Abstract
Probenecid and N-acetylcysteine (NAC) can preserve intracellular levels of the vital antioxidant glutathione (GSH) via two distinct biochemical pathways. Probenecid inhibits transporter-mediated GSH efflux and NAC serves as a cysteine donor for GSH synthesis. We hypothesized that probenecid and NAC alone would maintain intracellular GSH concentrations and inhibit neuronal death after traumatic stretch injury, and that the drugs in combination would produce additive effects. Sex-segregated rat primary cortical neurons were treated with probenecid (100 μM) and NAC (50 μM), alone and in combination (Pro-NAC), then subjected to mechanical stretch (10s−1 strain rate, 50% membrane deformation). At 24 h, both probenecid and NAC inhibited trauma-induced intracellular GSH depletion, lactate dehydrogenase (LDH) release, and propidium iodide (PI) uptake in both XY- and XX-neurons. Combined Pro-NAC treatment was superior to probenecid or NAC alone in maintenance of intracellular GSH and neuronal death assessed by PI uptake. Interestingly, caspase 3 activity 24 h after mechanical trauma was more prominent in XX-neurons, and treatment effects (probenecid, NAC, and Pro-NAC) were observed in XX- but not XY-neurons; however, XY-neurons were ultimately more vulnerable to mechanical stretch-induced injury than their XX counterparts, as was evidenced by more neuronal death detected by LDH release and PI uptake. In addition, after stretch injury in HT22 hippocampal cells, both NAC and probenecid were highly effective at reducing oxidative stress detected by dichlorofluorescein fluorescence. These in vitro data support further testing of this drug combination in models of traumatic neuronal injury in vivo.
Key words: : adenosine triphosphate binding cassette transporter C1, gender, multidrug resistance associated protein 1, primary cortical neurons, spinal cord injury, traumatic brain injury
Probenecid and N-acetylcysteine (NAC) can preserve intracellular levels of the vital antioxidant glutathione (GSH) via two distinct biochemical pathways. Probenecid blocks efflux of GSH and GSH conjugates via inhibition of the adenosine triphosphate (ATP)-binding cassette (ABC) membrane transporter ABCC1, also referred to as multidrug resistance-associated protein 1 (MRP1), including in neurons.1–3 By maintaining intracellular stores of GSH, probenecid reduces apoptosis in experimental models.4 Probenecid also blocks pannexin 1 channels,5 inhibits pyroptotic neuronal death in vitro,6 and protects against ischemic damage in mice after middle cerebral artery occlusion7 and in rats after global cerebral ischemia/reperfusion injury,8 although the effect of probenecid on GSH levels was not evaluated in these studies. NAC, on the other hand, can serve as a cysteine donor for GSH synthesis, thereby functioning as a substrate for the vital intracellular antioxidant.9 Although NAC is felt to be relatively impermeable to the intact blood–brain barrier (BBB),9 NAC could likely cross into the central nervous system (CNS) if the BBB is disrupted; for example, in cases of traumatic brain injury (TBI)10 or spinal cord injury (SCI).11 Treatment with NAC has been shown to improve outcomes in humans after blast-induced TBI,12 as well as in rodent models of TBI13,14 and SCI,15 alone or in combination with other drugs.16,17 Modifying NAC via esterification or amidation improves BBB penetration and has also been reported to be robustly protective in models of TBI.18,19 Here, we used an in vitro stretch model of neuronal trauma20 to evaluate the combinational strategy of probenecid and NAC.
