Abstract
In the current study, we investigated the effects of genistein on adipogenic differentiation of mouse bone marrow-derived mesenchymal stem cell (BMSC) cultures and its potential signaling pathway. The terminal adipogenic differentiation was assessed by western-blotting analysis of adipogenic-specific proteins such as PPARγ, C/EBPα, and aP2 and the formation of adipocytes. Treatment of mouse BMSC cultures with adipogenic cocktail resulted in sustained activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), which are members of the mitogen-activated protein kinase (MAPK) family, at the early phase of adipogenesis (from days 3 to 9). Inhibition of ERK1/2 activation by PD98059, a specific MEK inhibitor, reversed the induced adipogenic differentiation. Genistein dose-dependently decreased the phosphorylation of ERK1/2 in mouse BMSC cultures. Genistein incubation for the entire culture period, as well as that applied during the early phase of the culture period, significantly inhibited the adipogenic differentiation of mouse BMSC cultures. While genistein was incubated at the late stage (after day 9), no inhibitory effect on adipogenic differentiation was observed. BMSC cultures treated with genistein in the presence of fibroblast growth factor-2 (FGF-2), an activator of the ERK1/2 signaling pathway, expressed normal levels of ERK1/2 activity, and, in so doing, are capable of undergoing adipogenesis. Our results suggest that activation of the ERK1/2 signaling pathway during the early phase of adipogenesis (from days 3 to 9) is essential to adipogenic differentiation of BMSC cultures, and that genistein inhibits the adipogenic differentiation through a potential downregulation of ERK1/2 activity at this early phase of adipogenesis.
Keywords: genistein, mesenchymal stem cell, adipogenic differentiation, extracellular signal-regulated kinases 1 and 2 (ERK1/2)
Adipocytes in bone marrow are of mesenchymal origin and stem from a common precursor so called bone marrow-derived mesenchymal stem cells (BMSCs) that also give rise to osteoblasts. The trabecular bone and adipose tissue content in bone marrow are inversely related in postmenopausal osteoporosis due to lack of endogenous hormone 17β-estradiol (E2) [Gambacciani et al., 1997; Justesen et al., 2001], and an increased lipid accumulation in the bone marrow has been reported in association with age-related bone loss [Nuttall and Gimble, 2000] also imply an inverse relationship between osteoblastogenesis and adipogenesis. Genistein, the most abundant soy-derived isoflavone that shares structural similarities with the estrogen E2 has various biological actions, including weak estrogenic effects and inhibition of tyrosine kinase [Rickard et al., 2003]. Several studies have shown that genistein stimulates bone formation in vivo [Ishimi et al., 1999, 2000], which was also evidenced by our and other previous work in vitro [Heim et al., 2004; Pan et al., 2005]. More importantly, E2 and genistein have also been reported to stimulate osteogenesis and concurrently inhibits adipogenesis in mouse preosteoblast-like cell line (KS483) [Dang et al., 2002] and mouse BMSC line (ST2) [Okazaki et al., 2002], as well as human BMSCs [Heim et al., 2004]. However, the molecular mechanism of genistein repressing adipogenesis remains unclear.
The extracellular signal-regulated kinases 1 and 2 (ERK1/2) are members of mitogen-activated protein kinases (MAPK) and involved in signaling cascades that regulate a number of major cellular functions, such as cell proliferation and differentiation. Several laboratories have investigated the role of ERK1/2 signaling pathway in regulating adipogenesis, but the conclusions were controversial. Some studies claim that activation of ERK1/2 by various effectors promotes adipogenesis [Tang et al., 2005], while others show that ERK1/2 plays an inhibitory role on adipocyte differentiation in mouse pre-adipocyte 3T3-L1 cells [Tanabe et al., 2004]. We postulate that both of claims might be true based on the time of ERK1/2 activation during the stages of adipocyte differentiation process. In another word, ERK1/2 phosphorylation of the peroxisome proliferators-activated receptor γ (PPARγ), an adipogenic transcription factor during the early stage of adipocyte differentiation promotes adipogenesis, but activation of ERK1/2 during the late stage of adipocyte differentiation might, on the other hand, inhibits its differentiation.
There is increasing evidence showing the role of genistein on ERK1/2 activity. One research found that genistein at concentrations below 12.5 μM significantly increased ERK1/2 activity in a non-tumorigenic human prostate epithelial cell line (RWPE-1), while at concentrations above 50 μM significantly inhibited ERK1/2 activity [Wang et al., 2006]. But another study showed that at high concentrations (10–50 μM), genistein induced neuronal apoptosis by promoting ERK1/2 activity [Linford et al., 2001], suggesting that the effects of genistein on ERK1/2 activity is tissue-specific and dose-associated. Most recently, we have demonstrated that genistein at a physiological concentration (1 μM) stimulates osteoblastic differentiation of mouse BMSC cultures related to p38MAPK activity, another important member of MAPK family, but the role of genistein on ERK1/2 activity in BMSC cultures remains to be elucidated [Liao et al., 2007].
So, the goal of this study was to examine the effect of genistein on adipogenesis and explore whether the ERK1/2 signaling pathway involved this action by using mouse BMSC cultures, a population of pluripotent cells within the bone marrow microenvironment that has a capacity to undergo adipogenic differentiation in vitro.
