Abstract
Introduction: Cellular cardiomyoplasty has rapidly risen to prominence in the clinic following a myocardial infarction; however, low engraftment of transplanted cells limits the therapeutic benefit to these procedures. Recently, lineage-specific stem cells differentiated into cardiomyocytes have gained much attention to assist in the repair of an injured heart tissue; however, questions regarding the ideal cell source remain. In the present study, we have identified a source that is easy to extract stem cells from and show that the cells present have a high plasticity toward the cardiomyogenic lineage. We focused on the recently discovered neural crest stem cells residing in the periodontal ligament that can be easily obtained through dental procedures.
Materials and Methods: Neural crest stem cells were obtained from human excised third molars and differentiated in culture using a protocol for directed differentiation into cardiomyocytes. Differentiation of cells was assessed through gene expression and immunostaining studies. Optical stimulation using pulsed infrared radiation (IR) (λ = 1863 nm) was delivered to cell aggregates to study their contractile ability.
Results: We show that neural crest stem cells can be differentiated to a cardiomyogenic lineage, which was verified through immunostaining and gene expression. We observed a significant increase in cardiomyocyte-specific markers, NK2 homeobox 5 (NKX2.5) and troponin T type 2 (TNNT2), with positive changes in tropomyosin I (TPM1), gap junction protein alpha 1/Cx43 (GJA1/Cx43), and myocyte enhancement factor 2C (MEF2C). Furthermore, we were able to elicit and maintain pulse-by-pulse contractile responses in the derived cells, including in cardiospheres, with pulsed IR delivered at various radiant energies. The contractility in responses to IR could be maintained at different frequencies (0.25–2 Hz) and up to 10-min durations. While these cells did not maintain their contractility following cessation of IR, these cells demonstrated responses to the optical stimuli that are consistent with previous reports. We also found no evidence for irreversible mitochondrial depolarization in these cells following the long duration of infrared stimulation, suggesting the robustness of these cells.
Conclusions: Overall, these results suggest the merit of neural crest-derived stem cells for cardiomyogenic applications and a potential cell source for repair that should contribute to efforts to translate cell-based strategies to the clinic.
Introduction
Myocardial infarction (MI) remains a leading cause of death worldwide.1 Following MI, there is a significant decrease in the number of functional cardiomyocytes, and extracellular matrix deposition by infiltrating fibroblasts leads to adverse myocardial remodeling.2 Current treatments post-MI aim to slow the decline in cardiac function caused by this deposition, but are unable to reverse the remodeling process.3 Within the last 20 years, however, cellular cardiomyoplasty has emerged as a potential therapy to help reverse myocardial damage that occurs as a result of an infarction.4 These therapies apply the perfusion of stem or progenitor cells following an MI in an effort to repopulate the myocardium with functional myocytes. Clinically, patients receiving perfusions of these cells showed modest improvement in cardiac functional parameters, such as increased ejection fraction and decreased end-systolic volume.5
Mesenchymal stem cells (MSCs) were the first cell source to be investigated for cellular cardiomyoplasty.4 MSCs rapidly progressed through small and large animal preclinical models, and transplantation of MSCs showed gains in cardiac function following an MI; however, these results have been largely attributed to paracrine mechanisms as transplanted cells show low survival, engraftment, and differentiation in the host myocardium.6 The plasticity of MSCs toward a cardiomyogenic lineage has been debated in the literature, with several groups reporting the ability of MSCs to transdifferentiate into cardiomyocyte-like cells that exhibit a phenotype of cardiomyocytes, but fail to contract as functional cardiomyocytes.7–9
It has been proposed that to increase the clinical efficacy of cellular cardiomyoplasty applications, transplantation of cardiomyogenic precommitted stem cells may be utilized in future studies.10 This has been clinically verified with the use of cardiopoietic cells in the C-Cure trial. In this trial, MSCs treated with a growth factor cocktail became primed to cardiomyocyte lineage specification and exhibited the cardiomyocyte transcription factors, NKX2.5, MEF2C, and GATA4. These lineage-specific stem cells were found to be safe and effective and are now in later stage clinical trials.11
Ideally, an easily isolated cell source with high plasticity toward cardiomyogenic lineages would be used for cardiomyoplasty application. One such potential source of cells, which has been proposed, is stem cells derived from dental tissue.12 Dental tissue is known to harbor a population of stem cells, which are reportedly mainly mesenchymal in nature.13 Additionally, in vitro differentiation to cardiomyogenic lineages14 and perfusion studies on small animal models following MI using stem cells derived from dental tissue have been carried out.15 One tissue in which potential stem cells may be found is from periodontal ligament (PDL). For some time, the PDL has been known to have a population of resident stem cells, and the cardiomyogenic potential of these stem cells has been established.16
Recently, a stem cell subpopulation consisting of neural crest stem cells (NCSCs) residing in the PDL of excised impacted wisdom teeth has been identified. These cells are selected based on expression of migrating neural crest marker, connexin 43 (Cx43). These cells express the pluripotency markers, Oct4, Sox2, and Nanog, and will form teratomas in vivo.16,17 During development, cells from the neural crest undergo an epithelial-to-mesenchymal transformation and migrate to various postnatal tissues.18 These cells migrate as sheets and streams functionally coupled through gap junctions such as N-Cadherin and Cx43.19,20 The myocardium receives a postnatal contribution from the cardiac neural crest (CNC), which gives rise to the cardiac outflow tract, the heart valves, and the great arches.21 The cardiomyogenic potential of CNCs has been widely debated in the literature22,23; a recent report, however, shows that CNC cells possess full cardiomyocyte potential, but are undermined by changes in bone morphogenetic protein (BMP) and wnt signaling pathways.24 In addition to their contributions to the aforementioned structures, dormant NCSCs have also been identified in the postnatal myocardium. These cells have also been shown to migrate and differentiate into cardiomyocytes following injury such as MI.23,25 In addition to its role in neural crest migration, Cx43 is also the major gap junction protein in ventricular myocardium and is necessary for the electrical coupling and contractile wave propagation throughout the heart wall.26
The present study examined the potential of NCSCs for future applications in cellular cardiomyoplasty. We successfully differentiated NCSCs to a cardiomyogenic lineage using chemically defined media. Following differentiation, cells were analyzed using immunostaining for expression of sarcomeric proteins, α-skeletal muscle actin and cardiac troponin I. Additionally, genetic expression for GATA4, MEF2C, TPM1, and GJA1/Cx43 was analyzed. To test the functionality of the stem cell-derived cardiomyogenic cells, pulsed infrared radiation (IR) was used to stimulate NCSC-derived cardiomyogenic cells (NCSC-CMs). Recent research in photonics has highlighted the ability of IR stimulation to stimulate a wide variety of cells, including neurons,27 hair cells,28 cardiomyocytes,29 and other stem cell-derived cells.30 Multiple groups have reported that pulsed IR evokes intracellular Ca2+ responses and contraction in cardiomyocytes, which match the time course of the IR stimuli delivered.28,29,31,32 In the present study, the IR-evoked contraction of NCSC-CM cardiospheres was monitored. Furthermore, we studied whether the derived cells continue to respond to long periods of IR stimulation and whether such stimulation may cause any irreversible damage to the NCSC-CMs.
