Abstract
Significance: Regulation of Ca2+ signaling cascade by reactive oxygen species (ROS) is becoming increasingly evident and this regulation represents a key mechanism for control of many fundamental cellular functions. Canonical transient receptor potential (TRPC) 6, a member of Ca2+-conductive channel in the TRPC family, is widely expressed in kidney cells, including glomerular mesangial cells, podocytes, tubular epithelial cells, and vascular myocytes in renal microvasculature. Both overproduction of ROS and dysfunction of TRPC6 channel are involved in renal injury in animal models and human subjects. Although regulation of TRPC channel function by ROS has been well described in other tissues and cell types, such as vascular smooth muscle, this important cell regulatory mechanism has not been fully reviewed in kidney cells. Recent Advances: Accumulating evidence has shown that TRPC6 is a redox-sensitive channel, and modulation of TRPC6 Ca2+ signaling by altering TRPC6 protein expression or TRPC6 channel activity in kidney cells is a downstream mechanism by which ROS induce renal damage. Critical Issues: This review highlights how recent studies analyzing function and expression of TRPC6 channels in the kidney and their response to ROS improve our mechanistic understanding of oxidative stress-related kidney diseases. Future Directions: Although it is evident that ROS regulate TRPC6-mediated Ca2+ signaling in several types of kidney cells, further study is needed to identify the underlying molecular mechanism. We hope that the newly identified ROS/TRPC6 pathway will pave the way to new, promising therapeutic strategies to target kidney diseases such as diabetic nephropathy. Antioxid. Redox Signal. 25, 732–748.
Keywords: : reactive oxygen species, TRPC, calcium channel, kidney, diabetic nephropathy
Introduction
Canonical transient receptor potential (TRPC) proteins constitute one of the major subfamilies of the TRP superfamily first identified in Drosophila melanogaster (138). There are seven subtypes, designated TRPC1-7, of which TRPC2 is a pseudogene in humans. The remaining six proteins can be divided phylogenetically based on their structure, with TRPC1 most closely related to TRPC4 and TRPC5, and TRPC3 grouped with TRPC6 and TRPC7 (153, 161).
Over the past 20 years, the seven TRPC members have been the focus of intensive investigations in many cell types, including kidney cells. TRPCs may function as store-operated channels activated by depletion of intracellular Ca2+ stores (151) or as receptor-operated channels activated by G protein-coupled or receptor tyrosine kinase signaling pathways (79). In both cases, TRPCs act as nonselective cation channels, allowing Ca2+ influx. No matter whether their activation occurs via store-operated or receptor-activated mechanisms, increasing evidence demonstrates that TRPCs have functional significance in cellular Ca2+ signaling. This gives them the potential to contribute to the regulation of numerous Ca2+-dependent cellular functions from cell growth to myocyte contraction.
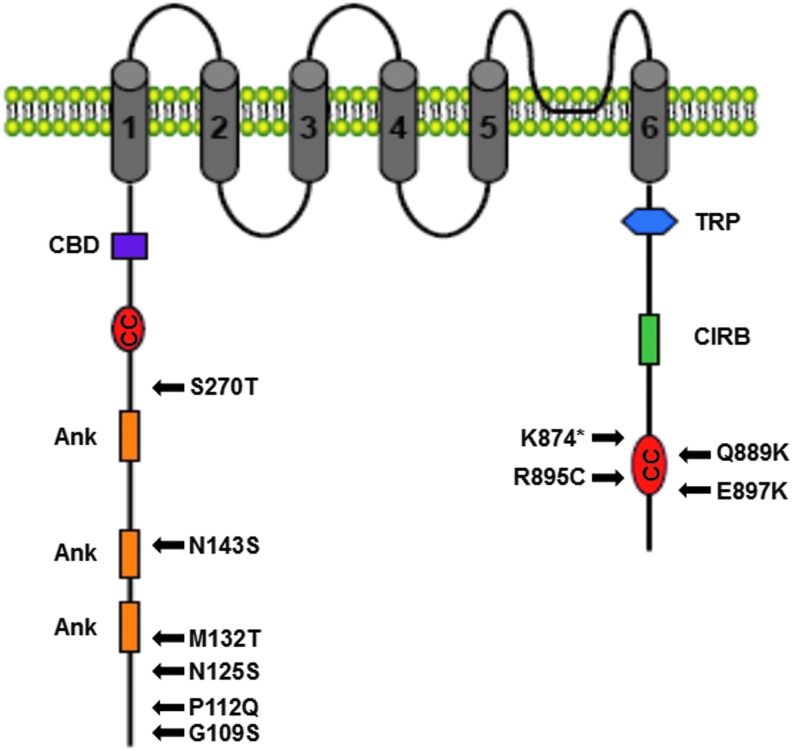
Like other TRPC family members, TRPC6 comprises six transmembrane spanning regions with intracellular N- and C-termini and a presumed pore-forming loop between transmembrane spanning regions 5 and 6. Other conserved regions include three ankyrin repeats, a coiled-coil domain, and caveolin binding region in the N-terminus. The C-terminus is characterized by a TRP motif (EWKFAR), another coiled-coil domain, a proline-rich motif, and a calmodulin/IP3R binding region (38, 205) (Fig. 1). TRPC6 is widely expressed in a variety of tissues and organs, including the kidney (2). Particularly, a link between an altered expression level or dysfunction of TRPC6 channel and renal diseases has been firmly established (38).
FIG. 1.
TRPC6 channel structure. TRPC6 is characterized by six transmembrane domains with a pore-forming region found between transmembrane domains 5 and 6. The N-terminus of TRPC6 contains three Ank, one CC, and one CBD, and the C-terminus contains one CIRB and highly conserved TRP box. Several disease-related point mutations (indicated by arrows) have been discovered in TRPC6. Amino acid changes are indicated by the single-letter code and a number for the exact position in the protein. *A stop codon (truncation mutant). CBD, caveolin-1 binding domain; CC, coiled-coiled domain; CIRB, calmodulin/IP3R binding domain; TRPC, canonical transient receptor potential.
Although TRPC6 is acknowledged as a lipid (such as dicylglycerol) and mechanosensitive channel (6, 7, 85, 91, 95, 113, 116, 188), evidence has accumulated that it is also a redox-sensitive channel (70). Recent studies have called attention to the modulation of TRPC6 channel by the cellular redox in kidney cells, which may underlie a new mechanism for development of some kidney diseases. This review summarizes recent studies on the importance of the ROS/TRPC6 signaling pathway in regulation of renal function and, importantly, the contribution of this intracellular pathway to kidney diseases. We hope that this review can provide new information for people in the field of both TRPC6 biology/pathology and renal physiology/pathology.
Distribution of TRPC6 in Renal Microvasculature
TRPC6 protein is widely expressed in blood vessels of varying sizes and plays a role in the regulation of vascular tone by contributing to smooth muscle contraction as well as endothelium-dependent vasorelaxation (6, 7, 39, 226). For instance, TRPC6 is expressed in myocytes of portal vein (6, 96), pulmonary artery (120, 207), cerebellar arteries (216), mesenteric arteries (7, 42), and aorta (42) and in the endothelial cells of pulmonary artery (108, 184) and aorta (32). TRPC6 in these vascular cells can regulate basal vascular tone, agonist-induced vasoconstriction and vasodilatation, vascular endothelial permeability, vascular endothelial cell migration, and vascular myocyte proliferation (2, 40, 63, 108, 213).
The findings in the extrarenal vasculature raise an interest in studying expression and function of TRPC6 channel in the renal microvasculature (Fig. 2). Different from microcirculation in other tissues/organs, renal microcirculation contains two sets of capillaries, termed glomerular and peritubular capillaries, which are arranged in series and separated by efferent arterioles. The afferent and efferent arterioles are major resistance vessels in the kidney and their contractile state controls the renal blood flow. Furthermore, the microcirculation of the kidney is regionally specialized. In the cortex, afferent and efferent arterioles govern the driving forces that promote glomerular filtration. A dense peritubular capillary plexus surrounds the proximal and distal convoluted tubules to accommodate enormous reabsorption of glomerular filtrates.
FIG. 2.
Distribution of TRPC6 in nephron and renal microvasculature.
By adjusting the resistance of the afferent and efferent arterioles, the kidneys can regulate the hydrostatic pressures in both the glomerular and the peritubular capillaries, thereby changing the rate of glomerular filtration and/or tubular reabsorption in response to body homeostatic demands. TRPC channels in the renal microvessels may help to regulate renal function by contributing to the regulation of driving force for glomerular filtration and tubular reabsorption, making their potential role in the renal microcirculatory system of particular interest.
Earlier studies have demonstrated that afferent and efferent arterioles expressed different isoforms of TRPC channels. TRPC1 is present in the efferent arterioles (198), but TRPC6 is expressed in the afferent arterioles. In the freshly isolated vascular smooth muscle cells derived from rat preglomerular vessels, Fellner and Arendshorst found that nifedipine or verapamil only partially inhibited vasopressin-induced Ca2+ entry (52). In the presence of the voltage-gated Ca2+ channel blockers, depletion of internal Ca2+ stores with cyclopiazonic acid or thapsigargin significantly raised intracellular Ca2+ concentration in response to Ca2+ readmission. They further found that TRPC blockade with 2-aminoethoxydiphenyl borane inhibited angiotensin II (Ang II)-induced Ca2+ influx, while flufenamic acid, which has been shown to activate TRPC6, resulted in an increased Ca2+ influx (51). The authors concluded that TRPC6 participated in agonist-evoked Ca2+ signaling in afferent arterioles.
It should be noted that several other subtypes of TRPCs (TRPC1, 3, 4, 5, and 7) were also detected at both mRNA and protein levels in the preglomerular resistance vessels, including afferent arterioles (47, 198). However, their role in contractile function of the afferent arterioles has not been studied yet.
Distribution of TRPC6 in Glomerular Cells
The glomerulus is the filtering unit of kidney. The mature glomerulus contains four cell types: parietal epithelial cells that form Bowman's capsule, podocytes that cover the outmost layer of the glomerular filtration membrane, endothelial cells that are lined along the luminal side of the wall of glomerular capillaries, and mesangial cells (MCs) that sit between the capillary loops (135, 190). Over the past 10 years, studies have been conducted to examine expression of TRPC6 in specific type of glomerular cells and the implications of TRPC6 in renal function. Although the results from different groups are not completely consistent, and some are even controversial, a consensus among the studies is that TRPC6 is expressed in glomerular cells and plays important roles in glomerular physiology and pathology (Fig. 2).