Sex-segregated primary cortical neuron cultures were prepared from 16–17-day-old Sprague–Dawley rat embryos as described.21,22 Dissociated cell suspensions were filtered through a 70 μm nylon cell strainer, then seeded on silicone membranes (0.051–0.13 mm thick, Specialty Manufacturing, Inc., Saginaw, MI) coated with poly-d-lysine (100 μg in 100 μL Hank's buffered salt solution [HBSS]) fixed to a stainless steel well at a density of 1.3 × 107 cells/cm2. Neurobasal medium supplemented with B27 (Gibco, Thermo Fisher Scientific, Waltham, MA) and GlutaMaxl (Sigma, St. Louis, MO) was used to produce neuron-enriched cultures.23 Neurons were incubated at 37°C in a humidified chamber containing 5% CO2. On the 2nd and 6th day in vitro (DIV), culture media (3 mL volume per dish) was replaced with fresh media. Neurons were subjected to severe, mechanical stretch on DIV 10 as described.24,25 Briefly, neurons grown on silicone membranes were placed in a custom-made, metal sealed chamber. The pressure within the chamber was rapidly increased, resulting in stretching of neurons adherent to the compliant membrane. The intake pressure was adjusted to reach a target strain rate of 10s−1 and 50% membrane deformation. Pressure waveforms were measured and collected on a data acquisition system to verify that these parameters were achieved. Probenecid (100 μM buffered to pH 7.4), NAC (50 μM dissolved in HBSS), or 100 μM probenecid plus 50 μM NAC (Pro-NAC), were added to media immediately before stretch-induced or sham injury. Intracellular GSH, lactate dehydrogenase (LDH) release, caspase 3 activity, and propidium iodide (PI) labeling (flow cytometry) were determined in neurons 24 h after stretch injury and in control neurons grown on silicone membranes as described.21,25 Data are presented as mean ± standard deviation (SD), and analyzed using two factor analysis of variance (ANOVA) with Tukey's post-hoc test, with treatment group and sex as the two factors.
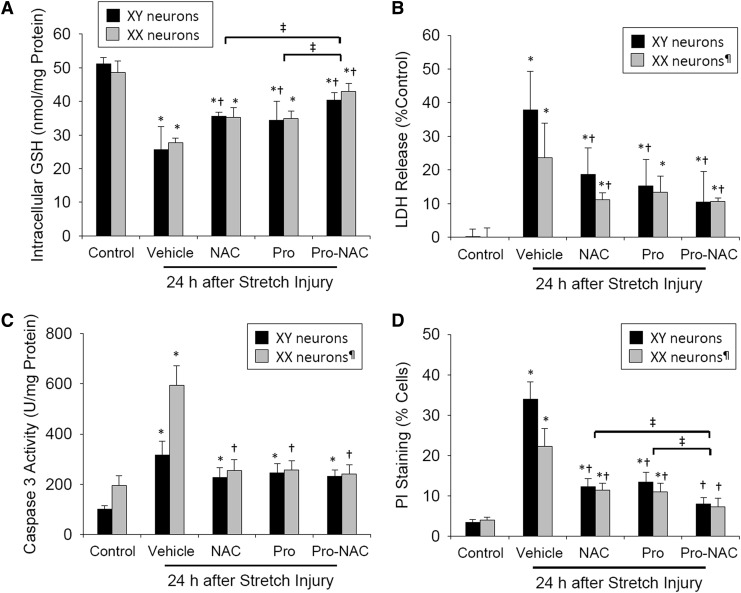
We first sought to establish whether probenecid and NAC, alone and in combination, prevent depletion of intracellular GSH after dynamic stretch-induced injury in neurons. Dishes were washed in phosphate-buffered saline (PBS), adherent neurons were scraped off membranes 24 h after stretch or sham injury, and protein concentration was measured for each sample using a standard assay and a spectrofluorophotometer capable of reading 96 well plates. GSH content in each cell homogenate was determined using a fluorescence assay (ThioGlo-1, EMD Millipore, Bedford, MA) and detected using a FilterMaxF5 Multi-Mode Microplate reader (Molecular Devices, Sunnyvale, CA) at excitation/emission wavelengths of 388 nM/500 nM. Stretch-injury reduced intracellular GSH levels by ∼50% in neurons from both male (XY-neurons) and female (XX-neurons) rats compared with sex-matched controls (Fig. 1A) (n = 4 independent wells/sex/group; both p < 0.001). Group differences were observed (F[4, 28] = 46.98, p < 0.0001). Intracellular GSH levels were partially preserved in both XY- and XX-neurons when treated with probenecid or NAC alone, and this effect was additive when the two drugs were used in combination (p = 0.007 probenecid alone vs. Pro-NAC; p = 0.023 NAC alone vs. Pro-NAC). A difference between XY- and XX-neurons in GSH was not observed (F[1, 28] = 0.150, p = 0.701).
FIG. 1.
Effects of N-acetylcysteine (NAC) (50 μM) and probenecid (100 μM), alone and in combination (Pro-NAC), on intracellular glutathione (GSH) stores and cell death when administered to sex-segregated primary cortical neurons immediately prior to mechanical stretch (10s−1 strain rate, 50% membrane deformation) or sham injury. (A) Intracellular GSH (n = 4/sex/group). (B) Lactate dehydrogenase (LDH) release (n = 5-8/sex/group). (C) Caspase 3/7-like activity (n = 4/sex/group). (D) Propidium iodide (PI) staining measured by flow cytometry (n = 4/sex/group). XY neurons harvested from male rats, XX neurons harvested from female rats; mean ± SD; *p < 0.01 versus control; †p < 0.04 versus vehicle; ‡p < 0.03 versus Pro-NAC; ¶p < 0.02 versus XY neurons.
