Abstract
Magnetic resonance imaging data were acquired at ∼24 h and ∼3 months post-injury on mild traumatic brain injury (mTBI; n = 75) and orthopedic injury (n = 60) cohorts. The mTBI subjects were randomly assigned to a treatment group with atorvastatin or a non-treatment mTBI group. The treatment group was further divided into drug and placebo subgroups. FreeSurfer software package was used to compute cortical thickness based on the three dimensional T1-weighted images at both time-points. Cross-sectional analysis was carried out to compare cortical thickness between the mTBI and control groups. Longitudinal unbiased templates were generated for all subjects and cortical thickness measurements were compared between baseline and follow-up scans in the mTBI group. At baseline, significant reduction in cortical thickness was observed in the left middle temporal and the right superior parietal regions in the mTBI group, relative to the control group (p = 0.01). At follow-up, significant cortical thinning was again observed in the left middle temporal cortex in the mTBI group. Further analysis revealed significant cortical thinning only in the non-treatment group relative to the control group. In the follow-up, small regions with significant but subtle cortical thinning and thickening were seen in the frontal, temporal, and parietal lobes in the left hemisphere in the non-treatment group only. Our results indicate that cortical thickness could serve as a useful measure in identifying subtle changes in mTBI patients.
Key words: : cortical thickness, FreeSurfer, longitudinal analysis, mTBI, orthopedic injury
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability in the U.S., with nearly 52,000 deaths and 2.2 million emergency department visits annually (www.braintrauma.org). More than 75% of TBI incidents are classified as mild (mTBI) (www.cdc.gov/traumaticbraininjury/pdf/BlueBook_factsheet-a.pdf). Some patients with mTBI experience a wide range of symptoms that include memory loss, irritability, dizziness/nausea, and other neurological deficits (www.cdc.gov/concussion/signs_symptoms.html). There are multiple causes for mTBI, including motor vehicle accidents, falls, assaults, sports-related injuries, and blast injuries in the combat zone. Even though the mechanism of mTBI-induced pathology is yet to be completely understood, there is evidence that axonal injury may be the primary cause.1 Early symptoms of mTBI might resolve over time, but studies showed that some patients suffer years after the actual trauma.2,3 Therefore, there is a need to develop sensitive methods to improve the diagnosis and prognosis of mTBI to help reduce long-term effects.
Cortical atrophy in mTBI has attracted attention in recent years due to improvements in neuroimaging techniques and post-processing methods. The development of methods to accurately measure cortical thickness has brought another dimension to studying cortical atrophy following mTBI. Animal studies have reported cortical atrophy following mTBI.4–6 A study on veterans reported negative correlations between post-traumatic stress disorder (PTSD) severity and cortical thickness in post-central and middle temporal gyri in blast-related mTBI.7 Another study in blast-induced mTBI reported cortical thinning in superior temporal and superior frontal gyri.8 Veterans exposed to early life trauma had reduced cortical thickness in paracentral and posterior cingulate regions.9
While the above studies focused on blast-related injury, other mechanisms leading to mTBI also have been studied. A recent study evaluated cortical thinning in sports-related mTBI in young subjects and found evidence of cortical thinning in the left dorsolateral prefrontal cortex and in the right inferior parietal cortex, compared with controls.10 Another recent study, based on 21 patients with mTBI from motor vehicle collisions, found cortical thinning in the left posterior middle temporal gyrus and cortical thickening in the right precuneus and left rostral middle frontal gyrus in the acute phase after injury.11 In light of these findings, changes in cortical thickness appear to be a pathologic feature in mTBI.
The main objective of this study is to evaluate the effect of mTBI on cortical thickness on a relatively large and well-characterized cohort on whom data were acquired in the acute phase (around 24 h post-injury) and in the recovery phase (around 3 months). Cortical thickness measurements were based on three-dimensional T1-weighted magnetic resonance imaging (MRI) scans.
Methods
This project is part of a larger study on mTBI supported by the Department of Defense (DoD). These studies were approved by the Institutional Review Board (IRB) and by the Human Research Protection Official Review of Research Protocols of the DoD. Patient data was acquired in compliance with the Health Insurance Portability and Accountability Act. Civilian patients from an ethnically diverse population were recruited from two emergency departments in Southwestern metropolitan–area hospitals.
Subjects
The patients were categorized into either the mTBI or the orthopedic injury group based on the guidelines by the DoD12 and the American Congress of Rehabilitation Medicine.13 Patients, irrespective of race, gender, and ethnicity, were recruited by healthcare professionals (registered nurses, physicians, and emergency medical technician-paramedics) who had prior clinical experience with brain injury patients and excellent interpersonal skills. The screening process comprised a review of electronic healthcare system data, consultation with emergency staff, and patient interviews. Under special provision from the IRB, the Galveston Orientation and Amnesia Test (GOAT) was administered prior to informed consent. Subjects needed to score 75 or higher in the test in order to provide informed consent and if not, a legally authorized representative had to provide consent.14 All subjects in this study had a GOAT score above 75. The following criteria were used for inclusion in the mTBI group: presence of a head injury, Glasgow Coma Scale score in the range of 13–15, a negative computed tomography (CT) scan, post-traumatic amnesia under 24 h, and loss of consciousness for under 30 min. Subjects with pre-existing psychological disorders, PTSD, substance abuse, and alcohol dependence were excluded from the study. In addition, subjects with either previous head injuries or with dominant left-handedness were excluded. Subjects in the orthopedic injury control group confirmed that they had not hit their head during the injury to the best of their recollection. In addition, they neither had visible signs of head injury nor had undergone any loss of consciousness or memory.
