenchymal transition (EMT). EMT is not only a key event for epithelial cells to acquire a motile and invasive phenotype, but also an essential process for anoikis resistance.8,18 Among the hallmarks of EMT in vitro and in vivo, the up-regulation of mesenchymal markers (N-cadherin and vimentin) and the down-regulation of epithelial markers (E-cadherin and cytokeratins) were frequently reported.19 In our results, the conversion from an epithelial into a mesenchymal phenotype, suggests that HCT-8 cells spontaneously undergo through an EMT process and acquire a metastatic potential. The loss of E-cadherin expression in fact, allows tumor cells to dissociate from the primary tumor, to overcome anoikis and disseminate.20 Successful metastatic colonization seems to require also the reverse process known as mesenchymal-epithelial transition (MET). Infact, to invade other sites, cancer cells, that have undergone EMT, must re-acquire epithelial characteristics to anchor in the surrounding tissue.21 Interestingly, our metastatic cells, only if seeded in cell-free wells, formed new, adherent, E-cadherin positive and vimentin negative colonies suggesting that the occurrence of MET might be regulated by a paracrine signal sent by adherent HCT-8 cells to the metastatic ones.
In recent years, exosomes have received increasing attention for their role in regulating and transferring molecules responsible for tumor progression and metastasis.22 We therefore investigated if exosomes might represent a mechanism trough which adherent HCT-8 cells communicate with neighboring disseminated cancer cells and modify their adhesion. The ability of HCT-8 cells to actually secrete exosomes was confirmed by transmission electron microscopy: cytoplasmic structures identical to those described in exosomes secreting cells were infact identified.22 Electron microscopy also demonstrated the presence of extracellular microvesicles nearby metastatic cells incubated with exosomes secrete by adherent HCT-8 cells. This finding may be indicative of both exosome release and uptake but the lack of intracellular multi-vesicle bodies enclosing exosomes in metastatic cells, strongly suggests the interaction with exogenous exosomes secreted from adherently growing HCT-8. This is also supported by the discovery that this interaction has a functional consequence on metastatic cells: infact, when exosomes isolated from primary HCT-8 culture media were administered to metastatic cells, the number of colonies formed by metastatic cells in cell-free wells, was significantly reduced. In the last few years, a number of studies have demonstrated that exosomes contribute to EMT, invasion and metastasis.20 The novelty of our findings is the documentation that exosomes modify the adhesion dynamic of neighboring metastatic cells and are involved in the MET process. Moreover, E-cadherin re-expression is usually induced by factors secreted by a secondary organ microenvironment.21 Here we demonstrated that the adhesion of metastatic cells and the re-expression of E-cadherin is also driven in a cell-free well where they were no longer in the presence of the originating HCT-8 cells. This finding suggests that in its own local microenvironment, the primary tumor itself, by secreting exosomes, hinders E-cadherin re-expression in nearby metastatic cells possibly to preserve its local permissive milieu.
To investigate the mechanism involved, we measured the expression of mir-210, known to be raised in colorectal cancer patients and to correlate to the occurrence of distant metastasis.12 miR-210 was significantly up-regulated in exosomes compared to its intracellular levels in adherently growing HCT-8 cells and correlated to anoikis resistance and EMT markers. Mudduluru and coworkers, recently performed a systematic analysis of the metastasis-associated miRNome.22 These authors demonstrated that colorectal cancer cell lines transfected with miR-210 displayed a significant decrease in E-cadherin expression and a parallel increase of N-cadherin expression, an increased migration and invasive capacity and an enhanced ability to metastasize in vivo. The expression of miR-210 was found to correlate with EMT markers also in human gastric and ovarian cancer cells.23-24
To better understand the characteristics of metastatic cells and of the new adherent colonies undergoing MET, a chemotherapy sensitivity assay was performed. The three different cell populations were exposed to 5FU or to a combination of 5FU plus oxaliplatin (5:1) to mimic FOLFOX treatment, commonly used strategies to treat colorectal cancer.25 We demonstrated that metastatic cells undergoing EMT were much more resistant to standard chemotherapy compared to the adherent cells. Studies using colorectal cell lines have shown that EMT inducers also increased resistance to chemotherapy.26 Conversely, oxaliplatin-resistant colorectal cell lines showed phenotypic and gene expression changes consistent with EMT.27 Recently, lung adenocarcinoma cell lines exhibiting TGF-β/FGF-2-induced EMT, were also chemoresistant to gefitinib and chemically induced EMT reversion only partly restored chemosensitivity.28 Studies investigating the potential survival advantage of cancer cells undergoing MET toward conventional chemotherapy are scarce and it is unclear whether EMT-driven therapy resistance is retained after cells undergo MET. We are among the first to demonstrate that cells undergoing MET are insensitive to conventional chemotherapy highlighting the importance to characterize this phenomenon to comprehend the EMT/MET axis in cancer and resistance to therapy. Infact, whereas the inhibition of EMT may be effective in preventing the invasive dissemination of organ-confined cancers, in the latter stages, it could also accelerate MET-dependent colonization of new sites of dissemination and increase chemoresistance.