We then evaluated the capacity for probenecid and NAC, alone and in combination, to prevent neuronal death after stretch injury as detected by LDH release, caspase 3 activity, and PI labeling. LDH release was quantified (LDH Cytotoxicity Detection Kit, Takara Bio, Mountain View, CA) in cell culture supernatant at 24 h after stretch or sham injury as previously described.16 Stretch injury resulted in 38% cell death in XY-neurons and 24% cell death in XX-neurons assessed by LDH release (Fig. 1B) (n = 5–8 independent wells/sex/group; p = 0.001 XY- vs. XX-neurons). Group (F[3, 43] = 17.38, p < 0.0001) and sex-dependent (F[1, 43] = 6.76, p = 0.013) differences were observed. LDH release was attenuated in both XY- and XX-neurons when treated with NAC alone and the two drugs used in combination (XY-neurons p < 0.001 vs. vehicle; XX-neurons p < 0.04 vs. vehicle). Probenecid alone attenuated LDH release in XY- but not XX-neurons (p < 0.001 and p = 0.135, respectively). Caspase 3-like activity was calculated using a fluorescent assay (Apo-ONE, Promega, Madison, WI) in cell homogenates as previously described.19 Stretch injury resulted in increased caspase 3 activity; however, in contrast to LDH release, the increase was more robust in XX-neurons (Fig. 1C) (n = 4 independent wells/sex/group; p < 0.001 XX- vs. XY-neurons). Group (F[4, 30] = 53.11, p < 0.0001) and sex-dependent (F[1, 30] = 36.18, p < 0.0001) differences were observed. Caspase 3 activity was attenuated only in XX-neurons when treated with probenecid or NAC alone, or the two drugs in combination, with equal effect (p < 0.001 vs. vehicle). Probenecid alone, NAC alone, or Pro-NAC in combination attenuated caspase 3 activation in XY-neurons (p > 0.05 vs. vehicle).
Neuronal cell death was confirmed using flow cytometry as previously described.16 Briefly, neurons were harvested from silicone membranes using trypsin- ethylenediaminetetraacetic acid (EDTA), labeled with PI (5 μg/mL; Sigma, St. Louis, MO), and analyzed using a tri-laser FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). Stretch injury resulted in 34% cell death in XY-neurons and 22% cell death in XX-neurons assessed by PI labeling (Fig. 1D) (n = 4 independent wells/sex/group; p < 0.001 XY- vs. XX-neurons). Group (F[4, 35] = 128.2, p < 0.0001) and sex-dependent (F[1, 35] = 15.56, p = 0.0004) differences were observed. Cell death assessed by PI labeling was attenuated in both XY- and XX-neurons when treated with probenecid alone, NAC alone, or the two drugs used in combination in both XY- and XX-neurons (p < 0.001 vs. vehicle). This effect was additive when the two drugs were used in combination (p = 0.015 probenecid alone vs. Pro-NAC; p = 0.017 NAC alone vs. Pro-NAC). A group difference between XY- and XX-neurons in PI uptake was observed (p < 0.001).