MATERIALS AND METHODS
Reagents and Antibodies
Alpha minimum essential medium (α-MEM), phenol red-free α-MEM, Dulbecoo’s modified eagle medium (DMEM), phenol red-free DMEM, fetal bovine serum (FBS), trypsin-EDTA, penicillin-streptomycin solution, and sodium dodecyl sulfate (SDS) were obtained from Gibco-BRL (Grand Island, NY). Genistein and fibroblast growth factor-2 (FGF-2) were purchased from Sigma Chemical Co (St. Louis, MO). Anti-phosphorylated ERK1/2 antibody and PD98059 were purchased from New England Biolabs (Beverly, MA). Anti-PPARγ antibody, anti-C/EBPα antibody, anti-aP2 anti-body, anti-β-actin antibody, and anti-NF-κB antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) Isolation, Expansion, and Culture
The BMSCs were obtained from 8- to 10-week-old female mice of the Kunming strain (Experimental Animal Center of Xiangya Medical College, Central South University, Changsha, China) as previously described [Pan et al., 2005]. Briefly, the femurs and tibias were dissected, the ends of the bones were cut, and the marrow was flushed out with 2 ml of ice-cold α-MEM containing 10% FBS (v/v) by using a needle and syringe. A suspension of bone marrow cells was obtained by repeated aspiration of the cell preparation through a 22 gauge needle and then the cells were counted with a hemocytometer. The cells were seeded onto 12-well plates or 60 mm plates at a density of 4 × 103 cells/cm2 and cultured for 5 days in α-MEM supplemented with 15% FBS (v/v), 100 U/ml sodium penicillin G, and 100 mg/L streptomycin sulfate in a humidified incubator with 5% CO2 and 95% air at 37°C. On day 5, all non-adherent cells were removed with the first medium change and then the adherent cells (representing BMSCs) were grown for additional periods of up to 21 days in the medium for inducing the adipogenic differentiation of BMSC cultures as described below.
Adipogenic Differentiation and Assays
For adipogenic differentiation, the cells were seeded at a density of 4 × 103 cells/cm2 and cultured in DMEM, 1% charcoal-treated FBS (serum free) supplemented with 200 nM dexamethasone (DEX) and 15 nM insulin (adipogenic cocktail) for up to 21 days. Treatments include vehicle (0.1% dimethylsulfoxide), genistein alone, and genistein concurrent with PD98059 or FGF-2. The cells are treated every 2 days and the medium was replaced every 4 days thereafter. For ERK1/2 signaling pathway analysis, the cells were harvested at the indicated time, protein analysis for PPARγ, C/EBPα, aP2, and adipocyte cell counting assays was performed on day 21.
Western Blotting Analysis
The cells were lysated in SDS sample buffer (62.5 mM Tris–HCl, 2% SDS, 10% glycerol, 50 mM dithiothreitol). After sonicated and denatured by boiling for 5 min, the protein concentration was measured by the method of Bradford. Twenty-five microgram proteins were resolved on 10% SDS–polyacrylamide gels and transferred to PVDF membrane. Non-specific binding sites on the membrane were blocked for 1 h in blocking buffer (50 mM Tris–HCL, 0.1% Tween 20, 5% non-fat milk). The membrane was first incubated with the indicated primary monoclonal antibody overnight at 4°C, and then with the anti-mouse or anti-goat IgG horseradish peroxidase-conjugated second antibody for 1 h. Detection was performed with enhanced chemiluminescence regent. Quantification of phosphorylation of ERK1/2 was determined by laser scanning densitometry (LKB).
Oil Red-O Staining and Cell Counting Assay
The cells were washed with PBS and fixed in 10% formalin for 20 min. After washing twice with PBS and once with 60% isopropyl alcohol, the cells were then stained with Oil Red-O solution (Sigma). The number of adipocytes was calculated by counting Oil Red O-positive cells in 15 separate fields, using three wells per condition.
Electrophoresis Mobility Shift Assay
Nuclear extracts were prepared from BMSC cultures at day 21, synthetic double-strand oligonucleotides of consensus PPARγ binding sequence 5'-GTC TTA CTG GAT CAG AGT TCA-3' was end-labeled with (γ-32P)dATP (PerkinElmer Life Sciences) using Klenow fragment of DNA polymerase. The binding reaction mixture contained 10,000 cpm radiolabeled oligonucleotide, 3 μg of nuclear protein, 20 mM Tris–HCl (pH 8), 50 mM NaCl, 3 mM EDTA, 1 mM dithiothreitol, 2 μg of poly(dI-dC) (Amersham Biosciences), and 10% glycerol in a final volume of 20 μl. The mixture was incubated for 30 min at room temperature, and the complexes were subjected to electrophoresis at room temperature on chilled 5% polyacrylamide gel. The gel was dried and visualized by autoradiography. For the supershift assays, 2 μg of the appropriate antibody was added to the reaction mixture for 30 min prior to the addition of the labeled probe. The samples were subjected to electrophoresis at room temperature on 5% polyacrylamide gel, which was dried and visualized by autoradiography.
Statistical Analysis
Data were analyzed using a paired Student’s t-test. Value of P < 0.05 was considered to be significant.