Expression of TRPC6 in glomerular MCs
Glomerular MCs and their matrix form the central stalk of the glomerulus. MCs comprise approximately one-third of the decapsulated glomerular cell population. In the glomerulus, MCs have many beneficial roles, which include secretion of growth factors that allow normal cell turnover, production of mesangial matrix to provide structural support for glomerular capillaries, modulation of glomerular hemodynamics through their contractile properties, and phagocytosis of apoptotic cells or immune complexes formed at or delivered to the glomerular capillaries (1, 130, 189). As in most cell types, Ca2+ signaling plays a central role in mediating and regulating those cellular processes in MCs. Ca2+ influx across the plasma membrane is a major component of MC Ca2+ response to a variety of stimuli (123). Among several types of Ca2+-conductive channels in the plasma membrane, TRPC channels have recently caught intensive attention. Over the past 10 years, we and others have extensively studied the function and regulatory mechanisms of TRPC channels, particularly TRPC6, in MCs.
In an earlier study, we identified the presence of TRPC6 protein in cultured human MCs (187). Expression of TRPC6 protein in human MCs was also reported by several other groups (87, 110, 119, 131, 158, 233). However, Goel et al. reported that rat MCs only expressed TRPC1 protein (61). The distinct results from different groups may be due to different antibodies used in those studies. It is also possible that the TRPC protein distribution in MCs differs among species.
MCs have a contractile phenotype similar to vascular smooth muscle cells. The contractile properties of MCs enable them to alter intraglomerular capillary flow and glomerular ultrafiltration surface area and thereby the glomerular filtration rate (41, 130, 189). MC contractile function is controlled by intracellular Ca2+ concentration, which is, to a large extent, attributed to Ca2+ influx through Ca2+ channels in the plasma membrane. TRPC6 is a Ca2+-conductive channel and can be activated by chemical (such as dicylglycerol) and physical factors (such as stretch) (85, 91, 95, 116, 188), both of which are physiological stimuli to MCs. Therefore, TRPC6 may regulate MC contractility. This speculation was confirmed by our recent study in which TRPC6 mediated Ca2+ entry and contraction of cultured human MCs in response to Ang II (72).
Diabetic nephropathy is a major and devastating complication of diabetes. At the very onset of diabetes, a dominant pathophysiological characteristic is the development of renal glomerular hyperfiltration (13, 214). This early hemodynamic phenotype provokes the subsequent demise of a diabetic kidney. The diabetic hyperfiltration is derived from a combined decreased responsiveness of both the renal afferent arterioles and glomerular MCs to vasoconstrictors (13, 27, 104, 144, 218). In diabetes, mesangial contractile function is impaired, and reduced Ca2+ influx is believed to be a major contributing factor to the hypocontractility (144, 218).
Since TRPC6 channel mediates Ca2+ response in MCs, it is conceivable that dysfunction of the channel may play a role in diabetic hyperfiltration. We recently found that expression of TRPC6, but not TRPC1 and TRPC3, was downregulated in glomeruli of streptozotocin (STZ) diabetic rats as well as in MCs treated with high glucose (71). Both high-glucose treatment and knockdown of TRPC6 attenuated Ang II-stimulated membrane currents and Ca2+ influx in MCs (71). This high-glucose/diabetes-induced decrease in TRPC6 protein expression was MC specific because this response was not observed in the aorta and the heart tissue isolated from those diabetic rats (72) and in high-glucose-treated podocytes (71).
Since our study was conducted in rats only 2 weeks after STZ injection, the decrease in abundance of TRPC6 protein might indicate an early change in the development of diabetic nephropathy. However, it is not clear whether the decrease of TRPC6 protein in MCs is the cause or consequence of diabetic kidney disease. Elucidation of this cause–effect relationship needs further studies, for instance, studies to investigate whether MC-specific knockout/knockdown of TRPC6 can initiate diabetic kidney disease and whether hyperfiltration in diabetic animals can be normalized by rescuing TRPC6 protein in MCs.
In addition to regulation of MC contraction, TRPC6 may also play a role in MC proliferation. Ca2+-sensing receptor is expressed in MCs and stimulation of the receptor promotes proliferation (112). Meng et al. found that activation of Ca2+-sensing receptor increased expression of TRPC6 in MCs. Knocking down of TRPC6 attenuated Ca2+-sensing receptor-mediated elevation of intracellular Ca2+ and MC proliferation (131), suggesting contributions of TRPC6 to the MC responses.
Ang II is a known proliferative factor in many cell types, including MCs (98, 171). Studies showed that Ang II stimulated MC proliferation by increasing the expression level of TRPC6 protein (158, 233). In addition, TRPC6 also contributed to phenylephrine (α1-adrenergic receptor agonist)-induced MC proliferation (110). Interestingly, the ERK signaling pathway can work as a mechanism both upstream (in Ang II signaling pathway) (233) and downstream (in α1-adrenergic receptor signaling pathway) (110) TRPC6 expression. Excessive proliferation of MCs is a characteristic pathological feature of glomerulosclerosis. Both an overactive Ang II system and sympathetic nervous hyperactivity (overactive α1-adrenergic receptor signaling pathway) in the kidney contribute to the pathogenesis of glomerulosclerosis in chronic kidney disease (22, 103, 133, 147, 206, 220). Therefore, TRPC6 may be used as a therapeutic target for treating renal fibrosis in chronic kidney disease.
Expression of TRPC6 in podocytes
Podocytes are pericyte-like cells with a complex cellular organization consisting of a cell body, major processes, and foot processes. The foot processes of neighboring podocytes interdigitate each other, forming filtration slits that are bridged by the glomerular slit diaphragm (75). Podocytes are highly differentiated cells with limited capability to undergo cell division in the adult, and the loss of podocytes is a hallmark of progressive kidney disease. Podocytes form the glomerular filtration barrier along with the fenestrated glomerular capillary endothelium and glomerular basement membrane and therefore play a significant role in the regulation of glomerular filtration (204). Disruption of the podocyte slit diaphragm is associated with severe proteinuria, including that associated with focal and segmental glomerulosclerosis (FSGS).
Like many other cell types, podocytes also express multiple subtypes of TRPC proteins. In an earlier study, TRPC3 and TRPC6 proteins were detected in rat podocytes (61). This was verified by a later study from Kim et al. who showed colocalizations of both TRPC3 and TRPC6 with large-conductance Ca2+-activated K+ channels (105). TRPC6 protein was also found in the podocytes of the freshly isolated rat glomeruli (93).
An enormous impetus of studying the importance of TRPC6 in podocyte biology, physiology, and pathology is derived from a breakthrough finding that a gain-of-function mutation of TRPC6 in podocytes was associated with familial FSGS, a pathologic lesion that frequently causes the nephritic syndrome and ensuing renal failure (136, 219). Through whole-genome linkage analysis, fine mapping, and candidate gene screening, Winn et al. identified a mutated trpc6 gene in a large family, most members of which progressed to end-stage renal disease (219). Subsequently, Reiser et al. identified TRPC6 mutations in five other unrelated families with autosomal dominant FSGS (163). More TRPC6 mutations were recently found in late-onset FSGS as well as childhood FSGS patients (57, 88, 235). So far, 14 different mutations in the trpc6 gene have been identified in nine families from different ethnic backgrounds and in five sporadic patients (57, 88, 163, 174, 219, 235). Most of these mutations were gain-of-function mutations that resulted in increased Ca2+ entry.
Although the associations of TRPC6 mutations in podocytes with FSGS have been firmly established, the underlying mechanisms are still unclear. Multiple mechanisms may be involved. For instance, increased intracellular Ca2+ derived from overactive TRPC6 channel may modify the contractile structure or actin cytoskeleton arrangement of podocyte foot processes, resulting in an alteration of the ultrafiltration coefficient (99, 137, 202). Another possible mechanism is that increased intracellular Ca2+ may cause loss of podocytes through apoptosis, detachment, or lack of proliferation, resulting in glomerulosclerosis (152). It is also possible that abnormally high intracellular Ca2+ limits normal podocyte proliferation (182).
Consistent with the role of hyperactive TRPC6 mutations in hereditary FSGS, an increased expression of TRPC6 was found in acquired forms of proteinuric kidney diseases, including minimal change disease and membranous glomerulonephritis (137). Overexpression of TRPC6 in podocytes disrupts actin cytoskeleton, which may result in slit diaphragm dysfunction and subsequent proteinuria in mice transiently overexpressing TRPC6 (137).
Because Ang II plays a critical role in the generation of proteinuria and progression of kidney injury (22, 103, 133, 147, 220), a role of TRPC6 channel in the Ang II signaling pathway in podocytes has been intensively studied. An earlier study by Zhang et al. demonstrated that Ang II-induced podocyte apoptosis resulted from augmentation of TRPC6-mediated Ca2+ influx (231). Ang II treatment increased TRPC6 protein expression through the ERK/NF-κB pathway (231), and the ERK1/2 pathway was activated by gain-of-function TRPC6 mutants in cultured podocytes (34). Therefore, there may be a positive feedback loop between ERK1/2 and TRPC6, which can accelerate Ang II-induced podocyte injury.
Nijenhuis et al. also reported that Ang II increased TRPC6 mRNA and protein expression levels in cultured mouse podocytes (142). They further showed that Ang II infusion enhanced glomerular and podocyte TRPC6 expression in rats. In rat models for human FSGS and increased renin–angiotensin system activity, glomerular and podocyte TRPC6 expression was increased in an Ang II-dependent manner and the increase in TRPC6 expression correlated with podocyte injury and glomerular damage (142). Using in vitro and in vivo settings, they demonstrated that Ang II induced podocyte injury by enhancing TRPC6 expression via a calcineurin/NFAT-positive feedback signaling pathway (142).
Indeed, several TRPC6 mutations previously shown to enhance channel activity caused constitutive activation of the calcineurin/NFAT pathway in podocytes (175), and activation of calcineurin increased TRPC6 expression in podocytes in a murine model of podocyte-specific constitutively active Gq α subunit (208). In another study, Eckel et al. infused TRPC6-deficient and wild-type control mice with Ang II for 28 days. Although both groups developed similar levels of hypertension, TRPC6-deficient mice had significantly less albuminuria (44), presumably due to lack of TRPC6-dependent Ca2+ influx upon Ang II stimulation. Therefore, TRPC6 adversely influences the glomerular filter and enhances Ang II-induced kidney injury.
In addition to increasing number of TRPC6 channels, Ang II may also increase activity of existing TRPC6 channels in podocytes. This has been demonstrated in podocytes of freshly isolated rat glomeruli (94). Therefore, Ang II has both chronic and acute effects on TRPC6 channels, the former is to increase abundance of TRPC6 proteins and the latter is to increase open probability of the channel.
Effects of TRPC6 on podocyte function may be context dependent. In contrast to those studies described above, Kistler et al. reported that overexpression of TRPC6 alone did not directly affect podocyte morphology and cytoskeletal structure, but protected podocytes from complement-mediated injury (109). Consistently, TRPC6 inactivation increased podocyte susceptibility to complement. They further showed that the TRPC6 effect was mediated by Ca2+/calmodulin-dependent protein kinase II mechanism (109). Their study suggests a dual and context-dependent role of TRPC6 in podocytes where acute activation protects from complement-mediated damage, but chronic overactivation leads to FSGS.
