Abstract
Cerebellar lesions can cause motor deficits and/or the cerebellar cognitive affective syndrome (CCAS; Schmahmann's syndrome). We used voxel-based lesion-symptom mapping to test the hypothesis that the cerebellar motor syndrome results from anterior lobe damage whereas lesions in the posterolateral cerebellum produce the CCAS. Eighteen patients with isolated cerebellar stroke (13 males, 5 females; 20–66 years old) were evaluated using measures of ataxia and neurocognitive ability. Patients showed a wide range of motor and cognitive performance, from normal to severely impaired; individual deficits varied according to lesion location within the cerebellum. Patients with damage to cerebellar lobules III–VI had worse ataxia scores: as predicted, the cerebellar motor syndrome resulted from lesions involving the anterior cerebellum. Poorer performance on fine motor tasks was associated primarily with strokes affecting the anterior lobe extending into lobule VI, with right-handed finger tapping and peg-placement associated with damage to the right cerebellum, and left-handed finger tapping associated with left cerebellar damage. Patients with the CCAS in the absence of cerebellar motor syndrome had damage to posterior lobe regions, with lesions leading to significantly poorer scores on language (e.g. right Crus I and II extending through IX), spatial (bilateral Crus I, Crus II, and right lobule VIII), and executive function measures (lobules VII–VIII). These data reveal clinically significant functional regions underpinning movement and cognition in the cerebellum, with a broad anterior-posterior distinction. Motor and cognitive outcomes following cerebellar damage appear to reflect the disruption of different cerebro-cerebellar motor and cognitive loops.
Keywords: Cerebellum, Stroke, MRI, Cognition, Ataxia, Cerebellar cognitive affective syndrome
Highlights
-
•
Different regions of the cerebellum support sensorimotor and cognitive functions.
-
•
We performed lesion-symptom mapping in cerebellar stroke patients.
-
•
Cerebellar motor syndrome resulted from damage to the cerebellar anterior lobe.
-
•
Poorer cognitive scores/CCAS were associated with cerebellar posterior lobe damage.
-
•
This is the first VLSM analysis of cognitive subscores in cerebellar stroke patients.
1. Introduction
Until recently, the cerebellum was considered solely a sensorimotor structure, with gait ataxia, appendicular dysmetria, and dysarthria being the hallmark clinical symptoms of cerebellar damage (Holmes, 1939). However, in the past 20 years, multiple lines of evidence support a role for the human cerebellum in cognition and emotion, and clinically the Cerebellar Cognitive Affective Syndrome (CCAS, Schmahmann and Sherman, 1998; also known as Schmahmann's syndrome, Manto and Marien, 2015) is characterized by deficits in language, visual spatial, and executive functions, and affective dysregulation. Theoretically, the type of processing that underlies the cerebellar contribution to movement could also be applied to cognitive functions (Ito, 2008, Schmahmann, 1991, Schmahmann, 1998), with damage leading to dysmetria of thought (Schmahmann, 1991) analogous to the dysmetric movements that characterize the cerebellar motor syndrome.
Anatomical connections link sensorimotor and association areas of the cerebral cortex in reciprocal loops with corresponding sensorimotor and cognitive regions of the cerebellum (Schmahmann and Pandya, 1997, Stoodley and Schmahmann, 2010, Strick et al., 2009). Primary and secondary sensorimotor homunculi in the cerebellar anterior lobe (lobules I/II through V) and lobule VIII, respectively, have been established through electrophysiological (e.g. Snider and Eldred, 1952) and human neuroimaging studies (e.g. Grodd et al., 2001). Predictably, these regions show resting state functional connectivity with somatomotor networks of the cerebral cortex (e.g. Buckner et al., 2011). The posterior lobes of the cerebellum (lobules VI through IX, which are greatly expanded in humans) interconnect with association cortices, supported by evidence from both tract-tracing (e.g. Middleton and Strick, 2001) and resting-state functional connectivity studies (Bernard et al., 2012, Buckner et al., 2011, Krienen and Buckner, 2009, O'Reilly et al., 2010). This functional topography is evident in task-based human neuroimaging data, in which sensorimotor tasks tend to engage the cerebellar anterior lobe and lobule VIII, and cognitive tasks activate the posterolateral cerebellar hemispheres (Keren-Happuch et al., 2014, Stoodley, 2012, Stoodley and Schmahmann, 2009, Stoodley et al., 2012).
Cerebellar functional topography may be critically important when considering clinical outcomes following cerebellar damage. For example, we have shown that the cerebellar motor syndrome is associated with damage to the anterior cerebellum (Schmahmann et al., 2009b), and other lesion-deficit studies have shown that limb and gait ataxia are more often associated with stroke involving the superior cerebellar artery as compared to posterior inferior cerebellar artery strokes (Kase et al., 1993, Timmann et al., 2008, Tohgi et al., 1993). Schoch et al. (2006) conducted detailed lesion-symptom mapping of ataxic signs in 90 patients, and also showed correlations between ataxia scores and anterior lobe damage (lobules II–V extending into VI). Dysarthria has been associated with damage to medial lobule VI, corresponding with the sensorimotor representation of the articulatory apparatus (Urban et al., 2003). In contrast, the CCAS is more likely to occur following cerebellar posterior lobe lesions (e.g. Levisohn et al., 2000, Schmahmann and Sherman, 1998). Several clinical studies have indicated an effect of lateralization of cerebellar damage on outcomes (e.g. Riva and Giorgi, 2000, Scott et al., 2001), reflecting the contralateral connections between the cerebellar hemispheres and the cerebral cortex. In particular, damage to right cerebellar regions results in a variety of language deficits, reflecting their interconnections with left cerebral hemisphere language areas (see Marien et al., 2014). The clinical consequences of cerebellar lesions on motor versus cognitive aspects of linguistic processing appear to be driven by cerebellar functional topography: ataxic dysarthria results from lesions in the cerebellar anterior lobe, whereas verbal fluency and working memory deficits are associated with posterolateral cerebellar damage (Bultmann et al., 2014, Ilg et al., 2013, Richter et al., 2007).
Here we further explore the structure-function correlation hypothesis within the human cerebellum by examining the relationship between the location of injury within the cerebellum and the motor versus cognitive outcomes. Patients with isolated cerebellar stroke completed a battery of motor and neuropsychological tasks to detect symptoms and signs of the cerebellar motor syndrome and the CCAS. Voxel-based lesion-symptom mapping was employed to determine which regions of the cerebellum were associated with behavioral deficits on a given task. We predicted that the cerebellar motor syndrome would be associated with damage to the sensorimotor representations in the anterior lobe and lobule VIII of the cerebellum, whereas impaired cognitive performance would be associated with damage to the cerebellar posterior lobe. These predictions, based on the established functional topography of the cerebellum and previous lesion mapping studies, enable us to assess whether damage to specific cerebellar regions are associated with cognitive subscores in a consistent manner. If cognitive deficits are indeed consistent with site of lesion, then these findings may be used to improve clinical diagnosis, management, and prognosis of cognitive outcomes following cerebellar stroke.
2. Materials and methods
2.1. Participants
Participants were 18 patients with a first ischemic stroke confined to the cerebellum admitted to the stroke service of the Massachusetts General Hospital during a four-year period. Exclusion criteria included age < 18 years, pregnancy, pre-existing neurological illness, underlying medical condition with active metabolic differences that may impair cognitive function, axis 1 or 2 psychiatric disorder, primary cerebellar hemorrhage, and cerebellar stroke showing signs of herniation requiring cerebellar excision with ventriculostomy placement. Patients with evidence (imaging or clinical examination) that the stroke involved brain areas outside the cerebellum, including the brainstem, were excluded. Patients provided written, informed consent and the study was approved by the Institutional Review Board of Partners Health Care.
Patients were thirteen males and five females (average age ± standard deviation of 46.8 ± 14.6 years; range 20–66 years). Each patient underwent a comprehensive medical and neurological examination and bedside mental state testing by a board certified neurologist (JDS). Neuroimaging, motor and neuropsychological testing were completed during the acute / subacute stage of recovery. The average times from date of stroke to behavioral assessment and MRI scan were 29.8 days (± 18.1 days, range 8–67 days) and 38.8 days (± 19.3 days, range 9–87 days), respectively. The average interval between behavioral assessment and MRI scan was 11.3 days (± 16.2, range 0–65 days).