Cancer cells manipulate their microenvironment to optimize conditions for growth and metastasis in multiple ways. Our hypothesis is that in our model, through the delivery of mir-210 to nearby metastatic cells, primary tumor gains a double evolutionary advantage: on one hand, it preserves its local cancer-growth permissive milieu in terms of space and nutrients and on the other hand, it also redirects metastatic homing to distant sites. Infact we demonstrated that disseminated cancer cells which leave the “primary site” revert to the epithelial state in the absence of exosomes containing mir-210. Thus, in the absence of the EMT-inducing signals received from the “primary site,” metastatic cancer cells may fall back into an epithelial state when entering into new, free sites of dissemination. This evolutionary vision of cancer agrees with the “Seed and soil” theory formulated by Paget29 in which metastatic cells are “seeds” and a particular microenvironment or niche, serves as the “soil.” In this vision, exosomes represent an instrument of communication which allows the primary tumor not only to create favorable conditions of “soil” in remote niches but also to prevent metastatic cells adhesion in its own “soil” avoiding unfavorable local conditions. In the last few years, this ecological vision of cancer has been suggested to be useful to understand the biology of metastasis and stimulate the discovery of new therapeutic strategies.30
As summarized in Fig. 5, we have demonstrated that exosomes containing miR-210 mediate the cross-talk between primary cancer cells and nearby disseminated cells, and redirect metastatic cells homing to free sites of dissemination, leading to the discovery of a new molecular mechanisms involved in the primary cancer niche preservation.
Figure 5.
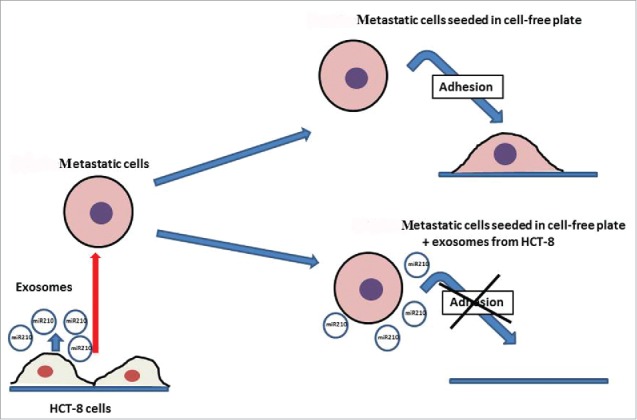
Schematic diagram depicting the major findings of this work.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors are very grateful to Prof. Piero Dolara for critical reading of the manuscript and his useful suggestions.
Funding
Supported by Ministry of Education, University and Research (MIUR) by the grant FIRB 2012 code RBFR12SOQ1: “Optimization of oncology therapy: novel drugs affecting multi drug resistance.”
Author's contribution
E.B. and C.L performed the experiments. E.B. analyzed the data, performed mir-210 expression and wrote the manuscript. D.G. was responsible of embedding and cutting for electron microscopy. L.C. performed electron microscopy analysis, planned and conceived the experiments and coordinated the research group.
References
- 1.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009; 1796(2):293-308; PMID:19683560; http://dx.doi.org/ 10.1016/j.bbcan.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 2.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013; 12(5):347-357; PMID:23584393; http://dx.doi.org/ 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- 3.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev 2013; 65:331-5; http://dx.doi.org/ 10.1016/j.addr.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al.. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014; 26(5):707-21; PMID:25446899; http://dx.doi.org/ 10.1016/j.ccell.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer 2014; 13:256; PMID:25428807; http://dx.doi.org/ 10.1186/1476-4598-13-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al.. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015; 17(6):816-26; PMID:25985394; http://dx.doi.org/ 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L, Lieberman J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest 2014; 124(12):5109-28; PMID:25401471; http://dx.doi.org/ 10.1172/JCI75695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 2011; 9(12):1608-20; PMID:21840933; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0568 [DOI] [PubMed] [Google Scholar]
- 9.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008; 68(19):7846-54; PMID:18829540; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1942 [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, et al.. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 2010; 12(3):247-56; PMID:20173740; http://dx.doi.org/ 10.1038/ncb2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu A, Du L, Yang Y, Liu H, Li J, Wang L, Liu Y, Dong Z, Zhang X, Jiang X, Wang H, et al.. Hypoxia-inducible MiR-210 is an independent prognostic factor and contributes to metastasis in colorectal cancer. PLoS One 2014; 9(3):e90952; PMID:24632577; http://dx.doi.org/ 10.1371/journal.pone.0090952 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Chen J, Wang W, Zhang Y, Chen Y, Hu T. Predicting distant metastasis and chemoresistance using plasma miRNAs. Med Oncol 2014; 31(1):799; PMID:24310813; http://dx.doi.org/ 10.1007/s12032-013-0799-x [DOI] [PubMed] [Google Scholar]