Recently, TRPC5 was found to promote podocyte motility by antagonizing the TRPC6 signaling pathway. Tian et al. demonstrated that podocytes contained two distinct molecular complexes of TRPC5-Rac1 and TRPC6-RhoA, which are spatially, biochemically, and functionally segregated (202). Both Rac1 and RhoA are the members of the Rho family of small guanosine triphosphatases, with Rac1 promoting cell motility and RhoA enhancing contractility (46). In accordance with the distinct functions of Rac1 and RhoA, TRPC5 promoted a motile phenotype, whereas TRPC6 maintained a contractile phenotype of podocytes (202). The antagonistic and mutually inhibitory TRPC5 and TRPC6 signaling is well coordinated and maintains the balance between contractility and motility of podocytes.
The findings from the Tian et al. study are consistent with the previous reports of increasing neuronal cell motility and vascular smooth muscle cell migration by TRPC5 (76, 222) and inhibiting endothelial cell migration by TRPC6 (184), thus highlighting complicated TRPC channel signaling in regulation of podocyte structure and function (74).
Although several other isoforms of TRPCs were also detected in podocytes either at the mRNA level or protein level, their physiological and pathological relevance in podocytes has not been studied, probably due to their low expression levels and/or no contribution to agonist-stimulated Ca2+ response in podocytes (93, 94, 163, 202).
Expression of TRPC6 in endothelial cells
Glomerular endothelial cells line the inner surface of the glomerular capillaries. Compared with the endothelial cells in most vascular beds, glomerular endothelial cells are extremely flat and perforated by dense arrays of transcellular pores, the fenestrae. This phenotype is critical for the high glomerular water and electrolyte permeability (145).
Many TRPC isoforms have been found in endothelial cells of extrarenal vasculature, as described previously (11, 32, 48, 55, 154, 184, 226, 227). However, studies on TRPC channels in the glomerular endothelial cells are scarce. Using immunofluorescent staining of rat kidney sections, Reiser et al. were able to detect TRPC6 protein expression in glomerular endothelial cells (163). However, the function of TRPC6 in the endothelial cells was not examined in that study. Since TRPC6 in endothelial cells plays an important role in vascular endothelial permeability (2, 40, 108), it may be worth studying the TRPC6 function in glomerular endothelial cells, which may provide new information on regulation of glomerular filtration.
Distribution of TRPC6 in Tubular Epithelial Cells
Renal tubules comprise four major segments, that is, the proximal tubule, Henle's loop, distal tubule, and collecting duct. Each segment is made up of a layer of epithelial cells that are uniquely suited to perform specific transport functions. By the processes of selective reabsorption of solutes and water and secretion of solutes, the renal tubules modulate the volume and composition of urine, which in turn allow the tubules to precisely control the volume, osmolality, composition, and pH of the extracellular and intracellular fluids. Accumulating evidence shows that TRPC6 is expressed in the epithelial cells of different segments and regulates water and solute transport in the tubules (Fig. 2).
Expression of TRPC6 in proximal tubule
TRPC6 (160) was found in cultured human-derived renal proximal tubular cell lines (HK-2) (172). A study further suggested that TRPC6 was involved in hepatocyte growth factor-induced growth, migration, cytoskeletal reorganization, and tubulogenesis of proximal tubular epithelial cells (160).
Expression of TRPC6 in distal tubules
TRPC6 expression in the distal tubules has been extensively studied. In cultured polarized MDCK cells, which resemble the intercalated cells of the renal cortical collecting duct (146), Bandyopadhyay et al. detected TRPC6, which was localized both apically and basolaterally (12). Goel et al. performed a series of studies in vivo (rats) and in the cultured mouse collecting duct cell line and comprehensively examined distributions and functions of TRPC6 in distal nephron. TRPC6 was detected in specific tubular cells of the cortex and both the outer and inner medullas (61). Using specific markers for different tubular segments, they further revealed that TRPC6 was expressed in principal cells of the collecting duct (61). Consistent with Bandyopadhyay's findings (12), Goel et al. also found that TRPC6 was expressed in both the basolateral and apical membranes in polarized cultures of M1 and IMCD-3 collecting duct cells (61).
Although both TRPC3 and 6 exist in principal cells of the collecting duct, only TRPC3-containing intracellular vesicles translocated to the apical membrane along with the water channel, aquaporin-2, upon stimulation with the antidiuretic hormone, arginine vasopressin (60, 62). TRPC3 channels were also responsible for transepithelial apical-to-basolateral Ca2+ flux stimulated by apical ATP in principal cells of the inner medullary collecting duct (59, 60). However, the function of TRPC6 channel in this tubule has not been studied.
Distribution of TRPC6 in Other Renal Cells
Juxtaglomerular (JG) cells of the kidneys are a group of specialized cells in renal microcirculation. They are modified smooth muscle cells located in the walls of the afferent arterioles immediately proximal to the glomeruli. The major function of the JG cells is to synthesize, store, and release renin into the blood flow for initiating the renin–angiotensin–aldosterone system. Ca2+ plays an unusual role in the control of renin secretion from the renal JG cells in the way that an increase in the Ca2+ concentration inhibits the exocytosis of renin. The transcript of TRPC6 was detected in mouse JG cells (149). TRPC6 might be responsible for adenosine-evoked inhibition of renin release (149).
Fibroblasts, when activated, undergo a phenotypic change to myofibroblasts, which is a key process of fibrosis. TRPC6 is expressed in renal fibroblasts and plays a role in growth and proliferation of the cells. In cultured rat renal fibroblasts, TRPC6 protein was found by forming a macromolecular complex with TRPC3 (173). However, functional studies showed that specific knockdown of TRPC6 did not inhibit agonist-stimulated Ca2+ entry in cultured fibroblasts (173). Therefore, the function of TRPC6 in renal fibroblasts needs to be further studied.
Reactive Oxygen Species and Ca2+ Signaling
Reactive oxygen species (ROS) are ubiquitous, highly reactive short-lived derivatives of oxygen metabolism produced in all biological systems that react with surrounding molecules at the site of formation. These species include free radicals, such as the superoxide anion (O2.−) and the hydroxyl radical (.OH), and nonradicals, such as H2O2. Compared with radicals, H2O2 has longer half-life and diffusion distance and is more stable. Different from O2.−, which remains near the site of its production, H2O2 can diffuse across membranes with the help of aquaporin proteins (15–17, 89, 132) and through the cytosol (10). Therefore, H2O2 has been focused on in most studies to investigate the roles of ROS in regulation of biological, physiological, and pathological processes.
It has been acknowledged that ROS, at a low level, play an essential role in multiple cellular signal transduction pathways. However, under conditions of oxidative stress, they contribute to a series of cellular dysfunctions. This is particularly true for H2O2. H2O2 has been identified as a second messenger molecule (90, 165) and initiates/regulates a diversity of cellular effects, such as cell shape changes, the formation of functional actomyosin structures, and vascular tone (42, 221). However, overproduction of H2O2 has been linked to metabolic syndrome (167), cardiovascular diseases (29, 77, 176), atherosclerosis (126, 185), hypertension (114, 180), and diabetic complications (65, 80, 102, 168). Like in all other organs/tissues, ROS not only play important roles in renal biology and physiology (41, 148, 177, 179) but also contribute to acute and chronic kidney injury (14, 64–67, 80, 97, 176, 199).
ROS are constitutively produced from the mitochondrial electron transport chain (30) and oxidases distributed in subcellular compartments (3, 25, 86, 101, 127), for example, NADPH oxidases (cytoplasm, plasma membrane, nuclei), xanthine oxidase (cytoplasm, extracellular), endoplasmic reticulum oxidase, sulfhydryl oxidases (nuclei, extracellular), thiol oxidases (plasma membrane, plasma), and monoamine oxidases (mitochondria outer membrane). Currently, there is substantial evidence that the phagocyte-like Nox family enzymes are a major source of ROS in the kidney (58, 64–68, 177). Particularly, associations of overproduction of ROS from activated NADPH oxidases with structural and functional deteriorations in diabetic nephropathy have recently been firmly established (19, 65–67, 97, 164, 178, 199, 228).
Multiple downstream pathways mediate ROS effects on cellular processes. These include direct and indirect oxidation of macromolecules (lipid, DNA, and proteins) (101, 183, 225), modulation of protein kinases (33, 56, 72, 83, 84, 191, 195), protein tyrosine phosphatases (37, 121, 165, 186, 197, 215), and transcription factors (41, 82, 100, 115, 166, 211). This is particularly true in the kidney. Alterations of activities of PKC, Akt, ERK1/2, and signaling pathways of TGF-β1, NF-κB, and Nrf-2 by ROS have been reported in glomerular cells and tubular epithelial cells (20, 41, 68, 69, 72, 82–84, 100, 115, 140, 166, 191, 211).
Among those ROS-associated pathways, the Ca2+ signaling pathway is an important target for modulation of cellular processes by ROS under physiological and pathological conditions. Intracellular Ca2+ represents a convergent point of many signal transduction pathways and modulates a diverse array of cellular activities ranging from contraction to cell growth. Cells generate Ca2+ signals through both internal and external Ca2+ sources. A major internal Ca2+ source is the ER/SR, which releases the stored Ca2+ in the lumen into the cytoplasm through IP3R or RyR. In terms of external source, several types of Ca2+-conductive ion channels in the plasma membrane are involved in controlling Ca2+ entry in response to a variety of stimuli. Elevated cytosolic Ca2+ is rapidly reduced back toward baseline by NCX (18) and PMCA in the plasma membrane and SERCA (26, 134, 150). The Ca2+ influx from the extracellular compartment and Ca2+ release from ER/SR, in concert with Ca2+ extrusion to outside the cell and Ca2+ sequestration into the ER/SR, maintain cytoplasmic Ca2+ homeostasis (Fig. 3).
FIG. 3.
Cellular components involved in Ca2+ homeostasis. All of those components are the targets of ROS. For simplicity, several other pathways, which also regulate the cytosolic Ca2+ level, are intentionally omitted, such as the nucleus and the mitochondria that contribute to remove Ca2+ from the cytoplasm. ROS, reactive oxygen species.
Enormous studies have demonstrated that all those Ca2+ homeostatic proteins are redox sensitive and thus are the targets of ROS (50, 78, 90, 196, 203) (Fig. 3). TRPC6, a Ca2+-permeable channel and widely expressed in a variety of tissues/organs, has been revealed to sense ROS and is involved in ROS-induced diverse physiological and pathological responses (111, 203). In the kidney, accumulating evidence has indicated that regulation of TRPC6 protein abundance or channel activity in different parts of the nephron may underlie ROS-induced changes in renal function.