2.2. Behavioral assessment
The motor task battery included the modified International Cooperative Ataxia Rating Scale (MICARS; Schmahmann et al., 2009a, Trouillas et al., 1997); the grooved pegboard test (Lewis and Rennick, 1979); and finger tapping test (Bornstein, 1985). These tests tap both gross and fine motor skills that could be affected by cerebellar damage. The MICARS assesses posture and gait, kinetic limb function, speech, and eye movements based on a 120-point scale, with higher scores associated with greater impairment. In the grooved pegboard task, patients are asked to place pegs into notched holes, first with their dominant hand and then with their non-dominant hand; performance is based on time to completion for each hand. During the finger tapping test, participants tapped their index finger on a finger tapping board for five 10-second intervals. Patients complete this task first with their dominant hand and then with their non-dominant hand. Scoring is based on the average number of taps across each 10-second interval for each hand. For both the grooved pegboard and finger tapping tasks, patient scores were converted into z-scores based on published norms.
The neuropsychological test battery was designed to test the cognitive aspects of the CCAS, and included measures of IQ, language, spatial processing, executive function, and working memory (see below). Premorbid intelligence was estimated using the Barona index (Barona et al., 1984).
Neuropsychological testing was conducted at the Massachusetts General Hospital Psychology Assessment Center by clinical neuropsychologists and psychometricians (supervised by JCS). Unless otherwise noted, all scores were converted into age-adjusted z-scores using the published norms for each measure. Selected subtests from the Wechsler Adult Intelligence Scale, version 3 (WAIS-3; Wechsler, 1997) were used to evaluate current verbal (Vocabulary, Similarities) and visual (Matrix Reasoning) cognitive functioning. Language processing was assessed with the Boston Naming Test (Goodglass and Kaplan, 2000) and phonemic (F-A-S) and semantic (animals) verbal fluency (Controlled Oral Word Association Test [COWAT (Tombaugh et al., 1999)]). Spatial processing was assessed with the copy and organization scores of the Rey complex figure test (Osterrieth, 1944), and on motor-free tests including the Judgment of Line Orientation test (Benton et al., 1975), and the Mental Rotations test (Vandenberg and Kuse, 1978). The Wisconsin Card Sorting Test (WCST; Heaton et al., 1993), the Cognitive Estimation Test (Axelrod and Millis, 1994) and the Trail Making test (A and B; Tombaugh, 2004) measured executive function. Working memory was assessed with the Wechsler Memory Scale-III (WMS-III; Wechsler, 2009), using the digit span and spatial span measures.
Clinical classification of patients as having diagnoses of the cerebellar motor syndrome, CCAS, or both, was conducted based on the following parameters. Patients with MICARS scores of > 13 out of a maximum of 120 were categorized as having the cerebellar motor syndrome on the basis of established cut-offs for that measure; patients with MICARS < 4 were classified as in the normal range (Schmahmann et al., 2009a). As our participants had a mean estimated premorbid IQ that was on average nearly 1 SD above the mean (Barona estimated mean z-score for the group was 0.9 ± 0.4, range 0.1–1.3), we considered performance > 1 SD below the mean score for the neuropsychological measures as being indicative of areas of relative deficit. Patients were categorized as high risk for CCAS if they had more than four z-scores that were > 1 SD below the mean score for the following tasks: in the language domain, Similarities from the WAIS-3, Animal fluency, F-A-S fluency, and the BNT; for visual-spatial processing, the Rey copy and organization scores; for executive function, the overall percent error score from the Wisconsin Cart Sorting Test and Trail Making B; and for working memory, digit span and spatial span from the WMS-III. While it is not unusual for normal healthy adults to score > 1 SD below average on a given neuropsychological measure as part of a larger battery, participants with average to above average IQ scores are less likely to have individual scores that fall within this range (Binder et al., 2009). For example, on the Halstead-Reitan Neuropsychological Battery, < 10% of healthy adults had 40% of their scores fall > 1 SD below the average (Binder et al., 2009). Therefore, our cutoffs for CCAS classification were based on the above-average estimated premorbid IQ of our patients and the low likelihood that 40% of scores would fall in this range in healthy individuals.
Finally, to rule out the possibility that post-stroke depression could explain poorer neuropsychological performance in this cohort, patients also completed the Structured Clinical Interview for the DSM (SCID; version 2.0, First et al., 1997) and the Beck Depression Inventory II (BDI; Beck et al., 1996). None of the patients had clinical depression based on the SCID. Two patients scored in the “moderate” range on the BDI, with the rest of the patients scoring in the “minimal” range for depression symptoms. Of the two patients scoring in the moderate range, one was classified as having the CCAS, but the other did not have significant neuropsychological impairment. Therefore, depression did not appear to be a major factor impacting the neuropsychological scores acquired in this sample, and would not be expected to significantly impact the lesion mapping results.
2.3. Neuroimaging
Neuroimaging was performed at the Athinoula A. Martinos Center for Biomedical Imaging (Charlestown, MA). Each patient underwent a T1 MP-RAGE MRI scan on a 3 T TimTrio scanner (Siemens, Erlangen, Germany). Slice acquisition was in the sagittal plane, with a voxel size of 1.3 × 1 × 1.3 mm3, and single-shot, interleaved acquisition of 128 slices, 256 mm field of view, repetition time (TR) 2530 ms, echo time (TE) 3.39 ms, and a flip angle of 7°. The total scan time was 8 min and 7 s.
2.4. Lesion symptom mapping
Voxel-based lesion-symptom mapping (VLSM) aims to identify regions in which damage is associated with a behavioral deficit by comparing behavioral performance between patients with and those without a lesion in a particular voxel in the brain on a voxel-by-voxel basis (Bates et al., 2003, Rorden et al., 2009), offering better resolution than grouping stroke lesions by the affected artery or conducting region of interest analyses. To improve cerebellar lobular alignment during normalization, we used the Spatially Unbiased Infratentorial (SUIT) template (Diedrichsen et al., 2009) implemented in Statistical Parametric Mapping 8 (SPM8; www.fil.ion.ucl.ac.uk/spm/software/spm8/). A cerebellar mask was created using each individual patient's MRI scan and the suit_isolate function. The lesion mask was drawn on the cropped, isolated image of the patient's cerebellum using MRIcron (www.mccauslandcenter.sc.edu/mricro/mricron/). Because large lesions can disrupt the normalization process, the lesion mask was included and ignored (with a margin of ~ 5 mm) during normalization. The lesion mask was then normalized into SUIT atlas space by applying the deformation parameters from the normalization procedure. This process was performed for each patient's lesion data, resulting in a set of lesion masks in SUIT atlas space that was used for VLSM.
First, to provide a broad test of our predictions as to the relationship between cerebellar lesion location and outcome, we used the clinical status of patients to examine the lesion maps associated with cerebellar motor syndrome and CCAS. Patient status was established as described above. The lesion maps of patients with symptoms of cerebellar motor syndrome and/or CCAS (as well as patients affected by both clinical disorders) were grouped and overlaid onto the SUIT cerebellar atlas template using MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/home).
