Abstract
The simultaneous deletion of six RNA molecules in mice has been found to cause respiratory and fertility defects, owing to improper assembly of structures called cilia on the cell surface.
Defects in the assembly of cilia — fine projections of the cell surface — are the cause of a plethora of human diseases1. One such disease is primary ciliary dyskinesia, a syndrome that severely compromises the function of the respiratory tract and reproductive system, resulting in airway infections and infertility. Identification of factors that control the assembly of cilia would constitute a major scientific advance. In this issue, Song et al.2 (page 115) implicate clusters of non-protein-coding RNA molecules in the regulation of cilium assembly, and show that deletion of these regulators in mice leads to a syndrome reminiscent of primary ciliary dyskinesia.
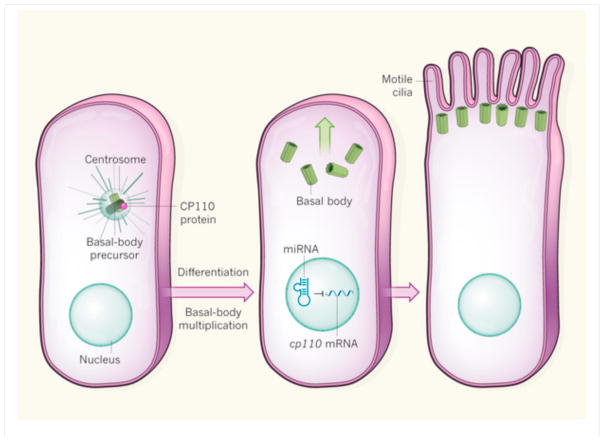
There are two types of cilium, non-motile and motile, both of which are essential for normal growth and development. Single, non-motile primary cilia are present on diverse cell types and function as sensors that provide the cell with information about the external environment. Motile cilia, meanwhile, function to coordinate fluid flow, and are found in large numbers on multiciliated cells (MCCs) in the respiratory tract and the Fallopian tubes of the female reproductive tract. Assembly of both types (a process known as ciliogenesis) is initiated by a cellular organelle called the basal body. A basal-body precursor, located inside another organelle, the centrosome, matures and migrates to the plasma membrane, extending microtubular structures into the extracellular space to form the axoneme — the skeleton of the cilium (Fig. 1). But how this process is controlled is unclear.
Figure 1. Ciliogenesis.
The production of multiciliated cells from precursors occurs through an orderly process known as ciliogenesis, which begins with a single precursor of an organelle called the basal body, located inside another organelle, the centrosome. As the cell differentiates, the basal-body precursor multiplies and matures. Basal bodies then migrate and dock to the surface of the cell, extending microtubular structures into the extracellular environment to form cilia. Song et al.2 report that, as precursors undergo differentiation, six miRNA molecules expressed by three related genetic regions regulate normal ciliogenesis in mice and frogs. The miRNAs inhibit translation of the messenger RNA that encodes the centrosomal protein CP110. The authors find that enhanced expression of CP110, brought about by simultaneous deletion of the six miRNAs, prevents normal docking of basal bodies to the cell surface (not shown).
A group of small, non-protein-coding RNAs called microRNAs (which regulate protein production by inhibiting the translation of messenger RNA) seem to be new-found regulators of motile ciliogenesis. The genetic region, or locus, known as miR-449 encodes three such miRNAs, and has been linked3, 4 to the development of a type of ciliated cell in the bronchi that is responsible for clearing mucus. A study5 of the role of miR-449 in two types of MCC — the mucociliary epidermis cells of embryonic frogs and the cells that line the human bronchi — showed that expression from this locus increases as cellular precursors differentiate into MCCs. The miR-449 locus shares sequence similarity with two other miRNA loci, miR-34a and miR-34b/34c. Together, these three loci encode six miRNAs in vertebrates.
The miR-34/449 miRNAs are remarkably abundant in tissues enriched with motile cilia, comprising around 50% of the miRNAs expressed in the embryonic epidermis of frogs and 13% of the miRNAs in the cells that line the human respiratory tract5. To test whether the miR-34/449 cluster is essential for the initiation of ciliogenesis, Song et al. deleted all three loci in mice by mating existing strains harbouring single deletions. Simultaneous deletion was necessary because the six miRNA molecules have similar functions, and so can compensate for one another if only one locus is deleted. Triple-mutant mice failed to thrive — only 40% reached adulthood, and those that did were half the size of controls. The mice exhibited severe respiratory distress owing to a failure to clear mucus, and were sterile, mirroring defects observed in people with primary ciliary dyskinesia.