RESULTS
Activation of ERK1/2 Signaling Pathway of BMSC Cultures Treated With Adipogenic Cocktail
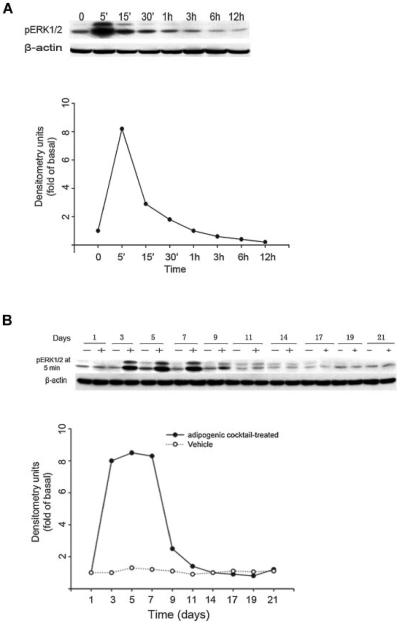
As several laboratories have investigated the role of ERK1/2 in regulating adipogenesis and got controversial conclusions, here, we examined ERK1/2 activation over the entire period of 21 days during treatment with adipogenic cocktail. ERK1/2 activation in adipogenic cocktail-treated cultures was determined by western blotting analysis using phosphospecific ERK1/2 antibody. As shown in Figure 1A, exposure of BMSC cultures to adipogenic cocktail resulted in rapid and sustained activation of ERK1/2, which reached its maximal activation at 5 min and even lasted for 3 h after exposure. However, this ERK1/2 activation can be achieved only after 3 days of adipogenic cocktail treatment, and maintained from days 3 to 9 (Fig. 1B). The maximal activation was observed at day 5, and it dropped to basal level after day 9 of culturing and stayed in this level from days 10 to 21 (Fig. 1B). So in the proceeding experiments, western blotting analysis for pERK1/2 was performed at day 5 after 5 min exposure to the adipogenic cocktail.
Fig. 1.
Adipogenic cocktail induces activation of ERK1/2 in mouse BMSC cultures. A: Mouse BMSC cultures were exposed to adipogenic cocktail, and lysates from day 5 during culture period were prepared at the indicated times after the exposure. Lysates were subjected to western blotting analysis using phosphospecific-ERK1/2 (pERK1/2) antibody. Anti-β-actin antibody was used as a control. Results are representative of three independent experiments. B: Mouse BMSC cultures were exposed (+) or not exposed (−) to adipogenic cocktail and at the indicated days during culture period, lysates were prepared at 5 min after the exposure to adipogenic cocktail or vehicle (0.1% dimethylsulfoxide). Lysates were subjected to western blotting analysis using phosphospecific-ERK1/2 (pERK1/2) antibody. Anti-β-actin antibody was used as a control. Results are representative of three independent experiments.
Inhibition of ERK1/2 Signaling Pathway Blocks Adipogenic Differentiation
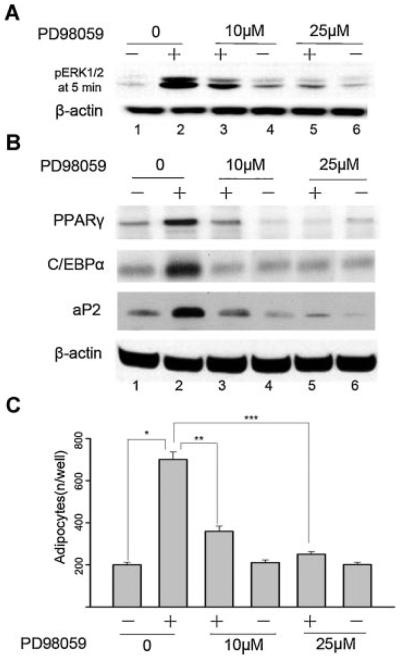
To explore whether ERK1/2 activation is necessary for adipogenic differentiation, PD98059, a selective inhibitor of MEK, was used to prevent the phosphorylation and activation of the ERK1/2. As shown in Figure 2A, PD98059 dose-dependently attenuated the adipogenic cocktail-induced pERK1/2 expression. PPARγ, CCAAT/enhancer-binding protein α (C/EBPα), and adipocyte-specific fatty acid-binding protein (aP2), which are known to be expressed in mature adipocytes, were also measured at day 21 of adipogenic cocktail-treated BMSC cultures by western blotting analysis. As show in Figure 2B, continuous incubation of BMSC cultures with adipogenic cocktail for 21 days significantly increased the expression of PPARγ, C/EBPα, and aP2 as compared with the non-treated control. In contrast, the expression of PPARγ, C/EBPα, and aP2 were significantly suppressed when BMSC cultures were added with adipogenic cocktail-containing PD98059 (Fig. 2B).
Fig. 2.
Effect of blockade of ERK1/2 activity on adipogenic differentiation of mouse BMSC cultures. Cells were cultured in control medium (−) or adipogenic cocktail (+) or adipogenic cocktail supplemented with PD98059. A: Western blotting assay for pERK1/2 was performed at day 5 after 5 min exposure to the adipogenic cocktail. B: Western blotting assay for PPARγ, C/EBPα, and aP2 were performed at day 21 as described under “Materials and Methods Section.” Anti-β-actin antibody was used as a control. Results are representative of three independent experiments. C: The number of Oil Red O-positive adipocytes was calculated at day 21. The data are mean ± SD. *P < 0.05 versus non-treated vehicle. **P < 0.05 versus adipogenic cocktail-treated alone. ***P < 0.05 versus adipogenic cocktail-treated alone. Results are representative of three independent experiments.
We also tested the effect of PD98059 on the formation of adipocytes by counting the number of Oil Red O-positive cells at day 21. As shown in Figure 2C, continuous incubation of BMSC cultures with adipogenic cocktail for 21 days significantly increased the number of adipocytes as determined by Oil Red O staining. This effect appears to be ERK1/2-dependent, as fewer adipocytes were seen in cultures treated with adipogenic cocktail concurrent with 10 μM PD98059, and more reduction with 25 μM PD98059 as compared with adipogenic cocktail-treated control.