Second, VLSM was conducted using NonParametric Mapping software (NPM; Rorden et al., 2007; www.cabiatl.com/mricro/npm/). The lesion masks for each patient were loaded into a design file with the corresponding motor or neuropsychological scores. For each voxel, behavioral scores were compared between patients with lesions in that voxel vs. those without lesions using either t-tests (for normally distributed data) or the Brunner Munzel rank-order statistic (for non-normally distributed data) with a permutation threshold given the small sample size (see Medina et al., 2010). Normality was determined using Shapiro-Wilk tests implemented in SPSS v22, with additional criteria of skewness between − 2 and 2 and kurtosis < 5. Using these criteria, only the Rey Copy z-score (Shapiro-Wilk p = 0.002, skewness − 2.2, kurtosis 6.4) and right-handed peg-moving z-score (Shapiro-Wilk p < 0.001, skewness − 3.7, kurtosis 14.8) were not normally distributed. Acute and subacute patients were included in one analysis, consistent with previous studies (e.g. Karnath et al., 2011). However, to examine whether patients in the acute stage of recovery had more severe neuropsychological deficits, we also examined the correlations between time from stroke (in days) and motor and neuropsychological scores for each measure. In VLSM, voxels were included in the analyses if at least 10% of patients had a lesion involving that voxel. The resulting Z-maps were thresholded at an uncorrected p < 0.01 (z > 2.33); additional findings were explored at a more lenient p < 0.05 (z > 1.65), consistent with previous cerebellar VLSM studies with a similar sample size (Ilg et al., 2013). For all VLSM analyses, a minimum cluster size of k = 20 was employed. The lobules and cerebellar nuclei involved in each cluster were identified using the SUIT atlas and the MRI Atlas of the Human Cerebellum (Schmahmann et al., 2000). To increase power, we also conducted a secondary analysis with all lesions on the right (see Schoch et al., 2006). As the lesions were equally distributed between the right and left cerebellum, the decision to flip left lesions to the right (rather than flipping right lesions to the left) was arbitrary, and based on a previous cerebellar VLSM analysis of working memory (Ilg et al., 2013). It is important to note that, while increasing statistical power due to improved lesion overlap, this analysis eliminates the ability to discern potential lateralization effects. The same parameters as described above were used in the analysis with all lesions on the right, with lesion masks for patients with left cerebellar lesions flipped to the right using LR Flip in MRICron.
3. Results
3.1. Lesion volumes and lesion maps
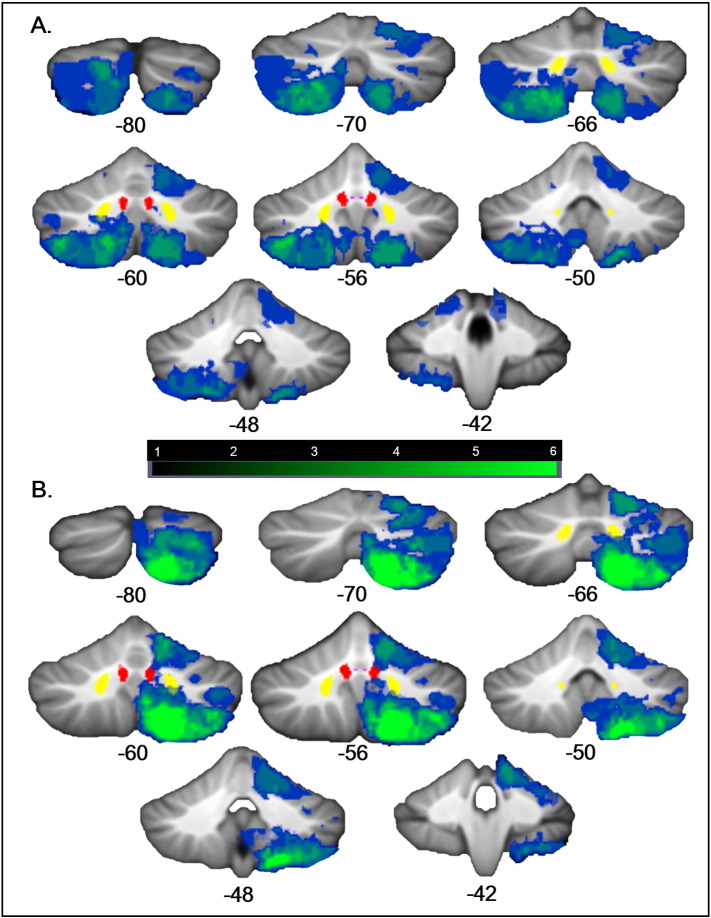
Table 1 provides gender, age, stroke location, and lesion volume information for individual patients. Lesion volumes ranged from 129 to 54,048 voxels in size (mean ± SD = 12,725 ± 1331 voxels). Lesions did not cover the entire cerebellum (Fig. 1A), and therefore we are not able to draw definitive conclusions about the potential functional significance of a lesion in unaffected regions. Flipping all lesions to the right increased the coverage of the cerebellum (Fig. 1B).
Table 1.
Patient and lesion characteristics. M = male, F = female; age is given in years; L = left, R = right; AICA = anterior inferior cerebellar artery, PICA = posterior inferior cerebellar artery, SCA = superior cerebellar artery.
| Case | Gender | Age at stroke | Stroke side/artery | Stroke volume (voxels) | Lesion extent |
|---|---|---|---|---|---|
| 1 | M | 59 | L-PICA | 2040 | Left VIIB/VIIIA/VIIIB/IX |
| 2 | F | 29 | L-SCA | 7168 | Left V/VI |
| 3 | M | 35 | R-SCA | 3620 | Right V/VI |
| 4 | M | 45 | L-PICA | 10,145 | Left VI/Crus I/Crus II; vermis VI-VIIIA |
| 5 | M | 48 | L-PICA | 7100 | Left VIIB/VIIIA |
| 6 | F | 56 | R-PICA | 7341 | Right Crus II/VIIB/VIIIA/VIIIB/IX |
| 7 | M | 66 | R-PICA | 212 | Right VIIIB/IX |
| 8 | M | 57 | R-PICA | 11,643 | Right Crus II/VIIB/VIIIA/VIIIB/IX |
| 9 | M | 51 | L-PICA | 18,923 | Left Crus I/Crus II/VIIIA/VIIIB/IX; ventral dentate |
| 10 | M | 28 | R-PICA | 16,103 | Right Crus I/Crus II/VIIIA/VIIIB/IX |
| 11 | M | 35 | L-SCA/L-AICA | 5182 | Left I-IV/V/VI/Crus I; dentate (primarily ventral) |
| 12 | F | 38 | R-PICA | 129 | Right VIIIB/IX |
| 13 | M | 66 | R-SCA | 11,263 | Right IV/V/VI into Crus I; interpositus and dorsal dentate |
| 14 | F | 65 | L-PICA | 54,048 | Left Crus I/Crus II/VIIB/VIIIA/VIIIB/IX |
| 15 | F | 20 | R-SCA/R-PICA | 11,081 | Right III/IV/V/VI/Crus I/Crus II/VIIB |
| 16 | M | 46 | L-PICA | 9661 | Left VIIB thru IX; ventral dentate |
| 17 | M | 34 | R-PICA | 17,169 | Right Crus I/II/VIIB/VIIIA/VIIIB/IX |
| 18 | M | 64 | L-PICA | 36,222 | Left Crus I/Crus II/VIIB/VIIIA/VIIIB/IX |
Fig. 1.
Lesion overlap maps. (A) The location of the lesions of the patients in the current study are mapped on coronal sections through the cerebellum from the SUIT atlas (numbers indicate y coordinates in MNI space). The blue-green scale indicates the number of patients with lesions in a particular region; green indicates regions of greater lesion overlap. The fastigial (violet), interpositus (red) and dentate (yellow) nuclei from the probabilistic cerebellar SUIT atlas (thresholded at 0.4, 0.6, and 0.6, respectively) are shown. The right cerebellum is shown on the right. (B) Lesion overlap maps with all lesions on the right; left-sided lesions were flipped to the right. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Behavioral scores
Table 2 reports the mean scores on the motor and neuropsychological tests. The mean of the WAIS subtest scores (mean z-score ± SD = 0.5 ± 0.6) was significantly lower than the Barona index pre-morbid estimated IQ score (mean z-score 0.9 ± 0.4; paired t-test p = 0.009), suggesting that there was a negative effect of cerebellar stroke on general cognition.
Table 2.
