Abstract
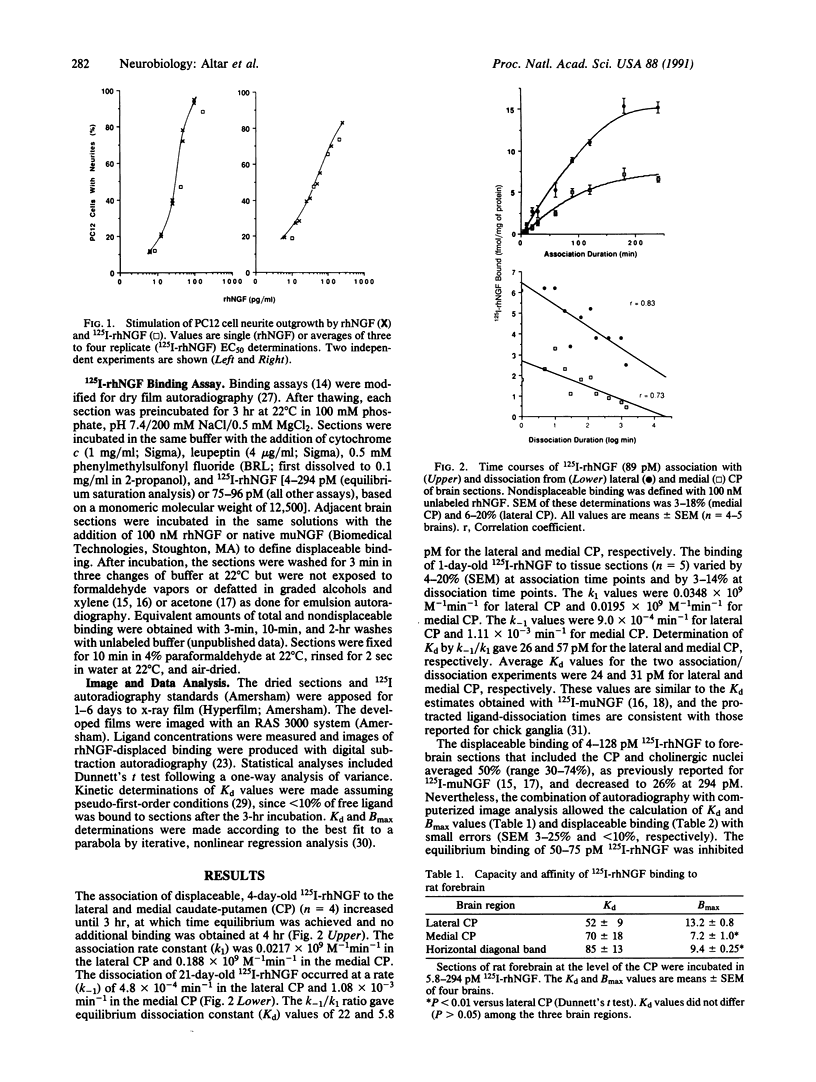
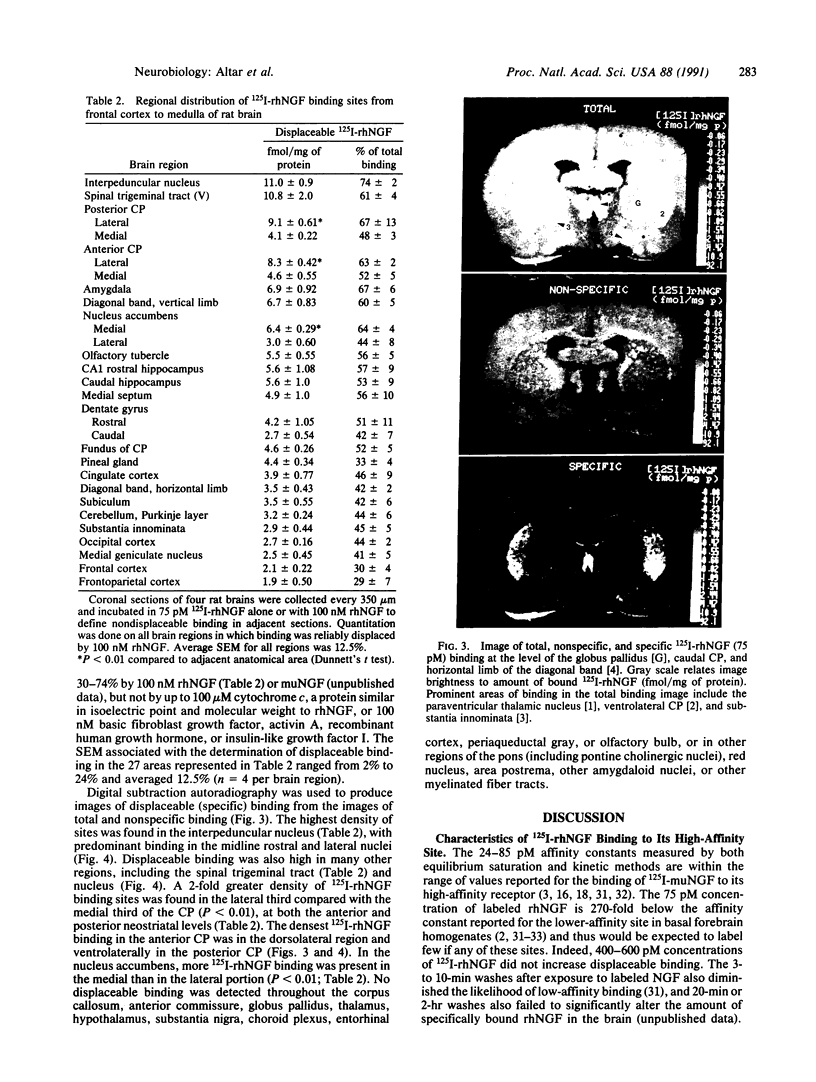
Iodinated recombinant human nerve growth factor (125I-rhNGF) stimulated neurite formation in PC12 cell cultures with a half-maximal potency of 35-49 pg/ml, compared with 39-52 pg/ml for rhNGF. In quantitative ligand autoradiography, the in vitro equilibrium binding of 125I-rhNGF to brain sections showed a 10-fold regional variation in density and was saturable, reversible, and specifically displaced by up to 74% with rhNGF or murine NGF (muNGF). At equilibrium, 125I-rhNGF bound to these sites with high affinity (Kd 52-85 pM) and low capacity (Bmax less than or equal to 13.2 fmol/mg of protein). Calculation of 125I-rhNGF binding affinity by kinetic methods gave average Kd values of 24 and 31 pM. Computer-generated maps revealed binding in brain regions not identified previously with 125I-muNGF, including hippocampus; dentate gyrus; amygdala; paraventricular thalamus; frontal, parietal, occipital, and cingulate cortices; nucleus accumbens; olfactory tubercle; subiculum; pineal gland; and medial geniculate nucleus. NGF binding sites were distributed in a 2-fold increasing medial-lateral gradient in the caudate-putamen and a 2-fold lateral-medial gradient in the nucleus accumbens. 125I-rhNGF binding sites were also found in most areas labeled by 125I-muNGF, including the interpedunucular nucleus, cerebellum, forebrain cholinergic nuclei, caudoventral caudate-putamen, and trigeminal nerve nucleus. 125I-rhNGF binding sites were absent from areas replete with low-affinity NGF binding sites, including circumventricular organs, myelinated fiber bundles, and choroid plexus. The present analysis provides an anatomical differentiation of high-affinity 125I-rhNGF binding sites and greatly expands the number of brain structures that may respond to endogenous NGF or exogenously administered rhNGF.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Marien M. R. [3H]vesamicol binding in brain: autoradiographic distribution, pharmacology, and effects of cholinergic lesions. Synapse. 1988;2(5):486–493. doi: 10.1002/syn.890020504. [DOI] [PubMed] [Google Scholar]
- Altar C. A., Walter R. J., Jr, Neve K. A., Marshall J. F. Computer-assisted video analysis of [3H]spiroperidol binding autoradiographs. J Neurosci Methods. 1984 Mar;10(3):173–188. doi: 10.1016/0165-0270(84)90054-2. [DOI] [PubMed] [Google Scholar]
- Bernd P., Martinez H. J., Dreyfus C. F., Black I. B. Localization of high-affinity and low-affinity nerve growth factor receptors in cultured rat basal forebrain. Neuroscience. 1988 Jul;26(1):121–129. doi: 10.1016/0306-4522(88)90131-5. [DOI] [PubMed] [Google Scholar]