Regulation of TRPC6 Channel by ROS in Kidney Cells
Regulation of TRPC6 by ROS in glomerular MCs
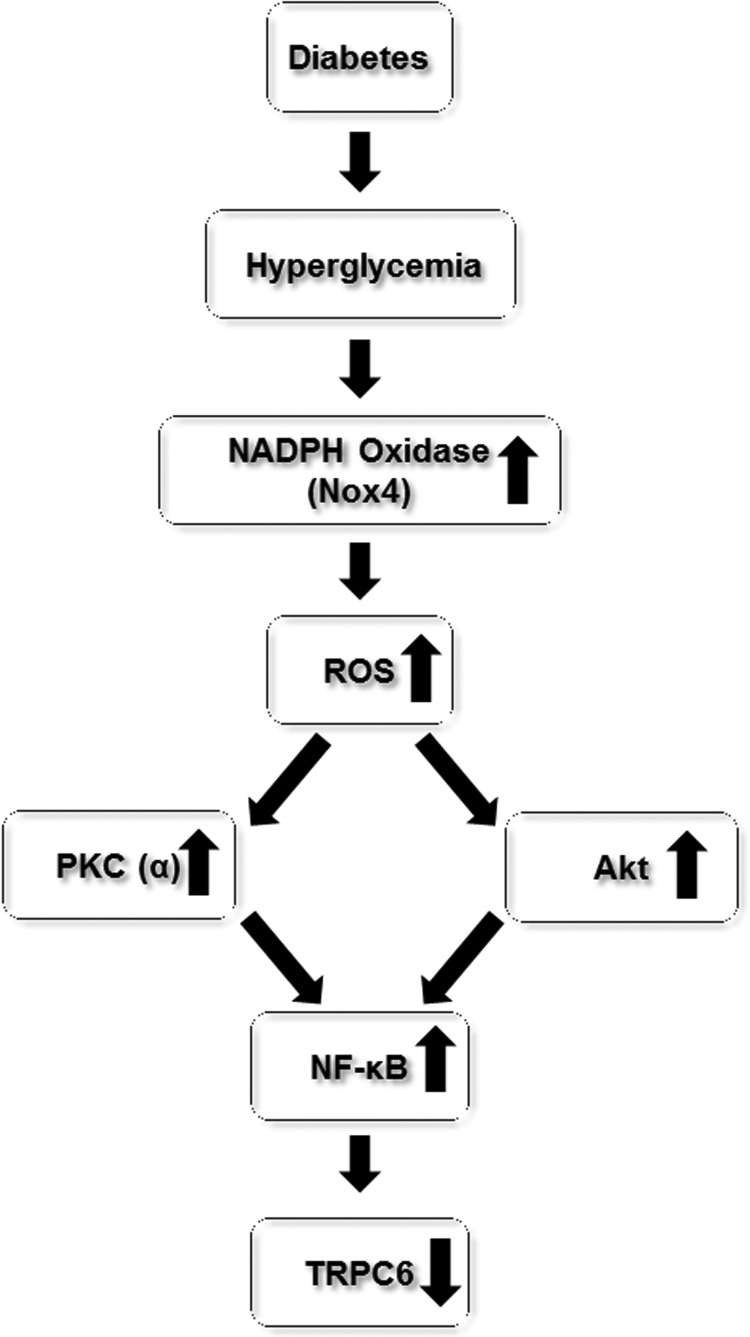
Our earlier study demonstrated that TRPC6 regulated contractile function of MCs (72) and a reduced abundance of TRPC6 protein in MCs might contribute to glomerular hyperfiltration in the early stage of diabetes (71). We recently carried out a series of studies to investigate the mechanism underlying the decrease in TRPC6 protein expression by diabetes. We found that the diabetes/high-glucose-induced downregulation of TRPC6 protein level was mediated by the elevated intracellular level of ROS (72). In cultured MCs, H2O2 or overexpression of Nox4 recapitulated the diabetes/high-glucose effect on TRPC6 expression in MCs, and antioxidant treatment or knockdown of Nox4 significantly suppressed high-glucose-induced decrease in TRPC6 expression (72). Importantly, in vivo antioxidant treatment significantly restored TRPC6 expression in glomerulus in diabetic rats (72, 122). We further showed that the ROS effect was mediated by activation of PKC, which phosphorylated NF-κB, resulting in repression of TRPC6 transcription (72, 211).
These findings were supported by Sun et al. who recently reported that astragaloside IV, a potent antioxidant, prevented high-glucose-induced decrease in TRPC6 protein expression through inhibition of the Nox4/Akt/NF-κB pathway (193). Therefore, there is a signaling cascade of high glucose/Nox4/ROS/PKC or Akt/NF-κB/TRPC6 in diabetic MCs, which leads to reduction of TRPC6 protein expression (Fig. 4).
FIG. 4.
Diagram depicting the pathways involved in TRPC6 protein downregulation in glomerular mesangial cells in diabetes.
However, a recent study by Liao et al. showed that chronic hypoxia increased TRPC6 protein expression in MCs (119). Because chronic hypoxia is usually accompanied with oxidative stress (124, 143, 155, 157), the results from the Liao et al. study seem to contradict with ours. However, multiple signaling pathways, such as Ang II signaling, are activated by chronic hypoxia (35, 54, 128, 129, 159). Thus, upregulation of TRPC6 expression might be attributed to an ROS independent mechanism. Indeed, Ang II has been shown to increase the TRPC6 protein expression level in MCs (158, 233).
It should be noted that all aforementioned studies are chronic effects of ROS, that is, altering abundance of TRPC protein. However, ROS can also acutely regulate TRPC6 channel function. In TRPC6-expressing HEK293 cells and vascular smooth muscle cells, H2O2 evoked TRPC6-mediated membrane currents in a few minutes by stimulating translocation of cytosolic TRPC6 protein to the cell surface (70). The ROS-induced acute activation of TRPC6 channels in vascular myocytes played an important role in controlling vasoconstrictor-regulated vascular tone (42). Therefore, when considering the overall effect of ROS on TRPC6 channels in MCs, both chronic and acute effects should be taken into account.
Regulation of TRPC6 by ROS in podocytes
As described previously, TRPC6 channels play a critical role in maintaining podocyte structural and functional integrity, and dysfunction of the channels can cause podocyte injury, leading to advanced kidney diseases such as FSGS and diabetic nephropathy (45, 57, 88, 92, 136, 137, 139, 162, 163, 194, 219, 232, 234, 235). Accumulating evidence indicates that TRPC6 expression and function in podocytes are under control of ROS.
Wang et al. were the first group to report increased TRPC6 protein expression by ROS in podocytes (212). In the puromycin aminonucleoside (PAN) rat model of FSGS, they found significant podocyte injury, which was accompanied by increased Nox4 expression and activity, and TRPC6 protein expression in glomeruli from PAN nephrosis rats. Apocynin, an inhibitor of NADPH oxidases, ameliorated the podocyte injury and reduced the PAN-induced TRPC6 expression (212). These in vivo findings were nicely recapitulated in cultured podocytes (212). Therefore, NADPH oxidase-derived ROS contributed to PAN-induced upregulation of TRPC6 expression and podocyte injury.
Diabetic nephropathy is characterized with podocyte injury (45, 92, 139, 162, 194, 232, 234). Increased TRPC6 channel expression and activity have been associated with impaired podocyte structure and function (136, 137, 163, 219, 235). High glucose or hyperglycemia is the major trigger of development of diabetic kidney disease (81, 200). In cultured human podocytes, Thilo et al. recently found that high-glucose treatment significantly increased TRPC6 expression at both mRNA and protein levels. These responses were blocked by tempol, a mimetic of superoxide dismutase, and mimicked by application of oxidative stress (201). Therefore, high glucose increased abundance of TRPC6 protein through ROS in podocytes.
Recent studies showed that insulin signaling to podocytes is crucial for the normal function of the entire glomerulus (23, 217). In cultured mouse podocytes, Kim et al. found that insulin evoked a rapid increase in the cell surface expression of functional TRPC6 (106). This response was mimicked by treating podocytes with H2O2. Furthermore, insulin treatment increased H2O2 production by mobilizing Nox4 to the cell surface. In addition, the insulin-stimulated surface expression of TRPC6 was reduced by pretreatment with diphenylene iodonium, an inhibitor of NADPH oxidases, by siRNA knockdown of Nox4, and by several antioxidants (106). This study suggests that insulin increases the surface expression of TRPC6 in podocytes through Nox4-derived ROS.
However, the conclusion from the in vitro studies may need to be verified in intact animals. In addition, the pathological relevance of the insulin-promoted cell surface translocation of TRPC6 in podocytes may need to be further elucidated. For instance, how this pathway contributes to podocyte injury in diabetic nephropathy, which is usually associated with insulin deficiency or impairment of insulin signaling. Nevertheless, this study at least revealed that ROS stimulate membrane trafficking of TRPC6 in podocytes, which is consistent with our findings in HEK293 cells (70) and vascular smooth muscle cells (42).
In addition to growth factor signaling (such as insulin signaling), G protein-coupled receptor signaling may also regulate TRPC6 expression and function through generation of ROS in podocytes. Studies demonstrated that Ang II and ATP activated TRPC6 channels through Gq-coupled AT1 and P2Y receptors, respectively, in cultured mouse podocyte cell lines and in primary rat podocytes in ex vivo glomeruli (9, 169). Both Ang II and ATP effects on TRPC6 relied on generation of ROS because pretreating podocytes with ROS quencher or NADPH oxidase inhibitors eliminated or reduced the Ang II and ATP activation of TRPC6 (9, 169). Since Ang II plays a major role in the progression of chronic kidney disease (22, 103, 133, 147, 220) and ATP is an important mediator of tubuloglomerular feedback (5, 24, 28, 49, 192), the findings that ROS mediated Ang II- and ATP-induced TRPC6 activation in podocytes may have important physiological and pathological relevance.
It has been widely accepted that NADPH oxidases are the major source of ROS in the kidney (58, 64–68, 177). Among seven isoforms of NADPH oxidases, Nox4 and Nox2 have been found in podocytes and both of them regulated TRPC6 channel function through ROS (45, 106, 107, 212). In the study described previously, TRPC6 protein expression was upregulated in PAN-treated podocytes. This response was significantly attenuated by knockdown of Nox4 (212). Similarly, knockdown of Nox4 significantly reduced insulin-stimulated cell surface expression of TRPC6 in podocytes (106). However, Nox4 might not be the only NADPH oxidase to regulate TRPC6 in podocytes. In a recent study, Nox2 was found to physically interact with TRPC6 channels in podocytes, and the complexes contributed to TRPC6 activation by diacylglycerol, a product generated in G protein-coupled and receptor tyrosine kinase receptor signaling cascades (107).