Motor and neuropsychological test z-scores. The percent of the sample that showed impaired performance on each measure is noted, with the exception of the Mental Rotation task, for which raw scores are given. This was based on published norms for MICARS, and a z-score of >− 1 for other measures.
| Category | Task | Mean ± SD | Range | % Impaired |
|---|---|---|---|---|
| Premorbid IQ | Barona premorbid estimated IQ | 0.9 ± 0.4 | 0.1–1.3 | 0% |
| Motor | MICARS ataxia scale | 9.1 ± 8.8 | 0–35 | 28% minimal impairment; 28% impaired |
| Grooved peg board R hand | − 1.3 ± 3.6 | − 15.1–1.4 | 33% | |
| Grooved peg board L hand | − 1.0 ± 1.4 | − 4.9–0.7 | 33% | |
| Finger tapping R hand | − 0.1 ± 1.2 | − 3.3–1.5 | 11% | |
| Finger tapping L hand | 0.2 ± 1.4 | − 2.0–2.9 | 22% | |
| Wechsler Adult Intelligence Test – 3 | Vocabulary | 0.5 ± 0.8 | − 0.7–2 | 0% |
| Similarities | 0.2 ± 0.8 | − 1–2 | 17% | |
| Matrix reasoning | 0.7 ± 0.7 | − 0.7–2 | 0% | |
| Mean WAIS subtest | 0.5 ± 0.6 | − 1.3–0.6 | 6% | |
| Mathematics raw score (spatial quantitative battery)⁎ | 61.2 ± 19.2 | 35–100 | N/A | |
| Executive function | Trails A | − 0.2 ± 1.4 | − 4.0–1.7 | 33% |
| Trails B | − 0.5 ± 1.0 | − 2.9–1.2 | 33% | |
| Wisconsin Card Sorting Task % errors⁎ | − 0.1 ± 0.8 | − 1.7–1.3 | 17% | |
| Wisconsin Card Sorting Task % non-perseverative errors⁎ | − 0.3 ± 0.91 | − 1.9–1.2 | 22% | |
| Wisconsin Card Sorting Task % perseverative errors⁎ | 0.14 ± 1.0 | − 1.5–1.8 | 11% | |
| Wechsler Memory Scale – 3 digit span | 0.2 ± 0.8 | − 1.3–2 | 6% | |
| Wechsler Memory Scale – 3 spatial span | 0.1 ± 0.9 | − 1.7–1.7 | 17% | |
| Cognitive Estimation Task | 0.6 ± 0.6 | − 0.9–1.7 | 0% | |
| Language | FAS letter fluency | − 0.6 ± 0.9 | − 1.8–1.7 | 33% |
| Animal semantic fluency | − 0.04 ± 1.0 | − 2.0–2.1 | 11% | |
| Boston Naming Test | − 0.04 ± 0.9 | − 1.9–1.3 | 28% | |
| Spatial | Benton Judgment of Line Orientation (raw) | 26.7 ± 3.7 | 17–31 | 5% |
| Mental rotation A (raw) | 8.5 ± 2.6 | 5–15 | N/A | |
| Mental rotation B (raw) | 7.7 ± 3.1 | 3–13 | N/A | |
| Mental rotation total (raw) | 16.2 ± 4.8 | 8–25 | N/A | |
| Rey figure copy | − 2.9 ± 2.8 | − 11.8–0.1 | 78% | |
| Rey figure organization | 0.3 ± 1.2 | − 2.8–1.5 | 17% |
n = 17.
There were no significant relationships between time from stroke and the majority of motor and neuropsychological test scores. Only one measure showed a significant correlation with time from stroke: there was a negative correlation between time from stroke to neuropsychological testing and Trails B scores, indicating that patients who were less acute had worse scores (Spearman's rho − 0.472, p = 0.05). These data suggest that patients in the more acute stage of recovery are not driving our symptom-location findings. In addition, lesion volume did not significantly correlate with performance on any of the motor or neuropsychological measures, with the exception of a positive correlation between lesion size and Spatial Span z-score (Spearman's rho 0.576, p = 0.012), suggesting that larger lesions were associated with better Spatial Span scores. This seemingly counter-intuitive finding emphasizes the importance of lesion location on task performance, given that even very large lesions were not associated with poorer performance on the spatial span task. The lack of relationship between lesion size and behavioral performance suggests that lesion location rather than size is an important factor in outcomes. In a similar vein, while very few of the tasks indicated impaired performance in the group as a whole (see Table 2), there was a broad range of performance: some individuals' performances were impaired and others' intact on the same measures. The goal of the lesion-symptom mapping was to investigate whether the patients with impaired performance on a given task had lesions in overlapping cerebellar regions.
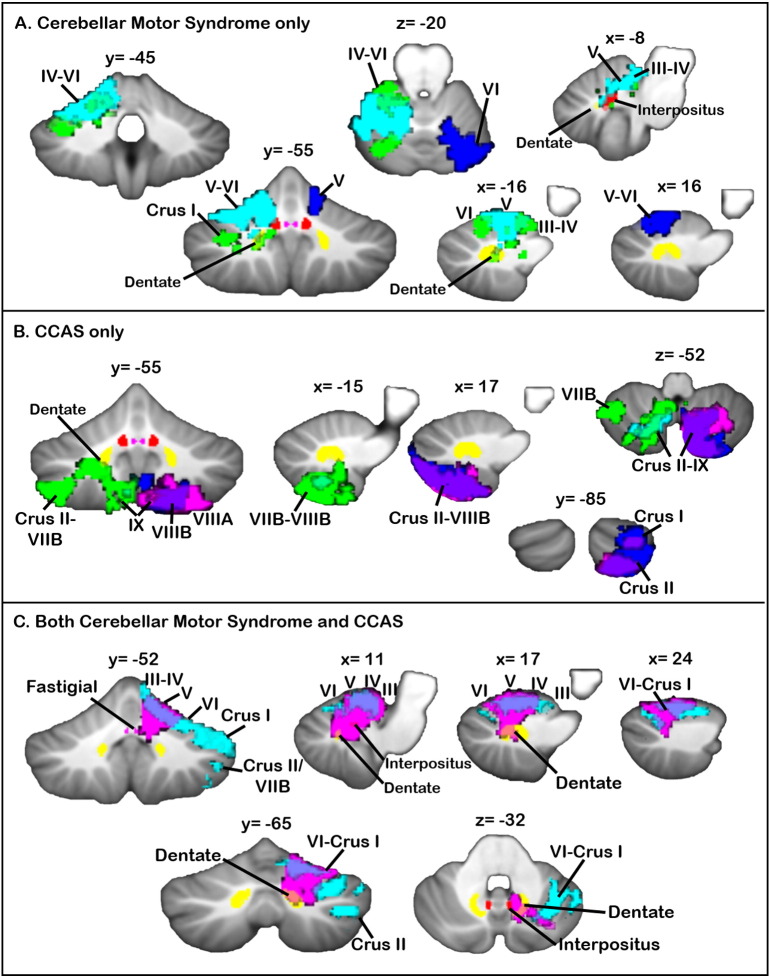
3.3. Lesion maps associated with clinical diagnoses
The lesion maps associated with patients diagnosed with the cerebellar motor syndrome, CCAS, or both are shown in Fig. 2. Patients with the cerebellar motor syndrome, but not CCAS, had damage to anterior cerebellar regions, extending into lobule VI, and included damage to the interpositus and dentate nuclei. Patients with the CCAS (but with normal motor performance) had damage in the cerebellar posterior lobe, including lobules VII and VIII, with limited damage to the deep nuclei. Patients with both the cerebellar motor syndrome and CCAS had lesions that spanned anterior sensorimotor regions and posterior cognitive cerebellar lobules, including damage to the interpositus and dentate nuclei. Only two patients had symptoms of neither the motor syndrome nor the CCAS; these two patients had extremely small lesions (212 and 2040 voxels) involving lobule IX.
Fig. 2.