MRI acquisition
All MRI data was acquired on a 3T Philips scanner using an eight-channel receive-only head coil. Three-dimensional T1-weighted images were acquired using the magnetization prepared rapid acquisition of gradient echoes (MPRAGE) sequence, with a repetition time (TR) of 8.1 msec and an echo time (TE) of 3.7 msec. The images were acquired with an isotropic resolution of 1 × 1 × 1 mm3. An automatic image quality assessment algorithm previously implemented elsewhere15,16 was applied to all the datasets. Images with inadequate quality (poor signal-to-noise ratio, image ghosting, incomplete brain coverage, etc.) were discarded from the analysis (n = 2).
Estimation of cortical thickness
Global and regional cortical thicknesses were estimated using FreeSurfer version 5.3.0 (https://surfer.nmr.mgh.harvard.edu/fswiki) on a Linux workstation. The FreeSurfer processing pipeline is described in numerous publications.17–20 Briefly, the preprocessing steps involve intensity normalization, transformation to the Talairach space, and a hybrid skull stripping algorithm based on watershed and deformable template models.21 The resulting mask is then labeled using a probabilistic atlas. Following the preprocessing, white matter segmentation is performed followed by generation of white and pial surfaces. The average distance between the gray-white boundary and the surface at each vertex is defined as the cortical thickness. In this study, the Desikan-Killiany atlas22 was used for neuro-anatomical labeling. Using this pipeline, cortical thickness was measured for all subjects at both time-points. The entire FreeSurfer pipeline was automated and the images were visually inspected for errors in preprocessing or segmentation.
In order to investigate group differences at the cross-sectional level, multiple comparisons analysis using FreeSurfer's Monte-Carlo simulation was performed with 5000 iterations, with a false discovery rate of p < 0.05. This analysis was repeated at both time-points.
Longitudinal analysis
The FreeSurfer longitudinal pipeline was used to evaluate cortical thickness differences over time within each of the study groups.23 Unbiased within-subject templates were created using data from both time-points with an inverse consistent registration algorithm.24,25 Within this template space, various processing steps, such as atlas registration and regional parcellation, were carried out. Because of the common information that is available in the within-subject template space, this pipeline increases reliability and statistical power. Following regeneration of surface maps based on the longitudinal pipeline, paired statistical analysis using FreeSurfer's generalized linear model analysis with a smoothing kernel of 10 mm was performed to evaluate the longitudinal differences.
Results
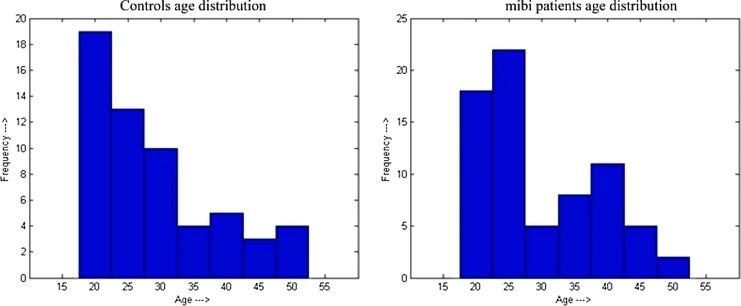
Injury mechanisms for both the mTBI group and the orthopedic control group are summarized in Table 1. Figure 1 illustrates the age distribution for the mTBI patients and control groups. The educational levels (mean ± standard deviation [SD]) of the control and the mTBI groups were 13.33 ± 2.59 years (range 6 to 19 years) and 13.21 ± 2.43 years (range 6 to 19 years), respectively. The educational levels between these two groups were not statistically different (t- test; p = 0.793). The socioeconomic indices (SEI) were calculated using a procedure described elsewhere.26 The SEI values (mean ± SD) for the control and mTBI groups were 0.07 ± 0.96 (range −1.72 to 2.84) and 0.04 ± 1.06 (range −1.69 to 2.39), respectively. The group differences in the socioeconomic index are not statistically significant (p = 0.86).
Table 1.
Summary of Injury Mechanisms in mTBI and Orthopedic Control Groups
| Injury mechanism | mTBI group (%) | Orthopedic control group (%) |
|---|---|---|
| Motor vehicle accident | 39.5 | 11.4 |
| Assault | 18.3 | 1.2 |
| Blow to head | 11.3 | 0 |
| Lacerations | 0 | 44.7 |
| Fall | 18.4 | 20.9 |
| Auto-pedestrian collision | 8.3 | 0 |
| Other | 4.2 | 21.8 |
mTBI, mild traumatic brain injury.
FIG. 1.
Age distribution of controls (left) and mild traumatic brain injury (mTBI) patients (right).