These results demonstrate that the adipogenic cocktail-induced expression of PPARγ, C/EBPα, and aP2, as well as the formation of Oil Red O-positive adipocytes, are associated with earlier ERK1/2 stimulation and, therefore, ERK1/2 might play an important role in adipogenic differentiation of BMSC cultures.
We did not observed any activation of p38MAPK, another MAPK pathway, during the mouse BMSC cultures with adipogenic cocktail, and SB203580, a selective inhibitor of p38MAPK, had no effects on the terminal adipogenenic differentiation, suggesting that p38MAPK pathway may not involve in the adipogenenic differentiation of mouse BMSC cultures (data not shown).
Genistein Dose-Dependently Inhibits Adipogenesis Through an Inhibition of ERK1/2 Activity in BMSC Cultures
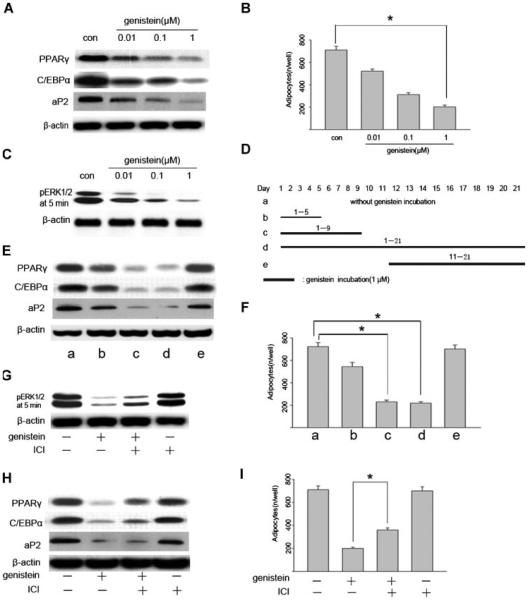
To evaluate the effects of genistein on adipogenesis, BMSC cultures were induced to differentiate into mature adipocytes in the presence of different concentrations of genistein. As shown in Figure 3A, the expression of PPARγ, C/EBPα, and aP2 were inhibited by genistein in a dose-dependent manner and 1 μM genistein was found to give the maximal inhibition, so genistein at a concentration of 1 μM was used for further investigation in this study. Consistent with adipogenic-specific proteins expression, the number of adipocytes was also decreased with genistein administration (Fig. 3B). Next, we tested if genistein had an effect on ERK1/2 signaling pathway. As shown in Figure 3C, genistein inhibited the ERK1/2 tyrosine phosphorylation in a dose-dependent manner (0.01–1 μM). Again, the inhibition of adipogenic differentiation was in accordance with the decrease in ERK1/2 phosphorylation, so we postulated that the inhibition of adipogenic differentiation by genistein was due to abrogation of ERK1/2 activity. Since ERK1/2 activity was promoted during the early stage (from days 3 to 9) of differentiation period and declined to basal level thereafter, genistein was expected to exert its inhibitory effect in this early stage. To test this possibility, we used 1 μM of genistein in different periods during adipogenic differentiation process as indicated in Figure 3D. The presence of genistein only in the first 9 days as well as in the entire experiment almost completely abolished the adipogenic differentiation (Fig. 3E), while genistein showed less or even no inhibition on adipogenic differentiation in the first 5 days or during the late stage of 11–21 days treatment (Fig. 3E). Similarly, the number of adipocytes was also significantly decreased during the earlier stage but not in the late stage treatment (Fig. 3F). These findings are consistent with the observations of ERK1/2 activation patterns using adipogenic cocktail in Figure 1.
Fig. 3.
Inhibitory effect of genistein on adipogenesis and ERK1/2 phosphorylation in BMSC cultures. A: Genistein dose-dependently inhibits adipogenic gene expression. Mouse BMSC cultures were exposed to adipogenic cocktail in the presence of increasing concentrations of genistein. Cells were harvested at day 21, and whole cell proteins were subjected to western blotting analysis. B: Genistein dose-dependently decreases the number of Oil Red O-positive adipocytes. The number of Oil Red O-positive adipocytes was calculated at day 21, the data are mean ± SD. *P < 0.05 versus control. C: Genistein dose-dependently inhibits the phosphorylation of ERK1/2. Cells were treated as above and harvested at day 5 after 5 min exposure to adipogenic cocktail, and whole cell protein were subjected to western blotting analysis. D: Schematic representation of the time points for the incubation of genistein during BMSC cultures. Genistein (1 μM) was added during the periods indicated as black lines. The preparation of whole cell proteins and Oil-red O staining were done at day 21. E: Western-blotting analysis of adipogenic gene expression at different conditions. F: The number of Oil Red O-positive adipocytes at different conditions. The data are mean ± SD, *P < 0.05 versus without genistein treatment control. G: ICI (10 μM) partially reversed the genistein (1 μM)-mediated inhibition of ERK1/2 activity. H,I: ICI (10 μM) partially reversed the inhibitory effect of genistein (1 μM) on adipogenic differentiation. *P < 0.05 versus genistein treated groups.
Since genistein was reported to bind to estrogen-receptor (ER), we then tested whether the effects of genistein on adipogenic differentiation were ER-dependent. In this regard, the cells were treated with genistein in the absence or presence of the ER antagonist, ICI182780. The inhibitory effects of genistein (1 μM) on ERK1/2 tyrosine phosphorylation and adipogenic differentiation of BMSC cultures were only partially reversed by co-incubation of ICI182780 (10 μM) (Fig. 3G–I), however, ICI182780 alone had no obvious effect. These data suggest that ER pathway contributes to the effects of genistein, and other mechanisms may also be involved.