Materials and Methods
Stem cell isolation and cardiomyocyte differentiation
Human Cx43+ NCSCs were obtained and cultured as previously described.17 All protocols received University of Miami IRB approval. NCSCs were seeded onto collagen-coated silicone membranes (FlexCell, Hillsborough, NC) at a density of 2000 cells/cm2. Membranes measured 5.58 cm2 and were placed in polystyrene six-well culture dishes (Falcon, Tewksbury, MA). Culture media consisted of Dulbecco's modification of Eagle's medium (DMEM, Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS; Atlanta Biologics, Flower Branch, GA), 1% penicillin/streptomycin (Life Technologies), and 0.1% amphotericin B (Thermo Scientific, Waltham, MA). Cells were cultured in these media overnight to promote adherence onto the silicone membranes. Once cells had adhered, cardiomyogenic differentiation was induced by replacing the previously defined medium with DMEM supplemented with 2% HI-FBS, 1% penicillin/streptomycin, 0.1% amphotericin B, 50 ng/mL, Dickkopf-related protein 1 (DKK-1; Pepperotech, Rocky Hills, NJ), 25 ng/mL fibroblast growth factor 4 (FGF-4; Pepperotech), 2 ng/mL transforming growth factor β3 (TGF-β3; Pepperotech), 10 ng/mL vascular endothelial growth factor (VEGF; Pepperotech), 5 ng/mL Activin A (Pepperotech), 10 ng/mL insulin-like growth factor-1 (IGF-1; Pepperotech), 10 ng/mL bone morphogenic protein 4 (BMP-4; Pepperotech), 250 nM cardiogenol C (Sigma-Aldrich, St. Louis, MO), and 10 nM oxytocin (Sigma-Aldrich). Differentiation media were replaced every 2 days for 2 weeks. To form aggregates, the cells were lifted using 0.05% Trypsin/EDTA (Life Technologies) and resuspended in DMEM supplemented with 10% FBS, 1% antibiotic–antimycotic, and 0.1% amphotericin B, at a concentration of 500,000 cells/mL. Ten-microliter droplets were placed on 9-mm polystyrene Petri dishes (VWR, Randor, PA). Petri dishes were inverted and cells were cultured according to the hanging drop aggregation assay for 2 days to promote three-dimensional aggregate formation. Aggregates were transferred to a 0.1% gelatin-coated six-well polystyrene dish for stimulation studies.
Immunohistochemical staining
NCSC-CMs were fixed in 10% neutral buffered formalin (VWR) at room temperature for 10 min. Following fixation, cells were washed three times in phosphate-buffered saline (PBS; Life Technologies). Cell membranes were permeabilized in either 0.2% Triton X-100 (Sigma-Aldrich) or with 0.25% Tween-20 (Sigma-Aldrich) in PBS at room temperature for 10 min. Cells were then washed three times and left in blocking buffer solution consisting of 0.05% Tween-20, 1% FBS, and 20 mg/mL bovine serum albumin (BSA; Life Technologies) in PBS for 1 h. After blocking, cells were incubated overnight at 4°C in 5 mg/mL BSA in PBS containing primary antibodies for cardiac troponin I (1:100; Abcam, Cambridge, MA), α-skeletal muscle actin (1:200; Abcam), and MEF2C (Abcam; 5 μg/mL). Cells were then washed three times in PBS, and incubated in 5 mg/mL BSA in PBS containing secondary antibodies at 1:500 for 2 h at room temperature. Secondary antibodies used were goat anti-mouse Alexa Fluor 488 (Life Technologies) and goat anti-rabbit Alexa Fluor 546 (Life Technologies). Cells were again washed three times in PBS, and one drop of VectaShield Mounting Media containing 1.5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA) was added to each well. Cells were mounted with a clean glass coverslip and imaged on a Nikon T2i inverted fluorescence microscope (Nikon, Melville, NY). Background was removed and brightness and contrast adjusted using ImageJ (NIH, Bethesda, MD) software. Care was taken to ensure that equivalent processing occurred for all images.
Gene expression
RNA isolation
Total RNA was isolated by means of TRIzol reagents following the manufacturers' recommended protocol and resuspended in RNase- and DNase-free water.
The RNA suspension was then frozen at −80°C overnight and quantified using a NanoDrop® spectrophotometer (Nanodrop Products, Wilmington, DE).