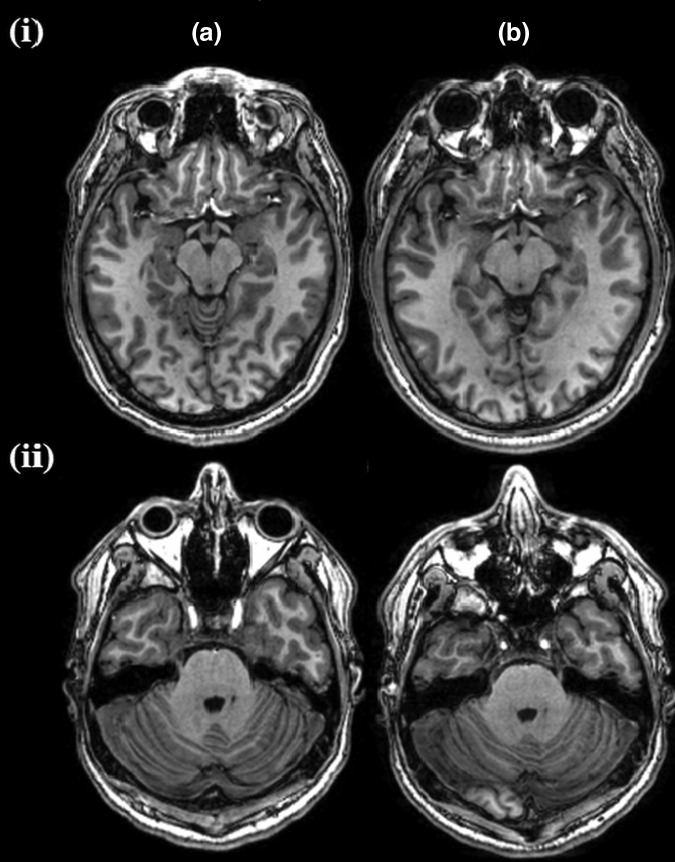
Table 2 summarizes patient demographics for all subjects. MRI data were acquired from a total of 135 subjects (mean age, 29.3 ± 8.8 years; range, 18–50 years; 96 males, 39 females); 60 subjects were categorized into the orthopedic injury group and 75 into the mTBI group. Two subjects in the mTBI group had Glasgow Coma Scale scores of 14 and the rest of the group had a score of 15. The average duration of loss of consciousness in the mTBI group was 3.95 ± 5 min (range, 1–20 min). MRI data acquisition for both groups was carried out in the acute phase and a follow-up session. The average times between injury and scan time was 25.5 ± 12.26 h (range, 5.8–46.0 h) for the mTBI group and 27.1 ± 13.7 h (range, 0.3–56.0 h) for the orthopedic injury group. For the follow-up session, the times from injury were 97.9 ± 17.57 days (range, 83.3–202.0 days) for the mTBI group and 96.7 ± 9.26 days (range, 82.9–126.9 days) for the orthopedic injury group. All 135 subjects were scanned at both time-points. As an example, typical T1-weighted axial images at the level of the orbitofrontal cortex and temporal poles (without any processing) at baseline and follow-up are shown in Figure 2. As can be appreciated from this figure, the image quality appears quite decent.
Table 2.
Subject Demographic Information
| Parameter | Group O (controls) | Group A (treated) | Group T (non-treated) |
|---|---|---|---|
| Number of participants | 58 | 33 | 38 |
| Age; mean ± SD, range, median | 28.95 ± 8.8, 20–50, 26.5 | 29.1 ± 9.9, 18–49, 25 | 30 ± 8.12, 18–47, 27 |
| Gender (M/F) | 43/15 | 21/12 | 29/9 |
SD, standard deviation; M, male; F, female.
FIG. 2.
Axial T1-weighted magnetic resonance images at the levels of orbitofrontal cortex (i) and temporal poles (ii) of one subject at baseline (A) and follow-up (B). The voxel size is 1 mm ×1 mm ×1 mm.
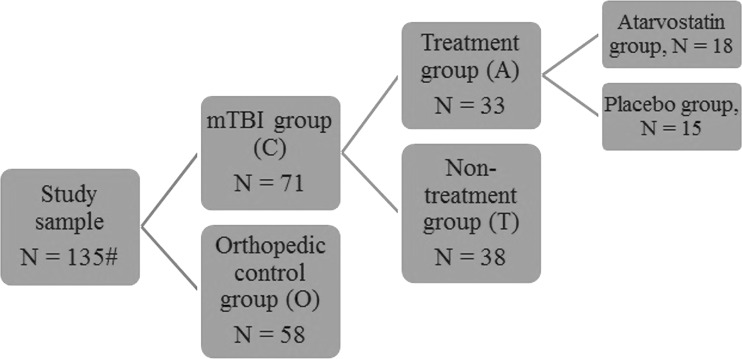
The mTBI group was further randomly divided into treatment (n = 35) and a non-treatment groups (n = 40). Further, the subjects from the treatment group were randomly assigned to two groups: treated with atorvastatin or placebo. Out of the 135 subjects, images with inadequate quality (poor signal-to-noise ratio, image ghosting, incomplete brain coverage, etc.) were discarded from the analysis (n = 2). Further, images that failed the visual inspection as part of the FreeSurfer pipeline were discarded from the study (n = 4). Figure 3 summarizes the categorization of the study sample into the subgroups.