FGF-2 Counteracts the Inhibitory Effects of Genistein on Adipogenic Differentiation
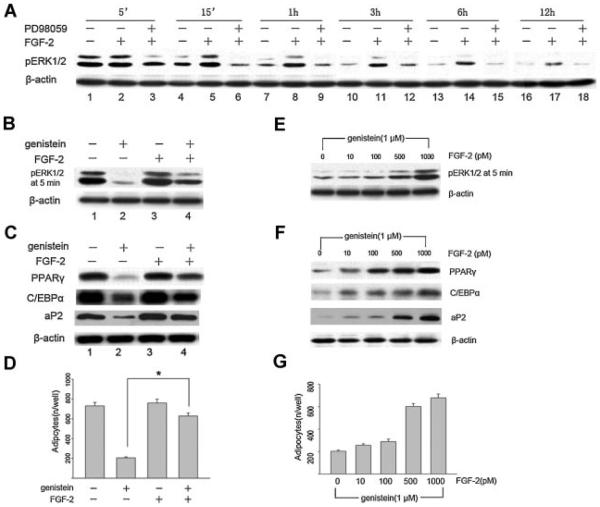
In order to further confirm that genistein inhibits adipogenic differentiation through downregulation of ERK1/2 activity, we explore whether FGF-2, a strong activator of the ERK1/2 signaling pathway, can counteracts the inhibitory effects of genistein by promoting ERK1/2 activity during adipogenesis. Figure 4A demonstrated that FGF-2 induced a sustained (>12 h) activation of ERK1/2 compared with the relative short (<3 h) activation in the control, and required more than 3 h of exposure to the MEK inhibitor PD98059 to completely block the FGF-2-induced phosphorylation of ERK1/2.
Fig. 4.
FGF-2 counteracts the inhibitory effect of genistein on adipogenic differentiation of BMSC cultures. A: Time-dependent effect of FGF-2 on ERK1/2 activity. Mouse BMSC cultures were grown to confluence, at day 5 the cells were pre-incubated with PD98059 (25 μM) or vehicle for 1.5 h prior to their induction to differentiate with adipogenic cocktail in the presence or absence of 1 nM FGF-2. Total cellular protein was harvested at the indicated times and subjected to western blotting analysis using the phosphospecific-ERK1/2 antibody. B–D: Effect of FGF-2 on the genistein-mediated inhibition of ERK1/2 activity and adipogenic differentiation in BMSC cultures. The Cells were treated with or without genistein (1 μM) and/or FGF-2 (1 nM), then harvested at day 5 after 5 min exposure (B, pERK1/2) or day 21 (C, adipogenic gene expression) and subjected to western blotting analysis. The number of Oil Red O-positive adipocytes were calculated at day 21, the data are mean ± SD, *P < 0.05 versus genistein treated groups (D). E–G: FGF-2 dose-dependently restores the genistein-mediated inhibition of ERK1/2 activity and adipogenic differentiation in BMSC cultures. The cells were treated under adipogenic cocktail with genistein (1 μM) plus different dose of FGF-2. Cells were harvested at day 5 after 5 min exposure (E, pERK1/2) or day 21 (F, adipogenic gene expression) and subjected to western blotting analysis. The number of Oil Red O-positive adipocytes was calculated at day 21 (G). Results are representative of three independent experiments.
Next, we also tested whether FGF-2 might be capable of attenuating the inhibitory effect of genistein on adipogenic differentiation by activating ERK1/2 signaling pathway. BMSC cultures were exposed to genistein in the presence or absence of FGF-2. Figure 4B showed that exposure of BMSC cultures to 1 μM of genistein resulted in a complete inhibition of phosphorylation of ERK1/2 as compared with the adipogenic cocktail control (Fig. 4B). In contrast, co-incubation 1 nM of FGF-2 with 1 μM of genistein restored adipogenic cocktail-induced ERK1/2 activation (Fig. 4B). Consistent with ERK1/2 activation, the adipocyte-specific protein and the number of Oil Red O-positive adipocytes were also up-regulated in BMSC cultures (Fig. 4C,D). To further confirm the result shown in Figure 4B–D, BMSC cultures were treated with increasing doses of FGF-2 in the presence of 1 μM of genistein, then the cells were harvested at day 5 after 5 min or at day 21 as described above. Again, the level of ERK1/2 activity at day 5 after 5 min post-induction in the presence of genistein (1 μM) is very low, exposure of BMSC cultures to genistein in the presence of FGF-2, however, restored the ERK1/2 activity to normal post-induction levels (Fig. 4E), as well as induced the expression of PPARγ, C/EBPα, and aP2 (Fig. 4F) and increased the number of Oil Red O-positive adipocytes in an FGF-2 dose-dependent manner (Fig. 4G).