Reverse transcription
Reverse transcription of RNA to cDNA was performed by means of the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Grand Island, NY) as per the manufacturer's recommended protocol. Briefly, 1 μg of RNA for each sample was converted to cDNA and then diluted to a final concentration of 10 ng/μL of PCR-grade water and used for gene expression analysis.
For MEF2C, GATA4, and TPM1, real-time PCR was carried out using the TaqMan® Fast universal PCR Master Mix (Applied Biosystems) and TaqMan probe predesigned primers (Applied Biosystems). For NKX2.5, TNNT2, and GJA1/Cx43, real-time PCR was carried out using SYBR Green PCR Master Mix (Applied Biosystems) and custom-designed primers (Table 1) (Eurofins MWG Operon, Louisville, KY). For all the genes, 20 ng of cDNA was used for each reaction. The reactions were performed using an Agilent Technologies Stratagene Mx3000p real-time PCR system. Each reaction was run in triplicate for each assay and gene expression quantification was carried out by means of the comparative Ct method with GAPDH used as an endogenous control.
Table 1.
Primers
| Gene | Accession no. | Forward primer 5′-3′ | Reverse primer 5′-3′ | Amplicon size (bp) | Amplification factor |
|---|---|---|---|---|---|
| CX43/GJA1 | NM_000165.4 | TATTGAAGAGCATGGTAAGG | TAGACTTGAAGAGAGATACTGA | 78 | 2 |
| TNNT2 | NM_000364.3 | GGTTACATCCAGAAGACAGA | TCCTCTCAGCCAGAATCT | 82 | 2.08 |
| NKX2.5 | NM_001166176.1 | CTAGAGCCCGAAAAGAAAG | AGCATTTGTAGAAAGTCA | 98 | 2.05 |
Ionomycin assessment of Ca2+ stores
Ca520 (UPharm Labratories, Parsippany, NJ) was stored in 10-μM aliquots. Ca520 was diluted to 5 μM in PBS and cells were incubated at 37°C for 90 min. Following dye loading, cells were washed with fresh Ca2+-free PBS and further incubated for 20 min at room temperature to allow for full acetoxymethyl (AM) ester cleavage. Imaging was performed at room temperature on a Leica SP5 confocal microscope with resonant scanner (Leica) with 20 × water immersion objective (14–28 fps). In a subset of the cultures, ionomycin (1 μM), a positive control of Ca 520 AM, was delivered through pipettes positioned over the cells and changes in intracellular Ca2+ were measured.
IR stimulation
In this study, pulsed IR was used to assess the functionality of NCSC-CMs and study the elicited aggregate contraction. A Capella pulsed IR laser (Lockheed Martin Aculight, Bothell, WA) coupled to a low-OH 400-μm diameter optical fiber (Ocean Optics, Dunedin, FL) was used to deliver the IR pulses (0.25–2 pps) to the cardiomyocytes. The output fiber was mounted onto a micromanipulator (Narishige, East Meadow, NY) and was positioned roughly 200–400 μm from the cells using a pilot light. The output wavelength was 1862 nm, the pulse width was 2 ms, and the energy output at the fiber tip was ∼668 mJ/cm2, as measured in air using a digital optical power/energy meter (FieldMaxII; Coherent, Santa Clara, CA). This wavelength was selected based on results of previous studies in cardiomyocytes and neurons.27–29,31
Contraction analysis
Analysis of contraction of NCSC cardiospheres was accomplished using PIVlab—Time-Resolved Digital Particle Image Velocimetry (PIV) Tool for MATLAB version: 1.4 on a computer running Mac OS X.33,34 Videos were captured in AVI format using a Nikon T2i microscope and Nikon NIS-Elements Advanced Research software on a computer running Microsoft Windows 7. Captured AVIs were converted to a stack of TIFF images using ImageJ on a computer running Mac OSX. TIFF stacks were then imported into MATLAB (Mathworks, Natick, MA) for analysis using PIVLab. Analysis was carried out over a region of interest surrounding the cardiosphere, which was drawn in the PIVLab graphical user interface. PIVlab results were calibrated using a scale bar obtained through still images from a Nikon T2i microscope and processed using Nikon NIS-Elements Advanced Research software on a computer running Microsoft Windows 7. Results from PIVLab were exported to a text file and imported into Microsoft Office Excel 2016 for Mac OSX to generate plots of velocity during stimulation. For spatial analysis, representative frames of contraction were chosen for each frequency investigated. A line vector was drawn using the PIVLab graphical user interface and displacement along the line was output as a text file. This text file was then imported into Microsoft Excel 2016 for plotting of data.
Long-duration stimulation and assessment of mitochondrial potential
Long-term stimulation was applied to NCSC-derived cardiomyocytes for 10 min (1 ms, 3 Hz, 661 mJ/cm2) on a cold pad in a laminar flow hood. Mitochondrial transmembrane potential was assessed at 24 h after stimulation by using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzamidazolylcarbocyanine iodide (JC-1; Life Technologies). JC-1 (1.5 μM) in PBS was loaded for 30 min, following which JC-1-containing loading medium was replaced with fresh PBS, and membranes were mounted onto glass microscope slides and kept on ice until imaging. Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; Sigma-Aldrich), a mitochondrial oxidative phosphorylation inhibitor, was used as a positive control for JC-1. FCCP was added to cells at 10 μM in PBS for 1 h and 30 min before JC-1 loading. After 1 h and 30 min, cells were washed in PBS and loaded for 30 min with 1.5 μM JC-1 in PBS. After 30 min, JC-1-containing loading medium was replaced with fresh PBS, and membranes were mounted onto glass microscope slides and kept on ice until imaging. Imaging of JC-1 fluorescence was performed on a Zeiss LSM700 with a 20 × objective or a 63 × oil immersion objective (Zeiss, Thornwood, NY).