- 13.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res 2011; 39(16):7223-33; PMID:21609964; http://dx.doi.org/ 10.1093/nar/gkr254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cinci L, Luceri C, Bigagli E, Carboni I, Paccosi S, Parenti A, Guasti D, Coronnello M. Development and characterization of an in vitro model of colorectal adenocarcinoma with MDR phenotype. Cancer Med 2016; 5(6):1279-91; PMID:27016279; http://dx.doi.org/ 10.1002/cam4.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011; 331(6024):1559-64; PMID:21436443; http://dx.doi.org/ 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Kuhlenschmidt TB, Li Q, Ali S, Lezmi S, Chen H, Pires-Alves M, Laegreid WW, Saif TA, Kuhlenschmidt MS. A mechanically-induced colon cancer cell population shows increased metastatic potential. Mol Cancer 2014; 13:131; PMID:24884630; http://dx.doi.org/ 10.1186/1476-4598-13-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan K, Goldstein D, Crowe P, Yang JL. Uncovering a key to the process of metastasis in human cancers: a review of critical regulators of anoikis. J Cancer Res Clin Oncol 2013; 139(11):1795-805; PMID:23912151; http://dx.doi.org/ 10.1007/s00432-013-1482-5 [DOI] [PubMed] [Google Scholar]
- 18.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68(10):3645-54; PMID:18483246; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2938 [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006; 172:973-81; PMID:16567498; http://dx.doi.org/ 10.1083/jcb.200601018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis 2008; 25:621-8; PMID:18600305; http://dx.doi.org/ 10.1007/s10585-008-9167-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vella LJ. The Emerging Role of Exosomes in Epithelial–Mesenchymal-Transition in Cancer. Front Oncol 2014; 4:361; PMID:25566500; http://dx.doi.org/ 10.3389/fonc.2014.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudduluru G, Abba M, Batliner J, Patil N, Scharp M, Lunavat TR, Leupold JH, Oleksiuk O, Juraeva D, Thiele W, et al.. A Systematic Approach to Defining the microRNA Landscape in Metastasis. Cancer Res 2015; 75(15):3010-9; PMID:26069251; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-0997 [DOI] [PubMed] [Google Scholar]
- 23.Yu P, Fan S, Huang L, Yang L, Du Y. MIR210 as a potential molecular target to block invasion and metastasis of gastric cancer. Med Hypotheses 2015; 84(3):209-12; PMID:25618442; http://dx.doi.org/ 10.1016/j.mehy.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Zhao L, Chen W, Liu T, Li Z, Li X. miR-210, a modulator of hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cell. Int J Clin Exp Med 2015; 8(2):2299-307; PMID:25932166 [PMC free article] [PubMed] [Google Scholar]
- 25.Aparicio J1, Fernandez-Martos C, Vincent JM, Maestu I, Llorca C, Busquier I, Campos JM, Perez-Enguix D, Balcells M. FOLFOX alternated with FOLFIRI as first-line chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 2005; 5(4):263-7; http://dx.doi.org/ 10.3816/CCC.2005.n.037 [DOI] [PubMed] [Google Scholar]
- 26.Hoshino H, Miyoshi N, Nagai K, Tomimaru Y, Nagano H, Sekimoto M, Doki Y, Mori M, Ishii H. Epithelial-mesenchymal transition with expression of SNAI1-induced chemoresistance in colorectal cancer. Biochem Biophys Res Commun 2009; 390(3):1061-5; PMID:19861116; http://dx.doi.org/ 10.1016/j.bbrc.2009.10.117 [DOI] [PubMed] [Google Scholar]
- 27.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res 2006; 12:4147-53; PMID:16857785; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-0038 [DOI] [PubMed] [Google Scholar]
- 28.Kurimoto R, Iwasawa S, Ebata T, Ishiwata T, Sekine I, Tada Y, Tatsumi K, Koide S, Iwama A, Takiguchi Y. Drug resistance originating from a TGF-β/FGF-2-driven epithelial-to-mesenchymal transition and its reversion in human lung adenocarcinoma cell lines harboring an EGFR mutation. Int J Oncol 2016; 48(5):1825-36; PMID:26984042; http://dx.doi.org/ 10.3892/ijo.2016.3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889; 133:571-3; PMID:2673568; http://dx.doi.org/ 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- 30.Pienta KJ, Robertson BA, Coffey DS, Taichman RS. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clin Cancer Res. 2013; 19(21):5849-55; PMID:24100626; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2158 [DOI] [PMC free article] [PubMed] [Google Scholar]