Genistein Inhibit the PPARγ Binding Activity
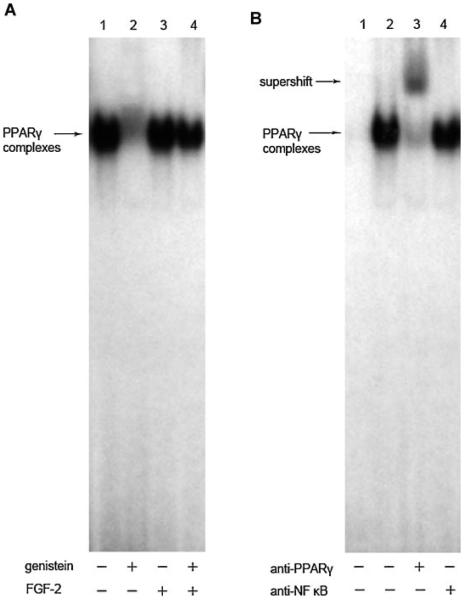
We also determined whether genistein affected the ability of PPARγ to bind to a PPARγ response element. Figure 5A showed PPARγ-consensus DNA binding activity was markedly decreased in the genistein-treated cells compared with the untreated control, and the decreased DNA binding activity can be restored by the addition of FGF-2 (1 nM) (Fig. 5A). The addition of the anti-PPARγ antibody resulted in an upper supershifted band and a decrease in the lower binding complex, indicating that the binding was apparently specific to PPARγ, since an irrelevant antibody (anti-NF-κB antibody) had no effect on the shift of the binding complex (Fig. 5B).
Fig. 5.
Effects of FGF-2 on the genistein-mediated inhibition of PPARγ DNA binding activity. A: Electrophoresis mobility shift assay (EMSA) were done using nuclear extracts from the BMSCs treated with or without genistein (1 μM) and/or FGF-2 (1 nM) at day 21 and incubated with the 32P-labeled oligonucleotide probe containing the PPARγ binding sequence as described under “Materials and Methods Section.” Lane 1, probe incubating with control extracts. Lane 2, probe incubating with genistein-treated extracts. Lane 3, probe with incubating with FGF-2-treated extracts. Lane 4, probe with incubating with genistein plus FGF-2-treated extracts. Three experiments were carried out with similar results. B: The super-shift of EMSA with or without anti-PPARγ antibody. Lane 1, probe alone without incubating with any extracts. Lane 2 without any antibody, lane 3 with 3 μl of the anti-PPARγ antibody, lane 4 with 3 μl of irrelevant anti-NF-κB antibody. The lower arrow indicates the specific band of total PPARγ DNA binding. The upper arrow indicates the super-shift complex of addition antibody against PPARγ. Three experiments were carried out with similar results.
DISCUSSION
In the present study, we have made the following three important findings: (1) Exposure of BMSC cultures to a mixture of adipogenic cocktail consisting of dexamethasone, insulin, and FBS activated ERK1/2 signaling pathway during the early phase (days 3–9) of culture period. Inhibition of this pathway by exposure of BMSC cultures to the MEK inhibitors PD98059 significantly attenuated the adipogenic differentiation as evidenced by downregulated expression of adipocyte-specific protein such as PPARγ, C/EBPα, and aP2 and decreased number of Oil Red O-positive adipocytes. (2) Genistein inhibited the ERK1/2 activation in a dose-dependent manner, and, exposure of BMSC cultures to a mixture of adipogenic cocktail in the presence of genistein significantly attenuated the adipogenic differentiation of BMSC cultures. (3) The attenuated adipogenic differentiation of BMSC cultures by genistein can be reversed by concurrent incubation of FGF-2, which is capable of inducing the activation of ERK1/2 signaling pathway. Based on these findings, we concluded that genistein inhibits the adipogenic differentiation of BMSC cultures through a potential downregulation of ERK1/2 activity.
The role of ERK1/2 signaling on adipogenesis is uncertain. Some studies have shown that drugs or effectors that block adipogenesis are also potent inducers of ERK1/2 activity [Shimba et al., 2001; Tanabe et al., 2004; Kim et al., 2007], and attenuation of ERK1/2 activity with the MEK inhibitors restores adipocyte differentiation [Hu et al., 1996; Font de Mora et al., 1997]. In contrast, others reported that the ERK1/2 signaling pathway enhancess adipogenesis [Park et al., 2004], and, in some cases, attenuation of ERK1/2 activity with MEK inhibitors blocks adipogenesis [Bost et al., 2002; Machinal-Quélin et al., 2002]. Those apparently contradictory findings can be explained by dual roles of the ERK1/2 signaling pathway in adipogenesis [Bost et al., 2005]. The differentiation of BMSC cultures into adipocytes proceeds through two steps, mitotic clonal expansion and terminal differentiation. If the ERK1/2 signaling pathway is essential for the mitotic clonal expansion stage, while prolonged and excessive activation may block the terminal differentiation. Our current data showed that ERK1/2 signaling pathway is activated during the early phase (days 3–9) following exposure of BMSC cultures to adipogenic cocktail. The peak of activity was found at day 5, and then ERK1/2 activity eventually subsided to basal level and kept in this level throughout the rest of the culture time. Clearly, our observations fit the above hypothesis; however, the phenomenon of ERK1/2 down regulation on a long-term exposure to the adipogenic cocktail in BMSC cultures remains unclear. It is known that the MAPK phosphatases (MKP)-1 that dephosphorylate and inactivate ERK1/2 is potential candidate for regulating ERK1/2 activity during adipogenesis. It was reported that expression of the MKP-1 gene was up-regulated during the late phase of adipogenesis [Sakaue et al., 2004], and therefore, it is conceivable, that adipogenic cocktail induces MKP-1 during the late phase of adipogenesis so that ERK activity is confined to the early phase of the differentiation process. In addition, inhibition of this activity in the early phase of the differentiation process by exposure of BMSC cultures to the MEK inhibitors PD98059 significantly attenuates expression of adipocyte-specific protein and decrease the number of Oil Red O-positive adipocytes, suggesting that stage-restricted burst of ERK1/2 activity promotes adipogenic differentiation of BMSC cultures (Fig. 2).