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Chandler C. E., Parsons L. M., Hosang M., Shooter E. M. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984 Jun 10;259(11):6882–6889. [PubMed] [Google Scholar]
- Cheney D. L., LeFevere H. F., Racagni G. Choline acetyltransferase activity and mass fragmentographic measurement of acetylcholine in specific nuclei and tracts of rat brain. Neuropharmacology. 1975 Nov;14(11):801–809. doi: 10.1016/0028-3908(75)90107-0. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S., Dreyfus C. F., Black I. B. Expression of high- and low-affinity nerve growth factor receptors by Purkinje cells in the developing rat cerebellum. Exp Neurol. 1989 Jul;105(1):104–109. doi: 10.1016/0014-4886(89)90177-5. [DOI] [PubMed] [Google Scholar]
- Dawbarn D., Allen S. J., Semenenko F. M. Coexistence of choline acetyltransferase and nerve growth factor receptors in the rat basal forebrain. Neurosci Lett. 1988 Nov 22;94(1-2):138–144. doi: 10.1016/0304-3940(88)90284-4. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Preston Y. A., Todaro G. J. Properties of a sarcoma-growth-factor-like peptide from cells transformed by a temperature-sensitive sarcoma virus. J Cell Physiol. 1981 Oct;109(1):143–152. doi: 10.1002/jcp.1041090116. [DOI] [PubMed] [Google Scholar]
- DiStefano P. S., Johnson E. M., Jr Nerve growth factor receptors on cultured rat Schwann cells. J Neurosci. 1988 Jan;8(1):231–241. doi: 10.1523/JNEUROSCI.08-01-00231.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger H. C. The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res. 1982 Nov;257(3):327–388. doi: 10.1016/0165-0173(82)90011-x. [DOI] [PubMed] [Google Scholar]
- Green S. H., Greene L. A. A single Mr approximately 103,000 125I-beta-nerve growth factor-affinity-labeled species represents both the low and high affinity forms of the nerve growth factor receptor. J Biol Chem. 1986 Nov 15;261(32):15316–15326. [PubMed] [Google Scholar]
- Guyenet P., Euvrard C., Javoy F., Herbert A., Glowinski J. Regional differences in the sensitivity of cholinergic neurons to dopaminergic drugs and quipazine in the rat striatum. Brain Res. 1977 Nov 18;136(3):487–500. doi: 10.1016/0006-8993(77)90073-7. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F., Cotman C. W., Nieto-Sampedro M. NGF receptor immunoreactivity in aged rat brain. Brain Res. 1989 Feb 13;479(2):255–262. doi: 10.1016/0006-8993(89)91626-0. [DOI] [PubMed] [Google Scholar]
- Hagg T., Hagg F., Vahlsing H. L., Manthorpe M., Varon S. Nerve growth factor effects on cholinergic neurons of neostriatum and nucleus accumbens in the adult rat. Neuroscience. 1989;30(1):95–103. doi: 10.1016/0306-4522(89)90356-4. [DOI] [PubMed] [Google Scholar]