Regulation of TRPC6 by ROS in tubular epithelial cells
As described previously, TRPC6 is highly expressed in principal cells of the collecting duct, located at both the apical (colocalized with aquaporin 2) and basolateral (colocalized with Na+-K+-ATPase) membranes (61). This existence suggests that TRPC6 may play a role in fine-tuning water and Na+ reabsorption in the collecting duct.
Using an ischemia–reperfusion rat model of acute kidney injury, Shen et al. found that the impaired water and Na+ excretion was associated with a reduced expression of TRPC6 protein in the collecting ducts (181). Pretreatment of the ischemia–reperfusion rats with recombinant human erythropoietin restored TRPC6 protein expression in the collecting ducts, which was accompanied with attenuation of acute renal tubular injury (181). Because ischemia–reperfusion injury is directly related to the formation of ROS (4, 53, 224), the decreased protein abundance of TRPC6 might be due to oxidative stress generated during the procedure. It has been well known that antioxidative stress is one of the major mechanisms for the cytoprotective effects of erythropoietin (36, 118, 229). An earlier study from our laboratory also showed that ROS reduced TRPC6 protein expression in MCs (72).
TRPC6 is also expressed in MDCK cells, a cell line of the intercalated cells of the renal cortical collecting duct (12). Treatment of MDCK cells with thymol, a natural essential oil present in many plants, elevated the intracellular ROS level and stimulated Ca2+ entry (31). The thymol-evoked Ca2+ influx was significantly inhibited by SK&F96365. Although the authors argued that the Ca2+ entry was mediated by store-operated Ca2+ channels, the compound of SK&F96365 in fact is a nonselective inhibitor of TRPC channels. Therefore, it is most likely that the thymol-induced Ca2+ entry was through one or more isoforms of TRPC channels. MDCK cells express redox-sensitive TRPC6 channels (12). Therefore, it is possible that TRPC6 channels were involved in the thymol-stimulated Ca2+ response.
It should be noted that MDCK cells also express TRPC1 and TRPC3 (12) and both may also be redox sensitive (56, 156, 223). Thus, it is uncertain whether TRPC6 was the only channel contributing to the thymol-induced increase in intracellular Ca2+.
In summary, ROS, primarily derived from NADPH oxidases, regulate TRPC6 signaling through multiple mechanisms in kidney cells. These mechanisms include alteration of TRPC6 protein abundance, stimulation of cell surface trafficking of cytosolic TRPC6 channel protein, and direct activation of the channel. The influence of ROS on TRPC6 channel function and the underlying mechanisms may be cell context dependent (Fig. 5).
FIG. 5.
Regulation of TRPC6 protein expression or channel activity by ROS in kidney cells.
Concluding Remarks
It is evident that the TRPC6-mediated signaling pathway in kidney cells is under control of ROS under both physiological and pathological conditions. However, the mechanism by which ROS regulate TRPC6 Ca2+ signals is poorly understood. ROS may directly or indirectly affect TRPC6 at any site of transcription, post-transcription, translation, and post-translation. If an indirect mechanism is involved, further study is needed to identify the intermediators, which may help search for a more specific target for treating ROS- and/or TRPC6-associated kidney diseases.
In terms of redox modulation of TRP channels, studies have focused on oxidative modifications of the redox-sensitive cysteine residues in proteins (125, 227). However, it is important to note that the oxidation of polyunsaturated fatty acids may also be a mechanism. This is particularly important for TRPC6 because some lipid messengers such as phosphoinositides and dicylglycerol are important regulators of TRPC6 channels (6, 7, 113, 116).
It should be noted that ROS have distinct effects on TRPC6 protein expression in different types of kidney cells. For instance, ROS decreased abundance of TRPC6 protein in MCs (72, 122), but increased TRPC6 protein expression in podocytes (212, 201, 106). Although the mechanism for the cell-type-specific effects of ROS is not known, one possibility is that ROS regulation of TRPC6 is cell context dependent. In MCs, the ROS/PKC or Akt/NF-κB pathway, which downregulates TRPC6 protein expression (211, 193), is predominant. However, this inhibitory pathway may not be very active in podocytes, instead the Ang II signaling pathway, which upregulates TRPC6 expression (9, 169, 231, 142), may play a major role.
In addition to TRPC6, several other isoforms of TRPC channels are also redox sensitive. These include TRPC1, TRPC3, TRPC4, and TRPC5 (56, 156, 223, 227). Although all those studies were carried out in nonrenal cells or the HEK293 cell line, the fact that those TRPC channels are also expressed in kidney cells and are involved in physiological processes in the kidney (12, 43, 59–62, 117, 141, 187, 202, 209, 210, 230) provides a possibility of those TRPC isoforms contributing to ROS-induced changes in renal structure and function.
Recent studies showed that reactive nitrogen species also regulated the function of TRPC channels (111, 203, 227). Like ROS, reactive nitrogen species such as nitric oxide may regulate TRPC signaling through site-specific modifications of amino acid residues such as the nitrosation or nitration of cysteine and tyrosine residues. Whether TRPC6 channel in kidney cells can be modulated by this type of redox is not known and needs to be further studied.
In this review, we focused on studies in which TRPC6 protein was a downstream target of ROS. However, emerging evidence suggests that there is a cross-talk between intracellular Ca2+ signals and ROS (21, 90, 203). Particularly, Duox 1 and 2 contain a Ca2+ binding domain (EF-hand) (8, 73, 170). It would be interesting to determine if this Ca2+-ROS interplay also exists between TRPC6 channel and ROS in kidney cells and, importantly, how it affects renal function.
Considering the ubiquitous nature of TRPC6 channels and ROS production in the kidney, it is conceivable that TRPC6 channel redox sensitivity may participate in multiple physiological and pathological processes. Hence, understanding the molecular mechanisms by which ROS alter TRPC6 channel activity and expression level will help develop specific drugs to treat TRPC6- and/or ROS-associated kidney diseases.
Abbreviations Used
- Ang II
angiotensin II
- Ank
ankyrin repeats
- CBD
caveolin-1 binding domain
- CC
coiled-coiled domain
- CD
collecting duct
- CIRB
calmodulin/IP3R binding domain
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- FSGS
focal and segmental glomerulosclerosis
- H2O2
hydrogen peroxide
- IC
intercalated cell
- IP3
inositol 1,4,5-trisphosphate
- IP3R
inositol 1,4,5-trisphosphate receptor
- JG
juxtaglomerular
- MC
mesangial cell
- MDCK
Madin–Darby canine kidney
- NCX
Na+-Ca2+ exchanger
- NFAT
nuclear factor of activated T cells
- PAN
puromycin aminonucleoside
- PC
principal cell
- PKC
protein kinase C
- PMCA
plasma membrane Ca2+ ATPase
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarcoplasmic and endoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- STZ
streptozotocin
- TRP
transient receptor potential
- TRPC
canonical transient receptor potential
References
- 1.Abboud HE. Mesangial cell biology. Exp Cell Res 318: 979–985, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Abramowitz J. and Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23: 297–328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Banerjee A, and Banerjee UC. Xanthine oxidoreductase: a journey from purine metabolism to cardiovascular excitation-contraction coupling. Criti Rev Biotech 31: 264–280, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Aksu U, Ergin B, Bezemer R, Kandil A, Milstein DMJ, Demirci-Tansel C, and Ince C. Scavenging reactive oxygen species using tempol in the acute phase of renal ischemia/reperfusion and its effects on kidney oxygenation and nitric oxide levels. Intensive Care Med Exp 3: 21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Mashhadi RH, Skøtt O, Vanhoutte PM, and Hansen PB. Activation of A2 adenosine receptors dilates cortical efferent arterioles in mouse. Kidney Int 75: 793–799, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Albert AP. and Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J Physiol 552: 789–795, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert AP, Saleh SN, and Large WA. Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. J Physiol 586: 3087–3095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, Al ghuzlan A, hartl D, Bidart JM, De Deken X, Miot F, Diallo I, de Vathaire F, Schlumberger M, and Dupuy C. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A 112: 5051–5056, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson M, Roshanravan H, Khine J, and Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol 229: 434–442, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Antunes F. and Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 474: 121–126, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Balzer M, Lintschinger B, and Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovas Res 42: 543–549, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay BC, Swaim WD, Liu X, Redman RS, Patterson RL, and Ambudkar IS. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. J Biol Chem 280: 12908–12916, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Bank N. Mechanisms of diabetic hyperfiltration. Kidney Int 40: 792–807, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Barnes JL. and Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79: 944–956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertolotti M, Bestetti S, García-Manteiga JM, Medraño-Fernandez I, Dal Mas A, Malosio ML, and Sitia R. Tyrosine kinase signal modulation: a matter of H2O2 membrane permeability? Antioxid Redox signal 19: 1447–1551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, and Jahn TP. Specfic aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Bienert GP, Schjoerring JK, and Jahn TP. Membrane transport of hydrogen proxide. Biochim Biophys Acta 1758: 994–1003, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Blaustein MP. and Lederer WJ. Sodium/Calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block K, Ricono JM, Lee DY, Bhandar B, Choudhury GG, Abboud HE, and Gorin Y. Arachidonic acid-dependent activation of a p22phox-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid Redox Signal 8: 1497–1508, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Bréchard S, Melchior C, Plancon S, Schenten V, and Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium 44: 492–506, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, and Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patient with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Brosius FC, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter E, Levi M, Mclndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, and Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown R, Olierstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, and Persson EG. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficinet mice. Am J Physiol Regul Integ Comp Physiol 281: R1362–R1367, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Bylund J, Brown KI, Movitz C, Dahlgren C, and Karlson A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and for? Free Rad Biol Med 49: 1834–1845, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Carafoli E. Calcium pump of the plasma membrane. Physiol Rev 71: 129–153, 1991 [DOI] [PubMed] [Google Scholar]