Genistein inhibits adipogenic differentiation via an inactivation of ERK1/2 activity in BMSC cultures. Genistein has also been demonstrated to inactivate ERK1/2 activity in immortalized human mammary epithelial cells [Frey and Singletary, 2003]. In our current study, genistein dose-dependently (0.01–1 μM) inhibited the phosphorylation of ERK1/2 (Fig. 3A), which accorded with the inhibition of the adipogenic differentiation in BMSC cultures (Fig. 3B,C). To test whether these inhibitory effects are corresponding to the stage of the cell cultures, we incubated genistein at different time of culture period and examine both the ERK1/2 activity and adipogenic differentiation. We found that incubation of genistein during the entire time significantly inhibit the adipogenic differentiation of mouse BMSC cultures. But if incubation of genistein was applied in the early stage of culture (from days 3 to 9), the complete inhibition of adipogenic differentiation can be achieved, consistent with a high ERK1/2 activity at this period of time. In contrast, the incubation of genistein was applied only during the late stage of culture, no significant inhibitory effect was observed on the terminal adipogenic differentiation in BMSC cultures because of a low basal ERK1/2 activity at that time (Fig. 3E,F). These observations indicate that the ERK1/2 activity of BMSC cultures is related to genistein inhibitory effect on adipogenic differentiation. To further confirm this hypothesis, we incubate genistein in the absence or presence of FGF-2, a strong activator of the ERK1/2 signaling pathway [Milasincic et al., 1996; Weyman and Wolfman, 1998], and then test the terminal adipogenic differentiation in BMSC cultures. As we expected, FGF-2 restored the level of phosphorylated-ERK1/2 that was inhibited by genistein, and this increased level of phosphorylated-ERK1/2 was enough to facilitate the terminal adipogenic differentiation of BMSC cultures (Fig. 4). These data suggest that genistein did inhibit adipogenic differentiation of BMSC cultures via downregulation of ERK1/2 activity. Genistein was reported to stimulate osteoblastic differentiation in BMSC cultures through ER-dependent mechanism [Pan et al., 2005], here we tested if the effects of genistein on adipogenic differentiation were also ER-dependent. In this regard, we found that the inhibitory effects of genistein (1 μM) on ERK1/2 tyrosine phosphorylation and adipogenic differentiation of BMSC cultures were only partially reversed by administration of ICI182780 (10 μM) (Fig. 3G–I), suggesting that ER pathway may play a role on genistein-suppressed adipogenic differentiation, and genistein anti-adipogenesis could be the combined effect of its ER activation and direct kinase inhibition.
Genistein promotes osteogenesis but inhibits adipogenesis in BMSC cultures via different regulation of MAPK pathways. As we know, accelerated adipogenesis in the bone marrow cavity appears to be associated with osteoporosis or aging [Ahdjoudj et al., 2004; Nuttall and Gimble, 2004], and this is also the case of high bone turnover in postmenopausal women. So, control the balance between osteogenesis and adipogenesis could be a direction for treatment of such osteoporosis. In this regard, the principal soy phytoestrogen, genistein has been shown to act as a regulator between osteogenesis and adipogenesis [Dang et al., 2003]. Our recent publication suggested that genistein stimulated osteoblastogenesis of mouse BMSC cultures via p38MAPK-Runx2 (Cbfa1) pathway [Liao et al., 2007]. Since it has been shown that the expression of PPARγ and C/EBPα plays an important role in the terminal differentiation of adipocytes [Loftus and Lane, 1997; Rosen et al., 2000], in this article we found that incubation of genistein during the entire culture significantly inhibits adipogenesis of BMSC cultures via an inhibition of ERK1/2 activity, which resulted in a markedly reduction of PPARγ and C/EBPα expression as well as a reduced level of aP2, an adipocyte-specific protein and the decreased number of Oil Red O-positive adipocytes (Fig. 3E,F), consistent with previous reports [Szkudelska et al., 2000; Dang et al., 2003]. In addition, we also observed that genistein significantly decrease the PPARγ DNA binding activity, which can also be reversed by FGF-2 administration (Fig. 5). So the molecular mechanism under-lying genistein-induced osteogenesis and anti-adipogenesis may be its ability to stimulate p38MAPK-Runx2 (Cbfa1) pathway but concurrently inhibit ERK1/2-PPARγ pathway. Since genistein was also reported to directly bind to PPARγ, we cannot exclude the direct role of genistein on PPARγ activity [Dang et al., 2003].
Taken together, our results suggest that the activation of ERK1/2 signaling pathway during the early stage of differentiation period is essential for adipogenesis and that genistein inhibits the adipogenic differentiation of BMSC cultures through a potential downregulation of ERK1/2 activity.
Acknowledgments
Grant sponsor: National Natural Science Foundation of China; Grant number: 30171085; Grant sponsor: Teaching and Research Award Program for Outstanding Young Teachers (TRAPOYT) in Higher Education Institutions of MOE, PRC; Grant number: 30040002; Grant sponsor: National Institute of Health, USA; Grant number: RO1-AR049712.