- Hefti F., Hartikka J., Salvatierra A., Weiner W. J., Mash D. C. Localization of nerve growth factor receptors in cholinergic neurons of the human basal forebrain. Neurosci Lett. 1986 Aug 15;69(1):37–41. doi: 10.1016/0304-3940(86)90410-6. [DOI] [PubMed] [Google Scholar]
- Hefti F., Mash D. C. Localization of nerve growth factor receptors in the normal human brain and in Alzheimer's disease. Neurobiol Aging. 1989 Jan-Feb;10(1):75–87. doi: 10.1016/s0197-4580(89)80014-4. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. B., Muth E. A., Jacobowitz D. M. A mapping of the distribution of acetycholine, choline acetyltransferase and acetylcholinesterase in discrete areas of rat brain. Brain Res. 1978 Sep 22;153(2):295–306. doi: 10.1016/0006-8993(78)90408-0. [DOI] [PubMed] [Google Scholar]
- Kiss J., McGovern J., Patel A. J. Immunohistochemical localization of cells containing nerve growth factor receptors in the different regions of the adult rat forebrain. Neuroscience. 1988 Dec;27(3):731–748. doi: 10.1016/0306-4522(88)90179-0. [DOI] [PubMed] [Google Scholar]
- Kiss J., Patel A. J. Characterization of neurons containing nerve growth factor receptors in the rat neostriatum. Neurosci Lett. 1989 Nov 6;105(3):251–256. doi: 10.1016/0304-3940(89)90629-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi R. M., Brownstein M., Saavedra J. M., Palkovits Choline acetyltransferase content in discrete regions of the rat brain stem. J Neurochem. 1975 Apr;24(4):637–640. [PubMed] [Google Scholar]
- Kordower J. H., Bartus R. T., Bothwell M., Schatteman G., Gash D. M. Nerve growth factor receptor immunoreactivity in the nonhuman primate (Cebus apella): distribution, morphology, and colocalization with cholinergic enzymes. J Comp Neurol. 1988 Nov 22;277(4):465–486. doi: 10.1002/cne.902770402. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J., Unnerstall J. R. In vitro labeling receptor autoradiography: loss of label during ethanol dehydration and preparative procedures. Brain Res. 1982 Jul 22;244(1):178–181. doi: 10.1016/0006-8993(82)90917-9. [DOI] [PubMed] [Google Scholar]
- Kása P. The cholinergic systems in brain and spinal cord. Prog Neurobiol. 1986;26(3):211–272. doi: 10.1016/0301-0082(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Marien M. R., Parsons S. M., Altar C. A. Quantitative autoradiography of brain binding sites for the vesicular acetylcholine transport blocker 2-(4-phenylpiperidino)cyclohexanol (AH5183). Proc Natl Acad Sci U S A. 1987 Feb;84(3):876–880. doi: 10.1073/pnas.84.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioro E. P., Cuello A. C. Purkinje cells of adult rat cerebellum express nerve growth factor receptor immunoreactivity: light microscopic observations. Brain Res. 1988 Jul 5;455(1):182–186. doi: 10.1016/0006-8993(88)90131-x. [DOI] [PubMed] [Google Scholar]