Lesion maps by clinical diagnosis. (A) Individual lesion maps of patients with cerebellar motor syndrome (without CCAS) show that damage to cerebellar anterior regions, extending into VI, is associated with cerebellar ataxia. Different colors represent the lesions of individual patients. (B) Individual lesion maps of patients with CCAS and normal motor performance suggest that posterior cerebellar damage involving lobules VII and VIII is a risk factor for cognitive deficits. Different colors represent the lesions of individual patients. (C) Individual lesion maps of patients with both the motor syndrome and CCAS reveal damage including both anterior sensorimotor regions and posterior cognitive-association regions of the cerebellum. Different colors represent the lesions of individual patients. The fastigial (violet), interpositus (red), and dentate (yellow) nuclei from the probabilistic cerebellar SUIT atlas (thresholded at 0.4, 0.6, and 0.6, respectively) are shown. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Voxel-based lesion-symptom mapping
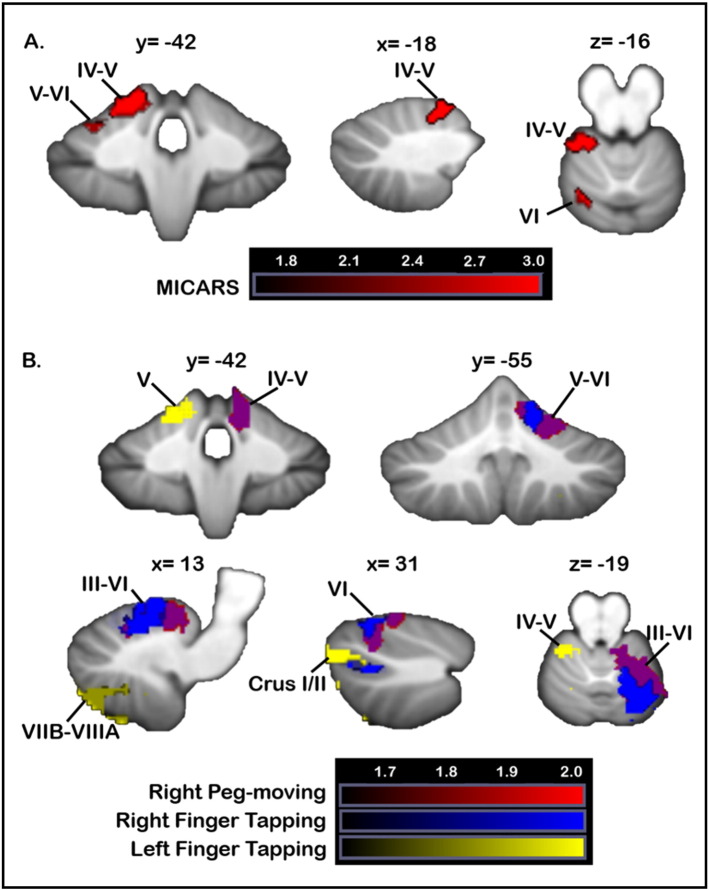
3.4.1. Motor tasks
Poorer MICARS scores were associated with damage to the left anterior lobe (lobules III-V) and lobule VI (Fig. 3A). Worse scores on right-handed finger tapping were associated with lesions in right lobule V–VI and a small cluster in right Crus I/II (Fig. 3B). Whereas left-handed finger tapping scores did not meet the z > 2.33 threshold, at a more lenient threshold (uncorrected p < 0.05; z > 1.65), poorer left-handed tapping was associated with lesions involving the left anterior lobe and right lobules VII, VIII and IX. Poorer right-handed grooved peg-board performance resulted from lesions involving the right anterior lobe (lobules IV–IV, V) extending into VI and Crus I (Fig. 3B). Impaired left-handed peg-board performance was associated with damage to a small cluster in left white matter only at the more lenient threshold (z > 1.65).
Fig. 3.
VLSM: motor performance. Data from the main VLSM analyses are shown. (A) Lesioned areas associated with significantly poorer MICARS scores are shown in red. (B) Regions where damage was associated with poorer scores on grooved pegboard with the right hand are shown (red). Voxels where lesions were associated with poorer finger tapping are shown in blue (right hand) and green (left hand). There was overlap between the regions where damage was associated with poorer performance on both right-handed pegboard and finger tapping (purple). X, y, and z values represent MNI coordinates. For visualization, data are shown at z > 1.65 (p < 0.05) and k > 20. The right cerebellum is shown on the right. Color bars represent the range of z scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
When lesions were all flipped to the right, more impaired MICARS scores were again associated with anterior cerebellar damage, extending into lobule VI and Crus I. Right-handed finger tapping scores were significantly worse in patients with lesions involving lobule V, with smaller clusters in lobules VI and VII; no significant clusters were found for left-handed tapping. For the Grooved Pegboard test, right-handed pegboard performance was worse when lesions impacted lobules V and VI, with some extension into Crus I; left-handed pegboard performance did not significantly differ between the lesioned and non-lesioned groups in any clusters. These findings were largely consistent with those shown in the primary VLSM analysis.
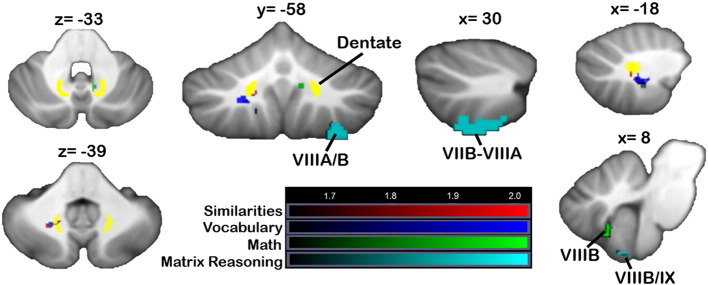
3.4.2. Neuropsychological tasks
Verbal reasoning scores (Similarities) reflected damage to a small cluster of white matter, encroaching on the left dentate nucleus, at z > 1.65 (Fig. 4, red). In the flipped analyses, at p < 0.05, patients with lesions in clusters in lobule V/VI and VI into Crus I had worse Similarities scores, but none of these regions survived at p < 0.01. Poorer Vocabulary scores did not show any significant relationship with lesion location at p < 0.01; at a more lenient threshold (z > 1.65, uncorrected p < 0.05), Vocabulary scores were associated with damage to small regions of left lobules VIIIB and IX, as well as similar regions of left hemisphere white matter as those associated with poorer Similarities performance (Fig. 4, blue; purple shows overlap). In the flipped analysis, a dentate cluster was significant at z > 2.3, and at z > 1.65 a large cluster in lobule VI was associated with lower Vocabulary scores, together with a small cluster in lobule IX. Lower scores on the Mathematics test (n = 17) were associated with damage to a small cluster involving right VIIIB and IX, but only at the more lenient threshold (z > 1.65, uncorrected p < 0.05; Fig. 4, green), and a small region of right white matter which did not involve the dentate or interpositus nuclei. The flipped analysis did not reveal any significant clusters for Mathematics scores. At p < 0.05, regions of damage associated with poorer Matrix Reasoning scores included right VIIB extending into VIIIA and a smaller cluster in VIIIB/IX. As with the Mathematics scores, the flipped analysis did not reveal any significant clusters for Matrix Reasoning.
Fig. 4.
VLSM: WAIS measures. Regions associated with poorer WAIS-3 performance (red = similarities, blue = vocabulary, green = mathematics, cyan = matrix reasoning). Data from the main VLSM analyses are shown. X, y, and z values represent MNI coordinates. For visualization, data are shown at z > 1.65 (p < 0.05) and k > 20. The right cerebellum is shown on the right. Color bars represent the range of z-scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
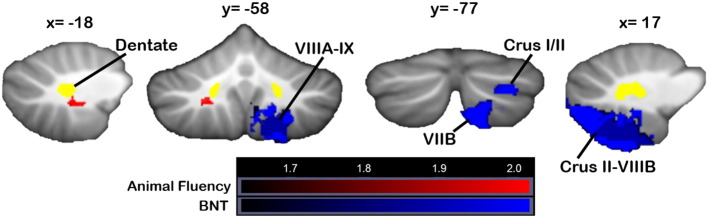
Poorer performance on language tasks was associated with damage to right-lateralized cerebellar regions overlapping with those related to Similarities and Vocabulary performance. Phonemic (FAS) letter fluency scores were not significantly worse in patients with lesioned vs. unlesioned voxels anywhere within the cerebellum; in the flipped analysis, only a very small region of lobule VIIB was associated with poorer scores at p < 0.05. Category (animal) fluency (Fig. 5, red) was impacted when damage involved the same areas associated with poorer Vocabulary scores at p < 0.05, namely left white matter. In the flipped analysis, a second cluster in lobule IX was associated with poorer category fluency (p < 0.05). Worse performance on the Boston Naming Test (BNT) was associated with damage to regions of right Crus I, Crus II, and VIIB (Fig. 5, blue). At p < 0.05, the right Crus II cluster extended through VIIB, VIIIA, and VIIIB.