FIG. 3.
Representation of patient categories and sample size. mTBI, mild traumatic brain injury.
Cross-sectional analysis—baseline (∼24 h after injury)
At baseline, both treatment and non-treatment groups were combined since treatment has not started prior to the initial scan. Cortical thickness was compared between the orthopedic control and mTBI groups. In the mTBI group, significant cortical thinning was observed in the middle temporal cortex in the left hemisphere and in the supramarginal cortex in the right hemisphere (p = 0.01) (Fig. 4). No significant thickening was observed in either hemisphere. The average cortical thickness values at baseline in various lobes (frontal, parietal, temporal, occipital, cingulate, and insula) are summarized in Table 3.
FIG. 4.
Regions of significant cortical thinning in mild traumatic brain injury patients, compared with normals, at baseline and at 3 months follow-up. Regions in the middle temporal cortex in the left hemisphere and inferior parietal cortex in the right hemisphere were significant at p = 0.01.
Table 3.
Average Cortical Thickness Values for Different Lobes in Each Subject Group at Two Time-Points of Acquisition
| Left hemisphere | ||||||
|---|---|---|---|---|---|---|
| Baseline | Frontal | Parietal | Temporal | Occipital | Cingulate | Insula |
| Controls | 2.53 ± 0.15 | 2.29 ± 0.59 | 2.87 ± 0.46 | 1.85 ± 0.40 | 2.55 ± 0.35 | 2.96 ± 0.47 |
| Treated | 2.52 ± 0.14 | 2.28 ± 0.57 | 2.83 ± 0.45 | 1.83 ± 0.39 | 2.55 ± 0.36 | 2.94 ± 0.44 |
| Non-treated | 2.50 ± 0.14 | 2.28 ± 0.54 | 2.82 ± 0.44 | 1.85 ± 0.39 | 2.53 ± 0.33 | 2.97 ± 0.42 |
| Follow-up | ||||||
| Controls | 2.52 ± 0.15 | 2.29 ± 0.59 | 2.87 ± 0.47 | 1.85 ± 0.40 | 2.55 ± 0.35 | 2.97 ± 0.48 |
| Treated | 2.52 ± 0.14 | 2.27 ± 0.58 | 2.83 ± 0.46 | 1.82 ± 0.39 | 2.58 ± 0.36 | 2.94 ± 0.46 |
| Non-treated | 2.51 ± 0.14 | 2.29 ± 0.56 | 2.84 ± 0.46 | 1.86 ± 0.39 | 2.54 ± 0.33 | 2.97 ± 0.45 |
| Right hemisphere | ||||||
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Controls | 2.54 ± 0.14 | 2.27 ± 0.6 | 2.91 ± 0.48 | 1.87 ± 0.40 | 2.50 ± 0.35 | 2.87 ± 0.49 |
| Treated | 2.53 ± 0.13 | 2.26 ± 0.58 | 2.86 ± 0.48 | 1.85 ± 0.39 | 2.53 ± 0.35 | 2.84 ± 0.46 |
| Non-treated | 2.50 ± 0.13 | 2.25 ± 0.56 | 2.85 ± 0.45 | 1.88 ± 0.38 | 2.50 ± 0.32 | 2.89 ± 0.47 |
| Follow-up | ||||||
| Controls | 2.53 ± 0.13 | 2.26 ± 0.61 | 2.90 ± 0.48 | 1.87 ± 0.40 | 2.51 ± 0.34 | 2.88 ± 0.5 |
| Treated | 2.51 ± 0.13 | 2.25 ± 0.59 | 2.87 ± 0.49 | 1.84 ± 0.40 | 2.52 ± 0.35 | 2.90 ± 0.48 |
| Non-treated | 2.52 ± 0.13 | 2.26 ± 0.58 | 2.87 ± 0.46 | 1.88 ± 0.40 | 2.50 ± 0.33 | 2.94 ± 0.48 |
Baseline (< 24 h after injury); follow-up (3 months after injury). Controls, n = 58; treated, n = 33; and non-treated, n = 38.