- 27.Carmines PK. and Fujiwara K. Altered electromechanical coupling in the renal microvasculature during the early stage of diabetes mellitus. Clin Exp Pharmacol Physiol 29: 143–148, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castrop H. Mediators of tubuloglomerular feedback regulation of glomerular filtration: ATP and adenosine. Acta Physiol 189: 3–14, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Cave AC, Brewer AC, Naryanapanicker A, Ray R, Grieve DJ, Walker S, and Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Chance B, Sies H, and Boveris A. Hydrogenperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 31.Chang HT, Chou CT, Liang WZ, Lu T, Kuo DH, Shieh P, Ho CM, and Jan CR. Effects of thymol on Ca2+ homeostasis and apoptosis in MDCK renal tubular cells. Chinese J Physiol 57: 90–98, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Chaudhuri P, Colles SM, Bhat M, Van Wagoner DR, Birnbaumer L, and Graham LM. Elucidation of a TRPC6-TRPC5 channel cascade that restricts endothelial cell movement. Mol Biol Cell 19: 3203–3211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Vita JA, Berk BC, and Keaney JF. c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves Src-dependent epidermal growth factor receptor transactivation. J Biol Chem 276: 16045–16050, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Chiluiza D, Krishna S, Schumacher VA, and Schlöndorff J. Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinase 1/2 (ERK1/2). J Biol Chem 288: 18407–18420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Silva AQ, Fontes MA, and Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Res 1368: 231–238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang J, Jia R, Tu Y, Xiao S, and Ding G. Erythropoietin prevents reactive oxygen species generation and renal tubular cell apoptosis at high glucose level. Biomed Pharmcother 64: 681–685, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Denu JM. and Dixon JE. Protein tyrosine phosphatases: Mechanisms of catalysis and regulation. Curr Opin Chem Biol 2: 633–641, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Dietrich A, Chubanov V, and Gudermann T. Renal TRPathies. J Am Soc Nephrol 21: 736–744, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Dietrich A, Chubanov V, Kalwa H, Rost BR, and Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112: 744–760, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Dietrich A, Kalwa H, Fuchs B, Grimminger F, Weissmann N, and Gudermann T. In vivo TRPC functions in the cardiopulmonary vasculature. Cell Calcium 42: 233–244, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Ding Y, Stidham RD, Bumeister R, Trevino I, Winters A, Sprouse M, Ding M, Ferguson DA, Meyer CJ, Wigley WC, and Ma R. The synthetic triterpenoid, RTA405, increases the glomerular filtration rate and reduces angiotensin II-induced contraction of glomerular mesangial cells. Kidney Int 83: 845–854, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wnag Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, and Ma R. Reactive oxygen species-mediated TRPC6 activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 286: 31799–31809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du J, Sours-Brothers S, Coleman R, Ding M, Graham S, Kong D, and Ma R. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J Am Soc Nephrol 18: 1437–1445, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G, Sparks M, Zhang ZS, Homstad A, Barisoni L, Birnbaumer L, Rosenberg P, and Winn MP. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol 22: 526–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, and Abboud HE. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Etienne-Manneville S. and Hall A. Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Facemire CS, Mohler PJ, and Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol 286: F546–F551, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, and Yuan JXJ. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1233–L1245, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Faulhaber-Walter R, Chen L, Oppermann M, Kin SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, and Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19:722–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Favero TG, Zable AC, and Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem 270: 25557–25563, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Fellner S. and Arendshorst WJ. Angiotensin II-stimulated calcium entry mechanism in afferent arterioles: role of transient receptor potential canonical channels and reverse Na+/Ca2+ exchange. Am J Physiol Renal Physiol 294: F212–F219, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Fellner SK. and Arendshorst WJ. Capacitative calcium entry in smooth muscle cells from preglomerular vessels. Am J Physiol 277: F533–F542, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Ferrari RS. and Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev 2015: 590987, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fletcher EC, Bao G, and Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Freichel M. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol 3: 121–127, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Gervásio OL, Whitehead NP, Yeung EW, Phillips WD, and Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase-role in Duchenne muscular dystrophy. J Cell Sci 121: 2246–2255, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Gigante M, Caridi G, Montemurno E, Soccio M, d'Apolito M, Cerullo G, Aucella F, Schirinzi A, Emma F, Massella L, Messina G, De Palo T, Ranieri E, Ghiggeri GM, and Gesualdo L. TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol 6: 1626–1634, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Gill PS. and Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Goel M. and Schilling WP. Role of TRPC3 channels in ATP-induced Ca2+ signaling in principal cells of the inner medullary collecting duct. Am J Physiol Renal Physiol 299: F225–F233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goel M, Sinkins WG, Zuo CD, Hopfer U, and Schilling WP. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol 293: F1476–F1488, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Goel M, Sinkins WG, Zuo CD, and Schilling WP. Identification and localization of TRPC channels in rat kidney. Am J Physiol Renal Physiol 290: F1241–F1252, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Goel M, Zuo CD, and Schilling WP. Role of cAMP/PKA signaling cascade in vasopressin-induced trafficking of TRPC3 channels in principal cells of the collecting duct. Am J Physiol Renal Physiol 298: F988–F996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Cobos J. and Trebak M. TRPC channels in smooth muscle cells. Front Biosci 15: 1023–1039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorin Y. Nox4 as a potential therapeutic target for treatment of uremic toxicity associated to chronic kidney disease. Kidney Int 83: 541–543, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorin Y. and Block K. Nox as a target for diabetic complications. Clin Sci 125: 361–382, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, and Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, and Abboud HE. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol 308: F1276–F1287, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorin Y, Ricono JM, Kim NH, Bhandar B, Choudhury GG, and Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, and Choudhury GG. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J 381: 231–239, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, and Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 285: 23466–23476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graham S, Ding M, Sours-Brothers S, Yorio T, Ma JX, and Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells. Am J Physiol Renal Physiol 293: F1381–F1390, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, Ding Y, and Ma R. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol 301: C304–C315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grasberger H. and Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281: 18269–18272, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Greka A. and Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol 22: 1969–1980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greka A. and Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greka A, Navarro B, Oancea E, Duggan A, and Clapham DE. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6: 837–845, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Griendling KK, Sorescu D, and Ushio-Fukai M. NAD(P)H oxidase, role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 78.Grover AK, Samson SE, and Fomin VP. Peroxide inactivates calcium pumps in pig coronary artery. Am J Physiol 263: H537–H543, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Gudermann T, Schnitzler M, and Dietrich A. Receptor-operated cation entry-more than esoteric terminology? Sci STKE 2004: e35, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Ha H, Hwang I, Park JH, and Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract 82S: S42–S45, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Ha H, Yu M. and Kim KH. Melatonin and taurine reduce early glomerulopathy in diabetic rats. Free Radic Biol Med 26: 944–950, 1999 [DOI] [PubMed] [Google Scholar]
- 82.Ha H, Yu MR, Choi YJ, Kitamura M, and Lee HB. Role of high glucose-induced nuclear factor-κB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol 13: 894–902, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Ha H, Yu MR, Choi YJ, and Lee HB. Activation of protein kinase C-δ and -ɛ by oxidative stress in early diabetic kidney. Am J Kidney Dis 38 (Suppl. 4): S204–S207, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Haneda M, Araki S, Togawa M, Sugimoto T, Isono M, and Kikkawa R. Mitogen-activated protein kinase cascade is activated in glomeruli of diabetic rats and glomerular mesangial cells cultured under high glucose conditions. Diabetes 46: 847–853, 1997 [DOI] [PubMed] [Google Scholar]
- 85.Hardie RC. Regulation of TRP channels via lipid second messengers. Annu Rev Physiol 65: 735–759, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Harrison R. Physiological roles of xanthine oxidoreductase. Drug Met Rev 36: 363–375, 2004 [DOI] [PubMed] [Google Scholar]
- 87.He F, Peng F, Xia X, Zhao C, Luo Q, Guan W, Li Z, Yu X, and Huang F. NiR-135a promotes renal fibrosis in diabetic nephropathy by regulating TRPC1. Diabetologia 57: 1726–1736, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, and Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4: e7771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henzler T. and Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculation and measurements with the pressure probe suggest transport of H2O2 across water channels. J Exp Bot 51: 2053–2066, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Hidalgo C. and Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 1275–1312, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, and Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999 [DOI] [PubMed] [Google Scholar]
- 92.Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, Nomoto K, and Nagata M. Podocyte injury promotes progressive nephropathy in Zuker diabetic fatty rats. Lab Invest 82: 25–35, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Ilatovskaya DV, Levchenko V, Ryan RP, and Cowley Jr. AW. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem Biophys Res Commun 408:242–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, and Staruschenko A. Acute effect of angiotensin II on TRPC6 channels in the podocytes of freshly isolated glomeruli. Kidney Int 86: 506–514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salmonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, and Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/ω-hydroxylase/20-HETE pathways. Circ Res 104: 1399–1409, 2009 [DOI] [PubMed] [Google Scholar]
- 96.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, and Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 97.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, Thallas-Bonke V, Wingler K, Szyndralewiez C, Heitz F, Touyz RM, Cooper ME, Schmidt HHHW, and Jandeleit-Dahm KA. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol 25: 1237–1254, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ji Z, Huang C, Liang C, Chen B, Chen S, and Sun W. Protective effects of of blocking renin-angiotensin system on the progression of renal injury in glomeruloscleorosis. Cell Mol Immunol 2: 150–154, 2005 [PubMed] [Google Scholar]
- 99.Jiang L, Ding J, Tsai H, Li L, Feng Q, Miao J, and Fan Q. Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp Biol Med 236: 184–193, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Jiang Z, Seo JY, Ha H, Yu MR, Kwon MK, and Lee HB. Reactive oxygen species mediated TGF-β1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun 309: 961–966, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295: C849-C868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kakehi T. and Yabe-Nishimura C. NOX enzymes and diabetic complications. Semin Immunopathol 30: 301–314, 2008 [DOI] [PubMed] [Google Scholar]
- 103.kennefick TM. and Anderson S. Role of angiotensin II in diabetic nephropathy. Semin Nephrol 17: 441–447, 1997 [PubMed] [Google Scholar]