Fig. 5.
VLSM: language measures. Locations in which damage resulted in poorer scores on language measures (red = animal fluency, blue = Boston Naming Test). X, y, and z values represent MNI coordinates. For visualization, data are shown at z > 1.65 (p < 0.05) and k > 20. The right cerebellum is shown on the right. Data from the main VLSM analyses are shown. Color bars represent the range of z-scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
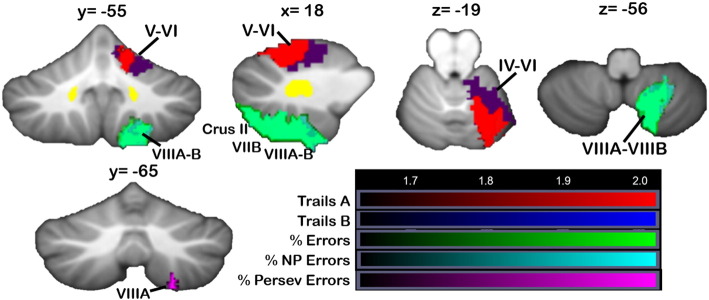
Fig. 6 shows the clusters in which damage produced poorer performance on executive function tasks, including Trails A and B (red and blue, respectively; overlap is shown in purple) and the Wisconsin Card Sorting Task (WCST; overall errors in green, non-perseverative errors in cyan, perseverative errors in violet). Trails A involves following “trail” from “a” to “b” to “c” (in connect-the-dots format) without the switching requirements of Trails B (“1” then “a” then “2” then “b”); performance is based on the speed at which the task is completed. Patients performing more slowly on Trails A had lesions involving the anterior cerebellum extending into VI, and analysis of Trails B showed significant regions overlapping with those associated with Trails A (p < 0.05; Fig. 6; overlap is shown in purple). Both tasks involved right Crus I at z > 1.65. Overall error score on the WCST (Fig. 6, green) and non-perseverative errors were associated with overlapping regions of right Crus II/VIIB, extending into lobules VIIIA and VIIIB. Perseverative errors were associated with a small cluster in right VIIIA, in the same region as the overall errors and non-perseverative errors (this cluster was only evident at p < 0.05 and is shown on its own in Fig. 6 at y = − 65). No regions showed significant differences between lesion and non-lesioned groups for the Cognitive Estimation Task.
Fig. 6.
VLSM: executive function tasks. Areas where damage led to poorer performance on executive function tasks (red = Trails A, blue = Trails B, green = WCST overall % errors, cyan = WCST % non-perseverative errors, violet = WCST % perseverative errors). Voxels where damage was associated with perseverative errors are shown at y = − 65. Data from the main VLSM analysis are shown. X, y, and z values represent MNI coordinates. For visualization, data are shown at z > 1.65 (p < 0.05) and k > 20. The right cerebellum is shown on the right. Color bars represent the range of z-scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
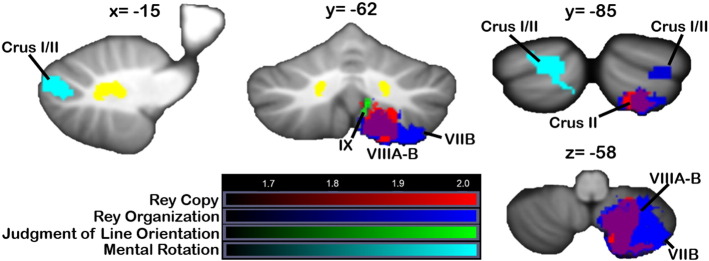
Regions where damage resulted in poorer scores on the spatial tasks are shown in Fig. 7. For Mental Rotation total score (Fig. 7, cyan), at p < 0.05 a cluster in left Crus I/II was associated with poorer scores. When all images were flipped to the right, poorer Mental Rotation Total scores were found with damage to an overlapping region of Crus I and II (p < 0.05). Impaired Rey figure copy accuracy (red) was associated with damage to a large cluster extending from right VIIB into VIIIB; a largely overlapping region (shown in purple) was associated with poorer Rey figure organization scores (blue; this cluster extends into VIIIA/VIIB compared with the cluster associated with Rey copy at p < 0.01). At p < 0.05, a second cluster associated with Rey organization was located in right Crus I/II. In the flipped analysis, the region associated with Rey figure organization extended more laterally into Crus I/II compared with the Rey copy cluster; this is also seen in the main analysis. Poorer performance on the Benton Judgment of Line Orientation (Fig. 7, green, at p < 0.05) was associated with damage to regions in VIIIB/IX, adjacent to those involved in Rey figure performance.
Fig. 7.
VLSM: visual-spatial tasks. Damage to regions from Crus I through IX was associated with poorer scores on measures of spatial processing (red = Rey figure copy, blue = Rey figure organization score, green = Judgment of Line Orientation, cyan = Mental Rotation Total score). Data from the main VLSM analyses are shown. X, y, and z values represent MNI coordinates. For visualization, data are shown at z > 1.65 (p < 0.05) and k > 20. The right cerebellum is shown on the right. Color bars represent the range of z-scores. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
At p < 0.05, a small cluster in right white matter between the dentate and interpositus nuclei was associated with poorer performance on digit span; poorer performance on spatial span was associated with a small region of right VI. In the flipped analysis, at the more lenient threshold (p < 0.05), spatial span performance was again poorer in patients with damage to lobule VI.
4. Discussion
We used voxel-based lesion-symptom mapping to examine the nature and severity of individual impairments in motor and cognitive function in relation to the location of lesions in adult patients with stroke confined to the cerebellum. Although group averages indicated only mild deficits in motor and cognitive functions, there was a wide range of performance, with individual impairments reflecting location of lesion within the cerebellum. Broadly speaking, there was a dichotomy between the pattern of cerebellar damage associated with the cerebellar motor syndrome (anterior lobe, extending into VI, causing ipsilateral motor deficit) and the damage associated with cognitive performance (posterior lobe, including lobules VII and VIII). When cognitive measures had a significant motor component, such as in the Trail Making test, anterior lobe lesions negatively impacted performance. These findings reflect the proposed functional topography in the healthy human cerebellum for motor and cognitive tasks (Stoodley and Schmahmann, 2009, Stoodley and Schmahmann, 2010, Stoodley et al., 2012), given that we found associations between performance on cognitive tasks without a significant motor component and damage to posterior regions of the cerebellum. Our results therefore provide further support for cerebellar involvement in cognition and the localization of the cerebellar regions involved in cognitive performance.
Our findings are consistent with earlier studies investigating outcome following cerebellar stroke. There is an established relationship between poorer ataxia scores and damage to the anterior lobe extending into lobule VI (Maderwald et al., 2012, Schmahmann et al., 2009b, Schoch et al., 2006). Further, as expected, our VLSM clusters for MICARS and performance on fine motor tasks (pegboard, finger tapping) localized to somatomotor networks based on resting-state functional connectivity mapping (Buckner et al., 2011), suggesting good agreement between functional connectivity and lesion-deficit methods. Lesion size was not associated with motor outcomes – even very large lesions did not produce significant impairment on motor performance if they did not extend into the anterior cerebellum.