Statistics
The data are expressed as mean ± SEM unless otherwise indicated. qPCR was analyzed using the ΔΔCT method.35 One-way analysis of variance (ANOVA) test, followed by a Dunnett post hoc test for multiple comparisons, was used for JC-1 analyses; p value of <0.05 was considered significant. All calculations were performed on a computer equipped with GraphPad Prism v 5.00c software for Mac OS X® (Graphad, La Jolla, CA), and graphs were generated using Microsoft Office Excel 2016 for Mac OS X.
Results
Growth factor treatment upregulates genetic expression and protein levels of cardiomyocyte-specific genes and proteins
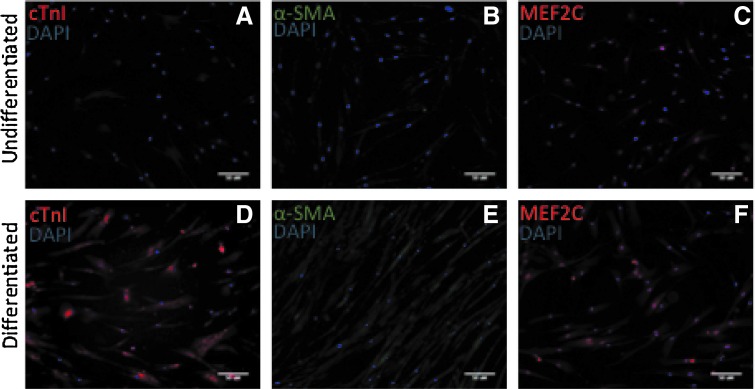
Initially, NCSCs exhibited a spindle fibroblast-like phenotype. During differentiation, the group receiving the growth factor cocktail cells appears to become more opaque and flattened, both of which are typical of cardiomyocytes. Furthermore, spatial arrangement of cells receiving the treatment appears to be more aligned and organized. After 2-week exposure to the growth factor cocktail, NCSCs showed upregulation of select cardiomyocyte-specific genes and proteins compared with those not receiving the cocktail. At the genetic level, a significant increase was observed in the cardiomyocyte markers, NK2 homeobox 5 (NKX2.5) gene and troponin T type 2 (TNNT2). Although not significant, we also see positive increases in tropomyosin I (TPM1), gap junction protein alpha 1/Cx43 (GJA1/Cx43), and myocyte enhancement factor 2C (MEF2C). (Fig. 1) NCSC-derived cardiomyogenic cells (NCSC-CMs) also stained positive for cardiomyocyte-specific proteins, cardiac troponin I (cTnI) and skeletal muscle actin (α-SMA), and showed greater expression and translocation of MEF2C. (Fig. 2) Cells that were not incubated with the growth factor cocktail stained negative for these sarcomeric proteins.
FIG. 1.
Gene expression. Significant upregulation seen in the early cardiomyocyte-specific transcription factor, NKX2.5 (*p < 0.05), and cardiac troponin T type 2 (**p < 0.01). Also seen is an upregulation, although not statistically significant in gap junction protein alpha 1/Cx43, tropomyosin 1, and a small increase in the cardiomyocyte-specific transcription factor, GATA4.
FIG. 2.
Immunohistochemical analysis of two important proteins: the sarcomeric proteins, cardiac troponin I (cTnl) and skeletal muscle actin (α-SMA), in NCSC-CMs after completion of the 2-week growth factor cocktail regime. Control cells grown under identical conditions, except for the addition of growth factors, do not express skeletal muscle actin or cardiac troponin I (A, B). However, the NCSC-CMs showed a robust expression of the two proteins (D, E). Differentiated cells also show increased expression and translocation of MEF2C (C, F). NCSCs, neural crest stem cells-derived cardiomyogenic cells.
Cardiomyocytes derived from NCSCs are excitable
Multiple studies have shown that infrared stimulation leads to an intracellular Ca2+ response and contraction in cardiomyocytes.28,29,31,32 In the present study, we analyzed if IR also elicits similar contraction of aggregate cardiospheres.
First, we sought to determine whether differentiated cells possessed stores of Ca2+, which could be stimulated for release. Intracellular Ca2+ transients in NCSC-CMs were monitored with Ca2+-sensitive Ca 520 AM dye. Ca2+ ionophore ionomycin was used to determine whether NCSC-derived cardiomyocytes possessed stores of Ca2+. Ionomycin depletes intracellular Ca2+ stores, leading to a rapid increase in cytosolic Ca2+ reflected on a significant increase in Ca2+ fluorescence. As expected, addition of ionomycin led to a large increase in normalized fluorescence lasting for a few minutes (Fig. 3).
FIG. 3.