Cross-sectional analysis at follow-up (∼3 months after injury)
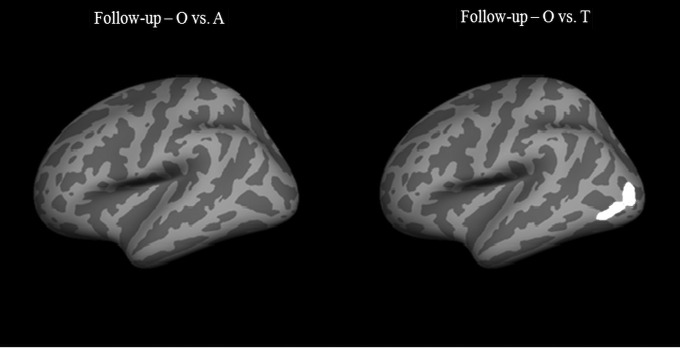
At follow-up, cortical thickness comparison was performed between: 1) orthopedic controls and mTBI subjects; 2) orthopedic controls and mTBI patients who received treatment; and 3) orthopedic controls and mTBI patients who did not receive treatment. Significant thinning was observed in mTBI patients, compared with orthopedic controls, in the left middle temporal cortex at p = 0.01 (Fig. 4). This was the same region that showed cortical thinning at baseline. No cortical thinning was observed in the right hemisphere and no cortical thickening was observed in either hemisphere. For the analysis involving orthopedic controls and the treatment group, no significant differences were observed in either hemisphere. The results from the comparison of orthopedic controls with the non-treatment group showed significant cortical thinning in the left middle temporal cortex, the same region that was involved in the whole group of mTBI patients. No significant differences were observed in the right hemisphere (Fig. 5). The average cortical thickness values in various lobes (frontal, parietal, temporal, occipital, cingulate and insula) at follow-up are summarized in Table 3.
FIG. 5.
Regions of significant cortical thinning in mTBI patients versus normal controls (O), separated by treatment group (A) and non-treatment group (T) at 3 months follow-up. Regions in the middle temporal cortex in the left hemisphere in the Group O vs. Group T comparison were significant at p = 0.01. No significant differences were observed in the right hemisphere.
In addition to the whole–group analysis, cortical thickness also was determined for the subgroups in the treatment group (placebo, n = 15 and drug, n = 18). We also compared these two subgroups separately with orthopedic controls in order to determine the effect of treatment on cortical thickness post injury. The subgroup that was administered the drug did not yield any significant differences, compared with the control group, either in thinning or thickening. In the comparison between the placebo and the orthopedic control groups, significant cortical thickening was observed in the cuneus region in both hemispheres (p = 0.01).
Longitudinal analysis
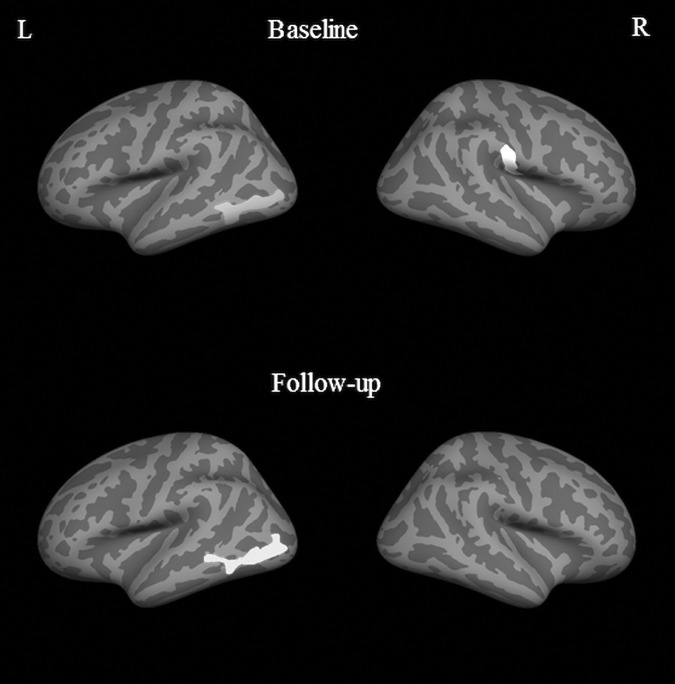
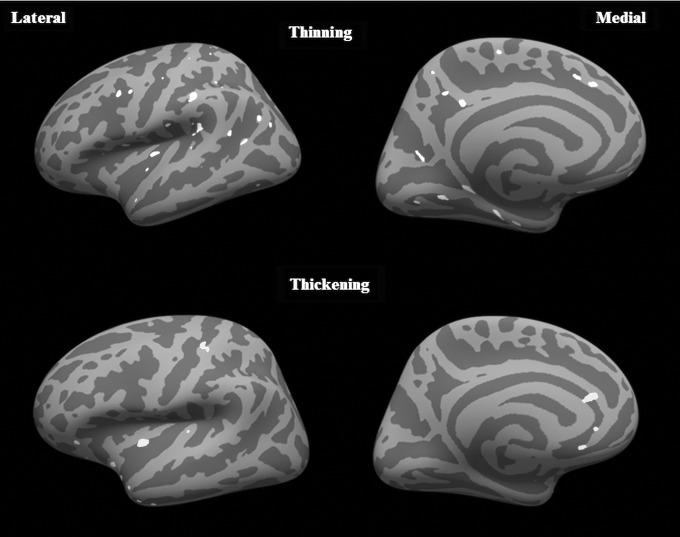
There were no significant differences (either thinning or thickening) in the orthopedic control group in either hemisphere. In the mTBI group, multiple clusters in the frontal, temporal and parietal lobes in the left hemisphere were observed with significantly thinner cortex at follow-up, compared with baseline (p = 0.01). Significant clusters showing cortical thickening also were observed at follow-up, compared with baseline within the insula, anterior cingulate, and superior parietal lobes. However, the cluster sizes with thicker cortices were much smaller, compared with the regions that showed thinning (Fig. 6). No significant differences were seen in any of the lobes in the right hemisphere. When the mTBI group was divided into treatment and non-treatment groups, there were no significant differences between baseline and follow-up in the treatment group. In the non-treatment group, at a significance level of p = 0.01, there were scattered regions in the left hemisphere spread over the frontal, temporal, and parietal cortex (all regions <100 mm2 each) for cortical thinning and a few small regions in the insular cortex for thickening. To ascertain if this was a trend, we lowered the threshold to p = 0.05 and found significant cortical thinning in parts of the temporal lobe, parietal lobe, frontal lobe, insula, and the cingulate cortex. In addition, significant cortical thickening was observed in the insula and parts of the inferior frontal lobe. No significant differences were found in the right hemisphere, even at this lower threshold.