It has been known since the initial description of the CCAS (Schmahmann and Sherman, 1998) that cognitive symptoms associated with cerebellar lesions can exist in the absence of motor deficits (e.g. Paulus et al., 2004), and that these deficits are associated with damage to the posterior inferior cerebellar artery territory in general, and Crus I and Crus II specifically (Tedesco et al., 2011). Here, we found that lesions involving lobules VIIB and VIII were also associated with cognitive performance following cerebellar stroke. While lobule VIII is the location of the second sensorimotor homunculus in the cerebellum, and is broadly associated with somatomotor networks (see Buckner et al., 2011), it is also engaged during cognitive tasks in functional neuroimaging studies (see Keren-Happuch et al., 2014, Stoodley and Schmahmann, 2009), including executive function and working memory measures. While we found no significant clusters associated with working memory (at p < 0.01), Kirschen and colleagues (Kirschen et al., 2008) reported that damage to left lobule VIII was associated with reduced verbal working memory in children following resection of cerebellar pilocytic astrocytomas. Right-lateralized posterolateral cerebellar lesions have consistently been associated with language difficulties (Marien et al., 2014, Marien et al., 2001), and we report that damage to right lobules VII through IX produced poorer scores on the Boston Naming Test. For spatial processing, mental rotation scores were significantly worse in patients with damage to a region of left Crus I/II, and Rey figure copy and organization scores were associated with large, right-lateralized regions extending from Crus II through VIIIB. The left cerebellar cluster associated with total mental rotation score was more lateral than activation patterns related to mental rotation in healthy young adults (Stoodley et al., 2012), although the cluster associated with the Mental Rotation B subscale (data not shown) was very close to the activation patterns previously reported. The regions associated with Rey figure scores encompass areas activated during both cognitive and motor tasks (see Stoodley and Schmahmann, 2009), which could reflect both the planning and the execution components of the task. Similarly, performance on the executive function Trail Making Test was associated with damage to anterior regions of the cerebellum that support somatomotor performance, consistent with the timed, motor aspects of the task; in contrast, executive function measured by the Wisconsin Card Sorting Test was associated with damage to posterior cerebellar regions. As expected, these findings suggest that performance on cognitive tasks in cerebellar patients is dictated both by lesion location and task demands – tasks with a significant motor component are impacted by a lesion in the anterior lobe, whereas performance on cognitive tasks with limited motor demands are more affected by lesions in cerebellar posterior lobe regions that are reciprocally interconnected with cerebral association cortices.
Another notable finding was the substantial overlap between regions of the cerebellum associated with cognitive task performance. This suggests that, although different activation patterns might be associated with particular cognitive tasks in neuroimaging studies (e.g. verb generation vs. working memory; see Stoodley et al., 2012), these regions are more generally involved in supporting cognitive functions, or shared aspects of these neuropsychological measures. A larger sample of patients will be required to investigate relative lesion patterns associated with different cognitive measures, as the small sample size of the current study provides insufficient power to compare lesion patterns between cognitive tasks.
Although there were some slight lateralization differences (e.g. mental rotation associated with lesions in left VII, Boston Naming Task localized to right VII), which were consistent with earlier studies (Levisohn et al., 2000, Scott et al., 2001), the majority of the cerebellar regions associated with poorer cognitive performance were right-lateralized. This is in line with the finding that right cerebellar damage leads to reduced grey matter in contralateral frontal, parietal and temporal cortices and is associated with lower scores on neuropsychological measures (Clausi et al., 2009). Clausi et al. reported that left cerebellar damage was not associated with neuropsychological or cerebral grey matter abnormalities, although only two of seven patients with left cerebellar damage in that study had lesions involving the dentate nucleus, whereas the majority (6/8) of the right cerebellar patients had dentate damage (Clausi et al., 2009). This is consistent with the finding that involvement of the cerebellar deep nuclei is a predictor of cognitive deficits in cerebellar lesions (Tedesco et al., 2011). Subdivision of the dentate nucleus into dorsal-rostral motor and ventral-caudal cognitive divisions is supported by neuroanatomical (Strick et al., 2009) and functional neuroimaging studies (Kuper et al., 2011). For example, upper limb ataxia has been shown to result from damage to lobule VI and the dorsal dentate nucleus (Maderwald et al., 2012), whereas damage to the ventral dentate was associated with poorer performance on a 3-back working memory task (Ilg et al., 2013). Here, although some lesions did involve the dentate, we were unable to accurately localize our VLSM findings within the dentate nucleus, and thus this remains a question for further study. An alternative or supplementary interpretation of the primarily right-lateralized findings is that damage to the right cerebellar-left cerebral cortical network supporting language function disproportionately impacts neuropsychological test performance, resulting in a general deleterious effect on cognitive scores.
This study has several limitations that must be considered when interpreting the results. First, because the lesions in the present data set do not cover the entire cerebellum, it was not possible to assess the effects of a lesion involving all cerebellar voxels; in the flipped analysis, we achieved better cerebellar coverage, but lost the ability to detect effects that are related to lateralized cerebellar damage. Future studies with complete cerebellar lesion coverage may reveal other regions that, when damaged, are associated with these motor and cognitive measures. Further, we acknowledge that the neuropsychological tests do not specifically isolate the nature of the cerebellar contribution to task performance, but they measure function within a cognitive domain, which was the goal of this structure-function correlation study. The VLSM method did not identify focal areas involved in working memory in this cohort. Working memory has bilateral representation in Crus I and Crus II, and this negative result may reflect the absence of patients with lesions in Crus I in this study. Future investigations with larger patient cohorts and utilizing experimental task measures are being designed to address these issues, and will further test the clinical impact of localized cerebellar damage. These limitations notwithstanding, we show a distinct pattern of localization of motor and cognitive performance deficits that reflects known cerebellar functional topography. Our findings, if confirmed in larger samples of cerebellar patients, could be relevant to establishing structural correlates supporting the clinical prognosis of the cerebellar motor syndrome, the CCAS, or both.
These findings provide further evidence that location of stroke damage within the cerebellum determines whether patients will develop the cerebellar motor syndrome, the cerebellar cognitive affective syndrome, or both. These results are consistent with the functional anatomy of the cerebellum established in anatomical, neurophysiological, and neuroimaging studies. They have implications for the evaluation and management of patients with cerebellar lesions, and for understanding the relevance of the cerebellum to the distributed neural circuits subserving movement and cognition.
Declaration of interest statement
Dr. Schmahmann serves as a consultant to Ataxion, Biogen, Biohaven, Pfizer, and Takeda. The other authors declare no conflicts of interest.
Acknowledgements
This work was supported in part by the National Institutes of Health [NIMH RO1MH67980, NCRR P41RR14075], and the MINDlink and Birmingham Foundations.
Contributor Information
Catherine J. Stoodley, Email: stoodley@american.edu.
Jeremy D. Schmahmann, Email: jschmahmann@partners.org.