Song and colleagues obtained tracheal tissue from the mutant mice and examined it using high-speed imaging. They observed abnormal ciliation in mutant tracheal MCCs, which resulted in reduced fluid flow. This was not due to a developmental defect in the MCC precursors, but rather to a substantial reduction in the length and number of cilia per cell.
Multi-ciliation is achieved through an ordered process, beginning with the multiplication of basal bodies that subsequently dock at the cell surface. The authors found no differences in basal-body replication in mutant and control cells. Instead, high-resolution structural studies revealed that many basal bodies remained undocked in mutant cells, and so failed to assemble cilia. Those that did dock generated significantly shorter, albeit structurally normal, axonemes.
Together, these data suggest that the miR-34/449 cluster might regulate basal-body docking. Song and co-workers screened a panel of genes for potential miR-34/449 targets, looking for genes that not only contained potential miR-34/449 binding sites but also showed downregulated expression during MCC differentiation (indicative of inhibition by highly expressed miRNAs). The authors identified more than 50 candidate genes from the screen, and chose the centrosomal protein CP110 as a focus for further investigation.
CP110 is an attractive target of miR-34/449 because previous work6 has shown that over-expression of CP110 suppresses primary ciliary assembly, and that destruction of CP110 at basal bodies is required to trigger early stages of ciliogenesis. MicroRNAs are known to have multiple targets7, and the miR-34/449 cluster may regulate other transcripts that also influence ciliogenesis — much like miR-129-3p, which targets both cp110 in ciliated cells and components of the cell’s structural skeleton in other cell types8. However, when Song et al. inhibited cp110 in frog embryos lacking miR-34/449 (which have increased levels of CP110), they found a substantial improvement in basal-body docking and fewer cilia defects, suggesting that excessive CP110 caused most of the observed defects. The current study, together with the previous report on miR-129-3p (ref. 8), unifies a rapidly growing body of evidence establishing an evolutionarily conserved regulatory role for CP110 in cells with primary and motile cilia.
It is worth noting that proliferating and differentiating cells dedicate a remarkable level of attention to the control of CP110 abundance, which is regulated by transcriptional9, post-transcriptional2, 3 and post-translational mechanisms6. Indeed, no fewer than seven miRNAs (miR-129 and the miR-34/449 cluster)2, 3, 8 and a pair of enzymes10, 11 have been identified as being involved in the robust regulation of cp110 levels. It is likely that the findings of Song and colleagues represent the tip of the iceberg, and it will be exciting to explore the roles that other miRNAs have in modulating the levels of ciliary proteins that require similar fine tuning.
More generally, it will be useful to understand how subtle changes in the regulation of proteins such as CP110 could give rise to human disease. Intriguingly, links between aberrant CP110 levels and respiratory illness have already been uncovered in a previous study12. That study and the work of Song and colleagues indicate the importance of exploring the role of genes such as cp110, which regulate basal body and centrosomal components, in diseases associated with defects in ciliogenesis, including primary ciliary dyskinesia and human respiratory disease.
Contributor Information
IRMA SÁNCHEZ, Email: irma.sanchez@med.nyu.edu.
BRIAN D. DYNLACHT, Email: brian.dynlacht@med.nyu.edu.
References
- 1.Tsang WY, Dynlacht BD. Cilia. 2013;2:9. doi: 10.1186/2046-2530-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song R, et al. Nature. 2014;510:115–120. doi: 10.1038/nature13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lizé M, Herr C, Klimke A, Bals R, Dobbelstein M. Cell Cycle. 2010;9:4579–4583. doi: 10.4161/cc.9.22.13870. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Anton A, et al. Am J Respir Cell Mol Biol. 2013;49:384–395. doi: 10.1165/rcmb.2012-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcet B, et al. Nature Cell Biol. 2011;13:693–699. doi: 10.1038/ncb2241. [DOI] [PubMed] [Google Scholar]
- 6.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Flynt AS, Lai EC. Nature Rev Genet. 2008;9:831–842. doi: 10.1038/nrg2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao J, et al. Nature Cell Biol. 2012;14:697–706. doi: 10.1038/ncb2512. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD. Dev Cell. 2002;3:339–350. doi: 10.1016/s1534-5807(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 10.Li J, et al. Nature. 2013;495:255–259. doi: 10.1038/nature11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Angiolella V, et al. Nature. 2010;466:138–142. doi: 10.1038/nature09140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai Y, et al. J Allergy Clin Immunol. 2011;128:1207–1215.e1. doi: 10.1016/j.jaci.2011.09.001. [DOI] [PubMed] [Google Scholar]