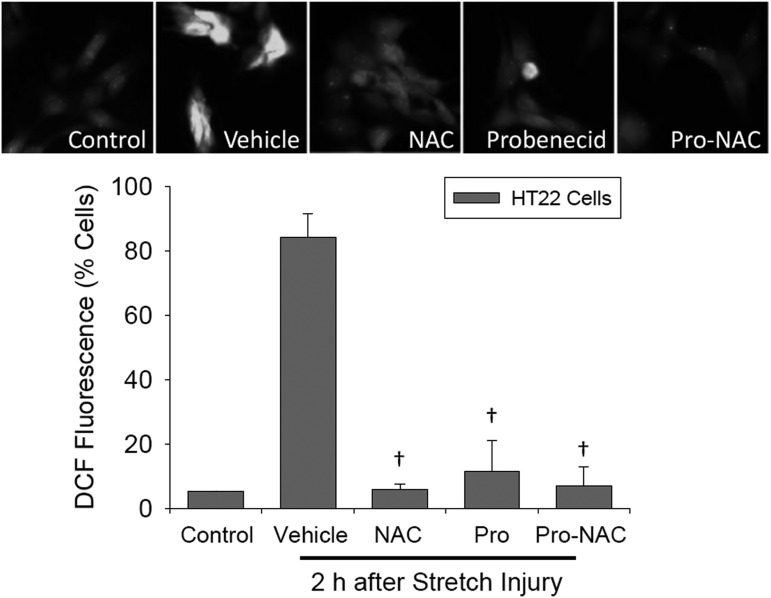
The effect of probenecid and NAC, alone or in combination, on oxidative stress after stretch injury was evaluated in HT22 hippocampal cells cultured in Dulbecco's modified Eagle's medium (ThermoFisher Scientific, Waltham, MA). Silicone membranes were coated with poly-d-lysine, seeded at a density of 2 × 106 cells/membrane, and incubated until 70% confluent. HT22 cells were subjected to stretch injury as described. Oxidative stress was evaluated by assessing redox sensitive intracellular conversion of dichlorodihydrofluorescein diacetate (DCFH-DA) (Cayman Chemical, Ann Arbor, MI; added with drug treatments) to dichlorofluorescein (DCF). Two hours after stretch injury, cells were harvested by trypsinization, re-suspended in PBS, and analyzed using flow cytometry (488 nm excitation/525 nm emission wavelengths for DCF). After stretch injury, 84% of HT22 cells were DCF positive versus 5.1% in control cells. All treatments reduced the number of DCF positive cells versus vehicle 2 h after stretch injury (Fig. 2) (n = 3 independent wells/treatment; F[4, 9] = 80.1, p < 0.0001; p < 0.05 NAC, probenecid, and Pro-NAC vs. vehicle). These data are consistent with oxidative stress after stretch injury in neural cells that is effectively inhibited by both NAC and probenecid in culture.
FIG. 2.
Effects of N-acetylcysteine (NAC) (50 μM) and probenecid (100 μM), alone and in combination (Pro-NAC), on intracellular reactive oxygen species (ROS) production detected by dichlorodihydrofluorescein (DCF) fluorescence in HT22 hippocampal cells 2 h after stretch injury (n = 3/group for treatments, n = 2 for control cells). The percentage of DCF positive cells was measured using flow cytometry (mean ± SD; †p < 0.05 vs. vehicle). Representative fluorescent images of HT22 cells for each group are shown.
Doses for probenecid2 and NAC21 were based on previous in vitro studies, and each alone was protective at the doses chosen. When used in combination, effects on intracellular GSH concentration and prevention of neuronal death assessed by PI uptake were additive and effective in neurons cultured from both male and female rats. Consistent with previous studies,21,22 sex-dependent effects were seen in the current study. XY-neurons, shown to be more vulnerable to oxidative stress and excitoxicity, were more vulnerable to stretch injury than their XX counterparts. In addition, caspase 3 activity was more robust in XX- than in XY-neurons after stretch injury, similar to XX- and XY-neurons exposed to the apoptosis-inducing agent staurosporine.21 Also of interest were the findings that probenecid and NAC alone and in combination were equally effective in terms of inhibiting caspase 3 activity in XX-neurons, but all were ineffective in terms of inhibiting caspase 3 activity in XY-neurons after stretch injury. This could be explained by initiation of multiple regulated cell death pathways in addition to apoptosis contributing to cell death after neural trauma, including pyroptosis, necroptosis, and ferroptosis;26 with synergy of probenecid and NAC in combination involving nonapoptotic cell death pathways, particularly in neurons from male rats. Alternatively, the temporal pattern of apoptosis after stretch injury may be sex dependent, and evaluating later time points after injury would be valuable. To our knowledge, other studies examining neuronal apoptosis after mechanical trauma have not utilized sex-segregated cultures. These data are consistent with a more prominent role for apoptosis in females after traumatic neuronal injury, with NAC and probenecid as potential therapies in both sexes.