REFERENCES
- Ahdjoudj S, Fromigué O, Marie PJ. Plasticity and regulation of human bone marrow stromal osteoprogenitor cells: Potential implication in the treatment of age-related bone loss. Histol Histopathol. 2004;19:151–157. doi: 10.14670/HH-19.151. [DOI] [PubMed] [Google Scholar]
- Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Binétury B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J. 2002;361:621–627. doi: 10.1042/0264-6021:3610621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Löwik CW. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res. 2002;17:394–405. doi: 10.1359/jbmr.2002.17.3.394. [DOI] [PubMed] [Google Scholar]
- Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Löwik CW. Peroxisome proliferator-activated receptor γ(PPARγ) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 2003;278:962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- Font de Mora J, Porras A, Ahn N, Santos E. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3-L1 adipocytic differentiation. Mol Cell Biol. 1997;17:6068–6075. doi: 10.1128/mcb.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey RS, Singletary KW. Genistein activates p38 mitogen-activated protein kinase, inactivates ERK1/ERK2 and decreases Cdc25C expression in immortalized human mammary epithelial cells. J Nutr. 2003;133:226–231. doi: 10.1093/jn/133.1.226. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, Hunziker W, Weber P, Martin I, Bendik I. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology. 2004;145:848–859. doi: 10.1210/en.2003-1014. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Miyaura C, Ohmura M, Onoe Y, Sato T, Uchiyama Y, Ito M, Wang X, Suda T, Ikegami S. Selective effects of genistein, a soybean isoflavone, on B-lymphopoiesis and bone loss caused by estrogen deficiency. Endocrinology. 1999;140:1893–1900. doi: 10.1210/endo.140.4.6663. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Arai N, Wang X, Wu J, Umegaki K, Miyaura C, Takeda A, Ikegami S. Difference in effective dosage of genistein on bone and uterus in ovariectomized mice. Biochem Biophys Res Commun. 2000;274:697–701. doi: 10.1006/bbrc.2000.3175. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kim JH, Wang Y, Sul HS. Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao QC, Xiao ZS, Qin YF, Zhou HH. Genistein stimulates osteoblastic differentiation via p38 MAPK-Cbfa1 pathway in bone marrow culture. Acta Pharmacol Sin. 2007;28:1597–1602. doi: 10.1111/j.1745-7254.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Yang Y, Cook DG, Dorsa DM. Neuronal apoptosis resulting from high dose of isoflavone genistein: Role for calcium and p42/44 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2001;299:67–75. [PubMed] [Google Scholar]
- Loftus TM, Lane MD. Modulating the transcriptional control of adipogenesis. Curr Opin Genet Dev. 1997;7:603–608. doi: 10.1016/s0959-437x(97)80006-8. [DOI] [PubMed] [Google Scholar]
- Machinal-Quélin F, Dieudonné MN, Leneveu MC, Pecquery R, Giudicelli Y. Proadipogenic effect of leptin on rat preadipocytes in vitro: Activation of MAPK and STAT3 signaling pathways. Am J Physiol Cell Physiol 282:C853–C863. 2002 doi: 10.1152/ajpcell.00331.2001. [DOI] [PubMed] [Google Scholar]
- Milasincic DJ, Calera MR, Farmer SR, Pilch PF. Stimulation of C2C12 myoblast growth by basic fibroblast growth factor and insulin-like growth factor 1 can occur via mitogen-activated protein kinase-dependent and -independent pathways. Mol Cell Biol. 1996;16:5964–5973. doi: 10.1128/mcb.16.11.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone. 2000;27:177–184. doi: 10.1016/s8756-3282(00)00317-3. [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) a or b. Endocrinology. 2002;143:2349–2356. doi: 10.1210/endo.143.6.8854. [DOI] [PubMed] [Google Scholar]
- Pan W, Quarles LD, Song LH, Yu YH, Jiao C, Tang HB, Jiang CH, Deng HW, Li YJ, Zhou HH, Xiao ZS. Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J Cell Biochem. 2005;94:307–316. doi: 10.1002/jcb.20308. [DOI] [PubMed] [Google Scholar]
- Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPβ at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard DJ, Monroe DG, Ruesink TJ, Khosla S, Riggs BL, Spelsberg TC. Phytoestrogen genistein acts as an estrogen agonist on human osteoblastic cells through estrogen receptors a and b. J Cell Biochem. 2003;89:633–646. doi: 10.1002/jcb.10539. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Sakaue H, Ogawa W, Nakamura T, Mori T, Nakamura K, Kasuga M. Role of MAPK Phosphatase-1 (MKP-1) in adipocyte differentiation. J Biol Chem. 2004;279:39951–39957. doi: 10.1074/jbc.M407353200. [DOI] [PubMed] [Google Scholar]
- Shimba S, Wada T, Tezuka M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentiation in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin. J Cell Sci. 2001;114:2809–2817. doi: 10.1242/jcs.114.15.2809. [DOI] [PubMed] [Google Scholar]
- Szkudelska K, Nogowski L, Szkudelski T. Genistein affects lipogenesis and lipolysis in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2000;75:265–271. doi: 10.1016/s0960-0760(00)00172-2. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K. Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARγ2. J Cell Sci. 2004;117:3605–3614. doi: 10.1242/jcs.01207. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein β by MAPK and glycogen synthase kinase 3β is required for adipogenesis. Proc Natl Acda Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Clubbs EA, Bomser JA. Genistein modulates prostate epithelial cell proliferation via estrogen-and extracellular signal-regulated kinase-dependent pathways. J Nutr Biochem. 2006;17:204–210. doi: 10.1016/j.jnutbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Weyman CM, Wolfman A. Mitogen-activated protein kinase kinase (MEK) activity is required for inhibition of skeletal muscle differentiation by insulin-like growth factor 1 or fibroblast growth factor 2. Endocrinology. 1998;139:1794–1800. doi: 10.1210/endo.139.4.5950. [DOI] [PubMed] [Google Scholar]