FIG. 6.
Regions of significant cortical thinning and significant cortical thickening in m traumatic brain injury patients (Group C) compared from baseline to 3 months follow-up using the longitudinal pipeline at p = 0.01. The lateral and medial surfaces of the left hemisphere are shown. No significant differences were observed in the right hemisphere.
In addition to the whole mTBI group, a longitudinal analysis also was performed to follow the subgroups (placebo and drug) from baseline to follow-up. Based on that analysis, no significant differences were found in the drug-treated group, whereas in the placebo group, significant thickening was observed in a small region of the middle temporal cortex at p = 0.01.
Discussion
We investigated changes in cortical thickness in mTBI relative to controls both cross-sectionally and longitudinally at two time-points (acute and recovery phase). Our results show significant cortical thinning in the left middle temporal region and right supramarginal region at baseline. At 3 months, the thinning observed in the supramarginal region disappeared but remained in the temporal region.
Within the mTBI group, the effect of treatment also was investigated. From this analysis, it appears that the cortical thinning found in the whole cohort is driven primarily by the non-treatment group, since there were no significant differences found between the treatment group and the control group. This trend of the non-treatment group being the contributing sample extended into the longitudinal analysis also, as the orthopedic control and treatment groups did not show any significant changes. The non-treatment group showed a trend of thinning and thickening in sporadic regions over the frontal, temporal, and parietal lobes.
While the literature on cortical thickness changes in mTBI is sparse, it still would be interesting to compare our results with the few published reports. Earlier studies on veterans with mTBI found an inverse correlation between cortical thickness in post-central and middle temporal gyri and the severity of PTSD.7 In our study, the middle temporal cortex also showed significant cortical thinning in mTBI, compared with the control group. Another recent study evaluated cortical thickness abnormalities in a small sample of blast-injured service members and reported significant thinning in the superior temporal and superior frontal regions.8 In our study, we did not find any significant differences in those regions. It is important to point out that the blast-injury mechanism is different from other injury mechanisms and that our study population consists primarily of motor vehicle collisions (∼40% of the sample).
Another study on sports-related injury in a small sample of young subjects reported significant cortical thinning in the left dorsolateral prefrontal cortex and the right inferior parietal cortex.10 In our study, we observed cortical thinning in the right inferior parietal cortex (supramarginal) at the baseline, but that disappeared at the 3 month follow-up. The above-mentioned study was performed at 3–6 months post-injury and also with a much younger cohort. Those factors might explain the differences between these two studies.
A recent publication investigated cortical thickness in mTBI in a sample of motor vehicle injury patients with data acquired at ∼48 h and at 3 months post injury.11 Their study found cortical thinning in the left middle temporal gyrus at baseline, but much less extensive than what was observed in the current study. In addition, their study reported significant cortical thickening in the mTBI group in the right precuneus and the left rostral middle frontal gyrus. In our study, we did not find any thickening in the mTBI group, compared with controls. Their analysis at a later time-point had fewer subjects (n = 11) and also did not use subject-based template method (longitudinal pipeline in FreeSurfer) but rather employed a region on interest (ROI)-based method, with the ROIs chosen from regions that showed significant changes at baseline. They observed significant reduction in cortical thickness in the left rostral middle frontal gyrus, which initially showed a pattern of thickening at baseline. They did not find any other significant differences. In our study at the follow-up time-point, the left middle temporal cortex still showed significant thinning in mTBI, compared with the controls, but the thinning of the right inferior parietal lobe had disappeared. Also, our sample size was the same at both time-points.