NCSC-CM response in Ca 520 AM fluorescence to ionomycin. Ionomycin is an ionophore-depleting intracellular Ca2+ store. As expected, addition of 1 μM ionomycin induced a transient rise in normalized fluorescence in NCSC-CMs, lasting for a few minutes. The arrow indicates addition of ionomycin. Data depicted as mean (red line) ± 95% CI (gray area bounded by black lines) for N = 10 NCSC-CMs. Color images available online at www.liebertpub.com/tec
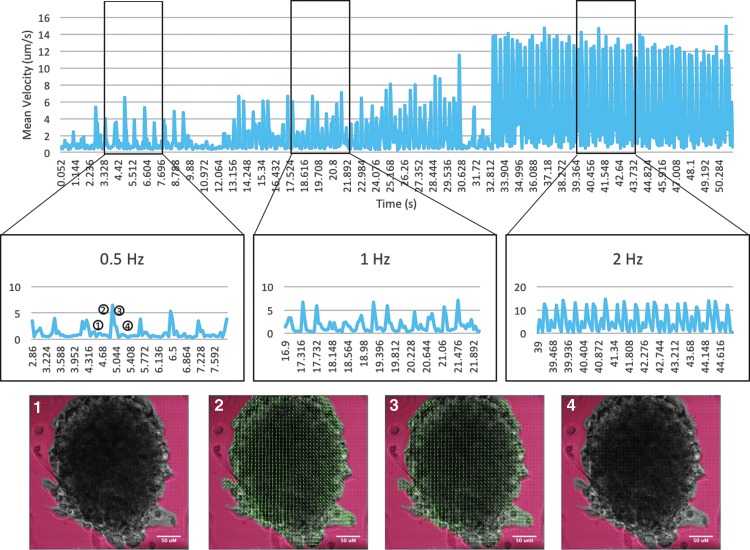
IR stimulation (0.25–2 pps, 2 ms, 661 mJ/cm2) delivered to NCSC-CM aggregates (N = 5, k = 2) elicited visible contractile behavior. Contraction was measured using PIV analysis (Fig. 4). The overall net direction of contraction is away from the IR source, which implies that distance from the IR source affects the velocity of induced contraction. To determine the spatial effects, mean velocity for the three frequencies studied was compared to the distance from the IR source. Data shown are from one trial for comparison purposes. Average velocity of contraction was larger near the bottom of the aggregate, which correlates with being spatially closer to the IR source, and declined as distance from the IR source increased (Fig. 5). This was also true for the relaxation vector. The mean velocities at the frequencies tested were 3.74 ± 1.59 μm/s (n = 12) at 0.5 Hz, 6.34 ± 1.81 μm/s (n = 30) at 1 Hz, and 11.5 ± 3.82 μm/s (n = 60) at 2 Hz. In the absence of applied stimulation, contractile behavior ceased.
FIG. 4.
PIV of NCSC-CM cardiosphere. Within NCSC-CM cardiospheres, visible contraction could be observed. Representative velocity vectors of an NCSC-CM cardiosphere undergoing IR-induced contraction (point 2) and relaxation (point 3) were computed using PIV analysis. Both contraction and relaxation are of larger magnitude on the periphery of the aggregate and close to the optical fiber. Images represent sample PIV results of aggregates before contraction (1), during contraction (2), during relaxation (3), and immediately after relaxation (4). IR, infrared radiation; PIV, particle image velocimetry.
FIG. 5.
Analysis of spatial effects: average velocity of contraction was measured along the beam path of the IR. The inset shows an NCSC-CM aggregate (the IR source was located in the lower right hand corner). Along the line from the source to the other side of the aggregate, the amplitude of the velocity vector decreased. Additionally, as frequency of stimulation is increased, the magnitude of the velocity of contraction increased. Color images available online at www.liebertpub.com/tec
Long-duration stimulation with IR
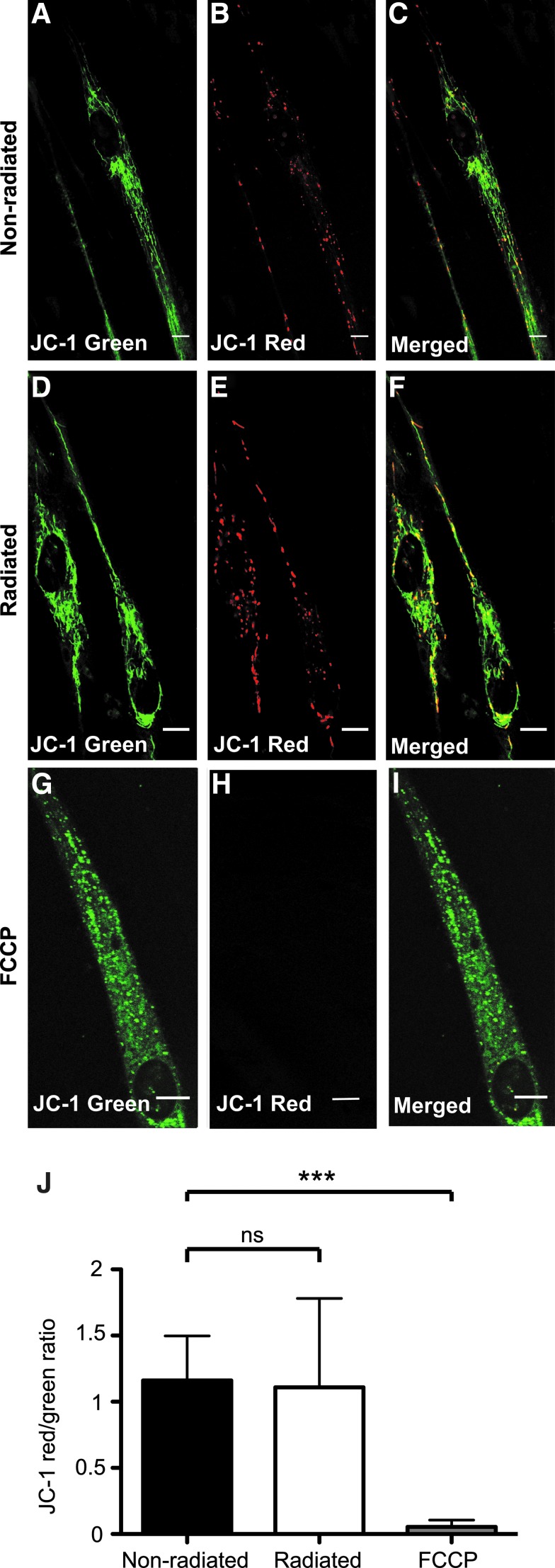
Previous studies have shown that the response to IR is dependent on mitochondrial Ca2+ cycling. We postulated that IR-induced Ca2+ cycling in NCSC-CMs would lead to excessive mitochondrial Ca2+ uptake, which may lead to membrane depolarization and permanent opening of the mitochondrial transition pore releasing apoptotic components to the cytosol (cytochrome c, smac Diablo, and ROS).36 To determine if a long period of IR stimulation would cause irreversible damage to the NCSC-CMs, we tested the efficacy of these cells to respond to and survive during long-duration stimulation. Mitochondrial function was assessed by investigating the state of mitochondrial membrane polarization. The NCSC-CMs were radiated for 10 min, and then incubated for 24 h before JC-1 staining. FCCP, a mitochondrial decoupling agent, was used as a positive control for these experiments. JC-1 red aggregates indicating normal resting values of membrane potential could be observed in both radiated (N = 18 cells) and nonradiated (N = 13) NCSC-CMs belonging to the same membrane with no significant difference (p > 0.05). As expected, FCCP (N = 16) led to a shift in JC-1 fluorescence emission to green, indicating loss of membrane potential (p < 0.001) (Fig. 6).