- 104.Kikkawa R, Kitamura E, Fujiwara Y, Arimura T, Haneda M, and Shigeta Y. Impaired contractile responsiveness of diabetic glomeruli to angiotensin II: a possible indication of mesangial dysfunction in diabetes mellitus. Biochem Biophys Res Commun 136: 1185–1190, 1986 [DOI] [PubMed] [Google Scholar]
- 105.Kim EY, Alvarez-Baron CP, and Dryer SE. TRPC3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes. Mol Pharmacol 75: 466–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim EY, Anderson M, and Dryer S. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol 302: F298–F307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim EY, Anderson M, Wilson C, Hagmann H, Benzing T, and Dryer SE. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex. Am J Physiol Cell Physiol 305: C960–C971, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Kini V, Chavez A, and Mehta D. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability and angiogenesis. J Biol Chem 285:33082–33091, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kistler AD, Singh G, Pippin J, Altintas MM, Yu H, Fernandez IC, Gu C, Wilson C, Srivasatava SK, Dietrich A, Walz K, Kerjaschki D, Ruiz P, Dryer S, Sever S, Dinda AK, Faul C, Shankland SJ, and Reiser J. Transient receptor potential channel 6 (TRPC6) protects podocytes during complement-mediated glomerular disease. J Biol Chem 288: 36598–36609, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong F, Ma L, Zou L, Meng K, Ji T, Zhang L, Zhang R, and Jiao J. Alpha1-adrenergic receptor activation stimulates calcium entry and proliferation via TRPC6 channels in cultured human mesangial cells. Cell Physiol Biochem 36: 1928–1938, 2015 [DOI] [PubMed] [Google Scholar]
- 111.Kozai D, Ogawa N, and Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal 21: 971–986, 2014 [DOI] [PubMed] [Google Scholar]
- 112.Kwak JO, Kwak J, Kim HW, Oh KJ, Kim YT, Jung SM, and Cha SH. The extracellular calcium sensing receptor is expressed in mouse mesanigal cells and modulates cell proliferation. Exp Mol Med 37: 457–465, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Kwon Y, Hofmann T, and Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell 25: 491–503, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lassègue B. and Griendling KK. Reactive oxygen species in hypertension. Am J Hypert 17: 852–860, 2004 [DOI] [PubMed] [Google Scholar]
- 115.lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, and Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 upregulation in mesangial cells and in diabetic kidney. Kidney Int 67: 1762–1771, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Lemonnier L, Trebak M, and Putney JW. Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium 43: 506–514, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Letaveriner E, Rodenas A, Guerrot D, and Haymann JP. Williams-Beuren syndrome hypercalcemia: is TRPC3 a novel mediator in calcium homeostasis? Pediatrics 129: e1626–e1630, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Li G, Ma R, Huang C, Tang Q, Fu Q, Liu H, Hu B, and Xiang J. Protective effect of erythropoietin on β-amyloid-induced PC12 cell death through antioxidant mechanisms. Neuroscien Lett 442: 143–147, 2008 [DOI] [PubMed] [Google Scholar]
- 119.Liao C, Yang H, Zhang R, Sun H, Zhao B, Gao C, Zhu F, and Jiao J. The upregulatipn of TRPC6 contributes to Ca2+ signaling and actin assembly in human mesangial cells after chronic hypoxia. Biochem Biophys Res Commun 421: 750–756, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Lin MJ, Leung GPH, Zhang WM, Yang XR, Yip KP, Tse CM, and Sham JSK. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, and Meng TC. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS J 275: 69–88, 2008 [DOI] [PubMed] [Google Scholar]
- 122.Luan JJ, Li W, Han J, Zhang W, Gong W, and Ma R. Renal protection of in vivo administration of tempol in streptozotocin-induced diabetic rats. J Pharmacol Sci 119: 167–176, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma R, Pluznick JL, and Sansom SC. Ion channels in mesangial cells: function, malfunction, or fiction. Physiology 20: 102–111, 2005 [DOI] [PubMed] [Google Scholar]
- 124.MacFarlane PM, Wilkerson JE, Lovett-Barr MR, and Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164: 263–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, and Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Madamanchi NR. and Runge MS. NADPH oxidases and atherosclerosis: unraveling the details. Am J Physiol Heart Circ Physiol 298: H1–H2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 342: 325–339, 2010 [DOI] [PubMed] [Google Scholar]
- 128.Marcus NJ, Li YL, Bird CE, Schultz HD, and Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171: 36–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marcus NJ, Philippi NR, Bird CE, Li YL, Schultz HD, and Morgan BJ. Effect of AT(1) receptor blockade on intermittent hypoxia-induced endothelial dysfunction. Respir Physiol Neurobiol 183: 67–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Menè P, Simonson MS, and Dunn MJ. Physiology of mesangial cell. Physiol Rev 69: 1347–1424, 1989 [DOI] [PubMed] [Google Scholar]
- 131.Meng K, Xu J, Zhang C, Zhang R, Yang H, Liao C, and Jiao J. Calcium sensing receptor modulates extracellular calcium entry and proliferation via TRPC3/6 channels in cultured human mesangial cells. PLoS One 9: e98777, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miller EW, Dickinson BC, and Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A 107: 15681–15686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mima A, Matsubara T, Arai H, Abe H, Nagai K, Kanamori H, Sumi E, Takahashi T, Iehara N, Fukatsu A, Kita T, and Doi T. Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab Invest 86: 927–939, 2006 [DOI] [PubMed] [Google Scholar]
- 134.Misquitta CM, Mack DP, and Grover AK. Sarco/endopasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium 25: 277–290, 1999 [DOI] [PubMed] [Google Scholar]
- 135.Misra RP. Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol 58: 135–139, 1972 [DOI] [PubMed] [Google Scholar]
- 136.Möller CC, Flesche J, and Reiser J. Sensitizing the slit diaphragm with TRPC6 ion channels. J Am Soc Nephrol 20: 950–953, 2009 [DOI] [PubMed] [Google Scholar]
- 137.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, and Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Montell C. TRP channels in Drosophila photoreceptor cells. J Physiol 567: 45–51, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nadarajah R, Milagres R, Dilauro M, Gutso A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batle D, and Burns K. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.New DD, Block K, Bhandhari B, Gorin Y, and Abboud HE. IGF-1 increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol 302: C122–C130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Niehof M. and Borlak J. Hepatic nuclear factor 4 alpha and the Ca-cahnnel TRPC1 are novel disease candidate genes in diabetic nephropathy. Diabetes 57: 1069–1077, 2008 [DOI] [PubMed] [Google Scholar]
- 142.Nijenhuis T, Sloan AJ, Hoenderop JGJ, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJM, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JFM, Berden JHM, Reiser J, Faul C, and van der Vlag J. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan THM, Mitchell PO, Sutliff RL, and Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol 40: 601–609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nutt LK. and O'Neil RG. Effect of elevated glucose on endothelin-induced store-operated and non-store-operated calcium influx in renal mesangial cells. J Am Soc Nephrol 11: 1225–1235, 2000 [DOI] [PubMed] [Google Scholar]
- 145.Obeidat M, Obeidat M, and Ballermann BJ. Glomerular endothelium: a porous sieve and formidable barrier. Exp Cell Res 318: 964–972, 2012 [DOI] [PubMed] [Google Scholar]
- 146.Oberleithner H, Steigner W, Silbernagl S, Vogel U, Gstraunthaler G, and Pfaller W. Madin-Darby canine kidney cells. Pflügers Arch 416: 540–547, 1990 [DOI] [PubMed] [Google Scholar]
- 147.Onozato ML, Tojo A, Goto A, Fujita T, and Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy:effects of ACEI or ARB. Kidney Int 61: 186–194, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Ortiz PA. and Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 149.Ortiz-Capisano MC, Atchison DK, Harding P, Lasley RD, and Beierwaltes WH. Adenosine inhibits renin release from juxtaglomerular cells via an A1 receptor-TRPC-mediated pathway. Am J Physiol Renal Physiol 305: F1209–F1219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parekh AB. and Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997 [DOI] [PubMed] [Google Scholar]
- 151.Parekh AB. and Putney JW. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 152.Pavenstadt H, Kriz W, and Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 153.Pedersen SF, Owsianik G, and Nilius B. TRP channels: an overview. Cell Calcium 38: 233–252, 2005 [DOI] [PubMed] [Google Scholar]
- 154.Peng G, Lu W, Li X, Chen Y, Zhong N, Ran P, and Wang J. Expression of store-operated Ca2+ entry and transient receptor potential canonical and vanilloid-related proteins in rat distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol Physiol 299: L621–L630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, and Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, and Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel-evidence for expression of native TRPC3/TRPC4 heteromeric channels in endothelial cells. J Biol Chem 281: 13588–13595, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Prabhakar NR, Kumar GK, Nanduri J, and Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal 9: 1397–1403, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Qiu G. and Ji Z. Ang II-induced glomerular mesangial cell proliferation inhibited by losartan via changes in intracellular calcium ion concentration. Clin Exp Med 14: 169–176, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rakusan K, Chvojkova Z, Oliviero P, Ostadalova I, Kolar F, Chassagne C, Samuel JL, and Ostadal B. Ang II type 1 receptor antagonist irbesartan inhibits coronary angiogenesis stimulated by chronic intermittent hypoxia in neonatal rats. Am J Physiol Heart Circ Physiol 292: H1237–H1244, 2007 [DOI] [PubMed] [Google Scholar]
- 160.Rampino T, Gregorini M, Guidetti C, Broggini M, Marchini S, Bonomi R, Maggio M, Roscini E, Soccio G, Tiboldo R, and Dal Canton A. KCNA1 and TRPC6 ion channels and NHE1 exchanger operate the biological outcome of HGF/scatter factor in renal tubular cells. Growth Fact 25: 382–391, 2007 [DOI] [PubMed] [Google Scholar]
- 161.Ramsey IS, Delling M, and Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 18.1–18.29, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Reddy GR, Kotlyarevska K, Ransom RF, and Menon RK. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens 17: 32–36, 2008 [DOI] [PubMed] [Google Scholar]
- 163.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, and Pollak MR. TRPC6 is a glomerular slit diaphram-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rhee EP. NADPH oxidase 4 at the nexus of diabetes, reactive oxygen species, and renal metabolism. J Am Soc Nephrol 27: 337–339, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rhee SG, Chang TS, Bae YS, Lee SR, and Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 14: S211–S215, 2003 [DOI] [PubMed] [Google Scholar]
- 166.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, and Lee HB. Role of reactive oxygen species in TGF-beta 1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 16: 667–675, 2006 [DOI] [PubMed] [Google Scholar]
- 167.Roberts CK. and Sindhu KK. Oxidative stress and metabolic syndrome. Life Scien 84: 705–712, 2009 [DOI] [PubMed] [Google Scholar]
- 168.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, and Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diab Metab Res Rev 17: 189–212, 2001 [DOI] [PubMed] [Google Scholar]
- 169.Roshanravan H. and Dryer SE. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G, Lu J, Antony S, and Doroshow JH. NADPH oxidases and cancer. Clin Sci (Lond) 128: 863–875, 2015 [DOI] [PubMed] [Google Scholar]
- 171.Ruiz-Ortega M, Rupérez M, Esteban V, Rodriguez-Vita J, Sánchez-López E, Carvajal G, and Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney disease. Nephrol Dial Transplant 21: 16–20, 2006 [DOI] [PubMed] [Google Scholar]