References
- Axelrod B.N., Millis S.R. Preliminary standardization of the Cognitive Estimation Test. Assessment. 1994;1:269–274. [Google Scholar]
- Barona A., Reynolds C., Chastain R. A demographically based index of pre-morbid intelligence for the WAIS-R. J. Consult. Clin. Psychol. 1984;52:885–887. [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., Dronkers N.F. Voxel-based lesion-symptom mapping. Nat. Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Beck Depression Inventory – II. [Google Scholar]
- Benton A., Hannay H.J., Varney N.R. Visual perception of line direction in patients with unilateral brain disease. Neurology. 1975;25:907–910. doi: 10.1212/wnl.25.10.907. [DOI] [PubMed] [Google Scholar]
- Bernard J.A., Seidler R.D., Hassevoort K.M., Benson B.L., Welsh R.C., Wiggins J.L., Jaeggi S.M., Buschkuehl M., Monk C.S., Jonides J., Peltier S.J. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L.M., Iverson G.L., Brooks B.L. To err is human: “abnormal” neuropsychological scores and variability are common in healhy adults. Arch. Clin. Neuropsychol. 2009;24:31–46. doi: 10.1093/arclin/acn001. [DOI] [PubMed] [Google Scholar]
- Bornstein R.A. Normative data on selected neuropsychological measures from a non-clinical sample. J. Clin. Psychol. 1985;41:651–659. [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultmann U., Pierscianek D., Gizewski E.R., Schoch B., Fritsche N., Timmann D., Maschke M., Frings M. Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait Posture. 2014;39:563–569. doi: 10.1016/j.gaitpost.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Clausi S., Bozzali M., Leggio M.G., Di Paola M., Hagberg G.E., Caltagirone C., Molinari M. Quantification of gray matter changes in the cerebral cortex after isolated cerebellar damage: a voxel-based morphometry study. Neuroscience. 2009;162:827–835. doi: 10.1016/j.neuroscience.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B.W., Benjamin L.S. American Psychiatric Press, Inc.; Washington, D.C.: 1997. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) [Google Scholar]
- Goodglass H., Kaplan E. Lippincott; Williams & Wilkins, Philadelphia: 2000. Boston Naming Test. [Google Scholar]
- Grodd W., Hulsmann E., Lotze M., Wildgruber D., Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R., Chelune G., Tailey J. Psychological Assessment Resources; Odessa, FL: 1993. The Wisconsin Card Sorting Test. [Google Scholar]
- Holmes G. The cerebellum of man. Brain. 1939;62:1–30. [Google Scholar]
- Ilg W., Christensen A., Mueller O.M., Goericke S.L., Giese M.A., Timmann D. Effects of cerebellar lesions on working memory interacting with motor tasks of different complexities. J. Neurophysiol. 2013;110:2337–2349. doi: 10.1152/jn.00062.2013. [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Rennig J., Johannsen L., Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain. 2011;134:903–912. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase C.S., Norrving B., Levine S.R., Babikian V.L., Chodosh E.H., Wolf P.A., Welch K.M. Cerebellar infarction: clinical and anatomic observations in 66 cases. Stroke. 1993;24:76–83. doi: 10.1161/01.str.24.1.76. [DOI] [PubMed] [Google Scholar]
- Keren-Happuch E., Chen S.H., Ho M.H., Desmond J.E. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp. 2014;35:593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen M.P., Davis-Ratner M.S., Milner M.W., Chen S.H., Schraedley-Desmond P., Fisher P.G., Desmond J.E. Verbal memory impairments in children after cerebellar tumor resection. Behav. Neurol. 2008;20:39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen F.M., Buckner R.L. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper M., Dimitrova A., Thurling M., Maderwald S., Roths J., Elles H.G., Gizewski E.R., Ladd M.E., Diedrichsen J., Timmann D. Evidence for a motor and a non-motor domain in the human dentate nucleus—an fMRI study. NeuroImage. 2011;54:2612–2622. doi: 10.1016/j.neuroimage.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Levisohn L., Cronin-Golomb A., Schmahmann J. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Lewis R.S., Rennick P.M. Ronald F; Lewis, Clinton Township, MI: 1979. Manual for the Repeatable Cognitive-Perceptual-Motor Battery. [Google Scholar]
- Maderwald S., Thurling M., Kuper M., Theysohn N., Muller O., Beck A., Aurich V., Ladd M.E., Timmann D. Direct visualization of cerebellar nuclei in patients with focal cerebellar lesions and its application for lesion-symptom mapping. NeuroImage. 2012;63:1421–1431. doi: 10.1016/j.neuroimage.2012.07.063. [DOI] [PubMed] [Google Scholar]
- Manto M., Marien P. Schmahmann's syndrome — identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias. 2015;2:2. doi: 10.1186/s40673-015-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P., Engelborghs S., Fabbro F., De Deyn P.P. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Marien P., Ackermann H., Adamaszek M., Barwood C.H., Beaton A., Desmond J., De Witte E., Fawcett A.J., Hertrich I., Kuper M., Leggio M., Marvel C., Molinari M., Murdoch B.E., Nicolson R.I., Schmahmann J.D., Stoodley C.J., Thurling M., Timmann D., Wouters E., Ziegler W. Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J., Kimberg D.Y., Chatterjee A., Coslett H.B. Inappropriate usage of the Brunner-Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia. 2010;48:341–343. doi: 10.1016/j.neuropsychologia.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F., Strick P. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly J.X., Beckmann C.F., Tomassini V., Ramnani N., Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth P. Le test de copie d'une figure complexe: contribution a l'étude de la perception et de la memoire, The test of copying a complex figure: a contribution to the study of perception and memory. Arch. Psychol. 1944;30 [Google Scholar]
- Paulus K., Magnano I., Conti M., Galistu P., D'Onofrio M., Satta W., Aiello I. Pure post-stroke cerebellar cognitive affective syndrome: a case report. Neurol. Sci. 2004;25:220–224. doi: 10.1007/s10072-004-0325-1. [DOI] [PubMed] [Google Scholar]
- Richter S., Gerwig M., Aslan B., Wilhelm H., Schoch B., Dimitrova A., Gizewski E.R., Ziegler W., Karnath H.O., Timmann D. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J. Neurol. 2007;254:1193–1203. doi: 10.1007/s00415-006-0500-9. [DOI] [PubMed] [Google Scholar]
- Riva D., Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123:1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Rorden C., Karnath H.O., Bonilha L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rorden C., Fridriksson J., Karnath H.O. An evaluation of traditional and novel tools for lesion behavior mapping. NeuroImage. 2009;44:1355–1362. doi: 10.1016/j.neuroimage.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D. An emerging concept. The cerebellar contribution to higher function. Arch. Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn. Sci. 1998;2:362–371. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. The cerebrocerebellar system. Int. Rev. Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Sherman J.C. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Doyon J., Toga A., Petrides M., Evans A. Academic Press; San Diego: 2000. MRI Atlas of the Human Cerebellum. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Gardner R., MacMore J., Vangel M.G. Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov. Disord. 2009;24:1820–1828. doi: 10.1002/mds.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Macmore J., Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–861. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch B., Dimitrova A., Gizewski E.R., Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. NeuroImage. 2006;30:36–51. doi: 10.1016/j.neuroimage.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Scott R.B., Stoodley C.J., Anslow P., Paul C., Stein J.F., Sugden E.M., Mitchell C.D. Lateralized cognitive deficits in children following cerebellar lesions. Dev. Med. Child Neurol. 2001;43:685–691. doi: 10.1017/s0012162201001232. [DOI] [PubMed] [Google Scholar]
- Snider R.S., Eldred E. Cerebrocerebellar relationships in the monkey. J. Neurophysiol. 1952;15:27–40. doi: 10.1152/jn.1952.15.1.27. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tedesco A.M., Chiricozzi F.R., Clausi S., Lupo M., Molinari M., Leggio M.G. The cerebellar cognitive profile. Brain. 2011;134:3672–3686. doi: 10.1093/brain/awr266. [DOI] [PubMed] [Google Scholar]
- Timmann D., Brandauer B., Hermsdorfer J., Ilg W., Konczak J., Gerwig M., Gizewski E.R., Schoch B. Lesion-symptom mapping of the human cerebellum. Cerebellum. 2008;7:602–606. doi: 10.1007/s12311-008-0066-4. [DOI] [PubMed] [Google Scholar]
- Tohgi H., Takahashi S., Chiba K., Hirata Y. Cerebellar infarction: clinical and neuroimaging analysis in 293 patients. Stroke. 1993;24:1697–1701. doi: 10.1161/01.str.24.11.1697. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N. Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- Trouillas P., Takayanagi T., Hallett M., Currier R., Subramony S., Wessel K., Bryer B., Deiner H., Massaquoi S., Gomez C., Coutinho P., Hamida M.B., Campanella G., Filla A., Schut L., Timmann D., Honnorat J., Nighoghossian N., Manyam B. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome: the ataxia neuropharmacology committee of the world federation of neurology. J. Neurol. Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Urban P.P., Marx J., Hunsche S., Gawehn J., Vucurevic G., Wicht S., Massinger C., Stoeter P., Hopf H.C. Cerebellar speech representation: lesion topography in dysarthria as derived from cerebellar ischemia and functional magnetic resonance imaging. Arch. Neurol. 2003;60:965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Vandenberg S.G., Kuse A.R. Mental rotations, a group test of three-dimensional spatial visualization. Percept. Mot. Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale — III. [Google Scholar]
- Wechsler D. NCS Pearson; San Antonio, TX: 2009. Wechsler Memory Scale: Administration and scoring manual. [Google Scholar]