FIG. 6.
JC-1 staining of NCSC-CMs after long-term IR stimulation. To test whether applied radiation causes permanent mitochondrial membrane depolarization, three different groups were tested using JC-1, a fluorescent sensor of mitochondrial membrane potential. (A–C) NCSC-CMs that were not exposed to any radiation; (D–F) NCSC-CMs grown on the same membrane as (A–C), but exposed to IR (30 pps, 1 ms, 661 mJ/cm2) for 10 min, and cultured for 24 h; (G–I) NCSC-CMs incubated with 10 μM FCCP, a mitochondrial oxidative phosphorylation inhibitor. Both radiated and nonradiated NCSC-CM mitochondria still fluoresce red, an indication that pulsed IR did not induce permanent mitochondrial depolarization in these experimental settings. In NCSC-CMs pretreated with FCCP, JC-1 red aggregates were absent, indicating loss of membrane potential. (J) Representation of JC-1 red/green signal ratios (mean ± SD). Red/green signal ratios were computed in ImageJ for the nonradiated (N = 13), radiated (N = 18), and FCCP-treated (N = 16) NCSC-CMs. Significant difference (***p < 0.001) was observed when comparing both radiated and nonradiated with FCCP-treated NCSC-CMs, but not between radiated and nonradiated (ns, p > 0.05). SD, standard deviation.
Discussion
This study shows the potential of NCSCs for cardiomyogenic application and the applicability of IR stimulation to generate contractile behavior in quiescent cell populations as we were able to engineer contracting tissue that expresses cardiac troponin and skeletal muscle actin. In addition, we show significant upregulation of NKX2.5, which is one of the earliest known markers of cardiac lineages.37 Additionally, NKX2.5 is known to interact with GATA4, which although not statistically significant, we do see a small upregulation of.14,37 The smaller than expected increase is not surprising as cells of the migrating neural crest origin are known to also express GATA4.38 This is also true of MEF2C; however, the translocation into the nucleus may show activation of the transcription factor.39 The smaller increase in GJA1/Cx43 is likely a result of our cells being selected on expression of connexin 43. Thus, with the increase in NKX2.5 coupled with the large increase in TNNT2 expression and immunohistochemical data, we hypothesize that NCSC-derived cardiomyocytes are similar to that of other MSCs where differentiated cells demonstrate upregulation of cardiomyocyte-specific genes and proteins, but do not exhibit cardiomyocyte functionality.40 To our knowledge, we are the first group to demonstrate in vitro differentiation of NCSCs and show that IR stimulation can initiate contractile behavior in stem cell-derived cardiomyogenic cells. Currently, one of the largest risks associated with cellular cardiomyoplasty procedures is the formation of arrhythmia.10 Current research suggests that transplanted cells lack proper gap junctions necessary for myocardial coupling. This in turn leads to transplanted cells becoming electrically isolated from the host myocardium and the generation of arrhythmia.26 As the most abundant gap junction in the myocardium is Cx43, it is possible that the neural crest cells isolated by our group may help to diminish this risk of arrhythmia. Overexpression of Cx43 in myoblasts has been investigated as a potential solution to the risk of arrhythmia formation.41–43 Roell et al. demonstrated that genetically engineered overexpression of Cx43 in skeletal myoblasts leads to protection against induced ventricular tachycardia in a murine infarct model.42 Fernandes et al. meanwhile report that genetically engineered myoblasts improved intracellular electrical coupling of myoblasts and cardiomycoytes.43 Preconditioning cells with IR stimulation may further reduce the risk of arrhythmia formation; however, it is also possible that transplantation of contracting cells may increase the risk of electrically isolated cells. This may be further evidenced by the increase in contraction velocity as the frequency is increased. We hypothesize that the additional energy leads to increased cellular recruitment, which may be a result of Cx43 selection.
Optical stimulation is advantageous as it does not require direct contact with cells, is spatially precise, and does not produce any stimulation artifact and, as shown in the study, can be applied for long durations without damaging the cells.28 Owing to these advantages, other approaches using optical stimulation have been investigated. This includes modifying the tissue to express photosensitive proteins (optogenetics) and controlling excitation and contraction by light,44,45 although it is likely that the required genetic manipulation has other deleterious effects.