Both probenecid and NAC are in clinical use and have favorable safety profiles, making them appealing in terms of “repurposing” for diseases involving neuronal trauma, such as TBI and SCI. Probenecid is currently in clinical use to treat uric acidemia and as an antibiotic adjunct, and was developed during World War II (Benemid) to increase the bioavailability of penicillin, in short supply at the time, to wounded soldiers.27,28 NAC is the antidote for acetaminophen/paracetamol overdoses, reducing the risk of hepatic necrosis and fulminant liver failure.29 A randomized clinical trial of systemically administered NAC after TBI has shown positive results.12 No previous experimental or clinical studies to our knowledge have reported probenecid and NAC in combination, although we have recently completed enrollment for a phase I pharmacokinetic study for the combination in pediatric TBI (ClinicalTrials.gov identifier NCT01322009) and results are forthcoming. With the obvious impact of the BBB in the intact brain, evaluation of these treatments on reactive oxygen species (ROS) production, neuronal death, and behavioral outcome after TBI and SCI in vivo, appears warranted. In the present study, the efficiency of NAC to cross the BBB and penetrate into the CNS is bypassed in vitro. Finally, given the presence of multiple transporters inhibited by probenecid on the BBB (e.g., organic anion transporters 1 and 3, ABCC1/MRP1) and cells in the CNS in mammals including humans,30,31 probenecid would have effects on multiple cell types and barriers after brain injury in vivo, which may result in synergistic or diametrically opposing actions.
In conclusion, these data provide proof of principle supporting the combination of probenecid and NAC as a neuroprotective strategy targeting preservation of intracellular GSH after traumatic neural injury. The results of these in vitro studies may extrapolate to in vivo models of TBI as well as models of SCI.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) R01 NS069247 and the Children's Hospital of Pittsburgh Scientific Program. We thank Dr. Qin Yang for providing the HT22 cells.
Author Disclosure Statement
No competing financial interests related to this study exist.
References
- 1.Cole S.P. (2014). Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem 289, 30,880–30,888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H., Miller D.W., and Elmquist W.F. (2001). Effect of probenecid on fluorescein transport in the central nervous system using in vitro and in vivo models. Pharm. Res. 18, 1542–1549 [DOI] [PubMed] [Google Scholar]
- 3.DeCory H.H., Piech–Dumas K.M., Sheu S.S., Federoff H.J., and Anders M.W. (2001). Efflux of glutathione conjugate of monochlorobimane from striatal and cortical neurons. Drug Metab. Dispos. 29, 1256–1262 [PubMed] [Google Scholar]
- 4.Hammond C.L., Marchan R., Krance S.M., and Ballatori N. (2007). Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J. Biol. Chem. 282, 14,337–14,347 [DOI] [PubMed] [Google Scholar]
- 5.Silverman W.R., de Rivero Vaccari J.P., Locovei S., Qiu F., Carlsson S.K., Scemes E., Keane R.W., and Dahl G. (2009). The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284, 18,143–18,151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamczak S.E., de Rivero Vaccari J.P., Dale G., Brand F.J., 3rd, Nonner D., Bullock M.R., Dahl G.P., Dietrich W.D., and Keane R.W. (2014). Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J. Cereb. Blood Flow Metab. 34, 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong X.X., Gu L.J., Shen J., Kang X.H., Zheng Y.Y., Yue S.B., and Zhu S.M. (2014). Probenecid protects against transient focal cerebral ischemic injury by inhibiting HMGB1 release and attenuating AQP4 expression in mice. Neurochem. Res. 39, 216–224 [DOI] [PubMed] [Google Scholar]
- 8.Wei R., Wang J., Xu Y., Yin B., He F., Du Y., Peng G., and Luo B. (2015). Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience 301, 168–177 [DOI] [PubMed] [Google Scholar]
- 9.Samuni Y., Goldstein S., Dean O.M., and Berk M. (2013). The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 1830, 4117–4129 [DOI] [PubMed] [Google Scholar]
- 10.Naziroglu M., Senol N., Ghazizadeh V., and Yuruker V. (2014). Neuroprotection induced by N-acetylcysteine and selenium against traumatic brain injury-induced apoptosis and calcium entry in hippocampus of rat. Cell Mol. Neurobiol. 34, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naziroglu M., Cig B., and Ozgul C. (2013). Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience 242, 151–160 [DOI] [PubMed] [Google Scholar]