- 172.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, and Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 173.Saliba Y, Karam R, Smayra V, Aftimos G, Abramowitz J, Birnbaumer L, and Farès N. Evidence of a role for fibroblast transient receptor potential canonical 3 Ca2+ channel in renal fibrosis. J Am Soc Nephrol 26: 1855–1876, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Santin S, Ars E, Rossetti S, Salido E, Silva I, Garcia-Maset R, Gimenez I, Ruiz P, Mendizabal S, Nieto J, Pena A, Camacho JA, Fraga G, Cobo MA, Bernis C, Ortiz A, de Pablos AL, Sanchez-Moreno A, Pintos G, Mirapeix E, Fernandez-Llama P, Ballarin J, and Torra R. TRPC6 mutational analysis in a large cohort of patients with focal segmental glomerulosclerosis. Nephrol Dial Transplant 24: 3089–3096, 2009 [DOI] [PubMed] [Google Scholar]
- 175.Schlöndorff J, del Camino D, Carrasquillo R, Lacey V, and Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296: C558–C569, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schnackenberg CG. Oxygen radicals in cardiovascular-renal disease. Curr Opin Pharmacol 2: 121–125, 2002 [DOI] [PubMed] [Google Scholar]
- 177.Schreck C. and O'Connor PM. NAD(P)H oxidase and renal epithelial ion transport. Am J Physiol Regul Integr Comp Physiol 300: R1023–R1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CRJ, Burns KD, Touyz RM, and Hébert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney-implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 179.Sedeek M, Nasrallah R, Touyz RM, and Hebert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 24: 1512–1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sedeek MH, Llinas MT, Drummond H, Fortepian L, Abram SR, Alexander BT, Reckelhoff JF, and Granger JP. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension 42: 806–810, 2003 [DOI] [PubMed] [Google Scholar]
- 181.Shen S, Jin Y, Li W, Liu X, Zhang T, Xia W, Wang Y, and Ma K. Recombinant human erythropoietin pretreatment attenuates acute renal tubular injury against ischemia-reperfusion by restoring transient receptor potential channel-6 expression and function in collecting ducts. Crit Care Med 42: e664–e672, 2014 [DOI] [PubMed] [Google Scholar]
- 182.Sherr CJ. and Roberts JM. CDK inhibitors:positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512, 1999 [DOI] [PubMed] [Google Scholar]
- 183.Sies H. Role of metabolic H2O2 generation: Redox signalling and oxidantive stress. J Biol Chem 289: 8735–8741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, and Mehta D. Gαq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem 282: 7833–7843, 2007 [DOI] [PubMed] [Google Scholar]
- 185.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, and Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 186.Soulsby M. and Bennett AM. Physiological signaling specificity by protein tyrosine phosphatases. Physiology 24: 281–289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sours S, Du J, Chu S, Zhou JX, Ding M, and Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am J Physiol Renal Physiol 290: F1507–F1515, 2006 [DOI] [PubMed] [Google Scholar]
- 188.Spassova MA, Hewavitharana T, Xu W, Soboloff J, and Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A 103: 16586–16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stockand JD. and Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev 78: 723–744, 1998 [DOI] [PubMed] [Google Scholar]
- 190.Striker GE. and Striker LJ. Biology of disease: Glomerular Cell Culture. Lab Invest 53: 122–131, 1985 [PubMed] [Google Scholar]
- 191.Studer RK, Craven PA, and DeRubertis FR. Antioxidant inhibition of protein kinase C-signaled incraeses in transforming growth factor-beta in mesangial cells. Metabolism 46: 918–925, 1997 [DOI] [PubMed] [Google Scholar]
- 192.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, and Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci U S A 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sun L, Li W, Li W, Xiong L, Li G, and Ma R. Astragaloside IV prevents damage to human mesangial cells through the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under high glucose conditions. Int J Mol Med 34: 167–176, 2014 [DOI] [PubMed] [Google Scholar]
- 194.Susztak K, Raff AC, Schiffer M, and Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 195.Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E, and Tamaki T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells. J Biol Chem 277: 9614–9621, 2002 [DOI] [PubMed] [Google Scholar]
- 196.Tabet F, Savoia C, Schiffrin EL, and Touyz RM. Differential calcium regulation by hyderogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol 44: 200–208, 2004 [DOI] [PubMed] [Google Scholar]
- 197.Tabet F, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, and Touyz RM. Redox-sensitive signaling by Angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res 103: 149–158, 2008 [DOI] [PubMed] [Google Scholar]
- 198.Takenaka T, Suzuki H, Okada H, Inoue T, Kanno Y, Ozawa Y, Hayashi K, and Saruta T. Transient receptor potential channels in rat renal microcirculation: actions of angiotensin II. Kidney Int 62: 558–565, 2002 [DOI] [PubMed] [Google Scholar]
- 199.Thallas-Bonke V, Jha JC, Gray SP, Barit D, Haller H, Schmidt HHHW, Coughlan MT, Cooper ME, Forbes JM, and Jandeleit-Dahm KAM. Nox-4 deletion reduces oxidative stress and injury by PKC-α-associated mechanisms in diabetic nephropathy. Physiol Rep 2: e12192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of daibetes on the development and progression of long-term complications in insulin-dependent daibetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 201.Thilo F, Lee M, Xia S, Zakrzewicz A, and Tepel M. High glucose modifies transient receptor potential canonical type 6 channels via increased oxidative stress and syndecan-4 in human podocytes. Biochem Biophys Res Commun 450: 312–317, 2014 [DOI] [PubMed] [Google Scholar]
- 202.Tian D, Jacobo SMP, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Hsu HH, Schlondorff J, Ramos A, and Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Trebak M, Ginnan R, Singer HA, and Jourd'heuil D. Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid Redox Signal 12: 657–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tryggvason K. and Wartiovaara J. How does the kidney filter plasma? Physiology 20: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 205.Vazquez G, Wedel BJ, Aziz O, Trebak M, and Putney JW. The mammalian TRPC cation channels. Biochim Biophys Acta 1742: 21–36, 2004 [DOI] [PubMed] [Google Scholar]
- 206.Vink EE, de Jager RL, and Blankestijin PJ. Sympathetic hyperactivity in chronic kidney disease: pathophysiology and (new) treatment options. Curr Hypertens Rep 15: 95–101, 2013 [DOI] [PubMed] [Google Scholar]
- 207.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, and Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 208.Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, Winn MP, and Spurney RF. Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest 125: 1913–1926, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang X, Pluznick JL, Settles DC, and Sansom SC. Association of VASP with TRPC4 in PKG-mediated inhibition of the store-operated calcium response in mesangial cells. Am J Physiol Renal Physiol 293: F1768–F1776, 2007 [DOI] [PubMed] [Google Scholar]
- 210.Wang X, Pluznick JL, Wei P, Padanilam BJ, and Sansom SC. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol 287: C357–C364, 2004 [DOI] [PubMed] [Google Scholar]
- 211.Wang Y, Ding M, Chaudhari S, Ding Y, Yuan J, Stankowska D, He S, Krishnamorthy R, Cunningham JT, and Ma R. Nuclear factor κB mediates suppression of canonical transient receptor potential 6 expression by reactive oxygen species and protein kinase C in kidney cells. J Biol Chem 288: 12852–12865, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang Z, Wei X, Zhang Y, Ma X, Li B, Zhang S, Du P, Zhang X, and Yi F. NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol Biochem 24: 619–626, 2009 [DOI] [PubMed] [Google Scholar]
- 213.Watanabe H, Murakami M, Ohba T, Takahashi Y, and Ito H. TRP channel and cardiovascular disease. Pharmacol Ther 118: 337–351, 2008 [DOI] [PubMed] [Google Scholar]
- 214.Wei P, Lane PH, Lane JT, Padanilam BJ, and Sansom SC. Glomerular structural and functional changes in a high-fat diet mouse model of early-stage type 2 diabetes. Diabetologia 47: 1541–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 215.Weibrecht I, Böhmer S, Dagnell M, Kappert K, Östman A, and Böhmer F. Oxidation sensitivity of the catalytic cystein of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Rad Biol Med 43: 100–110, 2007 [DOI] [PubMed] [Google Scholar]
- 216.Welsh DG, Morielli AD, Nelson MT, and Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Cir Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 217.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré Jm, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, and Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 12: 329–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Whiteside CI, Hurst RD, and Stevanovic ZS. Calcium signaling and contractile response of diabetic glomerular mesangial cells. Kidney Int 48: S28–S33, 1995 [PubMed] [Google Scholar]
- 219.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, and Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 220.Wolf G. and Ziyadeh FN. The role of angiotensin II in diabetic nephropathy: emphasis on nonhemodynamic mechanisms. Am J Kidney Dis 29: 153–163, 1997 [DOI] [PubMed] [Google Scholar]
- 221.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296: H539–H549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, and Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98: 1381–1389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, and Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature 451: 69–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yamaguchi M, Okamoto K, Kusano T, Matsuda Y, Suzuki G, Fuse A, and Yokota H. The effects of xanthine oxidoreductase inhibitors on oxidative stress markers following global brain ischemia reperfusion injury in C57BL/6 mice. PLoS One 10: e0133980, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yan LJ. Protein redox modification as a cellular defense mechanism against tissue ischemic injury. Oxid Med Cell Longev 2014: 343154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, Michael V, Yew DT-W, and Yao X. Expression of TRPC homologues in endothelial cells and smooth muscle layers of human arteries. Histochem Cell Biol 122: 553–561, 2004 [DOI] [PubMed] [Google Scholar]
- 227.Yoshida T, Inoue R, Mori T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, and Mori Y. Nitric oxide activates TRP channels by cystein S-nitrosylation. Nat Chem Biol 2: 596–607, 2006 [DOI] [PubMed] [Google Scholar]
- 228.You YH, Quach T, Saito R, Pham J, and Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol 27: 466–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yu T, Li L, Bi Y, Liu Z, Liu H, and Li Z. Erythropoietin attenuates oxidative stress and apoptosis in Schwann cells isolated from streptozotocin-induced diabetic rats. J Pharm Pharmacol 66: 1150–1160, 2014 [DOI] [PubMed] [Google Scholar]
- 230.Zhang D, Freedman BI, Flekac M, Santos E, Hicks PJ, Bowden DW, Efendic S, Brismar K, and Gu HF. Evaluation of genetic association and expression reduction of TRPC1 in the development of diabetic nephropathy. Am J Nephrol 29: 244–251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang H, Ding J, Fan Q, and Liu S. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-κB translocation. Exp Biol Med 234: 1029–1036, 2009 [DOI] [PubMed] [Google Scholar]
- 232.Zhang H, Schin M, Saha J, Burke K, Holzman LB, Filipiak W, Saunders T, Heiling CW, and Brosius III FC. Podocyte specific overexpression of GLUT1 surprisingly reduces mesangial matrix expansion in diabetic nephropathy in mice. Am J Physiol Renal Physiol 299: F91–F98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang N. and Ji Z. Effects of caveolin-1 and P-ERK1/2 on Ang II-induced glomerular mesangial cell proliferation. Ren Fail 35: 971–977, 2013 [DOI] [PubMed] [Google Scholar]
- 234.Zheng S, Carlson EC, Yang L, Kralik PM, Huang Y, and Epstein PN. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol 19: 2077–2085, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhu B, Chen N, Wang ZH, Pan XX, Ren H, Zhang W, and Wang WM. Identification and functional analysis of a novel TRPC6 mutation associated with late onset familial focal segmental glomerulosclerosis in Chinese patients. Mutat Res 664: 84–90, 2009 [DOI] [PubMed] [Google Scholar]