- Raivich G., Kreutzberg G. W. The localization and distribution of high affinity beta-nerve growth factor binding sites in the central nervous system of the adult rat. A light microscopic autoradiographic study using [125I]beta-nerve growth factor. Neuroscience. 1987 Jan;20(1):23–36. doi: 10.1016/0306-4522(87)90003-0. [DOI] [PubMed] [Google Scholar]
- Rea M. A., Simon J. R. Regional distribution of cholinergic parameters within the rat striatum. Brain Res. 1981 Aug 31;219(2):317–326. doi: 10.1016/0006-8993(81)90294-8. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Riopelle R. J. Distribution of neuronal receptors for nerve growth factor in the rat. J Neurosci. 1986 Aug;6(8):2312–2321. doi: 10.1523/JNEUROSCI.06-08-02312.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riopelle R. J., Richardson P. M., Verge V. M. Distribution and characteristics of nerve growth factor binding on cholinergic neurons of rat and monkey forebrain. Neurochem Res. 1987 Oct;12(10):923–928. doi: 10.1007/BF00966314. [DOI] [PubMed] [Google Scholar]
- Satoh K., Armstrong D. M., Fibiger H. C. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull. 1983 Dec;11(6):693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Schatteman G. C., Gibbs L., Lanahan A. A., Claude P., Bothwell M. Expression of NGF receptor in the developing and adult primate central nervous system. J Neurosci. 1988 Mar;8(3):860–873. doi: 10.1523/JNEUROSCI.08-03-00860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J. B. Nerve growth factor receptor-mediated transport from CSF labels cholinergic neurons: direct demonstration by a double-labeling study. Brain Res. 1989 Jun 26;490(2):390–396. doi: 10.1016/0006-8993(89)90260-6. [DOI] [PubMed] [Google Scholar]
- Springer J. E. Nerve growth factor receptors in the central nervous system. Exp Neurol. 1988 Dec;102(3):354–365. doi: 10.1016/0014-4886(88)90231-2. [DOI] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Taniuchi M., Schweitzer J. B., Johnson E. M., Jr Nerve growth factor receptor molecules in rat brain. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1950–1954. doi: 10.1073/pnas.83.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Weiland G. A., Molinoff P. B. Quantitative analysis of drug-receptor interactions: I. Determination of kinetic and equilibrium properties. Life Sci. 1981 Jul 27;29(4):313–330. doi: 10.1016/0024-3205(81)90324-6. [DOI] [PubMed] [Google Scholar]
- Woolf N. J., Gould E., Butcher L. L. Nerve growth factor receptor is associated with cholinergic neurons of the basal forebrain but not the pontomesencephalon. Neuroscience. 1989;30(1):143–152. doi: 10.1016/0306-4522(89)90360-6. [DOI] [PubMed] [Google Scholar]