Our results are qualitatively similar to Dittami et al. who show a rapid increase in intracellular Ca2+ as a result of IR stimulation and contraction of neonatal cardiomyocytes. Neonatal cardiomyocytes are known to qualitatively differ from the adult myocyte in Ca2+ handling and special arrangement of sarcoplasmic reticulum (the primary site of Ca2+ storage in the mycoyte) and mitochondria.28,46 Furthermore, Dittami et al. did report that in spontaneously contracting cells, IR stimulation caused a cessation of spontaneous events and return of Ca2+ to baseline levels. They hypothesize that this response may be the result of the action of IR stimulation on the mitochondrial calcium uniport (mCU) to clear Ca2+. These data were further verified by the inhibition of IR-evoked responses in the presence of Ruthenium Red, an inhibitor of the mCU. Pulsed IR is also known to cause a capacitive photothermal membrane current.47–49 However, the relatively small amplitude depolarization reported would not be sufficient to trigger the responses observed here. Pulsed IR clearly evokes large changes in the intracellular calcium in cultured spiral and vestibular ganglion neurons.27 This is in agreement with previous studies reporting that IR applied to the cell body modulates intracellular (Ca2+) and that this signaling plays a major role in somatic IR excitability.32,50 Previous results have shown that inhibition of transmembrane Ca2+ channels or removal of extracellular Ca2+ did not impact the response to IR.27 This is further evidenced as all our stimulation experiments were performed in Ca2+-free buffer. Pharmacological data from this study showed that IR likely activates the endoplasmic reticulum (ER) Ca2+ release with a dependence on mitochondrial Ca2+ cycling.27,28 In unpublished recent studies, we have observed that IR may induce Ca2+ releases from the ER in neurons by activating IP3, ryanodine receptor channels, and/or through a mechanism that increases the probability of opening of these channels such as the degree of Ca2+ loading into the ER. The Ca2+ release from the ER was fully reversible and completely blocked by application of caffeine, indicating that IP3 and/or ryanodine channels are necessary for its release. Additionally, mitochondria close to endoplasmic or sarcoplasmic Ca2+ release sites (e.g., ryanodine receptors, RyRs) are exposed to higher Ca2+, making them likely to have higher rates of Ca2+ uptake.51 It is possible that IR modulates the ΔΨm component of the electrochemical gradient potential that drives mitochondrial Ca2+ uptake.27,52 However, the mechanisms by which pulsed IR modulates mitochondrial membrane potential, Ψm, plasma membrane potential, Ψp, or significant changes in cytosolic Ca2+ and the events controlling the IR effects on cells remain to be fully elucidated.
It could also be hypothesized that IR-induced contraction is related to thermal effects elicited by stimulation as has been shown.53 It has been shown that pulsed IR induces a transient increase in temperature up to ∼22.2°C for a 10-ms pulse (7.3 mJ, 5.8 J/cm).49 In the current study, the maximum radiant energy was 0.839 mJ, far less than that reported in previous studies investigating IR-evoked temperature transients. Coupled with previous results, which have shown that cooling or heating extracellular fluid does not alter the observed Ca2+ transients, we do not believe that the observed contractions are a result of temperature transients induced by IR stimulation.27,28
Conclusions
This study shows the applicability of IR radiation to induce contraction in stem cell-derived cardiomyocytes. As clinicians have suggested, cardiac committed precommitted cells may be used in future studies to improve clinical outcomes. By preconditioning these cells with IR stimulation, the risk of arrhythmia formation may also be improved. This merits further in vitro work to fully characterize the electrophysiological properties of cells undergoing IR stimulation. In vivo work should be performed to determine if preconditioned cells are able to integrate into the host electrical network properly. IR has many advantages, including its spatial precision and lack of stimulation artifacts, and should be investigated further for cardiomyocyte stimulation/applications.
Disclosure Statement
No competing financial interests exist.
References
- 1.The top 10 causes of death: World Health Organization, 2014. Available from www.who.int/mediacentre/factsheets/fs310/en/ Last viewed June 6, 2016
- 2.van den Borne S.W., Diez J., Blankesteijn W.M., Verjans J., Hofstra L., and Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol 7, 30, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Matar A.A., and Chong J.J. Stem cell therapy for cardiac dysfunction. SpringerPlus 3, 440, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elnakish M.T., Hassan F., Dakhlallah D., Marsh C.B., Alhaider I.A., and Khan M. Mesenchymal stem cells for cardiac regeneration: translation to bedside reality. Stem Cells Int 2012, 646038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Rendon E., Brunskill S.J., Hyde C.J., Stanworth S.J., Mathur A., and Watt S.M. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 29, 1807, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Mohsin S., Siddiqi S., Collins B., and Sussman M.A. Empowering adult stem cells for myocardial regeneration. Circ Res 109, 1415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose R.A., Jiang H., Wang X., Helke S., Tsoporis J.N., Gong N., et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells 26, 2884, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Qian Q., Qian H., Zhang X., Zhu W., Yan Y., Ye S., et al. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cells Dev 21, 67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q., Guo M., Jiang X., Hu X., Wang Y., and Fan Y. A cocktail method for promoting cardiomyocyte differentiation from bone marrow-derived mesenchymal stem cells. Stem Cells Int 2014, 162024, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menasche P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol 50, 258, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Bartunek J., Behfar A., Dolatabadi D., Vanderheyden M., Ostojic M., Dens J., et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol 61, 2329, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Xin L.Z., Govindasamy V., Musa S., and Abu Kasim N.H. Dental stem cells as an alternative source for cardiac regeneration. Med Hypotheses 81, 704, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Mayo V., Sawatari Y., Huang C.Y., and Garcia-Godoy F. Neural crest-derived dental stem cells—where we are and where we are going. J Dent 42, 1043, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Arminan A., Gandia C., Bartual M., Garcia-Verdugo J.M., Lledo E., Mirabet V., et al. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev 18, 907, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gandia C., Arminan A., Garcia-Verdugo J.M., Lledo E., Ruiz A., Minana M.D., et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 26, 638, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Huang C.Y., Pelaez D., Dominguez-Bendala J., Garcia-Godoy F., and Cheung H.S. Plasticity of stem cells derived from adult periodontal ligament. Regen Me 4, 809, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Pelaez D., Huang C.Y., and Cheung H.S. Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev 22, 2906, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Bronner M.E. Formation and migration of neural crest cells in the vertebrate embryo. Histochem Cell Biol 138, 179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo C.W., Waldo K.L., and Kirby M.L. Gap junction communication and the modulation of cardiac neural crest cells. Trends Cardiovasc Med 9, 63, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Xu X., Li W.E., Huang G.Y., Meyer R., Chen T., Luo Y., et al. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol 154, 217, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoller J.Z., and Epstein J.A. Cardiac neural crest. Semin Cell Dev Biol 16, 704, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Keith M.C., and Bolli R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res 116, 1216, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura Y., Matsumura K., Sano M., Tabata H., Kimura K., Ieda M., et al. Neural crest-derived stem cells migrate and differentiate into cardiomyocytes after myocardial infarction. Arterioscler Thromb Vasc Biol 31, 582, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Hatzistergos K.E., Takeuchi L.M., Saur D., Seidler B., Dymecki S.M., Mai J.J., et al. cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci U S A 112, 13051, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calderone A. Nestin+ cells and healing the infarcted heart. Am J Physiol Heart Circ Physiol 302, H1, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62, 309, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lumbreras V., Bas E., Gupta C., and Rajguru S.M. Pulsed infrared radiation excites cultured neonatal spiral and vestibular ganglion neurons by modulating mitochondrial calcium cycling. J Neurophysiol 112, 1246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dittami G.M., Rajguru S.M., Lasher R.A., Hitchcock R.W., and Rabbitt R.D. Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J Physiol 589, 1295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins M.W., Wang Y.T., Doughman Y.Q., Watanabe M., Cheng Y., and Rollins A.M. Optical pacing of the adult rabbit heart. Biomed Opt Express 4, 1626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bas E., Van De Water T.R., Lumbreras V., Rajguru S., Goss G., Hare J.M., et al. Adult human nasal mesenchymal-like stem cells restore cochlear spiral ganglion neurons after experimental lesion. Stem Cells Dev 23, 502, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins M.W., Duke A.R., Gu S., Chiel H.J., Fujioka H., Watanabe M., et al. Optical pacing of the embryonic heart. Nat Phot 4, 623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith N.I., Kumamoto Y., Iwanaga S., Ando J., Fujita K., and Kawata S. A femtosecond laser pacemaker for heart muscle cells. Opt Express 16, 8604, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Thielicke W. The Flapping Flight of Birds—Analysis and Application. Rijksunviersiteit Groningen, 2104 [Google Scholar]
- 34.Thielicke W. ‘The Flapping Flight of Birds: Analysis and Application’, Doctoral Thesis, University of Groningen; 2014 [Google Scholar]
- 35.Livak K.J., and Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Dorn G.W., II Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res 81, 465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lien C.L., Wu C., Mercer B., Webb R., Richardson J.A., and Olson E.N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 126, 75, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Pilon N., Raiwet D., Viger R.S., and Silversides D.W. Novel pre- and post-gastrulation expression of Gata4 within cells of the inner cell mass and migratory neural crest cells. Dev Dyn 237, 1133, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Verzi M.P., Agarwal P., Brown C., McCulley D.J., Schwarz J.J., and Black B.L. The transcription factor MEF2C is required for craniofacial development. Dev Cell 12, 645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel G., Krause P., Wohrle S., Nowak P., Ayturan M., Kluba T., et al. Bone marrow-derived human mesenchymal stem cells express cardiomyogenic proteins but do not exhibit functional cardiomyogenic differentiation potential. Stem Cells Dev 21, 2457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham M.R., Henrikson C.A., Tung L., Chang M.G., Aon M., Xue T., et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res 97, 159, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Roell W., Lewalter T., Sasse P., Tallini Y.N., Choi B.R., Breitbach M., et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature 450, 819, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Fernandes S., van Rijen H.V., Forest V., Evain S., Leblond A.L., Merot J., et al. Cardiac cell therapy: overexpression of connexin43 in skeletal myoblasts and prevention of ventricular arrhythmias. J Cell Mol Med 13, 3703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyle P.M., Williams J.C., Ambrosi C.M., Entcheva E., and Trayanova N.A. A comprehensive multiscale framework for simulating optogenetics in the heart. Nat Commun 4, 2370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arrenberg A.B., Stainier D.Y., Baier H., and Huisken J. Optogenetic control of cardiac function. Science 330, 971, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Sobie E.A. Getting heart cells on the same wavelength: infrared triggering of Ca2+ transients in cardiac myocytes. J Physiol 589, 1243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Q., Frerck M.J., Holman H.A., Jorgensen E.M., and Rabbitt R.D. Exciting cell membranes with a blustering heat shock. Biophys J 106, 1570, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okunade O., and Santos-Sacchi J. IR laser-induced perturbations of the voltage-dependent solute carrier protein SLC26a5. Biophys J 105, 1822, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro M.G., Homma K., Villarreal S., Richter C.P., and Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun 3, 736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwanaga S., Kaneko T., Fujita K., Smith N., Nakamura O., Takamatsu T., et al. Location-dependent photogeneration of calcium waves in HeLa cells. Cell Biochem Biophys 45, 167, 2006 [DOI] [PubMed] [Google Scholar]
- 51.David G., Barrett J.N., and Barrett E.F. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol 509, 59, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szabadkai G., and Duchen M.R. Mitochondria: the hub of cellular Ca2+ signaling. Physiology 23, 84, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Oyama K., Mizuno A., Shintani S.A., Itoh H., Serizawa T., Fukuda N., et al. Microscopic heat pulses induce contraction of cardiomyocytes without calcium transients. Biochem Biophys Res Commun 417, 607, 2012 [DOI] [PubMed] [Google Scholar]