In our follow-up analysis, since the mTBI group was sub-divided into a treatment group and a non-treatment group, we compared the control group with the two subgroups separately. The results showed that the treatment group did not have any significant differences, whereas the non-treatment group showed significant cortical thinning in the left middle temporal gyrus. This might suggest that the group differences found at follow-up was primarily driven by the non-treatment group. Within the treatment group, there were once again two subgroups—the group receiving atorvastatin and the placebo group. Based on comparison of cortical thickness between these subgroups and the control sample, we found no significant differences in the group receiving atorvastatin but significant cortical thickening of the precuneus region in the left hemisphere in the placebo sample. From these results, it appears that the effect of the drug is only subtle. However, it should be pointed out that at this level of analysis, the sample size is reduced by a factor of 4 from the initial mTBI subject pool. It is worth pointing out that a recent study evaluated the sample size required to detect changes in cortical thickness. This study reported that with less than 20 subjects in the sample, only cortical thickness differences greater than 4% were detectable.27
The other important aspect of our analysis was the use of longitudinal pipeline. Our study sample consisted of matched data points from the same subject at both time-points that enabled us to use FreeSurfer's longitudinal analysis pipeline to create an unbiased subject-specific template.18 A subject-specific template would have higher stability if it were generated from multiple data points. Even with two data points, since each subject had his/her own template for analysis, anatomic variabilities between the two time-points are absent. From our results, the orthopedic and treatment groups did not show any significant differences between baseline and follow-up. The non-treatment group showed a trend at p = 0.01, and when the threshold was lowered to p = 0.05, sporadic regions in the frontal, temporal, and parietal lobes showed both thinning and thickening. To our knowledge, this is the first study in mTBI to include a truly longitudinal component of a significant sample size at two time-points. When divided into individual treatment groups, our sample sizes reduced considerably and therefore limit the possibility of a split-sample validation. This type of analysis would further benefit from the availability of a larger cohort, and more time-points would also aid in creating stable unbiased templates.
To determine whether motor vehicle collisions had a greater impact on cortical thickness in mTBI, we selected subjects whose injury was caused by motor vehicle collisions (n = 28) and compared them with the orthopedic control group. No significant differences were observed in this comparison. When separated into the treatment (n = 15) and non-treatment (n = 13) groups, orthopedic controls versus the treatment group showed significant thickening in the left posterior temporal region at baseline and the left superior parietal region at follow-up. These differences were observed at a p value of 0.05 and they did not survive at a p value of 0.01. This finding once again underlines the importance of sample size when making meaningful inferences.
In our study, we still observed significant cortical thinning even in the acute phase (∼24 h post-injury) in the mTBI group, compared with the control group. It is surprising that such a difference could be detected that early in the post-injury timeline. We could only speculate that this finding is perhaps an indicator to premorbid structural differences post-mTBI.
The false discovery rate analysis used here has certain limitations. Ideally, split sample analysis would verify the findings of this study. However, such an analysis was not performed in the present study due to sample size limitation. Thus, the findings of this study should be considered hypothesis generating rather than conclusive.
Limitations
This study does have a few limitations. First, the study could definitely benefit from a larger sample size. The current sample size, though larger than most other studies, does not allow for robust quantification when divided into subgroups. Secondly, the longitudinal analysis could be transformed into a powerful tool if the study included more time-points along the recovery of the patients. This would enable a higher stability of the unbiased template and also provide a way to monitor the changes in the brain through the recovery phase. Thirdly, our mTBI population consisted of injuries from multiple causes (motor vehicle collisions, assault, fall, etc.). A homogenous study population would provide better inferences since injury models and mechanisms would be different for different types of injury. Also, 3 months may not be long enough to observe substantial cortical thinning and more data further down the recovery phase needs to be collected. In this study, we did not assess the heterogeneity of injury. A z-score–based approach, in which individual subject is compared with the control group, could be a powerful way to address the injury heterogeneity. Our future studies will include such an analysis. The results of this study were not correlated with any neurobehavioral measures. It would be interesting to evaluate if the cortical thickness measures had any relationship with behavioral scores. Our MRI data was acquired at a voxel size of 1 mm3. However, the estimation of cortical thickness can be improved by acquiring data at sub-millimeter resolution.
Conclusions
We performed cross-sectional and longitudinal analysis of cortical thickness differences in mTBI patients, compared with orthopedic-injured controls. The study comprised an acute time-point (∼24 h post injury) and a recovery phase time-point (∼3 months post injury). Our results show significant cortical thinning in the middle temporal gyrus at both time-points in the left hemisphere. No cortical thickening was observed. Within the mTBI group, the group treated with atorvastatin did not show any significant differences, whereas the non-treated patients showed significant thinning of the middle temporal gyrus. The results of longitudinal analysis showed a trend of cortical thinning and thickening in the non-treatment group between the two time-points but no other significant changes. To our knowledge, this is the first study to evaluate cortical thickness differences in mTBI on a large sample size with a truly longitudinal component.