- 12.Hoffer M.E., Balaban C., Slade M.D., Tsao J.W., and Hoffer B. (2013). Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One 8, e54163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eakin K., Baratz–Goldstein R., Pick C.G., Zindel O., Balaban C.D., Hoffer M.E., Lockwood M., Miller J., and Hoffer B.J. (2014). Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One 9,e90617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong Y., Peterson P.L., and Lee C.P. (1999). Effect of N-acetylcysteine on mitochondrial function following traumatic brain injury in rats. J. Neurotrauma 16, 1067–1082 [DOI] [PubMed] [Google Scholar]
- 15.Guo J., Li Y., Chen Z., He Z., Zhang B., Hu J., Han M., and Xu Y. (2015). N–acetylcysteine treatment following spinal cord trauma reduces neural tissue damage and improves locomotor function in mice. Mol. Med. Rep. 12, 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel Baki S.G., Schwab B., Haber M., Fenton A.A., and Bergold P.J. (2010). Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS One 5, e12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karalija A., Novikova L.N., Kingham P.J., Wiberg M., and Novikov L.N. (2012). Neuroprotective effects of N-acetyl-cysteine and acetyl-L-carnitine after spinal cord injury in adult rats. PLoS One 7, e41086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai Y., Hickey R.W., Chen Y., Bayir H., Sullivan M.L., Chu C.T., Kochanek P.M., Dixon C.E., Jenkins L.W., Graham S.H., Watkins S.C., and Clark R.S. (2008). Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J. Cereb. Blood Flow Metab. 28, 540–550 [DOI] [PubMed] [Google Scholar]
- 19.Pandya J.D., Readnower R.D., Patel S.P., Yonutas H.M., Pauly J.R., Goldstein G.A., Rabchevsky A.G., and Sullivan P.G. (2014). N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 257, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith D.H., Wolf J.A., Lusardi T.A., Lee V.M., and Meaney D.F. (1999). High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J. Neurosci. 19, 4263–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du L., Bayir H., Lai Y., Zhang X., Kochanek P.M., Watkins S.C., Graham S.H., and Clark R.S.B. (2004). Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J. Biol. Chem. 279, 38,563–38,570 [DOI] [PubMed] [Google Scholar]
- 22.Du L., Hickey R.W., Bayir H., Watkins S.C., Tyurin V.A., Guo F., Kochanek P.M., Jenkins L.W., Ren J., Gibson G., Chu C.T., Kagan V.E., and Clark R.S. (2009). Starving neurons show sex difference in autophagy. J. Biol. Chem. 284, 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brewer G.J. (1995). Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci. Res. 42, 674–683 [DOI] [PubMed] [Google Scholar]
- 24.Ji J., Kline A.E., Amoscato A., Samhan–Arias A.K., Sparvero L.J., Tyurin V.A., Tyurina Y.Y., Fink B., Manole M.D., Puccio A.M., Okonkwo D.O., Cheng J.P., Alexander H., Clark R.S., Kochanek P.M., Wipf P., Kagan V.E., and Bayir H. (2012). Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 15, 1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji J., Tyurina Y.Y., Tang M., Feng W., Stolz D.B., Clark R.S., Meaney D.F., Kochanek P.M., Kagan V.E., and Bay R.H. (2012). Mitochondrial injury after mechanical stretch of cortical neurons in vitro: biomarkers of apoptosis and selective peroxidation of anionic phospholipids. J. Neurotrauma 29, 776–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanden Berghe T., Linkermann A., Jouan–Lanhouet S., Walczak H., and Vandenabeele P. (2014). Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 15, 135–147 [DOI] [PubMed] [Google Scholar]
- 27.Boger W.P., Pitts F.W., and Gallagher M.E. (1950). Benemid and carinamide: comparison of effect on para-aminosalicylic acid (PAS) plasma concentrations. J. Lab. Clin. Med. 36, 276–282 [PubMed] [Google Scholar]
- 28.Frisk A.R., Diding N., and Wallmark G. (1952). Influence of probenecid on serum penicillin concentration after oral administration of penicillin. Scand. J. Clin. Lab. Invest. 4, 83–88 [DOI] [PubMed] [Google Scholar]
- 29.Kanter M.Z. (2006). Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am. J Health Syst. Pharm. 63, 1821–1827 [DOI] [PubMed] [Google Scholar]
- 30.Nigam S.K., Bush K.T., Martovetsky G., Ahn S.Y., Liu H.C., Richard E., Bhatnagar V., and Wu W. (2015). The organic anion transporter (OAT) family: a systems biology perspective. Physiol. Rev. 95, 83–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willyerd F.A., Empey P.E., Philbrick A., Ikonomovic M.D., Puccio A.M., Kochanek P.M., Okonkwo D.O., and Clark R.S. (2016). Expression of ATP-binding cassette transporters B1 and C1 after severe traumatic brain injury in humans. J. Neurotrauma 33, 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]