Acknowledgments
This study was funded by the Congressionally Directed Medical Research Program of the Department of Defense (W81XWH-08-2-0133, W81XWH-08-2-0135, W81XWH-08-2-0138, W81XWH-08-2-0132, W81XWH-08-2-0149, W81XWH-08-2-0142, W81XWH-08-2-0140, and W81XWH-08-2-0131). The purchase of the MRI scanner was supported in part by the NIH grant S10 RR19186.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Blumbergs P.C., Scott G., Manavis J., Wainwright H., Simpson D.A., and McLean A.J. ( 1995). Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J. Neurotrauma 12, 565– 572 [DOI] [PubMed] [Google Scholar]
- 2. Iverson G.L. ( 2005). Outcome from mild traumatic brain injury. Curr. Opin. Psychiatry 18, 301– 317 [DOI] [PubMed] [Google Scholar]
- 3. Belanger H.G., Vanderploeg R.D., Curtiss G., and Warden D.L. ( 2007). Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 19, 5– 20 [DOI] [PubMed] [Google Scholar]
- 4. Weber J.T. ( 2007). Experimental models of repetitive brain injuries. Prog. Brain Res. 161, 253– 261 [DOI] [PubMed] [Google Scholar]
- 5. Bigler E.D. ( 2008). Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 14, 1– 22 [DOI] [PubMed] [Google Scholar]
- 6. Signoretti S., Vagnozzi R., Tavazzi B., and Lazzarino G. ( 2010). Biochemical and neurochemical sequelae following mild traumatic brain injury: summary of experimental data and clinical implications. Neurosurg. Focus 29, E1 [DOI] [PubMed] [Google Scholar]
- 7. Lindemer E.R., Salat D.H., Leritz E.C., McGlinchey R.E., and Milberg W.P. ( 2013). Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. NeuroImage Clin. 2, 601– 611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tate D.F., York G.E., Reid M.W., Cooper D.B., Jones L., Robin D.A., Kennedy J.E., and Lewis J. ( 2014). Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 8, 102– 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corbo V., Salat D.H., Amick M.M., Leritz E.C., Milberg W.P., and McGlinchey R.E. ( 2014). Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Res. Neuroimaging 223, 53– 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keightley M., Sinopoli K.J., Chen J., Ptito A., Taha T., Wells G., and Fait P. ( 2014). Cortical thinning following sports-related mTBI: the relationship between MRI findings and dual-task performance in youth. Arch. Phys. Med. Rehabil. 95, e68 [Google Scholar]
- 11. Wang X., Xie H., Cotton A.S., Tamburrino M.B., Brickman K.R., Lewis T.J., McLean S.A., and Liberzon I. ( 2015). Early cortical thickness change after mild traimatic brain injury following motor vehicle collision. J. Neurotrauma 32, 455– 463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Assistant Secretary of Defense for Health Affairs. ( 2007). Health Affairs Memorandum. Traumatic Brain Injury; Definition and Reporting.
- 13. Kay T. ( 1993). Mild traumatic brain injury committee of the head interdisciplinary special interest group of the American congress of rehabilitation medicine. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 86– 87 [Google Scholar]
- 14. Levin H.S., O'Donnell V.M., and Grossman R.G. ( 1979). The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J. Nerv. Ment. Dis. 167, 675– 684 [DOI] [PubMed] [Google Scholar]
- 15. Narayana P. A, Govindarajan K. A, Goel P., Datta S., Lincoln J. A, Cofield S.S., Cutter G.R., Lublin F.D., and Wolinsky J.S. ( 2012). Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. NeuroImage. Clin. 2, 120– 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hasan K.M., Wilde E.A., Miller E.R., Kumar Patel V., Staewen T.D., Frisby M.L., Garza H.M., McCarthy J.J., Hunter J. V, Levin H.S., Robertson C.S., and Narayana P.A. ( 2014). Serial atlas-based diffusion tensor imaging study of uncomplicated mild traumatic brain injury in adults. J. Neurotrauma 31, 466– 475 [DOI] [PubMed] [Google Scholar]
- 17. Fischl B., Sereno M.I., Tootell R.B.H., and Dale A.M. ( 1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8, 272– 284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fischl B., Sereno M.I., and Dale A.M. ( 1999). Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195– 207 [DOI] [PubMed] [Google Scholar]
- 19. Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., and Dale A.M. ( 2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341– 355 [DOI] [PubMed] [Google Scholar]
- 20. Fischl B., Liu A., and Dale A.M. ( 2001). Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 20, 70– 80 [DOI] [PubMed] [Google Scholar]
- 21. Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., and Fischl B. ( 2004). A hybrid approach to the skull stripping problem in MRI. Neuroimage 22, 1060– 1075 [DOI] [PubMed] [Google Scholar]
- 22. Desikan R.S., Segonna F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., and Killiany R.J. ( 2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968– 980 [DOI] [PubMed] [Google Scholar]
- 23. Reuter M., Rosas H.D., and Fischl B. ( 2012). Longitudinal FreeSurfer for reliable imaging biomarkers. Available at: http://reuter.mit.edu/blue/papers/reuter-miccai12/reuter-miccai12.pdf Accessed February3, 2016
- 24. Reuter M., Schmansky N.J., Rosas H.D., and Fischl B. ( 2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402– 1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reuter M., Rosas H.D., and Fischl B. ( 2010). Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181– 1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeates K.O. and Taylor H.G. ( 1997). Predicting premorbid neuropsychological functioning following pediatric traumatic brain injury. J. Clin. Exp. Neuropsychol. 19, 825– 837 [DOI] [PubMed] [Google Scholar]
- 27. Iscan Z., Jin T.B., Kendrick A., Szeglin B., Lu H., Trivedi M., Fava M., McGrath P.J., Weissman M., Kurian B.T., Adams P., Weyandt S., Toups M., Carmody T., McInnis M., Cusin C., Cooper C., Oquendo M.A., Parsey R.V., and DeLorenzo C. ( 2015). Test-retest reliability of FreeSurfer measurements within and between sites: effects of visual approval process. Human Brain Mapping. 36, 3472– 3485 [DOI] [PMC free article] [PubMed] [Google Scholar]